Abstract
Purpose
We have reported previously nonsense inactivating mutations of the phosphodiesterase 11A (PDE11A) gene in patients with micronodular adrenocortical hyperplasia and Cushing syndrome. The aim of this study is to investigate the presence of somatic or germ-line PDE11A mutations in various types of adrenocortical tumors: ACTH-independent macronodular adrenocortical hyperplasia (AIMAH), adrenocortical adenoma (ACA), and adrenocortical cancer (ACC).
Experimental Design
PDE11A was sequenced in 117 adrenocortical tumors and 192 controls subjects; immunohistochemistry for PDE11A and tumor cyclic AMP levels were studied in a subgroup of adrenocortical tumors.
Results
One PDE11A inactivating mutation (R307X) was found in one ACA, 22 germ-line missense variants (18.8%) were found in adrenocortical tumors, and only 11 missense variants (5.7%) were found in controls. By comparing the common mutations, a higher frequency of mutations in adrenocortical tumors than in age/sex-matched controls were observed [16% versus 10% in ACC, 19% versus 10% in ACA, and 24% versus 9% in AIMAH; odds ratio (OR), 3.53; P = 0.05]. Somatic DNA from adrenocortical tumors with missense variants showed a wild-type allelic loss. A significant difference between ACC and controls was observed for a polymorphism in exon 6 (E421E; OR, 2.1; P = 0.03) and three associated polymorphisms located in intron 10-exon 11-intron 11 (OR, 0.5; P = 0.01). In AIMAH/ACA, cyclic AMP levels were higher than in normal adrenals and decreased PDE11A immunostaining was present in adrenocortical tumors with PDE11A variants.
Conclusions
The present investigation of a large cohort of adrenocortical tumors suggests that PDE11A sequence defects predispose to a variety of lesions (beyond micronodular adrenocortical hyperplasia) and may contribute to the development of these tumors in the general population.
In the last decade, the study of the genetics of adrenocortical tumors has led to major advances in the understanding of the pathogenesis of adrenocortical tumors. The identification of germ-line molecular defects in hereditary syndromes responsible for adrenocortical tumors has played a major role toward this goal 1–4. Several types of adrenocortical tumors that lead to corticotrophin (ACTH)–independent Cushing syndrome may be caused by genetic alterations of key components of the cyclic AMP (cAMP) pathway 2, 5, 6. An activating germ-line mutation of the ACTH receptor (the MC2R gene) that displays high levels of basal activity has been reported in a case of bilateral adrenocortical hyperplasia associated with Cushing syndrome 7. Somatic GNAS mutations are associated with ACTH-independent macronodular adrenocortical hyperplasia (AIMAH) in McCune-Albright syndrome 8. Primary pigmented nodular adrenocortical disease (PPNAD) as part of the Carney complex or isolated may be caused by germ-line PRKAR1A-inactivating mutations 9, 10, and secreting adrenocortical adenoma (ACA) can be due to PRKAR1A somatic mutations 11. In the last years, it has become apparent that several forms of adrenocortical hyperplasia are not caused by mutations in PRKAR1A or GNAS 5.
Recently, we reported a whole-genome association study that led to the discovery of a new factor of the cAMP pathway that is apparently involved in adrenocortical tumorigenesis: inactivating mutations of the phosphodiesterase 11A (PDE11A) gene were found in a subgroup of patients with Cushing syndrome due to micronodular adrenocortical hyperplasia 12. The PDE11A gene is mapped to the 2q31-35 chromosomal region and loss of heterozygosity in 2q was observed in adrenal tumors from micronodular adrenocortical hyperplasia patients with PDE11A-inactivating mutations; three PDE11A nonsense mutations and two functional missense substitutions, R804H and R867G, were identified. In vitro studies of the R804H and R867G substitutions showed altered enzymatic activity and higher levels of cAMP and/or cyclic GMP in HeLa and HEK293 cells. It was suggested that these variants, in addition to their association with adrenocortical hyperplasia, could contribute to a genetic predisposition to other adrenocortical tumors, which were, however, also present in the general population 13. Interestingly, in the same study, normal subjects that were prospectively followed for the development of cancer was identified to carry (at a very low frequency) some of the previously identified PDE11A inactivating mutations.
The aim of the present study was to investigate the role of genetic alterations of PDE11A in various types of adrenocortical tumors that can be associated with Cushing syndrome: AIMAH (a bilateral benign disease), unilateral ACA, and unilateral adrenocortical cancer (ACC) at both somatic and germ-line levels. This cohort was also compared with a large cohort of consecutively recruited controls who were carefully screened to rule out Cushing syndrome and associated clinical abnormalities. Our data were very interesting: missense PDE11A variants were frequently found in patients with all types of adrenocortical tumors, whereas only few were found and with a low frequency in controls. There were no significant differences in the number of variants in each diagnostic group, indicating that PDE11A sequence defects may confer susceptibility to the types of development of all types of adrenocortical lesions associated with ACTH-independent Cushing syndrome.
Materials and Methods
Patients
Leukocyte samples from 117 patients with adrenocortical tumors were collected: 45 ACC, 43 ACA consisting of 18 cortisol-secreting adenomas and 25 nonsecreting or responsible for mild form of Cushing syndrome (preclinical), and 29 AIMAH. Diagnostic confirmation of benign versus malignant unilateral tumors was made by the application of the Weiss score (with a cutoff score of 3) and testing for 17p13 allelic losses as reported previously 14. All patients signed an informed consent for the analysis of leukocyte and tumor DNA and for access to the collected data. The study was approved by an institutional review board (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale, Hôpital Cochin, Paris).
Tumor samples
Corresponding tumor samples were collected for 108 of the patients analyzed at the germ-line DNA level (20 AIMAH, 45 ACC, and 43 ACA). Frozen adrenocortical tumors tissues were collected at the Hôpital Cochin as described previously 15, 16: fragments obtained during surgery were immediately frozen and stored in liquid nitrogen until DNA extraction. For diagnosis and scoring, tumors were fixed in formalin and embedded in paraffin and 4 μm sections were cut and stained with H&E. The adrenal tumors in this study were negative for PRKAR1A mutations, screened as described previously 11.
Controls
Controls were collected in the same center as part of a program dedicated to the genetic predisposition to endocrine tumors. All volunteers were examined by a senior endocrinologist to exclude personal or family history or clinical signs suggestive of a genetic endocrine neoplasia syndrome and clinical signs of pituitary, thyroid, or adrenal tumors or Cushing syndrome 17. All controls signed an informed consent for the analysis of leukocyte DNA and access to their clinical data. The study was approved by an institutional review Board (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale, Hôpital Cochin, Paris). Leukocytes from 192 consecutive control subjects were collected. The control group was divided into three groups sex-age (±5 years) matched with each group of adrenocortical tumors (ACA, ACC, and AIMAH): 45 ACC cases and 157 matched controls, 43 ACA cases and 107 matched controls, and 29 AIMAH cases and 96 matched controls, respectively.
DNA preparation and sequencing studies
DNA was extracted from surgically removed adrenocortical tumors and from blood samples as described previously 15. The 20 coding exons (exons 3–23) and the flanking intronic sequences of the PDE11A gene (Ensembl protein coding gene: ENSG00000128655) were amplified in the cohort of adrenocortical tumors and in the control group leukocyte by PCR using specific primers as described previously 12. All amplified samples were examined by agarose gel electrophoresis to confirm successful amplification of each exon. Direct sequencing of the purified fragments was then done using the Genetic Sequencer ABI3100 Applied Biosystems apparatus.
cAMP assay of adrenocortical tumors samples
Quantitative determination of cAMP levels in cell lysates from tissue samples (AIMAH and normal adrenal tissues) was done using commercially available assays (Direct cAMP kit; Assay Design). The assay is based on competitive binding, in which endogenous cAMP levels compete with a fixed amount of alkaline phosphate–labeled cyclic nucleotides. The assays are colorimetric and absorbance is read at 405 nm.
Immunohistochemistry
Immunohistochemistry for PDE11A was done in a subset of available tissue sections: 16 wild-type adrenocortical tumors (6ACC, 6 ACA, and 4 AIMAH) and 12 adrenocortical tumors with PDE11A missense mutations (5 ACC, 6 ACA, and 1 AIMAH). Tissue from normal testis was used as positive control. Sections of 4 μm from formalin-fixed tissue embedded in paraffin were mounted on Superfrost/Plus glass slides. The paraffin was eliminated by incubating the sections in xylene and then rehydrating them. For antigen retrieval, sections were heated in a microwave for a total of 40 min in Target Retrieval Solution at pH 9.0 (Dako), 10 nmol sodium citrate (pH 6.0) as described previously 18. The slides were incubated with a polyclonal anti-antibody specific for PDE11A4 as directed by the manufacturer (ab14624; Abcam) at a dilution of 1:100 for 60 min at room temperature. Sections were then incubated with the streptavidin-biotin-peroxidase complex, and the marker labeling was detected by the enzymatic precipitation of the 3,3-diaminobenzidine tetrahydrochloride in 0.5 mmol Tris Dako Real Detection System Peroxidase/DAB+ (Dako). The slides were counterstained with Mayer hematoxylin. To reduce bias, immunostaining was assessed by two independent investigators blinded to the PDE11A gene status. PDE11A expression was considered as being decreased when at least 95% of the cells presented an absence of expression or a weak expression compared with normal adrenocortical gland.
Statistical analysis
Conditional logistic regression was used to estimate the odds ratios (OR) and 95% confidence intervals (95% CI) for the association between each polymorphism and each type of adrenocortical tumors (ACC, ACA, and AIMAH). The analyses were done using three logistic models based on dominant, recessive, and codominant effects (inheritance model). In the dominant model, the heterozygous variant and rare homozygous variant were combined in a dummy variable. In the recessive model, the variant was defined in a dummy variable as only the rare homozygous genotype. In the multiplicative codominant model, a dose-response effect was tested on the variable counting the number of copies of the variant. Multivariate models were constructed to test the polymorphisms simultaneously and to examine the interactions between the polymorphisms. All tests were two sided, with an α level of 0.05 considered to indicate statistical significance.
Results
PDE11A nonsense mutation/missense variants in adrenocortical tumors and controls
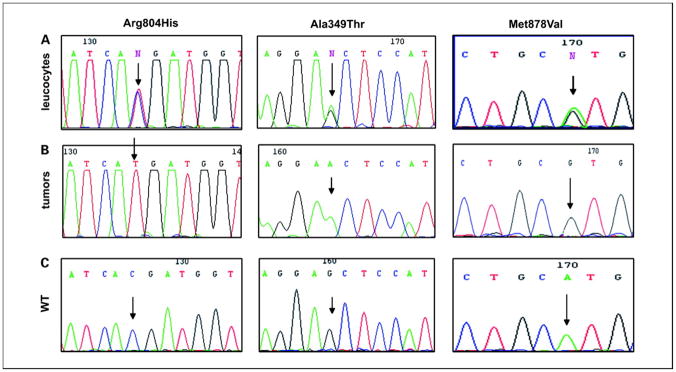
By analyzing the entire PDE11A coding sequence in the germ-line DNA of 117 patients with adrenocortical tumors, one heterozygous inactivating mutation was found in one nonsecreting ACA (R307X). A total of 22 heterozygous missense mutations were also found: in 7 of the 45 ACC patients (16%; 1 patient having two different missense mutations), in 7 of the 29 AIMAH patients (24%), and in 8 of the 43 ACA patients (19%; Table 1). Figure 1 shows the position of these point mutations on the PDE11A gene. All point mutations were found in tumor DNA and allelic loss of the wild-type allele was observed in three adrenocortical tumors: two ACC and one ACA that harbored the following point mutations (present as hemizygous in the tumor and heterozygous in the germ-line DNA; Figure 2): R804H, A349T, and M878V.
Table 1.
Results of PDE11A mutations found in the 117 patients with adrenocortical tumors.
| Tumor no. | Tumoral type | Exon no. | Base | Amino acid | |
|---|---|---|---|---|---|
| 1 | ACC | 3 | c.613 T>C | Phe205Leu | Arg804His |
| 19 | c.2411 G>A | ||||
| 2 | ACC | 4 | c.1045 G>A | Ala349Thr | |
| 3 | ACC | 17 | c.2190A>G | Tyr727Cys | |
| 4 | ACC | 17 | c.2190A>G | Tyr727Cys | |
| 5 | ACC | 29 | c.2411 G>A | Arg804His | |
| 6 | ACC | 19 | c.2411 G>A | Arg804His | |
| 7 | ACC | 22 | c.2599 C>G | Arg867Gly | |
| 8 | ACA secreting | 17 | c.2190A>G | Tyr727Cys | |
| 9 | ACA secreting | 19 | c.2411 G>A | Arg804His | |
| 10 | ACA secreting | 22 | c.2599 C>G | Arg867Gly | |
| 11 | ACA nonsecreting | 4 | c.919 C>T | Arg307stop | |
| 12 | ACA nonsecreting | 19 | c.2411 G>A | Arg804His | |
| 13 | ACA nonsecreting | 19 | c.2411 G>A | Arg804His | |
| 14 | ACA nonsecreting | 22 | c.2599 C>G | Arg867Gly | |
| 15 | ACA nonsecreting | 22 | c.2950 A>G | Met878Val | |
| 16 | AIMAH | 4 | c.1045 G>A | Ala349Thr | |
| 17 | AIMAH | 14 | c.1825 A>G | Asp609Asn | |
| 18 | AIMAH | 15 | c.1992 C>G | His664Gln | |
| 19 | AIMAH | 17 | c.2190A>G | Tyr727Cys | |
| 20 | AIMAH | 19 | c.2411 G>A | Arg804His | |
| 21 | AIMAH | 19 | c.2411 G>A | Arg804His | |
| 22 | AIMAH | 22 | c.2599 C>G | Arg867Gly |
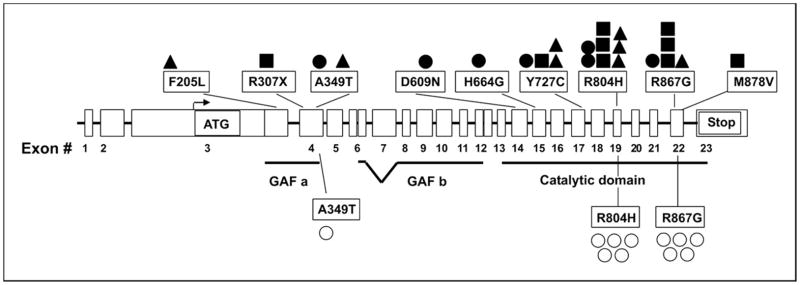
Figure 1. Localization of mutations and missense variants on the PDE11A gene.
The PDE11A gene contains 23 exons; coding starts from exon 3 and ends with exon 23; exons 3 to 12 sequences code for the GAF a and GAF b domain (cGMP binding PDE) and exons 14 to 22 code for the catalytic domain. ▲, ACC; ■ ACA; ●, AIMAH; ○, controls.
Figure 2. Example of mutations in 2 ACC and 1 ACA.
A and B, missense mutations in 2 ACC (R804H and A349) and 1 ACA (M878V) in leukocyte DNA (heterozygous state) and in the corresponding tumor (hemizygous state). In the tumor sample, the wild-type allele is lost and only the mutated allele is present. C, wild-type sequence.
We found the previously reported missense variants (R804H in exon 19 and R867G in exon 22) in 8 and 4 patients, respectively: 4 ACC, 5 ACA (2 secreting and 3 nonsecreting), and 3 AIMAH patients. Furthermore, we found several new missense mutations: T727C in ACA (n = 1) and AIMAH (n = 1), F205L in ACC (n = 1), A349T in AIMAH (n = 1) and ACC (n = 1), D609N in AIMAH (n = 1), H664G in AIMAH (n = 1), and M878V in ACA (n = 1).
To investigate the frequency of these point mutations, we sequenced the entire PDE11A coding region in 192 controls that had been screened previously for endocrine disease. No inactivating mutations were found in the control group. The two previously reported missense variants (R804H and R867) were found each in 5 of 192 subjects; one missense variant of unknown significance (A349T) was observed in one control subject.
If we consider the frequency of all PDE11A mutations in all adrenocortical tumors, we found a significantly higher number of changes in patients with adrenocortical tumors compared with those in the 192 control subjects (18.8% versus 5.7%). To improve the stringency of the statistical analysis, we compared each adrenocortical tumors group with sex- and age-matched control subjects. Again, a higher frequency of missense and nonsense mutations in all three adrenocortical tumors group than in controls was observed (16% versus 10% in ACC, 19% versus 10% in ACA, and 24% versus 9% in AIMAH). The difference was statistically significant in the AIMAH group with an OR of 3.53 (P = 0.05; Tables 2–4). The lack of statistical difference in the ACC and ACA group is probably due to the small size of these series.
Table 2.
Main characteristics and PDE11A sequence changes in the ACA (n = 43) and controls (n = 107).
| ACA (n = 43) | Control group (n = 107) | OR* (95% CI) | P | |
|---|---|---|---|---|
| Sex (F/M) | 34/9 | 85/22 | NS | |
| Age, y(mean ± SD) | 49.7 ± 12.9 | 46.5 ± 12.3 | NS | |
| c.147A>C/p.L49L (exon 3) | ||||
| A/A | 43 (100) | 107 (100) | — | — |
| A/C | 0 (0) | 0 (0) | ||
| C/C | 0 (0) | 0 (0) | ||
| c.354G>A/p.Q118Q(exon 3) | ||||
| G/G | 43 (100) | 107 (100) | — | — |
| G/A | 0 (0) | 0 (0) | ||
| A/A | 0 (0) | 0 (0) | ||
| c.480G>A/p.L160L (exon3) | ||||
| G/G | 42 (98) | 107 (100) | — | — |
| G/A | 1 (2) | 0 (0) | ||
| A/A | 0 (0) | 0 (0) | ||
| c.690C>T/p.C230C (exon 3) | ||||
| C/C | 43 (100) | 106 (99) | — | — |
| C/T | 0 (0) | 1 (1) | ||
| T/T | 0 (0) | 0 (0) | ||
| c.1072-3C>T (intron 4) | ||||
| C/C | 32 (74) | 90 (84) | (d) (c) 1.58 (0.61–4.02) | 0.39 |
| C/T | 11 (26) | 17 (16) | ||
| T/T | 0 (0) | 0 (0) | ||
| c.1263A>G/p.E421E (exon 6) | ||||
| A/A | 30 (70) | 87 (81) | (c) 1.69 (0.78–3.79) | 0.21 |
| A/G | 11 (25) | 19 (18) | (d) 1.70 (0.69–4.15) | 0.28 |
| G/G | 2 (5) | 1 (1) | — | |
| c.1577-3C/T (intron10) | ||||
| c.1626A>G/p.A542A(exon 11) | ||||
| c.1644+26insGTTTATA(intron 11) | ||||
| C/C | 22 (51) | 46 (43) | (c) 0.75 (0.43–4.29) | 0.34 |
| C/T | 14 (33) | 43 (40) | (d) 0.62 (0.26–1.40) | 0.29 |
| T/T | 7 (16) | 18 (17) | (r) 0.79 (0.24–2.24) | 0.83 |
| c.2758_2760insTCC/p.S920ins (exon 23) | ||||
| A/A | 8 (19) | 15 (14) | (c) 1.02 (0.58–1.85) | 1 |
| A/B | 13 (30) | 47 (44) | (d) 0.73 (0.23–2.3) | 0.7 |
| B/B | 22 (51) | 45 (42) | (r) 1.28 (0.54–3.10) | 0.66 |
| Presence of missense/nonsense mutations | Total n of mutations (%) | |||
| Yes | 8 (19) | 11 (10) | 2.68 | 0.16 |
| No | 35 (81) | 96 (90) | ||
Frequency of different synonymous variations (polymorphisms) and missense/nonsense mutations in 43 ACA and sex- and age- matched controls. Values are numbers (percentages) unless otherwise stated.
OR (95% CI) are given for recessive (r), codominant (c), and dominant (d) models, wherever possible.
Table 4.
Main characteristics and PDE11A sequence changes in the ACC (n = 45) and controls (n = 157).
| ACC (n = 45) | Control group (n = 157) | OR* (95% CI) | P | |
|---|---|---|---|---|
| Sex (F/M) | 36/9 | 113/44 | — | — |
| Age, y (mean ± SD) | 44.4 ±16.8 | 39.4 ± 14.9 | — | — |
| c.147A>C/p.L49L (exon 3) | ||||
| A/A | 45 (100) | 156 (99) | — | — |
| A/C | 0 (0) | 1 (1) | ||
| C/C | 0 (0) | 0 (0) | ||
| c.354G>A/p.Q118Q(exon 3) | ||||
| G/G | 42 (93) | 157 (100) | — | — |
| G/A | 3 (7) | 0 (0) | ||
| A/A | 0 (0) | 0 (0) | ||
| c.480G>A/p.L160L (exon3) | ||||
| G/G | 45 (100) | 157 (100) | — | — |
| G/A | 0 (0) | 0 (0) | ||
| A/A | 0 (0) | 0 (0) | ||
| c.690C>T/p.C230C (exon 3) | ||||
| C/C | 43 (95) | 153 (97) | — | — |
| C/T | 2 (5) | 4 (3) | ||
| T/T | 0 (0) | 0 (0) | ||
| c.1072-3C>T (intron 4) | ||||
| C/C | 35 (78) | 127 (81) | (c) (d) 1.23 (0.47–3.1) | 0.76 |
| C/T | 10 (22) | 30 (19) | ||
| T/T | 0 (0) | 0 (0) | ||
| c.1263A>G/p.E421E (exon 6) | ||||
| A/A | 30 (67) | 127 (81) | (c) 2.1 (1.1–3.95) | 0.03 |
| A/G | 10 (22) | 28 (18) | (d) 2.06 (0.89–4.78) | 0.09 |
| G/G | 5 (11) | 2 (1) | ||
| c.1577-3C/T (intron 10) | ||||
| c.1626A>G/p.A542A(exon 11) | ||||
| c.1644+26insGTTTATA(intron 11) | ||||
| C/C | 28 (62) | 67 (43) | (c) 0.5 (0.25–0.89) | 0.01 |
| C/T | 14 (31) | 64 (41) | (d) 0.4 (0.20–0.93) | 0.03 |
| T/T | 3 (7) | 26 (17) | (r) 0.3 (0.03–1.36) | 0.16 |
| c.2758_2760insTCC/p.S920ins (exon 23) | ||||
| A/A | 10 (22) | 18 (12) | (c) 0.84 (0.47–1.47) | 0.6 |
| A/B | 15 (33) | 76 (48) | (d) 0.38 (0.12–1.12) | 0.08 |
| B/B | 20 (45) | 63 (40) | (r) 1.23 (0.54–2.77) | 0.7 |
| Presence of missense/nonsense mutations | Total n of mutations (%) | |||
| Yes | 7 (16) | 15 (10) | 2.24 | 0.22 |
| No | 38 (84) | 142 (90) | ||
Frequency of different synonymous variations (polymorphisms) and missense/nonsense mutations in 45 ACC and sex- and age- matched controls. Values are numbers (percentages) unless otherwise stated.
OR (95% CI) are given for recessive (r), codominant (c), and dominant (d) models, wherever possible.
PDE11A intronic and synonymous polymorphisms in adrenocortical tumors and controls
We found several synonymous and intronic PDE11A polymorphisms that have not been reported. Their frequencies varied between patients and controls: some polymorphisms were quite rare (L49L, Q118Q, L160L, and C230C in exon 3) and others were more frequent (c.1072-3C>T in intron 4, E421E in exon 6, c.1577-3C/T in intron 10, A542A in exon 11, c.1644+26insGTTTATA in intron 11, and S920ins in exon 23). To look for an association of these polymorphisms with a given adrenocortical tumors, a statistical analysis was done taking into account different possible models of inheritance (recessive, codominant, and dominant; Tables 2–4). When we compared AIMAH and ACA, the observed differences did not reach statistical significance; a significant difference was found between ACC and controls for a synonymous polymorphism in exon 6 (E421E, OR, 2.1 and 2.06 for the codominant and dominant models, respectively). Similarly, a significant difference was found for an intronic and a synonymous polymorphism in intron 10-exon 11-intron 11, with OR of 0.5 and 0.4 for the codominant and dominant models, respectively. By a binary regression study, we then studied the possible association due to linkage disequilibrium between different polymorphisms in each adrenocortical tumors and control groups: a perfect association between intronic and synonymous polymorphisms in intron 10, exon 11, and intron 11 was observed (Tables 2–4). To test the possible interactions between synonymous and intronic polymorphisms, a multivariate conditional logistic regression was used: a significant association in ACC group between the polymorphism E421E (exon 6) in a codominant model and the three polymorphisms at intron 10, exon 11, and intron 11 in a dominant model was found (OR, 1.88; P = 0.05).
cAMP levels and PDE11A immunohistochemistry
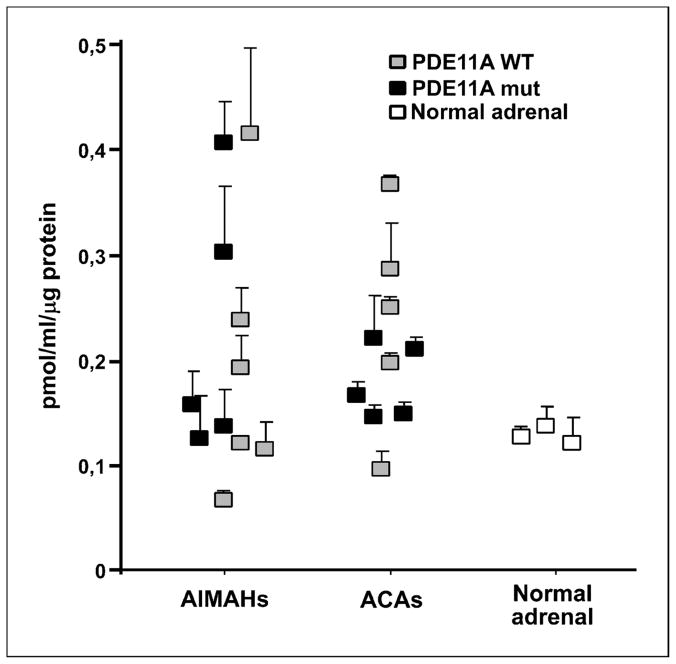
cAMP levels in tissue homogenates from 11 AIMAH, 10 ACA, and 3 normal adrenals were measured to test the effect of PDE11A variants on PDE activity. Variable cAMP levels were observed in both AIMAH and ACA, often much higher than in the normal adrenal. Indeed, in some PDE11A variants, cAMP levels were much higher than in the normal adrenal as shown in Figure 3.
Figure 3. Effect of PDE11A variants in cAMP levels.
cAMP activity in adrenal tissue lysates of 6 AIMAH and 5 ACA wild-type for PDE11A (). cAMP activity in adrenal tissue lysates of 5 AIMAH and 5 ACA with PDE11A variants (R804H, H664G, A349T, D609N, and R867G and R307X, M878V, R804H, R867G, and T727C, respectively; ■). Tissue lysates from three normal adrenal glands were assayed separately for cAMP content (□). All experiments were repeated at least twice and each sample was run in duplicate.
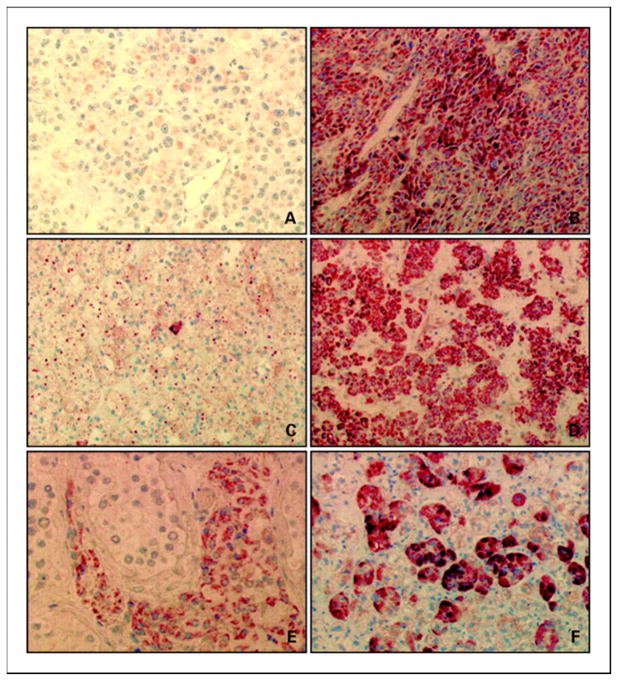
Immunohistochemistry was used to examine PDE11A expression in 16 wild-type adrenocortical tumors for PDE11A (6 ACC, 6 ACA, and 4 AIMAH) and 12 adrenocortical tumors with PDE11A missense variants (5 ACC, 6 ACA, and 1 AIMAH). Tissue from normal testis was used as positive control, as PDE11A is highly expressed in Leydig cells (Figure 4). Interestingly, decreased PDE11A immunostaining was present in adrenocortical tumors with missense variants, in both ACC and ACA, compared with the adrenocortical tumors having a wild-type PDE11A sequence, suggesting decreased PDE11A protein levels in adrenocortical tumors harboring PDE11A missense variants. In these cases, immunohistochemistry documented low or absent PDE11A expression in at least 95% of tumor cells.
Figure 4. PDE11A immunohistochemistry.
Decreased PDE11A protein in two adrenocortical tumors with PDE11A missense substitutions: one ACC (A) and one ACA (C). PDE11A immunostaining in three wild-type adrenocortical tumors: one ACC (B), one ACA (D), and one AIMAH (F). Immunopositivity for PDE11A in Leydig cell from normal testis tissue was used as positive control (E). Magnification, ×400 (A and E), ×200 (B and F), and ×100 (C and D).
Discussion
Genetic susceptibility to common diseases could result from the combined effects of many genetic variants, each of which have a modest effect individually 19, 20. The polygenic model of cancer susceptibility suggests that multiple alleles contribute to excess familial risk 20–22. Candidate gene association studies have been a commonly used approach in the search for such alleles in many benign and malignant tumors as shown by the identification of the involvement of PDE11A in micronodular adrenocortical hyperplasia 12. Phosphodiesterase expression and activity in brain has also led to investigation of these genes in common brain and neurologic disorders 23. Wong et al. 24 showed possible association of PDE11A with major depression and response to antidepressant treatment.
These observations led us to test the hypothesis of a possible association of PDE11A variants with susceptibility to common adrenocortical tumors. We collected a large cohort of 117 adrenocortical tumors that included benign lesions, such as AIMAH, ACA, and malignant ones, such as ACC, to examine the role of the PDE11A gene in susceptibility to adrenocortical tumorigenesis. In this study, all controls were carefully screened to rule out biochemical or clinical evidence of adrenal lesions. Our data showed that a nonsense inactivating PDE11A mutation that was already described by Horvath et al. was found in one ACA; as expected from such a carefully screened control group, no inactivating mutations was found in control subjects.
We also found several coding sequence variants in 22 of 117 adrenocortical tumors, with a higher frequency in AIMAH (24%) than in ACA (19%) and in ACC (16%). The two previously described functional variants R804H and R867H 13 were also present in 8 and 4 adrenocortical tumors, respectively. We identified several new coding variants, localized in the GAF and catalytic region of the PDE11A enzyme. Most of these variants have not been reported previously in the general population, suggesting that they are very rare variants of PDE11A. Indeed they were either absent or present at a lower frequency in controls. If we add all these rare nonsense and missense variants, their frequency was much higher in adrenocortical tumors than in controls. If one breaks down the diagnostic groups, AIMAH patients had a significantly higher number of PDE11A variants (OR, 3.53).
AIMAH is a bilateral form of adrenocortical tumors and one would expect that a germ-line genetic predisposing factor would indeed favor bilateral (versus unilateral) disease. These data are in agreement with several studies reviewed by Kryukov et al. 25 and report the implication of most rare and deleterious missense mutations in complex diseases. The effects of nonsynonymous polymorphisms have been widely characterized; because these variations directly influence protein function, they are relatively easy to study statistically and experimentally. Interestingly, many examples of genetic susceptibility are reported also in different types of human cancers: a genetic variant of a gene encoding a member of the Ras superfamily, ARLTS1, was found predisposing patients to familial cancer 26. Recently, rare missense variants within the human melanocortin 1 receptor (MC1R), another important actor in the cAMP pathway, have been associated with a susceptibility to basal cell carcinoma of the skin27.
In our study, frequent synonymous or intronic polymorphisms were also found in both adrenocortical tumors and controls: the polymorphism E421E and associated sequences located in intron 10-exon 11-intron 11 were more frequent in ACC. Recently, Nackley et al. 28 showed the functional significance of synonymous genetic variations of human cathecol-O-methyltransferase and have also suggested the importance of haplotypes over single nucleotide polymorphisms for analysis of genetic variations. The synonymous E421E variant and the intron 10/intron11 variants could therefore play a role in the predisposition to ACC development as a whole haplotype such as MC1R polymorphisms in cutaneous melanoma 29, the codon 72 polymorphic variants in TP53 gene in different types of tumors 30, 31, or polymorphisms in the promoter region of MDM2, a key negative regulator of TP53, in lung 32 or breast cancer 33, 34.
Allelic losses of the wild-type PDE11A allele in ACC with PDE11A missense mutations supports a role as a tumor suppressor gene for PDE11A and the hypothesis that these variants could play a role in tumor development. This was also observed previously in micronodular adrenocortical hyperplasia by Horvath et al. 12, 13 and was confirmed by immunohistochemistry. In AIMAH and ACA, the high cAMP levels compared with normal adrenals also suggest that these PDE11A variants inhibit enzymatic activity leading to increased cyclic nucleotide levels, as Horvath et al. 13 showed in vitro for the two variants (R804H and R867G). One could also postulate that these mutant proteins could act in different signaling pathways than the wild-type PDE11A.
In conclusion, this study adds new evidence that PDE11A may function as a tumor suppressor gene in the adrenal cortex. Clearly, inactivating nonsense mutations were initially observed in patients with micronodular adrenocortical hyperplasia, a rare form of bilateral adrenocortical tumor responsible for Cushing syndrome in children; less deleterious germ-line genetic alterations of PDE11A are apparently part of a genetic predisposition to the development of various other forms of benign as well as malignant adrenocortical tumors diagnosed most often in adults. One could speculate that PDE11A sequence defects may underlie at least part of the commonly found in the general population adrenal incidentaloma.
Table 3.
Main characteristics and PDE11A sequence changes in the AIMAH (n = 29) and controls (n = 96).
| AIMAH (n = 29) | Control group (n = 96) | OR* (95% CI) | P | |
|---|---|---|---|---|
| Sex (F/M) | 24/5 | 75/21 | — | — |
| Age, y (mean ± SD) | 48.7 ± 9.2 | 47.1 ± 0.7 | — | — |
| c.147A>C/p.L49L (exon 3) | ||||
| A/A | 28 (96) | 96 (100) | — | |
| A/C | 1 (4) | 0 (0) | ||
| C/C | 0 (0) | 0 (0) | ||
| c.354G>A/p.Q118Q(exon 3) | ||||
| G/G | 29 (100) | 96 (100) | — | |
| G/A | 0 (0) | 0 (0) | ||
| A/A | 0 (0) | 0 (0) | ||
| c.480G>A/p.L160L (exon3) | ||||
| G/G | 29 (100) | 96 (100) | — | |
| G/A | 0 (0) | 0 (0) | ||
| A/A | 0 (0) | 0 (0) | ||
| c.690C>T/p.C230C (exon 3) | ||||
| C/C | 28 (96) | 96 (100) | — | — |
| C/T | 1 (4) | 0 (0) | ||
| T/T | 0 (0) | 0 (0) | ||
| c.1072-3C>T (intron 4) | ||||
| C/C | 20 (69) | 79 (82) | (c) (d) 2.01 (0.67–5.9) | 0.23 |
| C/T | 9 (31) | 17 (18) | ||
| T/T | 0 (0) | 0 (0) | ||
| c.1263A>G/p.E421E (exon 6) | ||||
| A/A | 18 (62) | 78 (81) | (c) 1.94 (0.73–5.13) | 0.19 |
| A/G | 11 (34) | 17 (18) | (d) 2.25 (0.78–6.5) | 0.14 |
| G/G | 0 (0) | 1 (1) | ||
| c.1577-3C/T (intron 10) | ||||
| c.1626A>G/p.A542A(exon 11) | ||||
| c.1644+26insGTTTATA(intron 11) | ||||
| C/C | 14 (48) | 42 (44) | (c) 0.91 (0.47–1.7) | 0.89 |
| C/T | 11 (38) | 39 (40) | (d) 0.86 (0.33–2.22) | 0.91 |
| T/T | 4 (14) | 15 (16) | (r) 0.9 (0.2–3.36) | 1 |
| c.2758_2760insTCC/p.S920ins (exon 23) | ||||
| A/A | 3 (11) | 11 (11) | (c) 0.72 (0.34–1.50) | 0.44 |
| A/B | 16 (55) | 41 (43) | (d) 0.91 (0.2–5.67) | 1 |
| B/B | 10 (34) | 44 (46) | (r) 0.56 (0.18–1.58) | 0.34 |
| Presence of missense/nonsense mutations | Total n of mutations (%) | |||
| Yes | 7 (24) | 9 (9) | 3.53 | 0.05 |
| No | 22 (76) | 87 (91) | ||
Frequency of different synonymous variations (polymorphisms) and missense/nonsense mutations in 29 AIMAH and sex- and age- matched controls. Values are numbers (percentages) unless otherwise stated.
OR (95% CI) are given for recessive (r), codominant (c), and dominant (d) models, wherever possible.
Acknowledgments
We thank Anne Audebourg, Christine Klein, Jocelyne Daugabel, Patricia Morinière, and Pierre Launay for excellent technical assistance in immunohistochemistry study (Department of Pathology, Hôpital Cochin) and Franck Letourneur (Plate-forme sequencage et génomique, Hôpital Cochin) and Prof. Eric Clauser (Oncogenetic Unit, Hôpital Cochin) for the help in sequencing study.
Grants support
Plan Hospitalier de Recherche Clinique grants AOR 01093 and AOM06179, ANR GIS-INSERM Institut des Maladies Rares grant ANR-06-MRAR-002, National Institute of Child Health and Human Development Intramural Program (C.A. Stratakis and A. Horvath), and NIH intramural project Z01-HD-000642-04 (C.A. Stratakis).
References
- 1.Sidhu S, Sywak M, Robinson B, Delbridge L. Adrenocortical cancer: recent clinical and molecular advances. Curr Opin Oncol. 2004;16:13–8. doi: 10.1097/00001622-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Libe R, Bertherat J. Molecular genetics of adrenocortical tumours, from familial to sporadic diseases. Eur J Endocrinol. 2005;153:477–87. doi: 10.1530/eje.1.02004. [DOI] [PubMed] [Google Scholar]
- 3.Beuschlein F, Reincke M. Adrenocortical tumorigenesis. Ann N Y Acad Sci. 2006;1088:319–34. doi: 10.1196/annals.1366.001. [DOI] [PubMed] [Google Scholar]
- 4.Libe R, Fratticci A, Bertherat J. Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer. 2007;14:13–28. doi: 10.1677/erc.1.01130. [DOI] [PubMed] [Google Scholar]
- 5.Stratakis CA, Boikos SA. Genetics of adrenal tumors associated with Cushing’s syndrome: a new classification for bilateral adrenocortical hyperplasias. Nat Clin Pract Endocrinol Metab. 2007;3:748–57. doi: 10.1038/ncpendmet0648. [DOI] [PubMed] [Google Scholar]
- 6.Groussin L, Jullian E, Perlemoine K, et al. Mutations of the PRKAR1A gene in Cushing’s syndrome due to sporadic primary pigmented nodular adrenocortical disease. J Clin Endocrinol Metab. 2002;87:4324–9. doi: 10.1210/jc.2002-020592. [DOI] [PubMed] [Google Scholar]
- 7.Swords FM, Baig A, Malchoff DM, et al. Impaired desensitization of a mutant adrenocorticotropin receptor associated with apparent constitutive activity. Mol Endocrinol. 2002;16:2746–53. doi: 10.1210/me.2002-0099. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–95. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 9.Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 10.Groussin L, Kirschner LS, Vincent-Dejean C, et al. Molecular analysis of the cyclic AMP-dependent protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene in patients with Carney complex and primary pigmented nodular adrenocortical disease (PPNAD) reveals novel mutations and clues for pathophysiology: augmented PKA signaling is associated with adrenal tumorigenesis in PPNAD. Am J Hum Genet. 2002;71:1433–42. doi: 10.1086/344579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertherat J, Groussin L, Sandrini F, et al. Molecular and functional analysis of PRKAR1A and its locus (17q22-24) in sporadic adrenocortical tumors:17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res. 2003;63:5308–19. [PubMed] [Google Scholar]
- 12.Horvath A, Boikos S, Giatzakis C, et al. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet. 2006;38:794–800. doi: 10.1038/ng1809. [DOI] [PubMed] [Google Scholar]
- 13.Horvath A, Giatzakis C, Robinson-White A, et al. Adrenal hyperplasia and adenomas are associated with inhibition of phosphodiesterase 11A in carriers of PDE11A sequence variants that are frequent in the population. Cancer Res. 2006;66:11571–5. doi: 10.1158/0008-5472.CAN-06-2914. [DOI] [PubMed] [Google Scholar]
- 14.Gicquel C, Bertagna X, Gaston V, et al. Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Res. 2001;61:6762–7. [PubMed] [Google Scholar]
- 15.Libe R, Groussin L, Tissier F, et al. Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity. Clin Cancer Res. 2007;13:844–50. doi: 10.1158/1078-0432.CCR-06-2085. [DOI] [PubMed] [Google Scholar]
- 16.Tissier F, Cavard C, Groussin L, et al. Mutations of β-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65:7622–7. doi: 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- 17.Cazabat L, Libe R, Perlemoine K, et al. Germline inactivating mutations of the aryl hydrocarbon receptor-interacting protein gene in a large cohort of sporadic acromegaly: mutations are found in a subset of young patients with macroadenomas. Eur J Endocrinol. 2007;157:1–8. doi: 10.1530/EJE-07-0181. [DOI] [PubMed] [Google Scholar]
- 18.Tissier F, Louvel A, Grabar S, et al. Cyclin E correlates with malignancy and adverse prognosis in adrenocortical tumors. Eur J Endocrinol. 2004;150:809–17. doi: 10.1530/eje.0.1500809. [DOI] [PubMed] [Google Scholar]
- 19.Pharoah PD, Dunning AM, Ponder BA, Easton DF. Association studies for finding cancer-susceptibility genetic variants. Nat Rev Cancer. 2004;4:850–60. doi: 10.1038/nrc1476. [DOI] [PubMed] [Google Scholar]
- 20.Pharoah PD, Tyrer J, Dunning AM, Easton DF, Ponder BA. Association between common variation in 120 candidate genes and breast cancer risk. PLoS Genet. 2007;3:e42. doi: 10.1371/journal.pgen.0030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meijers-Heijboer H, van den Ouweland A, Klijn J, et al. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002;31:55–9. doi: 10.1038/ng879. [DOI] [PubMed] [Google Scholar]
- 22.Antoniou AC, Easton DF. Polygenic inheritance of breast cancer: implications for design of association studies. Genet Epidemiol. 2003;25:190–202. doi: 10.1002/gepi.10261. [DOI] [PubMed] [Google Scholar]
- 23.Menniti FS, Faraci WS, Schmidt CJ. Phosphodiesterases in the CNS: targets for drug development. Nat Rev Drug Discov. 2006;5:660–70. doi: 10.1038/nrd2058. [DOI] [PubMed] [Google Scholar]
- 24.Wong ML, Whelan F, Deloukas P, et al. Phosphodiesterase genes are associated with susceptibility to major depression and antidepressant treatment response. Proc Natl Acad Sci U S A. 2006;103:15124–9. doi: 10.1073/pnas.0602795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kryukov GV, Pennacchio LA, Sunyaev SR. Most rare missense alleles are deleterious in humans: implications for complex disease and association studies. Am J Hum Genet. 2007;80:727–39. doi: 10.1086/513473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calin GA, Trapasso F, Shimizu M, et al. Familial cancer associated with a polymorphism in ARLTS1. N Engl J Med. 2005;352:1667–76. doi: 10.1056/NEJMoa042280. [DOI] [PubMed] [Google Scholar]
- 27.Scherer D, Bermejo JL, Rudnai P, et al. MC1R variants associated susceptibility to basal cell carcinoma of skin: interaction with host factors and XRCC3 polymorphism. Int J Cancer. 2008;122:1787–93. doi: 10.1002/ijc.23257. [DOI] [PubMed] [Google Scholar]
- 28.Nackley AG, Shabalina SA, Tchivileva IE, et al. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–3. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- 29.Stratigos AJ, Dimisianos G, Nikolaou V, et al. Melanocortin receptor-1 gene polymorphisms and the risk of cutaneous melanoma in a low-risk southern European population. J Invest Dermatol. 2006;126:1842–9. doi: 10.1038/sj.jid.5700292. [DOI] [PubMed] [Google Scholar]
- 30.Papadakis EN, Dokianakis DN, Spandidos DA. p53 codon 72 polymorphism as a risk factor in the development of breast cancer. Mol Cell Biol Res Commun. 2000;3:389–92. doi: 10.1006/mcbr.2000.0241. [DOI] [PubMed] [Google Scholar]
- 31.Koushik A, Platt RW, Franco EL. p53 codon 72 polymorphism and cervical neoplasia: a meta-analysis review. Cancer Epidemiol Biomarkers Prev. 2004;13:11–22. doi: 10.1158/1055-9965.epi-083-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Miao X, Guo Y, et al. Genetic polymorphisms in cell cycle regulatory genes MDM2 and TP53 are associated with susceptibility to lung cancer. Hum Mutat. 2006;27:110–7. doi: 10.1002/humu.20277. [DOI] [PubMed] [Google Scholar]
- 33.Boersma BJ, Howe TM, Goodman JE, et al. Association of breast cancer outcome with status of p53 and MDM2 SNP309. J Natl Cancer Inst. 2006;98:911–9. doi: 10.1093/jnci/djj245. [DOI] [PubMed] [Google Scholar]
- 34.Toyama T, Zhang Z, Nishio M, et al. Association of TP53 codon 72 polymorphism and the outcome of adjuvant therapy in breast cancer patients. Breast Cancer Res. 2007;9:R34. doi: 10.1186/bcr1682. [DOI] [PMC free article] [PubMed] [Google Scholar]