Abstract
Background
Lymphoid tissues constitute basic structures where specific immune responses take place. This leads to the development of germinal centres (GCs), migration of cells and the generation of memory cells. Here, we have compared human tumour reactive lymph nodes and tonsils with control lymph nodes.
Results
The study by flow cytometry shows that in control lymph nodes the majority of cells were naive T-lymphocytes (CD45RA+/CD7+). In reactive nodes, although the percentage of CD45RO+ T cells remains constant, there is an increase in the number of B-lymphocytes, and a reduction in naive T cells. The percentage of cells expressing CD69 was similar in reactive nodes and in controls. In both cases, we have found two populations of B cells of either CD69- or CD69dull. Two populations of T cells, which are either negative for CD69 or express it in bright levels (CD69bright), were also found.
The analysis of tissue sections by confocal microscopy revealed differences between control, tonsils and tumor reactive lymph nodes. In control lymph nodes, CD19 B cells are surrounded by a unique layer of CD69bright/CD45RO+ T cells. GCs from tonsils and from tumour reactive nodes are mainly constituted by CD19 B cells and have four distinct layers. The central zone is composed of CD69- B cells surrounded by CD69bright/CD45RO+ T cells. The mantle region has basically CD69dull B-lymphocytes and, finally, there is an outer zone with CD69-/CD45RO+ T cells.
Conclusions
Human secondary lymphoid organs react with an increase in the proportion of B lymphocytes and a decrease in the number of CD45RA+ T cells (naive). In tonsils, this is due to chronic pathogen stimulation, whereas in lymph nodes draining head and neck carcinomas the reaction is prompted by surrounded tumors. During this process, secondary lymphoid organs develop secondary follicles with a special organization of T and B cells in consecutive layers, that are described here by confocal microscopy. This pattern of cellular distribution may suggest a model of cell migration into the secondary lymphoid follicles.
Background
Efficient interactions between T, B and antigen-presenting cells in T-dependent immune responses take place at the secondary lymphoid organs [1-3]. T cells are located mainly in the paracortical zone, which includes the interfollicular regions. B cells are placed in small primary follicles in the cortex, which become secondary follicles or germinal centres (GCs) after antigenic stimulation [4].
Most recent studies of the GC reaction focused either on B cells, centroblasts and centrocytes, or on follicular dentritic cells. It is known that T cells have a crucial role in the development of the GC reaction, mediated by both cellular contacts and humoral factors (interleukins). GC T cells express CD40L (CD154) [5,6], a molecule which allows the interactions with CD40+ B-lymphocytes. High affinity B cells, selected by antigen retained in the surface of follicular dendritic cells (FDCs) [1,7], become antibody producing plasma cells or memory B cells. This distinction is determined by the signals of the CD40/CD40L interaction and by the type of interleukins secreted by T cells [5,6,8,9]. Non-selected B cells, however, die by apoptosis [2,8,10,11,12]. The activation and generation of memory T cells in the secondary follicles of lymphoid tissues remain unclear, although much is known about these processes in B cells. Some authors have shown that, in mice, T cells migrating to follicles are also able to proliferate the developing GC [13,14]. During the GC reaction, T cells become concentrated both, at and near the junction of the follicular mantle with the light zone. Some cells remain there after the end of the GC reaction [13].
The origin, migration and role of intra-GC T cells in human follicles is not accurately known, as it is not possible to study the kinetic of the GC reaction in humans. This study compares control lymph nodes with human tonsils and tumour reactive lymph nodes from patients with head and neck's carcinomas.
The identification and distribution of cells in these nodes has been achieved through the study of several markers and other membrane antigens. The markers used were CD69, which is a very early activation antigen, CD45RA, a marker mostly associated to virgin cells and CD45RO, a marker associated to memory cells. In addition to the differences between control and reactive lymph nodes, an interesting distribution of the B and T cells in several layers was found, when tissue sections were performed.
These results suggest a speculative model of the cellular traffic into the GC, giving a crucial role to the T cells in the regulation of the GC reaction.
Results
Decrease of CD45RA+ T cells in tonsils and tumour reactive lymph nodes
Lymphoid subpopulations from human control lymph nodes, tonsils and tumour reactive lymph nodes have been compared. Flow cytometry was used to analyse a total of 28 control lymph nodes, 9 tonsils and 65 head and neck tumour reactive lymph nodes. In all cases several markers were used (Table 1). These included markers related to specific T or B cells, others, like CD69, to the activation level and, finally, CD45RA naive or CD45RO memory, which are associated with the state of maturation.
Table 1.
Lymphocyte subpopulations from control lymph nodes, tonsils and reactive lymph nodes to head and neck carcinoma
| Control | lymph | Reactive lymph nodes to lead | ||||||
| nodes | Tonsils | and neck carcinoma | ||||||
| ANTIGEN | N | Mean ± | N | Mean ± | Pa) (Tonsil | N | Pa)(reactive | |
| SD | SD | Vs control) | Mean ± SD | Vs control) | ||||
| CD7 | 27 | 68 ± 10 | 9 | 48 ± 11 | 0.0002 | 62 | 62 ± 11 | 0.01 |
| CD19 | 26 | 24 ± 10 | 9 | 40 ± 15 | 0.004 | 63 | 30 ± 11 | 0.05 |
| CD4 | 26 | 58 ± 10 | 9 | 42 ± 10 | 0.0005 | 65 | 56 ± 12 | NS |
| CD8 | 27 | 12 ± 5 | 9 | 8 ± 1 | 0.002 | 65 | 9 ± 4 | 0.03 |
| CD45RA | 28 | 63 ± 9 | 9 | 54 ± 17 | NS | 64 | 58 ± 12 | NS |
| CD45RO | 28 | 29 ± 8 | 9 | 31 ± 9 | NS | 63 | 33 ± 10 | NS |
| CD7/CD45RA | 25 | 37 ± 14 | 9 | 16 ± 4 | 0.0004 | 60 | 29 ± 10 | 0.004 |
| CD7/CD45RO | 26 | 24 ± 8 | 9 | 27 ± 9 | NS | 60 | 28 ± 8 | NS |
| CD69 | 8 | 14 ± 12 | 9 | 29 ± 10 | 0.02 | 14 | 23 ± 10 | NS |
| CD3/CD69 | 7 | 12 ± 11 | 9 | 21 ± 10 | NS | 14 | 19 ± 8 | NS |
a) P < 0.05 by Kruskal-Wallis test considered significant. NS means no significant.
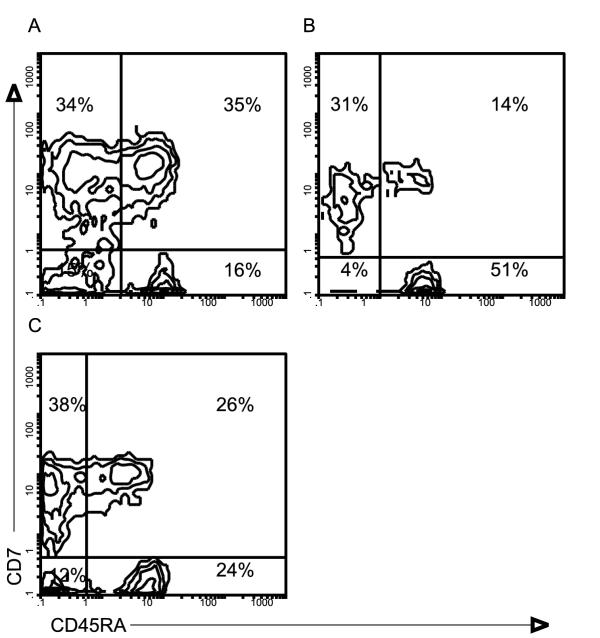
The results show that T cells are the main population in all groups analysed. However, there is a decrease in the number of T cells, both CD4 and CD8 lymphocytes, in tumour reactive lymph nodes and in tonsils, compared to controls (Table 1). To study the expression of the molecule CD45, double immunofluorescence was used. Both the CD45RA isoform, associated to the naive phenotype, and the CD45RO-related to the memory phenotype [15-18] were analysed. This revealed a decrease in the percentage of CD7+/CD45RA+ in tonsils and in tumour reactive lymph nodes compared to controls (Figure 1). No percentual change was detected in the expression of CD45RO within tonsils and nodes. However, an increase in the percentage of B cells in tonsils and in tumour reactive lymph nodes, was observed in comparison with control lymph nodes.
Figure 1.
Two color analysis of lymphocytes from human control lymph nodes (A), tonsils (B) and reactive lymph nodes to head and neck carcinoma (C). Cells were stained with anti-CD45RA-FITC and anti-CD7-PE monoclonal antibodies.
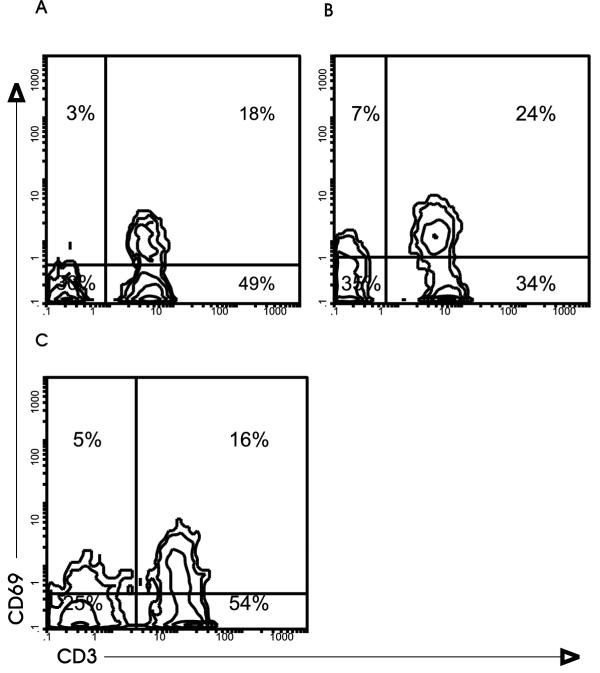
The analysis of CD69, described as an early activation marker [19,20], indicates that it is expressed in a fraction of lymphocytes in all lymph nodes analysed (Table 1). Although slightly higher in tonsil B cells, there are no significant differences in the expression of CD69 between reactive and control lymph nodes. The differences in the intensity of this marker are worth noting. CD69bright is expressed in a fraction of T cells, but B cells are mainly CD69- or dull (Figure 2).
Figure 2.
Expression of antigen CD69 in human lymphocytes from control lymph nodes (A), tonsils (B) and reactive lymph nodes to head and neck carcinoma (C). Cells were stained with anti-CD3-FITC and anti-CD69-PE monoclonal antibodies.
Histological distribution of lymphoid subpopulations in control lymph nodes, tonsils and head and neck tumour reactive lymph nodes
As described in Materials and Methods tissue sections were analysed by confocal microscopy. Subpopulations of cells with specific monoclonal antibodies against different membrane molecules were identified. Nuclear staining was performed with 7-AAD (in blue). As was expected, in control lymph nodes there are mainly primary follicles constituted by accumulations of B-lymphocytes surrounded by T cells. However, GCs appear in both, tumour reactive lymph nodes and tonsils. By confocal microscopy, B cells in control lymph nodes were mainly CD19dull. This contrasted with GC B-lymphocytes from tonsils and tumour reactive lymph nodes that expressed on their membrane higher levels of the CD19 antigen (Figure 3). Although other causes cannot be completely rule out, the variations found in the fluorescence intensity of the marker CD19 could be due to the larger size of the GC-B cells
Figure 3.
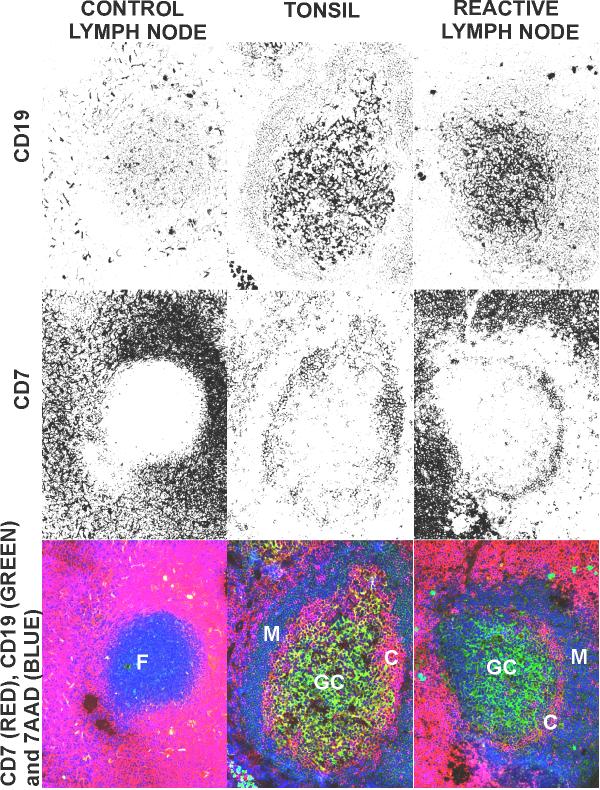
Distribution of T (CD7+) and B cells (CD19+) in frozen sections from human control lymph node, tonsil and reactive lymph node to head and neck carcinoma. Sections were stained with anti-CD7- PE and anti-CD19-FITC monoclonal antibodies and with 7 AAD and analyzed by confocal microscopy. F: primary follicle; GC: germinal center; M: mantle of B cells; C: centrogerminal layer of T cells.
In tonsils and in tumour reactive lymph nodes, secondary follicles or GCs showed a particular lymphocyte distribution in four layers. From the internal to the outer part of the GC, layers of B / T / B and, finally, again T-lymphocytes can be seen. Although some CD7+ T cells are also present (Figure 3), the internal layer is formed mainly by CD19+ B cells. It is possible to see a thin layer of CD7+ T cells as a crown of cells, surrounding these B cells (C in Figure 3). The third layer, external to these T cells, is constituted by small B-lymphocytes, which form the follicular mantle region. Among these follicular mantle B cells there are some CD7+ T cells located in a radial disposition. The final layer is formed by CD7+ T-lymphocytes that spread to the interfollicular zones. Despite the lower number of secondary follicles found in tumour reactive lymph nodes as compared to those in tonsils, both showed the same distribution in layers of T and B cells (Figure 3).
The tissue location of cells expressing the CD69 antigen (in red) together with two T cell markers, CD3 or CD45RO (both in green), are shown in Figures 4 and 5 respectively. The images obtained from tonsils and tumour reactive lymph nodes reveal that the T cell layer in the GC located under the mantle (C in Figures 4 and 5), is CD3+/CD45RO+/CD69bright. However, follicular mantle B cells are weakly positive for CD69 staining (CD69dull). Scattered T-lymphocytes CD3+/CD45RO+ with a variable expression of CD69 antigen appear in the mantle zone: cells near the T cell layer (region C in Figures 4 and 5) are CD69dull, while those close to the outer zone of the mantle are CD69 - (fig 5). Very few CD69+ cells were found in the interfollicular T zones.
Figure 4.
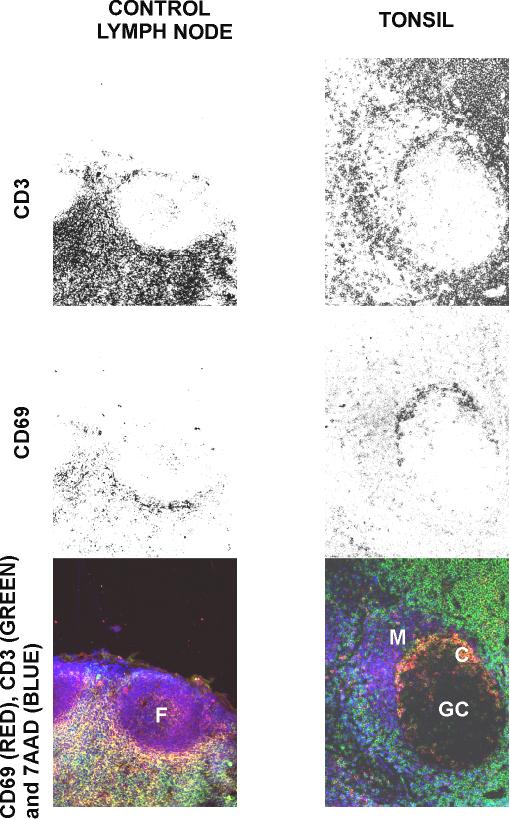
Comparison of the distribution of activated and memory T cells in frozen sections from human control lymph node and tonsil. Sections were stained with 7 AAD, anti-CD69-PE and with anti-CD3-FITC monoclonal antibodies. F: primary follicle; GC: germinal centre; M: mantle of B cells; C: centrogerminal layer of T cells.
Figure 5.
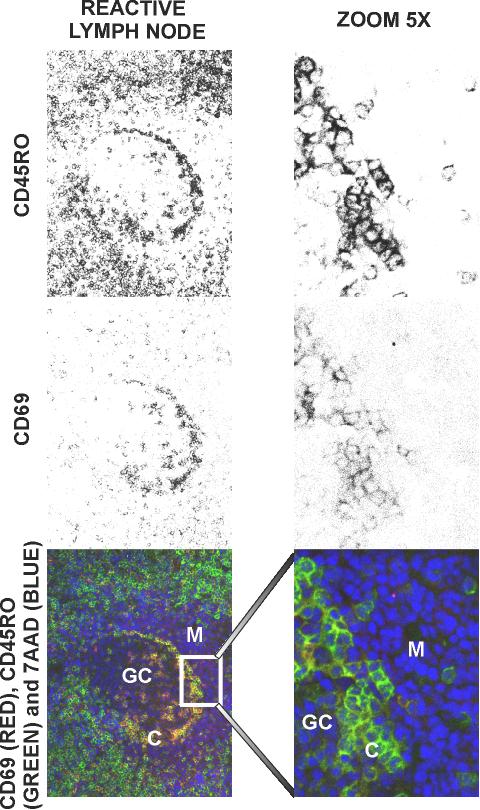
Distribution of activated and memory T cells in frozen sections from human reactive lymph node to head and neck carcinoma. Sections were stained with 7 AAD, anti-CD69-PE and with anti-CD45RO-FITC monoclonal antibodies. F: primary follicle; GC: germinal centre; M: mantle of B cells; C: centrogerminal layer of T cells.
In control lymph nodes it is possible to find some CD3+/CD45RO+/CD69+ cells in a discrete and defined area around B cells in the primary follicle. Follicular B cells appear weakly positive to CD69, similar to what is found in the secondary follicle mantle B cells.
Discussion
The quantitative and histological changes, at the cellular level, that take place in human secondary lymphoid tissues are described in this paper. These result from either chronic stimulation by pathogens in tonsils, or by tumour stimulation in the draining lymph nodes of human head and neck squamous carcinomas. An increase in the percentage of B-lymphocytes in these organs compared to control lymph nodes, due to the generation of GCs after antigen stimulation, was observed by flow cytometry. In tissue sections, the phenotype and morphology of the primary follicle B cells, in control lymph nodes, were seen to be similar to the secondary follicle mantle B cells in tumor reactive lymph nodes or in tonsils. Several authors consider that after the stimulation, during the immune response, the human B cells placed outside the follicles, in the T cell areas, migrate to the region of B cells and FDCs that form the primary follicle [1,4,21,22]. The stimulated B cells proliferate giving rise to centroblasts, which push the naive B cells to the periphery of the GC, thus generating the follicular mantle [2,4,22].
Regarding the T cell population, the most significant data is the decrease of naive T-lymphocytes (CD45RA+) in reactive lymph nodes. Previous studies in other species have demonstrated that naive circulating lymphocytes (L-selectin+) penetrate in the lymph node by the blood vessels and go out by efferent lymphatics [15,16,23]. This decrease in naive T cells could be due to a change of phenotype (from CD45RA+ naive T-lymphocytes to CD45RO+memory cells) or to the dilution of this population due to the higher number of reactive lymph node cells. In addition, it has been described that memory cells are retained more often in the sites were antigen is present [15,24]. In fact, our studies in tissue sections show an increase of CD45RO+ cells compared to control lymph nodes. These CD45RO+ cells are located mainly in two zones: 1) between the follicular mantle and the light zone of the GC and 2) surrounding the secondary follicle.
Surprisingly, in the three types of tissues studied, CD69 antigen was only highly expressed in a T-lymphocyte fraction, while B-lymphocytes showed either a negative or a low expression. CD69 has been reported to be an early activation marker [19,20], therefore its presence was expected only in reactive lymph nodes and tonsils but not in controls. The study by confocal microscopy confirmed the flow cytometry results. CD69dull B cell population is constituted by cells that form the primary follicles in control lymph nodes and also the GC follicular mantle in reactive lymph nodes and tonsils. It has been described that neither primary follicle B cells nor follicular mantle B cells proliferate during the immune response [6]. This could imply that both have the same origin. The small increase in the CD69 expression found in tonsils, by flow cytometry analysis, is probably due to the greater number of GCs in this tissue, which obviously provokes an increase in the number of mantle cells.
CD69bright T-lymphocytes surround B-lymphocytes in primary follicles and are in close contact with the internal part of the CD19/CD69dull mantle B cells in the secondary follicles. This suggests an unknown functional association between these CD69 positive T-lymphocytes and mantle B cells. As the CD69 ligand is still unknown, it is possible to speculate that this molecule could be involved in interactions between these two lymphocyte subpopulations.
By confocal microscopy, GCs in tonsils and in tumour reactive lymph nodes can be seen with a distribution of cells in several layers: a CD19bright B cell zone, a CD69bright/CD45RO+ intra-GC T cell layer, a follicular mantle B cell layer and an external T cell zone. A group of T-lymphocytes CD3+/CD69bright/CD45RO+ is organized between the light zone of the GC and the follicular mantle, as a crown of cells. Their phenotype supports findings reported by other authors in mice [5]. Moreover, it is similar to the narrow T cell layer that surrounds the primary follicles in human control lymph nodes (Figure 4). It seems possible that these T cells (CD3+/CD69bright/CD45RO+), already observed in the primary follicle, could give place to the GC T cells when an immune response is initiated.
This distribution in a four layer-structure of different lymphoid populations (B / T / B / T cells), indicates a very organized structure. From our results and with data from several authors, a speculative model of migration and activation of lymphocytes in the GCs is suggested (Figure 6). Some authors [4,25] support the hypothesis that T-B interaction takes place in the area that surrounds the primary follicle and that, later, stimulated B and T cells migrate to the follicle, where they proliferate [13], forming the GCs. In the inner part of the GC, these CD69bright/CD45RO+ T-lymphocytes could be generated by T-B cellular interaction, migrating to the marginal zone of GC, in close contact with the mantle (Figures 4 and 5). In this model, it is proposed that proliferating memory T cells could continuously be exported to the CD45RO+ layer that surrounds the secondary follicle and that follicular mantle B-lymphocytes could participate in this migration. The CD69 molecule might participate in this process. Moreover, scattered memory T-lymphocytes were found among mantle B cells. The expression of CD69 varies in these T-lymphocytes, it diminishes moving out from the internal part of the GC and it is lacking in cells located near to the outside of the follicle. It is likely that these GC T-lymphocytes could interact with mantle B cells, and also could express all the necessary molecules to be able to interact with B-lymphocytes [6]. Interactions with mantle B cells may provoke an exit of T cells to the mantle outer zone. This may avoid continuous GC T-lymphocyte generation and expansion, which would lead to an unregulated or exacerbated immune response. T cells would join the physiological barrier created by mantle B cells and stop the immune response, keeping a resting memory T-lymphocyte store outside the follicle.
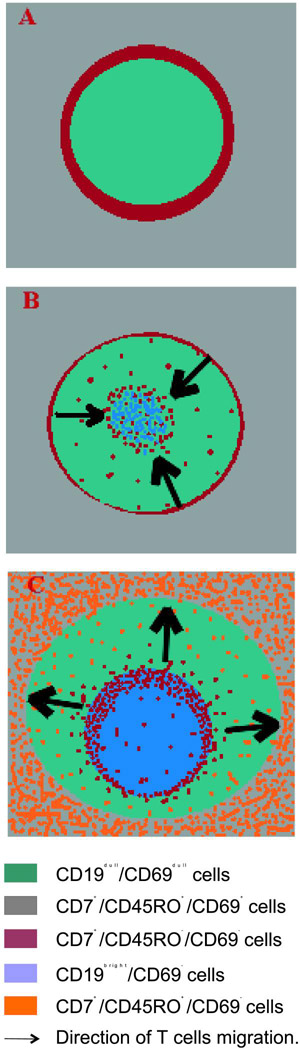
Figure 6.
Proposed model of T cell migration during the development of a secondary follicle. A. primary follicle. The CD7+/CD45RO+/CD69bright are disposed surrounding the primary follicle. B. After antigenic challenge the CD7+/CD45RO+/CD69bright cells migrate into the follicle, where they proliferate. C. The T cells originated into the germinal centre migrate out of the secondary follicle. The expression of CD69 diminishes going out from the internal part of the GC and it is lacking in cells outside of the follicle.
To confirm some of these hypotheses, experiments are currently being carried out in our laboratory. These include the study of whether the memory T lymphocytes from the outer layer are progeny of the lymphocytes placed in the inner layers.
Conclusions
Human control lymph nodes have a higher number of T lymphocytes than tonsils or tumor reactive lymph nodes. These are mostly T cells with naive phenotype (CD45RA+). Histologically, control lymph nodes only show primary lymph follicles. Tonsils and tumor reactive lymph nodes display an increase in the number of B cells and a decrease in the number of CD45RA+ T lymphocytes. Studies in tissue sections show the formation of secondary lymphoid follicles with a very organized structure constituted by alternative layers of subpopulations of B and T lymphocytes: CD19bright B cell zone, CD69bright CD45RO+ intra- GC T cell layer, a follicular mantle B cell layer and an external T cell zone. The characteristics of mantle B cells are nearly identical to the primary follicle B cells. We propose a hypothetical model of lymphocyte migration in the GC.
Materials and methods
Tumour reactive lymph nodes
We have analysed 65 lymph nodes from 44 patients suffering from head and neck squamous carcinomas, with ages ranging between 30 to 65 years. Nodes from patients treated with radiotherapy or chemotherapy were not included in this study. Enlarged reactive lymph nodes were selected randomly from the region draining the area of the tumour and surgically excised. One to three nodes were obtained from each patient.
Control lymph nodes
The study of truly non-reactive nodes is limited by ethical and practical considerations. Therefore, cystic nodes from patients undergoing surgery for cholelithiasis were taken. Neck lymph nodes were also taken from patients suffering from a thyroid pathology. Inflammatory or tumoral pathologies were excluded. We consider that these nodes represent the closest approximation to normality that can be achieved in a study of this type. Nineteen patients aged between 25- 65 years were studied.
Tonsils
Nine tonsils were obtained from surgical operations on patients suffering from recurrent tonsillitis. The patients were between 8 and 38 years of age.
Cell preparations
Single cell suspensions of lymphoid cells were prepared by pressing each lymph node through a stainless steel mesh. The suspensions were placed in cold medium (RPMI 1640) supplemented with 2% FCS and 0.02% sodium azide.
Monoclonal Antibody Staining
FITC or PE-conjugated monoclonal antibodies were purchased from Coulter, Immunotech and Boehringer Ingelheim. Details of the panel antibodies used are given in Table 2. The staining technique involved direct two-colour immunofluorescence. A million cells were incubated 45 min at 4°C with optimal amounts of FITC and PE-conjugated mAb, washed twice (RPMI 2% FCS) and resuspended in medium. Samples were fixed by adding 200 μl of 0.1% paraformaldehyde solution in PBS to the cellular suspension.
Table 2.
Monoclonal antibodies used
| Official name | Clone | Population recognized |
| CD3 | UCHT1 | T cell lineage |
| CD4 | T4 | Helper/ inducer T cells |
| CD7 | 3A1 | T cell lineage |
| CD8 | T8 | Cytotoxic T cells |
| CD19 | B4 | B cells |
| CD25 | 1HT44H3 | Activated T and B cells |
| CD45RA | ALB 11 | B and T cell subset |
| CD45RO | UCHL1 | T cell subset |
| CD69 | CH/4 | Activated T and B cells |
Flow-cytometric Analysis
An ELITE flow cytometer (Coulter Electronics, Hialeah, FL) equipped with an argon ion laser (488 nm) was used. Lymphocytes were gated using forward and orthogonal light scatter, and the percentage of positive cells for each marker was measured. A minimum of 5,000 cells was analysed for each sample.
Data Analysis
The lymph nodes were classified as control tonsils or reactive nodes to head and neck carcinoma. The control lymph nodes were cystic nodes and neck nodes from patients with non-inflammatory pathologies. The results obtained by flow cytometry were statistically analysed with the SPSS program. The standard error of the mean was determined for each group of nodes. The non-parametric Kruskal-Wallis test was used to assess the statistical significance. This test was chosen because of the difficulty to discern the normal distribution of our data due to the number of samples analyzed.
Tissue sections
Frozen tissue sections of secondary lymphoid organs were fixed in acetone. The staining technique involved three-colour direct immunofluorescence. FITC and PE-conjugated monoclonal antibodies were from Coulter, Immunotech and Boehringer Ingelheim, and 7-aminoactinomycin D (7AAD) was from Sigma. To avoid unspecific binding the sections with RPMI+2.5% FCS were incubated for 30 minutes. The sections were washed with PBS and incubated 2 hours in a humid chamber in the dark with enough amounts of antibodies and the 7AAD (1 μg/ml) to cover the tissue. Samples were washed twice with PBS and then prepared with Mounting Medium (Inova Diagnostics Inc.). The sections were maintained at 4°C until analysed.
Histological study with confocal microscopy
Three-colour signals were collected separately on a BioRad MRC-1024-ES confocal system equipped with a krypton/argon ion laser (property of C.A.C.T.I., Vigo University). Photomicrographs were taken from electronic overlays.
Abbreviations
7AAD, 7- aminoactinomycin D; CD40L, CD40 ligand; FDC, follicular dendritic cell; GC, germinal center.
Acknowledgments
Acknowledgements
This work was supported by grant 94/0955 from Fondo de Investigaciones Sanitarias de la Seguridad Social and XUGA90501B95 from Xunta de Galicia, Spain. We thank to the Services of Othorrinolaringology and General Surgery (Meixoeiro Hospital, Vigo) for providing tonsils and lymph nodes. We are also grateful to Mrs. Maria Valeiras-Puga and Mrs. Amalia Estevez-Santos for their technical assistance. We also wish to express our thanks to Mr Ted Cater and Dr Teresa Carretero for reviewing the manuscript.
Contributor Information
Berta Vidal-Rubio, Email: bvidal@excite.com.
Marta Sanchez-Carril, Email: elbebelorzas@hotmail.com.
Josefina Oliver-Morales, Email: fgambon@cesga.es.
África González-Femandez, Email: africa@uvigo.es.
Francisco Gambón-Deza, Email: F.gambon@globalmail.net.
References
- Kosco-Vilbois MH, Zentgraft H, Gerdes J, Bonnefoy JY. To 'B' or not to 'B' a germinal center? Immunol Today. 1997;18:225–230. doi: 10.1016/S0167-5699(97)01048-7. [DOI] [PubMed] [Google Scholar]
- Kelsoe G. Life and death in germinal centers (redux). Immunity. 1996;4:107–111. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- Thorbecke GJ, Amin AR, Tsiagbe VK. Biology of germinal centers in lymphoid tissue. FASEB J. 1994;8:832–840. doi: 10.1096/fasebj.8.11.8070632. [DOI] [PubMed] [Google Scholar]
- Casamayor-Palleja M, Gulbranson-Judge A, MacLennan ICM. T cells in the selection of germinal center B cells. In Human B cell populations Edited by Ferrarini M and Caligaris-Cappio F Basel, Karger: Chem Immunol. 1997. pp. 27–44. [DOI] [PubMed]
- Secord EA, Rizzo LV, Barroso EWS, Umetsu DT, Thorbecke GJ, DeKruyff RH. Reconstitution of germinal center formation in nude mice with Th1 and Th2 clones. Cell Immunol. 1996;174:173–179. doi: 10.1006/cimm.1996.0307. [DOI] [PubMed] [Google Scholar]
- Vyth-Dreese FA, Dellemijn TAM, Majoor D, de Jong D. Localization in situ of the co-stimulatory molecules B7.1, B7.2, CD40 and their ligands in normal human lymphoid tissue. Eur J Immunol. 1995;25:3023–3029. doi: 10.1002/eji.1830251106. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997;156:111–126. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- Liu YJ, de Bouteiller O, Fugier-Vivier I. Mechanisms of selection and differentiation in germinal centers. Curr Opinion Immunol. 1997;9:256–262. doi: 10.1016/S0952-7915(97)80145-8. [DOI] [PubMed] [Google Scholar]
- Galibert L, Burdin N, Barthélémy C, Meffre G, Durand I, Garcia E, Garrone P, Rousset F, Banchereau J, Liu YJ. Negative selection of human germinal center B cells by prolonged BCR cross-linking. J Exp Med. 1996;183:2075–2085. doi: 10.1084/jem.183.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ. Reuse of B lymphocytes in germinal centers. Science. 1997;278:238–239. doi: 10.1126/science.278.5336.238. [DOI] [PubMed] [Google Scholar]
- Shokat KM, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375:334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Kannourakis G, Nouri S, Smith KGC, Nossal GJV. Soluble antigen can cause enhanced apoptosis of germinal-center B cells. Nature. 1995;375:331–334. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- Gulbranson-Judge A, MacLennan ICM. Sequential antigen-specific growth of T cells in the T zones and follicles in response to pigeon cytochrome c. Eur J Immunol. 1996;26:1830–1837. doi: 10.1002/eji.1830260825. [DOI] [PubMed] [Google Scholar]
- Zheng B, Han S, Zhu Q, Goldsby R, Kelsoe G. Alternative pathways for the selection of antigen-specific peripheral T cells. Nature. 1996;384:263–266. doi: 10.1038/384263a0. [DOI] [PubMed] [Google Scholar]
- Westermann J, Pabst R. How organ-specific is the migration of 'naive' and 'memory' T cells?. Immunol Today. 1996;17:278–282. doi: 10.1016/0167-5699(96)80545-7. [DOI] [PubMed] [Google Scholar]
- Swain SL, Croft M, Dubey C, Haynes L, Rogers P, Zhang X, Bradley LM. From naive to memory T cells. Immunol Rev. 1996;150:143–167. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Bell EB, Sparshott SM, Bunce C. CD4+ T-cell memory, CD45R subsets and the persistence of antigen - a unifying concept. Immunol Today. 1998;19:60–64. doi: 10.1016/S0167-5699(97)01211-5. [DOI] [PubMed] [Google Scholar]
- Shaw AS, Dustin ML. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity. 1997;6:361–369. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- Cebrián M, Yagüe E, Rincón M, López-Botet M, de Landázuri MO, Sánchez-Madrid F. Triggering of T cell proliferation through AIM, an activation inducer molecule expressed on activated human lymphocytes. J Exp Med. 1988;168:1621–1637. doi: 10.1084/jem.168.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testi R, Phillips JH, Lanier LL. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. J Immunol. 1989;142:1854–1860. [PubMed] [Google Scholar]
- Kosco-Vilbois MH, Bonnefoy JY, Chvatchko Y. The physiology of murine germinal center reactions. Immunol Rev. 1997;156:127–136. doi: 10.1111/j.1600-065x.1997.tb00964.x. [DOI] [PubMed] [Google Scholar]
- Camacho SA, Kosco-Vilbois MH, Berek C. The dynamic structure of the germinal center. Immunol Today. 1998;19:511–514. doi: 10.1016/S0167-5699(98)01327-9. [DOI] [PubMed] [Google Scholar]
- Young AJ, Marston WL, Dessing M, Dudler L, Hein WR. Distinct recirculating and non-recirculating B-lymphocyte pools in the peripheral blood are defined by the coordinated expression of CD21 and L-selectin. Blood. 1997;90:4865–4875. [PubMed] [Google Scholar]
- Liu YJ, Barthélémy C, de Bouteiller O, Arpin C, Durand I, Banchereau J. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7-l and B7-2. Immunity. 1995;2:239–248. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Garside P, Ingulli E, Merica RR, Jonhson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]