Abstract
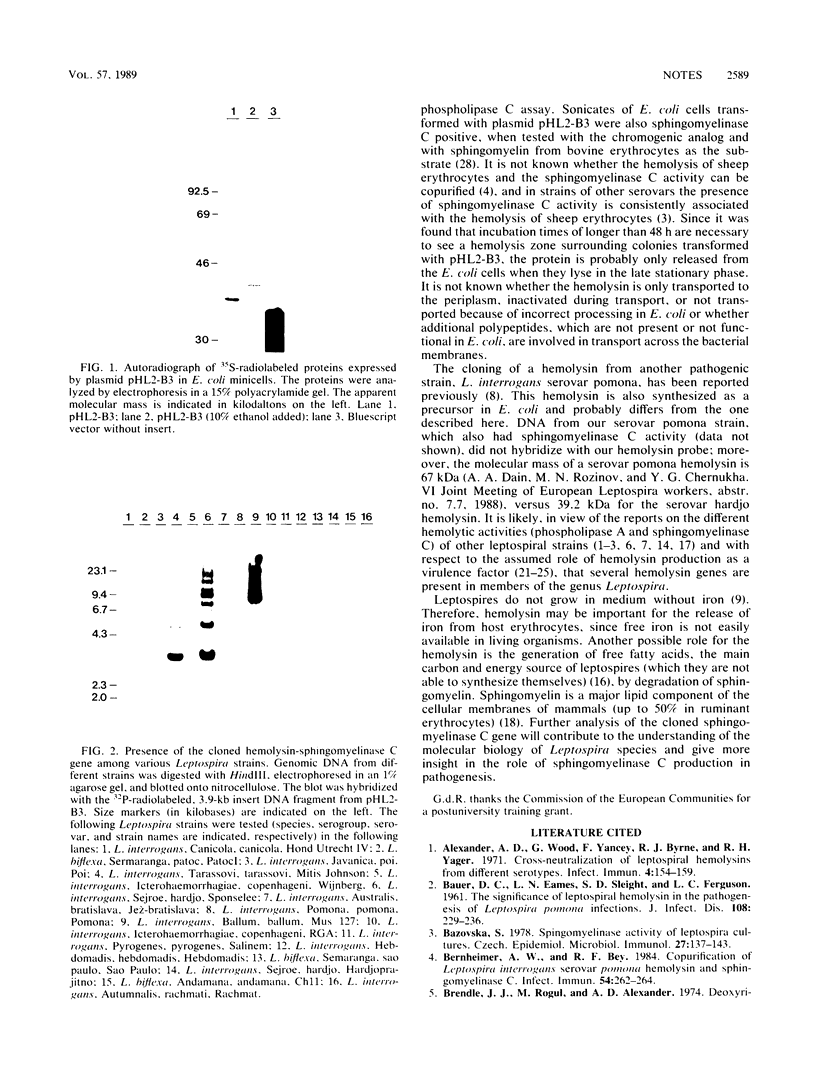
A DNA fragment encoding both hemolysin and sphingomyelinase C activity was cloned from the pathogenic bacterium Leptospira interrogans serovar hardjo. Initial clones were obtained by screening a genomic library in EMBL3 for hemolytic activity. Both hemolytic and sphingomyelinase C activities were coded for by a 3.9-kilobase BamHI fragment. The hemolysin was expressed from its own promoter in Escherichia coli K-12. Similar DNA sequences were also present in the serovars tarassovi and ballum.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander A. D., Wood G., Yancey F., Byrne R. J., Yager R. H. Cross-neutralization of leptospiral hemolysins from different serotypes. Infect Immun. 1971 Aug;4(2):154–159. doi: 10.1128/iai.4.2.154-159.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUER D. C., EAMES L. N., SLEIGHT S. D., FERGUSON L. C. The significance of leptospiral hemolysin in the pathogenesis of Leptospira pomona infections. J Infect Dis. 1961 Mar-Apr;108:229–236. doi: 10.1093/infdis/108.2.229. [DOI] [PubMed] [Google Scholar]
- Bazovská S. Sfingomyelinázova aktivita leptospírových kultúr. Cesk Epidemiol Mikrobiol Imunol. 1978 Jun;27(3):137–143. [PubMed] [Google Scholar]
- Bernheimer A. W., Bey R. F. Copurification of Leptospira interrogans serovar pomona hemolysin and sphingomyelinase C. Infect Immun. 1986 Oct;54(1):262–264. doi: 10.1128/iai.54.1.262-264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAINE S. Iron as a growth requirement for pathogenic Leptospira. J Gen Microbiol. 1959 Apr;20(2):246–251. doi: 10.1099/00221287-20-2-246. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gal A. E., Brady R. O., Barranger J. A., Pentchev P. G. The diagnosis of type A and type B Niemann Pick disease and detection of carriers using leukocytes and a chromogenic analogue of sphingomyelin. Clin Chim Acta. 1980 May 21;104(1):129–132. doi: 10.1016/0009-8981(80)90143-6. [DOI] [PubMed] [Google Scholar]
- Garcia E., Bergmans H. E., Van den Bosch J. F., Orskov I., Van der Zeijst B. A., Gaastra W. Isolation and characterisation of dog uropathogenic Escherichia coli strains and their fimbriae. Antonie Van Leeuwenhoek. 1988;54(2):149–163. doi: 10.1007/BF00419202. [DOI] [PubMed] [Google Scholar]
- Hathaway S. C., Marshall R. B. Haemolysis as a means of distinguishing between Leptospira interrogans serovars balcanica and hardjo. J Med Microbiol. 1980 Aug;13(3):477–481. doi: 10.1099/00222615-13-3-477. [DOI] [PubMed] [Google Scholar]
- Kasărov L. B. Degradiation of the erythrocyte phospholipids and haemolysis of the erythrocytes of different animal species by leptospirae. J Med Microbiol. 1970 Feb;3(1):29–37. doi: 10.1099/00222615-3-1-29. [DOI] [PubMed] [Google Scholar]
- Kojima T., Yanagihara Y., Mifuchi I. Characterization of inhibitor to leptospiral hemolysin present in bovine serum. Microbiol Immunol. 1984;28(3):291–302. doi: 10.1111/j.1348-0421.1984.tb00681.x. [DOI] [PubMed] [Google Scholar]
- Kurioka S., Matsuda M. Phospholipase C assay using p-nitrophenylphosphoryl-choline together with sorbitol and its application to studying the metal and detergent requirement of the enzyme. Anal Biochem. 1976 Sep;75(1):281–289. doi: 10.1016/0003-2697(76)90078-6. [DOI] [PubMed] [Google Scholar]
- Stalheim O. H. Virulent and avirulent Leptospires: biochemical activities and survival in blood. Am J Vet Res. 1971 Jun;32(6):843–849. [PubMed] [Google Scholar]
- Thompson J. C., Marshall R. B. In vitro studies of haemolysis by Leptospira interrogans serovars pomona and ballum. Vet Microbiol. 1986 Mar;11(3):285–292. doi: 10.1016/0378-1135(86)90030-1. [DOI] [PubMed] [Google Scholar]
- Trowbridge A. A., Green J. B., 3rd, Bonnett J. D., Shohet S. B., Ponnappa B. D., McCombs W. B., 3rd Hemolytic anemia associated with leptospirosis. Morphologic and lipid studies. Am J Clin Pathol. 1981 Oct;76(4):493–498. doi: 10.1093/ajcp/76.4.493. [DOI] [PubMed] [Google Scholar]
- Volina E. G., Levina L. F., Soboleva G. L. Phospholipase activity and virulence of pathogenic leptospirae. J Hyg Epidemiol Microbiol Immunol. 1986;30(2):163–169. [PubMed] [Google Scholar]
- Yelton D. B., Charon N. W. Cloning of a gene required for tryptophan biosynthesis from Leptospira biflexa serovar patoc into Escherichia coli. Gene. 1984 May;28(2):147–152. doi: 10.1016/0378-1119(84)90251-8. [DOI] [PubMed] [Google Scholar]
- Zuerner R. L., Charon N. W. Nucleotide sequence analysis of a gene cloned from Leptospira biflexa serovar patoc which complements an argE defect in Escherichia coli. J Bacteriol. 1988 Oct;170(10):4548–4554. doi: 10.1128/jb.170.10.4548-4554.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B., Comfurius P., van Deenen L. L. Organization of phospholipids in human red cell membranes as detected by the action of various purified phospholipases. Biochim Biophys Acta. 1975 Sep 16;406(1):83–96. doi: 10.1016/0005-2736(75)90044-9. [DOI] [PubMed] [Google Scholar]