Abstract
To study the long term the effects of chronic exposure to P. falciparum malaria on Epstein–Barr virus (EBV) reactivation in children, EBV-specific antibody levels were measured in a cross-sectional survey of two groups of Kenyan children with divergent malaria exposure, varying in age from 1 to 14 years. A total of 169 children were analyzed within three age groups (1–4 years, 5–9 years and 10–14 years). Using a Luminex assay, elevated levels of IgG to EBV lytic and latent antigens were observed in children from the holoendemic malaria area; these remained elevated for each age group studied. In comparison, children from the sporadic malaria area had lower levels of EBV-specific IgG antibodies and these levels declined across age groups. These data suggest that chronic exposure to malaria may lead to long-term EBV reactivation.
Keywords: EBV antibodies, co-infection, Plasmodium falciparum, Burkitt's lymphoma
Introduction
Endemic Burkitt's lymphoma is the most prevalent childhood cancer in equatorial Africa, with a peak incidence in children aged 6–7 years [Mwanda et al., 2004]. Infection by Epstein–Barr virus (EBV) and a high degree of exposure to Plasmodium falciparum malaria are recognized generally as pre-requisites in the etiology of endemic Burkitt's lymphoma [Dalldorf et al., 1964; Kafuko and Burkitt, 1970; de-The et al., 1978; Rochford et al., 2005]. While additional factors are likely to be essential in the pathogenesis of endemic Burkitt's lymphoma, both reactivation of EBV by P. falciparum infection [Rochford et al., 2005; Donati et al., 2006b; Chene et al., 2007], as well as suppression of EBV-specific T-cell responses by P. falciparum [Moormann et al., 2007] are thought to be major factors leading to the development of endemic Burkitt's lymphoma.
A number of recent publications have described the effects of both acute, as well as chronic P. falciparum infection on EBV reactivation and control. It was shown recently that plasma EBV DNA levels decrease after treatment of acute P. falciparum malaria [Donati et al., 2006a], and that red blood cells infected with P. falciparum can reactivate directly EBV from B cells through the interaction of CIDR1α with the Fcγ receptor [Chene et al., 2007]. It has been shown earlier that EBV DNA levels are increased in healthy children living in malaria holoendemic areas, as compared with children living in areas with sporadic transmission of malaria [Moormann et al., 2005], whether they are infected acutely or not [Rasti et al., 2005]. Evidence that chronic P. falciparum-induced reactivation of EBV can be a major factor leading to endemic Burkitt's lymphoma derives from early prospective studies in Uganda, where it was discovered that children with endemic Burkitt's lymphoma had elevated levels of IgG to the EBV viral capsid antigen (VCA) before the onset of disease [de-The et al., 1978; Geser et al., 1982]. In addition, two recent studies in Uganda and Malawi have shown a strong association between endemic Burkitt's lymphoma, recent exposure to malaria, and elevated titers of EBV-specific antibodies [Carpenter et al., 2008; Mutalima et al., 2008].
Typically, in healthy EBV carriers, both the EBV VCA and Epstein–Barr nuclear antigen (EBNA)1-specific IgG are detectable for life, whereas the early antigen diffuse (EAd)-specific IgG falls relatively more rapidly after primary infection [Miller, 1990]. Detection of EAd at high levels in latent EBV infection has been associated with reactivation of EBV [Rahman et al., 1991]. Z trans-activation antigen (Zta) (also known as ZEBRA) is an EBV immediate early protein, which does not usually induce IgG in healthy individuals. However, the presence of Zta-specific IgG has been associated with EBV reactivation in several disease settings [Miller, 1990; Tedeschi et al., 1995; Chan et al., 2003; Donati et al., 2006a]. Little is known whether Zta-specific IgG is detectable in children.
In earlier studies, an interesting discrepancy was observed between the age at which EBV DNA is elevated in young children from a holoendemic malaria area (i.e., 1–4 years), and the age at EBV-specific T-cell responses were inhibited (namely 5–9 years) [Moormann et al., 2005, 2007]. The latter also corresponds to the age of peak incidence of endemic Burkitt's lymphoma [Mwanda et al., 2004]. Thus, it was hypothesized that the levels of EBV DNA in children from malaria endemic areas may not be the best reflection of the degree of reactivation of EBV virus over long periods of time. In contrast, EBV-specific antibody levels may provide a more accurate image of EBV reactivation over time, and a better understanding of the elusive pattern of age distribution of endemic Burkitt's lymphoma. However, there is little data on the patterns of EBV-specific antibodies in children with divergent exposure to P. falciparum malaria. To evaluate how exposure to holoendemic P. falciparum malaria affects the levels of EBV-specific IgG, a comparison was made between EBV-specific antibodies in children from the Kisumu region of Kenya, where there is holoendemic malaria transmission, and in children from the Nandi region with sporadic malaria transmission. To enable the measurement of IgG to several EBV antigens within limited plasma samples from young children, a Luminex-based suspension bead assay was developed, allowing the determination of levels of IgG to four different EBV antigens (EBNA, VCA, EAd and Zta) within the same sample. In this publication serological data are presented which suggest that EBV reactivation persists at a higher level across increasing age groups in children exposed chronically to P. falciparum, as compared to children with sporadic exposure to P. falciparum.
Materials and Methods
Study Participants and Area
Samples were collected from study participants in 2 epidemiologically distinct areas of western Kenya [Moormann et al., 2005]. In the present study, a subset of samples described in an earlier study was used based on availability of samples; this did not alter significantly the age composition of the groups [Moormann et al., 2005]. The first study site was in Nyanza Province, Kisumu District, in the sublocation of Kanyawegi (herein referred to as “Kisumu”) where malaria transmission is holoendemic, and which is a high-risk area for endemic Burkitt's lymphoma [Rainey et al., 2007]. The second study site was in Rift Valley Province, Nandi District, in the sublocation of Kipsamoite (herein referred to as “Nandi”). Malaria transmission in this area is unstable and associated with periodic outbreaks of malaria morbidity. In this study, plasma from children 1 to 14 years of age was analyzed, with a roughly equal representation of children 1–4, 5–9, and 10–14 years of age from each study site, as indicated in Table I. Children were recruited amongst healthy children within the communities of the study areas. All children were healthy at time of sampling. Approval for the study was obtained from the Kenya Medical Research Institute (KEMRI) National Ethical Review Committee and the Institutional Review Board for Human Studies at University Hospitals of Cleveland, CWRU, University of Michigan, and SUNY Upstate Medical University. Written, informed consent was obtained from the guardians of study participants.
TABLE I. Numbers of Children in Each Study Site.
| Site | Total no. of children | Mean agea | Age group | No. of children per age group |
|---|---|---|---|---|
| 1–4 | 19 | |||
| Kisumu | 67 | 7.1 | 5–9 | 30 |
| 10–14 | 18 | |||
| 1–4 | 31 | |||
| Nandi | 102 | 7.4 | 5–9 | 39 |
| 10–14 | 32 |
P-value of t-test = 0.520.
EBV Antigens
EBV-specific IgG was detected using 4 synthetic peptides covering immunodominant epitopes of the viral capsid antigen P18 (VCA), EBV nuclear antigen 1 (EBNA1), diffuse early antigen complex (EAd) and immediate early protein (Zta) antigens of EBV [Fachiroh et al., 2006 and unpublished data by Jaap Middeldorp].
Luminex Assay
Carboxylated microspheres (Luminex, Austin, TE) were coupled to EBV antigens using the manufacturers protocol, and as described by others [Komatsu et al., 2004], with slight modifications. Briefly, 20 μg of peptide was coupled to 1×106 pre-activated microspheres in 500 μl of 100 mM MES pH 6.0 buffer, beads were washed and stored in PBS, 0.1% BSA, 0.02% Tween-20, 0.05% Azide, pH 7.4 at 4°C until use. Antigen-specific IgG was detected by incubating 1,000 beads of each antigen per well with plasma diluted at 1:100, in a final volume of 100 μl. After washing, a 1:200 dilution of PE-conjugated Goat F(ab)2 Anti Human IgG (Biosource, Camarillo, CA) was added. At least 75 beads of each region/antigen were then acquired on a Bioplex reader (Bio-Rad, Hercules, CA). EBV seronegative and positive controls were used on each plate. The results of the assay were expressed as the mean fluorescence intensity (MFI) of at least 75 beads for each EBV antigen. The assay was validated against conventional ELISA [Fachiroh et al., 2006], with good correlations between ELISA results and Luminex results for all four EBV antigens (Spearman's correlation 0.752–0.983, P < 0.005).
Statistics
Levels of antigen-specific IgG in the groups of children from Kisumu and Nandi were compared using Mann–Whitney U test. Correlations between levels of antigen-specific IgG and age were calculated using Spearman's rho test. All tests of statistical significance assume two-tailed alpha = 0.05 and used SPSS version 16.0.
Results and Discussion
Increased Levels of EBV-Specific IgG in Children From Kisumu Compared to Nandi Children
Using the luminex-based assay, EBV-specific IgG levels for VCA, EAd, Zta, and EBNA were determined in plasma obtained from Kisumu and Nandi children, as summarized in Table II. Median VCA-specific IgG levels were 27,558 MFI in Kisumu versus 25,540 MFI in Nandi (Fig. 1A), median EBNA-specific IgG levels 26,750 MFI in Kisumu versus 24,490 MFI in Nandi (Fig. 1B), median Zta-specific IgG levels 1,190 MFI in Kisumu versus 519 MFI in Nandi (Fig. 1C) and median EAd-specific IgG levels 2,212 MFI in Kisumu versus 584 MFI in Nandi (Fig. 1D). These results demonstrate that IgG specific for the EBV lytic antigen Zta is readily detectable in children. Using the Mann–Whitney U test, it was found that EBV-specific antibody levels were significantly elevated in children from Kisumu as compared to Nandi for all four EBV antigens (P-values <0.005) (Fig. 1). This difference was also found when analyzing age groups separately, with levels of EBV-specific antibodies being more elevated in Kisumu children as compared to Nandi children across all three age groups as defined in Table I (data not shown). These results suggest that there may be an increased production of EBV-specific IgG in response to EBV reactivation in children living in a holoendemic malaria area compared to children from an area with sporadic malaria transmission.
TABLE II. Median Levels and Interquartile Range of IgG Specific for the Different EBV Antigens.
| Site | VCA IgGa | EBNA1 IgGa | Zta IgGa | EAd IgGa |
|---|---|---|---|---|
| Kisumu (n = 67) | 27,558 (25,170-30,924) | 26,750 (25,051–30,537) | 1,190 (387–8,975) | 2,212 (1,024–8,881) |
| Nandi (n =102) | 25,540 (20,471–26,807) | 24,490 (10,500–25,839) | 519 (141–1,829) | 584 (212–1,232) |
| P-valueb | <0.005 | <0.005 | <0.005 | <0.005 |
Results shown represent the median of the median fluorescent intensity (MFI of ≥75 beads) for each EBV antigen. The interquartile range is given between brackets for each antigen.
Comparison of Kisumu with Nandi results, using Mann–Whitney U test.
Fig. 1.
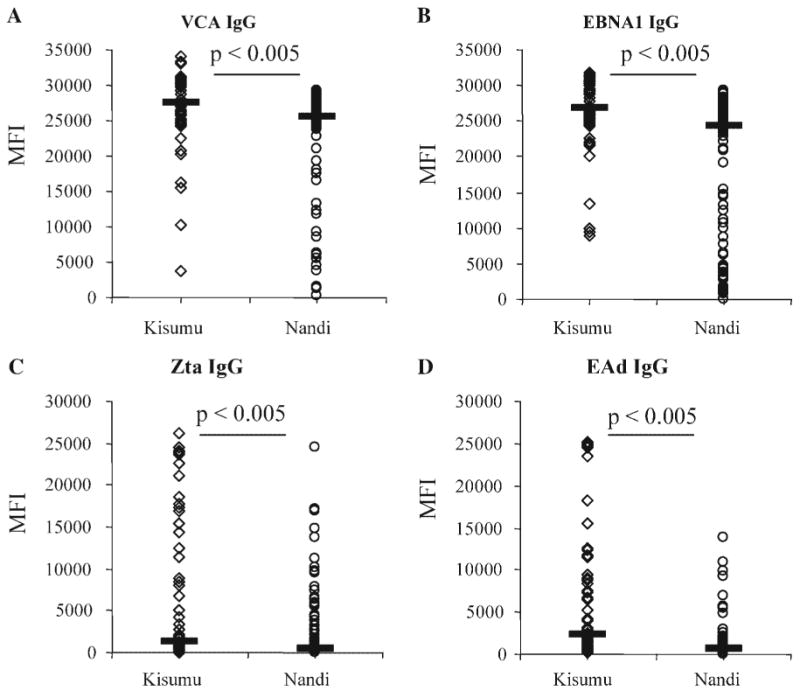
Relative levels of EBV-specific IgG in children from Kisumu compared to Nandi, across all age groups. A: VCA-specific IgG, (B) EBNA1-specific IgG, (C) Zta-specific IgG, (D) EA-specific IgG. Y-axis: MFI, median fluorescence intensity of ≥75 Luminex beads for each antigen tested. P-values of Mann–Whitney U test are indicated in the figures. Horizontal black bars indicate median value per site.
High Levels of EBV-Specific IgG Are Maintained in Kisumu Children With Increasing Age, But Decrease Across Age Groups in Nandi Children
In a previous study, it was observed that EBV DNA load is significantly higher in the youngest age group (e.g., 1–4 years old) in Kisumu compared to same age group in Nandi [Moormann et al., 2005]. To study whether antibody levels were also correlated with age, antibody levels were correlated with age in the Kisumu and Nandi children separately, using Spearman's correlation statistic (Fig. 2). Interestingly, a positive correlation of EAd-specific IgG with age was found in children from Kisumu, with an increase in antibody levels correlating with an increase in age (EAd: rho = 0.285, P = 0.021, Rho 95% confidence interval (Rho CI) = 0.045–0.494). However, no significant correlation between age and antibody levels was found in the Kisumu children for IgG to VCA, EBNA1, or Zta (Kisumu VCA: rho = −0.098, n.s.; EBNA1: −0.220, n.s.; Zta: 0.225, n.s.). Clinically, elevated levels of EAd are indicative of EBV reactivation [Rahman et al., 1991]. These data suggest that over time, the children in Kisumu experience greater incidence of events that induce EBV reactivation. In contrast, in children from Nandi, an inverse correlation was observed between age and IgG levels for all four EBV antigens tested, with a decrease in antibody levels as age increased (Nandi VCA: −0.203, P = 0.040*, Rho CI =−0.382 to −0.009; EBNA1: −0.332, P < 0.005*, Rho CI= −0.494 to −0.148; Zta: −0.231, P = 0.020*, Rho CI = −0.407 to −0.039; EAd: −0.247, P = 0.012*, Rho CI = −0.421 to −0.056).
Fig. 2.

Correlation between levels of EBV-specific IgG and age. Subparts (A,B) for VCA-specific IgG; (C,D) for EBNA-specific IgG; (E,F) for Zta-specific IgG; (G,H) for EAd-specific IgG. Data from Kisumu are presented in (A,C,E,G), data from Nandi in (B,D,F,H). Spearman's Rho correlation coefficient and P-values are indicated in the figures. Significant correlations are indicated by an asterisk.
In summary, this study shows that levels of EBV-specific antibodies in children chronically exposed to P. falciparum malaria remain high across groups with increasing age, while they decrease with age in children from an area with sporadic malaria transmission. This would suggest that the likelihood for persistent reactivation of EBV is increased in Kisumu children, possibly caused by chronic P. falciparum infection. In contrast, EBV-specific antibodies decrease with age in children from an area with sporadic malaria transmission who are not submitted to such an intense pressure of repeated P. falciparum infections. Interestingly, in earlier studies on EBV DNA levels in children from this area [Moormann et al., 2005], a decrease of EBV viral load with age was noted in children from Kisumu which contrasts to the increasing EBV-specific antibody titers. These data support the hypothesis that measurement of EBV-specific IgG may provide a better image of the long-term effects of malaria on reactivation of EBV. Furthermore, they reinforce the view that repeated stimulation of the B-cell pool and subsequent reactivation of EBV from these cells are an essential part of the pathogenesis of endemic Burkitt's lymphoma [de-The et al., 1978; Donati et al., 2004, 2006b; Chene et al., 2007]. Together, these data support a model for the development of endemic Burkitt's lymphoma whereby long-term, chronic malaria infection and corresponding reactivation of EBV are prerequisite factors in the development of disease [de-The et al., 1978; Geser et al., 1982; Rochford et al., 2005; Carpenter et al., 2008; Mutalima et al., 2008].
Acknowledgments
We could like to acknowledge the study participants and their parents for their participation in this study; the field workers for their help with sample collection and Dave McNamara for his help with setting up the Luminex-based assay. This work was done with the permission of the Director of the Kenya Medical Research Institute.
Grant sponsor: NIH; Grant numbers: R01 CA102667 (to R.R.), K08 AI 51565 (to A.M.); Grant sponsor: Elizabeth Crosby Award (to R.R.).
Footnotes
The authors do not have a commercial or other association that might pose a conflict of interest. JM is owner and CEO of Cyto-Barr BV, but declares no commercial interest in this study.
References
- Carpenter LM, Newton R, Casabonne D, Ziegler J, Mbulaiteye S, Mbidde E, Wabinga H, Jaffe H, Beral V. Antibodies against malaria and Epstein-Barr virus in childhood Burkitt lymphoma: A case-control study in Uganda. Int J Cancer. 2008;122:1319–1323. doi: 10.1002/ijc.23254. [DOI] [PubMed] [Google Scholar]
- Chan KH, Gu YL, Ng F, Ng PS, Seto WH, Sham JS, Chua D, Wei W, Chen YL, Luk W, Zong YS, Ng MH. EBV specific antibody-based and DNA-based assays in serologic diagnosis of nasopharyngeal carcinoma. Int J Cancer. 2003;105:706–709. doi: 10.1002/ijc.11130. [DOI] [PubMed] [Google Scholar]
- Chene A, Donati D, Guerreiro-Cacais AO, Levitsky V, Chen Q, Falk KI, Orem J, Kironde F, Wahlgren M, Bejarano MT. A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathogens. 2007;3:e80. doi: 10.1371/journal.ppat.0030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalldorf G, Linsell CA, Barnhart FE, Martyn R. An epidemiologic approach to the lymphomas of African children and Burkitt's Sacroma of the Jaws. Perspect Biol Med. 1964;7:435–449. doi: 10.1353/pbm.1964.0023. [DOI] [PubMed] [Google Scholar]
- de-The G, Geser A, Day NE, Tukei PM, Williams EH, Beri DP, Smith PG, Dean AG, Bronkamm GW, Feorino P, Henle W. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt's lymphoma from Ugandan prospective study. Nature. 1978;274:756–761. doi: 10.1038/274756a0. [DOI] [PubMed] [Google Scholar]
- Donati D, Zhang LP, Chene A, Chen Q, Flick K, Nystrom M, Wahlgren M, Bejarano MT. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect Immun. 2004;72:5412–5418. doi: 10.1128/IAI.72.9.5412-5418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati D, Espmark E, Kironde F, Mbidde EK, Kamya M, Lundkvist A, Wahlgren M, Bejarano MT, Falk KI. Clearance of circulating Epstein-Barr virus DNA in children with acute malaria after antimalaria treatment. J Infect Dis. 2006a;193:971–977. doi: 10.1086/500839. [DOI] [PubMed] [Google Scholar]
- Donati D, Mok B, Chene A, Xu H, Thangarajh M, Glas R, Chen Q, Wahlgren M, Bejarano MT. Increased B cell survival and preferential activation of the memory compartment by a malaria polyclonal B cell activator. J Immunol. 2006b;177:3035–3044. doi: 10.4049/jimmunol.177.5.3035. [DOI] [PubMed] [Google Scholar]
- Fachiroh J, Paramita DK, Hariwiyanto B, Harijadi A, Dahlia HL, Indrasari SR, Kusumo H, Zeng YS, Schouten T, Mubarika S, Middeldorp JM. Single-assay combination of Epstein-Barr Virus (EBV) EBNA1- and viral capsid antigen-p18-derived synthetic peptides for measuring anti-EBV immunoglobulin G (IgG) and IgA antibody levels in sera from nasopharyngeal carcinoma patients: Options for field screening. J Clin Microbiol. 2006;44:1459–1467. doi: 10.1128/JCM.44.4.1459-1467.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser A, de The G, Lenoir G, Day NE, Williams EH. Final case reporting from the Ugandan prospective study of the relationship between EBV and Burkitt's lymphoma. Int J Cancer. 1982;29:397–400. doi: 10.1002/ijc.2910290406. [DOI] [PubMed] [Google Scholar]
- Kafuko GW, Burkitt DP. Burkitt's lymphoma and malaria. Int J Cancer. 1970;6:1–9. doi: 10.1002/ijc.2910060102. [DOI] [PubMed] [Google Scholar]
- Komatsu N, Shichijo S, Nakagawa M, Itoh K. New multiplexed flow cytometric assay to measure anti-peptide antibody: A novel tool for monitoring immune responses to peptides used for immunization. Scand J Clin Lab Invest. 2004;64:535–545. doi: 10.1080/00365510410007008. [DOI] [PubMed] [Google Scholar]
- Miller G. Epstein-Barr virus: Biology, pathogenesis, and medical aspects. In: Fields BN, Knipe DM, Chanock RM, Melnick JL, Hirsch MS, Monath TP, Roizman B, editors. Virology. New York: Raven Press, Ltd; 1990. pp. 1921–1958. [Google Scholar]
- Moormann AM, Chelimo K, Sumba OP, Lutzke ML, Ploutz-Snyder R, Newton D, Kazura J, Rochford R. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J Infect Dis. 2005;191:1233–1238. doi: 10.1086/428910. [DOI] [PubMed] [Google Scholar]
- Moormann AM, Chelimo K, Sumba PO, Tisch DJ, Rochford R, Kazura JW. Exposure to holoendemic malaria results in suppression of Epstein-Barr virus-specific T cell immunosurveillance in Kenyan children. J Infect Dis. 2007;195:799–808. doi: 10.1086/511984. [DOI] [PubMed] [Google Scholar]
- Mutalima N, Molyneux E, Jaffe H, Kamiza S, Borgstein E, Mkandawire N, Liomba G, Batumba M, Lagos D, Gratrix F, Boshoff C, Casabonne D, Carpenter LM, Newton R. Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: Results from a case-control study. PLoS ONE. 2008;3:e2505. doi: 10.1371/journal.pone.0002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwanda OW, Rochford R, Moormann AM, Macneil A, Whalen C, Wilson ML. Burkitt's lymphoma in Kenya: Geographical, age, gender and ethnic distribution. East Afr Med J. 2004;8(Suppl):S68–S77. doi: 10.4314/eamj.v81i8.9210. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Kingsley LA, Atchison RW, Belle S, Breinig MC, Ho M, Rinaldo CR., Jr Reactivation of Epstein-Barr virus during early infection with human immunodeficiency virus. J Clin Microbiol. 1991;29:1215–1220. doi: 10.1128/jcm.29.6.1215-1220.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey JJ, Mwanda WO, Wairiumu P, Moormann AM, Wilson ML, Rochford R. Spatial distribution of Burkitt's lymphoma in Kenya and association with malaria risk. Trop Med Int Health. 2007;12:936–943. doi: 10.1111/j.1365-3156.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- Rasti N, Falk KI, Donati D, Gyan BA, Goka BQ, Troye-Blomberg M, Akanmori BD, Kurtzhals JA, Dodoo D, Consolini R, Linde A, Wahlgren M, Bejarano MT. Circulating Epstein-Barr virus in children living in malaria-endemic areas. Scand J Immunol. 2005;61:461–465. doi: 10.1111/j.1365-3083.2005.01589.x. [DOI] [PubMed] [Google Scholar]
- Rochford R, Cannon MJ, Moormann AM. Endemic Burkitt's lymphoma: A polymicrobial disease? Nature Rev. 2005;3:182–187. doi: 10.1038/nrmicro1089. [DOI] [PubMed] [Google Scholar]
- Tedeschi R, Foong YT, Cheng HM, dePaoli P, Lehtinen T, Elfborg T, Dillner J. The disease associations of the antibody response against the Epstein-Barr virus transactivator protein ZEBRA can be separated into different epitopes. J Gen Virol. 1995;76:1393–1400. doi: 10.1099/0022-1317-76-6-1393. [DOI] [PubMed] [Google Scholar]


