Abstract
Bovine tuberculosis (bTB) is diagnosed in naturally infected populations exposed to a wide variety of other pathogens. This study describes the cell-mediated immune responses of cattle exposed to Mycobacterium avium subspecies paratuberculosis (Map) and Mycobacterium avium subspecies avium with particular reference to routine antefmortem Mycobacterium bovis diagnostic tests. The IFN-γ released in response to stimulated blood was found to peak later in the Map-exposed group and was more sustained when compared to the Maa-exposed group. There was a very close correlation between the responses to the purified protein derivatives (PPD) used for stimulation (PPDa, PPDb, and PPDj) with PPDa and PPDj most closely correlated. On occasion, in the Map-infected cattle, PPDb-biased responses were seen compared to PPDa suggesting that some Map-infected cattle could be misclassified as M. bovis infected using this test with these reagents. This bias was not seen when PPDj was used. SICCT results were consistent with the respective infections and all calves would have been classed skin test negative.
1. Introduction
Mycobacterium avium subspecies paratuberculosis (Map), a gram positive bacterium, is the causative agent of Johne's disease in cattle. It has a wide host range which includes cattle, sheep, goats, deer, and other nonruminants such as rabbits [1]. The recognised transmission routes are via the faecal-oral route through the consumption of contaminated milk or colostrum and in utero [2]. Infection can be cleared by the immune system or progress to subclinical or clinical disease. The proportion of animals that progress to disease will probably vary depending upon the host, the infective dose, and the age of exposure [3–5]. Cattle are most susceptible to infection during their first months of life although older animals can be infected [5–7]. Clinical signs do not usually appear for 3–5 years after infection [4, 6]. Clinical signs include chronic enteritis, diarrhoea, weight loss, and eventual death [8] making it an important constraint on animal health, welfare, and productivity. The direct costs of the disease are difficult to measure, however, in dairy herds it has been estimated that subclinical infection can depress milk production by 10% [9, 10]. However, if infection confounds the diagnosis of other diseases such as M. bovis, the actual national cost of infection may be grossly underestimated.
Early Map diagnosis is difficult because of the length of time taken for cattle to develop an antibody response or for clinical signs to develop therefore many infected animals remain on farms where they can readily shed organisms and possibly infect others. Current antibody serology tests vary widely in terms of sensitivity and specificity making comparisons difficult [11–13]. However, with the complete sequencing of the Map genome [14] new antigens are being discovered which may improve the performance of serology tests [15]. The gold standard of disease diagnosis is tissue/faeces culture which, although accurate, can take months to complete and is likely to be insensitive in preclinically infected animals [16] so is not ideal for disease eradication programs. An alternative diagnostic tool is measuring the Map-specific early cell-mediated immune response (CMI) [17]. This is already routinely used in many countries to diagnose bTB infections in cattle in conjunction with the skin test and is recognised by the Office International des Epizooties for diagnosing M. bovis infections [18]. Several studies have demonstrated that the IFN-γ assay is more effective at detecting early Map infections than antibody tests [19, 20]. One of the limitations with these diagnostic tests is the possible misdiagnosis due to cross reactivity with other Mycobacteria. Álvarez et al. have shown that a dual Map and M. bovis infection in cattle may reduce the sensitivity of the IFN-γ test to detect bTB [21]. To eliminate this cross reactivity, it may become essential to develop more specific tests, for example, using ESAT-6/CFP10 in the IFN-γ assay to help differentiate between M. bovis and Map infections [22, 23].
Map infection in cattle is global. While many studies have been undertaken to determine prevalence within a country, they are not easily comparable due to the different methods used and the cattle tested in the studies not reflecting the overall population. Herd level prevalences within European countries have been estimated to be between 3 and 68% [24]. A 2009 UK government report has estimated that the UK dairy herd prevalence of Johne's disease is 34.7% (95% ci 27.6%–42.5%) [25]. Given this, it is very likely that Johne's disease is present in virtually all countries which currently have bTB. Bovine TB eradication programs are heavily dependent on diagnostic tools to accurately identify affected animals and to distinguish them from animals exposed to environmental mycobacteria. Most current diagnostic tests depend upon the purified protein derivatives (PPDs) of M. bovis (PPDb) and Maa (PPDa). Current bTB eradication programs compare the delayed-type hypersensitivity (DTH) reactions of these two tuberculins in the single intradermal comparative cervical tuberculin (SICCT) skin test. Given that these are crude, poorly characterised, can vary with manufacturer [26] and that the Map, Maa, and M. bovis mycobacteria are genetically similar [27], there is a strong likelihood that an infection with Map may give responses to either reagent that could affect both the sensitivity and specificity of current tests [21, 23].
The purpose of this paper is to assess the CMI response of cattle experimentally infected with either Maa or Map and if there is any impact on the specificity of current M. bovis diagnostics.
2. Materials and Methods
2.1. Animals and Experimental Design
All calves used were male Friesian-Holsteins sourced in Northern Ireland from M. bovis and Map free herds and were tested for exposure to M. bovis, Map, and Maa using the IFN-γ release assay beforehand. Throughout each experiment, the calves were housed in a secure, level 2 containment facility and were blood sampled weekly. In the first experiment, 12 calves aged approximately 16 weeks (ranging from 15 to18 weeks) were infected with Map and kept for 39 weeks before being euthanized and examined at postmortem [28]. In the second experiment, 6 calves were infected with Maa at approximately 20 weeks (ranging from 19 to 22 weeks) of age and then kept for 64 weeks after which they were euthanized and examined at postmortem. Both groups were kept for the maximum length of time practically possible in a level 2 containment facility. In the third experiment, 12 calves were infected with Maa at 18 weeks of age (ranging from 17–19 weeks old) and were kept for 8 weeks before postmortem examination. All experiments complied with UK Home Office approved licence conditions and were subject to regular inspections.
2.2. Inoculation of Calves
The strain of Map used was a local, low-passage bovine strain courtesy of Dr. Grant, Queens University Belfast, and the strain of Maa used was NCTC 8559. The Map culture was grown in 7H9 Middlebrook media supplemented with OADC and mycobactin J (BD Diagnostics), and the Maa culture was grown in 7H9 Middlebrook media supplemented with OADC (BD Diagnostics). Each inocula was made using cultures harvested in the log phase of growth, and a count was performed using a wet weight method [29]. The innocula were resuspended in buffered saline and the calves infected orally. Calves were administered 108 CFU of Map in experiment 1 (optimised by a titration in an earlier study—data not shown) and 109 CFU of Maa in experiments 2 and 3. In the absence of other evidence, 109 CFU was chosen as the Maa dose to ensure a sufficient immune response.
2.3. IFN-γ Release Assay
Blood samples were taken by jugular venepuncture into heparinised Vacutainer tubes (BD Diagnostics) and transported to the laboratory within 1 hour. The blood was then immediately stimulated with either PBS (nil control), avian-purified protein derivatives (PPDa) at 4 μg/mL final concentration (Veterinary Laboratories Agency (VLA)), bovine-purified protein derivative (PPDb) at 8 μg/mL final concentration (VLA), and johnin-purified protein derivative (PPDj) at 4 μg/mL final concentration (Central Veterinary Institute (CVI)), ESAT-6 at 2 μg/mL final concentration (Statens Serum Institut), and pokeweed mitogen (Sigma) (positive control). After 24 hours, the plasma was removed and tested in duplicate using a sandwich ELISA for the detection of bovine IFN-γ (Bovigam, Prionics). The IFN-γ released is expressed as a Net OD (OD of antigen minus OD of PBS). The cut-off for an animal to be classed as bTB-positive animal in Northern Ireland is if PPDb OD minus PBS OD is greater than 0.1 and PPDb OD minus PPDa OD is greater than 0.05 OD. This cutoff has been used in the following results and discussion.
2.4. SICCT Test
A single intradermal comparative cervical tuberculin (SICCT) test was conducted. Three discreet sites were clipped on each animal and measured for skin thickness by callipers and injected intradermally with 100 μL PPDa (VLA), PPDb (VLA), and PPDj (CVI) tuberculins. Each injection site was checked to ensure intradermal delivery. The skin thickness of each site was measured 72 hours later. Using standard test criteria, an animal is classified as bTB skin test positive if there is a skin reaction to PPDb at least 5 mm greater than that to PPDa. All injections and measurements were performed by the same person to limit operator variation.
2.5. Statistics
Data analysis was carried out using GenStat 12th edition (VSN international). Skin test reactions were compared between infection groups using the Kolmogorov-Smirnov test. The comparisons were between PPDa and PPDb, PPDa and PPDj, and PPDj and PPDb. Correlation coefficients for IFN-γ responses to the three tuberculins were estimated using Kendall's Rank Correlation Coefficients. Graphs were produced using FigSys, (Biosoft).
3. Results
3.1. Experiment 1—Map Infection
The purpose of experiment 1 was to expose 12 calves to a known dose of Map and to measure the IFN-γ released in vitro by antigen stimulated PBMCs to study CMI responses. In summary, all calves elicited a CMI response after infection which remained over the course of the experiment. Figure 1(a) shows the mean IFN-γ released in response to blood stimulated with PPDa, b, and j tuberculins. The graph indicates there is a high degree of cross reactivity between the tuberculins with all three showing a similar pattern of response over time with the peak IFN-γ response achieved between weeks 14 and 25 postinfection. All tuberculin comparisons of IFN-γ responses were significantly correlated (P < .001) with the response to PPDa and PPDj most closely correlated. However, in 6 out of the 12 calves there was a PPDb bias over PPDa at some time points (Figure 2(a)) suggesting that these animals may, on occasion, be misclassified as bTB positive using the current cut-offs used in Northern Ireland (see Section 2.3 for further details). 8% of incidences would have been misclassified with one animal (K) accounting for most (44% of its time points); although, in preinfection bleeds the animal would have tested negative. Importantly, the overall percentage of incidences was less than 5% if the comparison was between PPDb and PPDj (Figure 2(b)). In general the greatest immune response was seen with PPDj, followed by PPDa and then PPDb. No calf was misclassified using the SICCT (Figure 3(a)), and there were no significant differences between PPDa and PPDj skin reactions but there was a significant response difference between PPDa and PPDb (P = .05).
Figure 1.
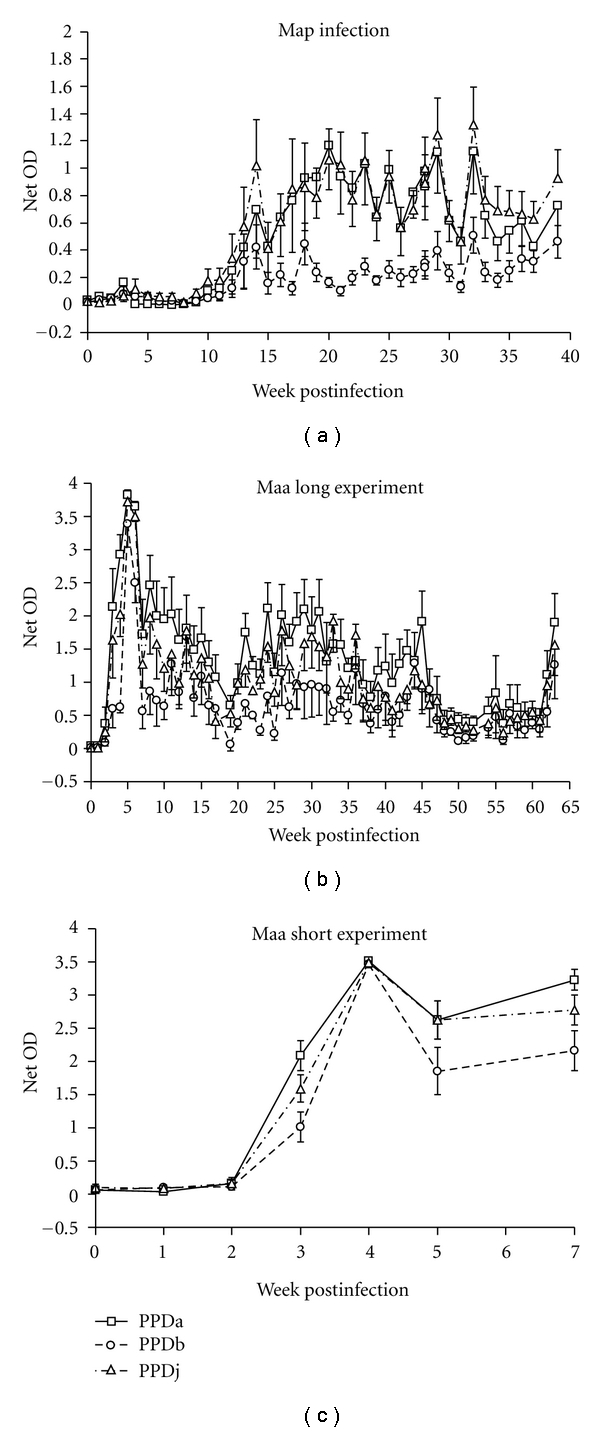
(a) Mean IFN-γ net OD values from whole blood stimulated with PPDa, PPDb, and PPDj tuberculins from 12 Map-exposed calves over 39 weeks. For clarity, only one side of the standard error of measurement bar is shown for PPDa and PPDj data points. (b) Mean IFN-γ net OD values from whole blood stimulated with PPDa, PPDb, and PPDj tuberculins from 6 Maa-exposed calves over 64 weeks. (c) Mean IFN-γ net OD values from whole blood stimulated with PPDa, PPDb, and PPDj tuberculins from 12 Maa-exposed calves over 7 weeks.
Figure 2.
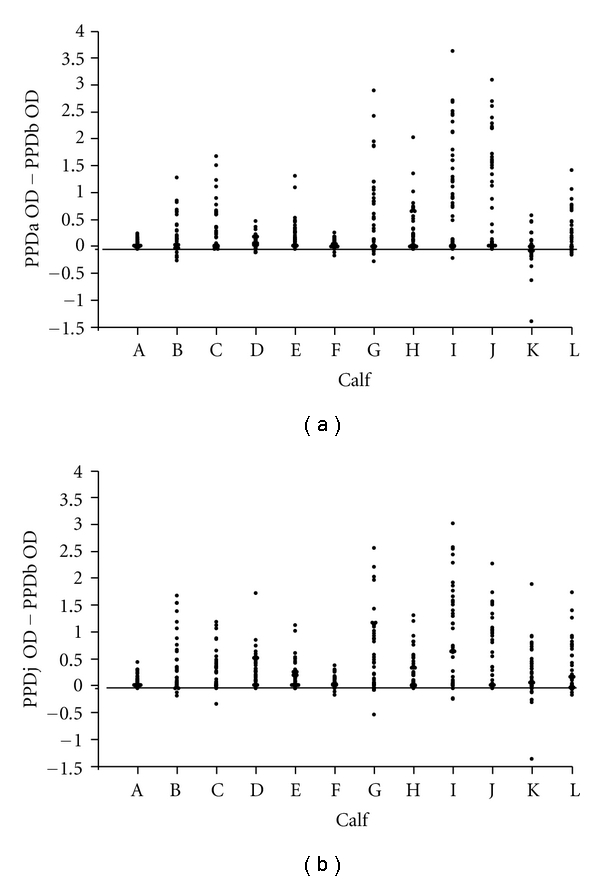
(a) Difference between IFN-γ responses to PPDa and PPDb over the course of experiment 1 at weekly intervals with Map exposed cattle. A cut-off of −0.05 OD has been applied to highlight cattle with a higher response to PPDb than PPDa. (b) Difference between IFN-γ responses to PPDa and PPDb over the course of experiment 1 at weekly intervals with Map-exposed cattle. A cut-off of −0.05 OD has been applied to highlight cattle with a higher response to PPDb than PPDj.
Figure 3.
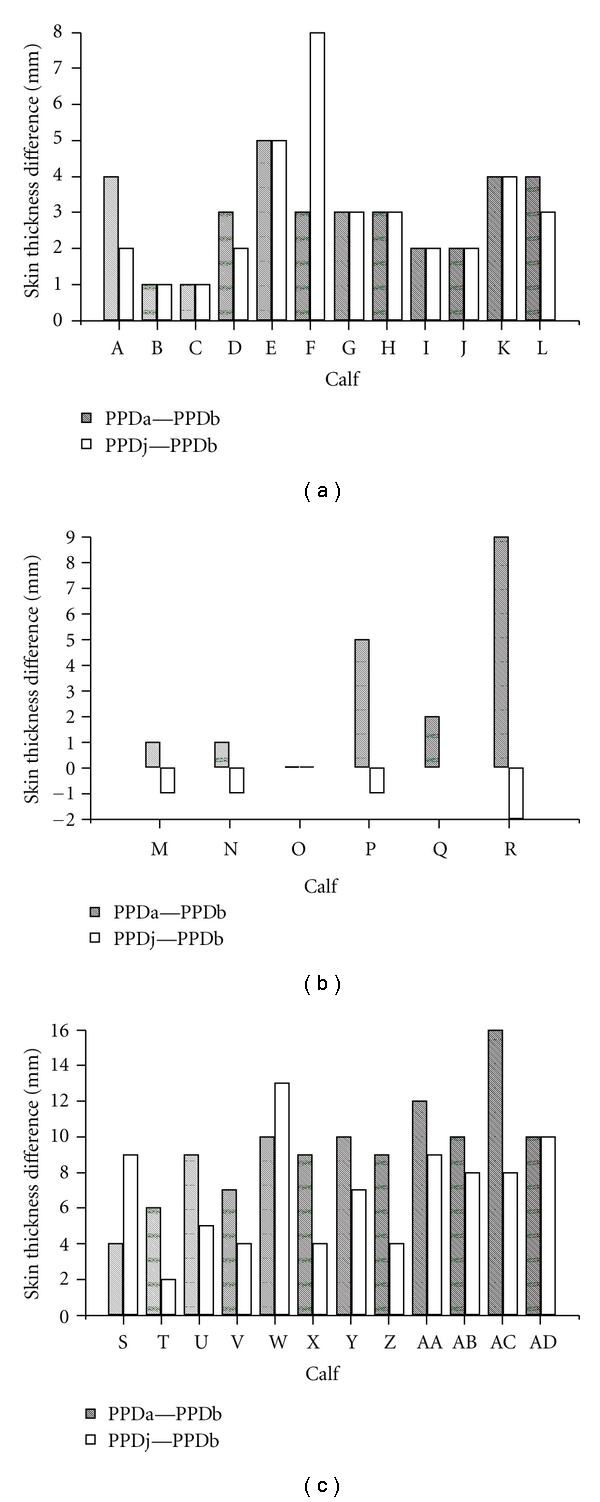
(a) Mean differences in skin thickness of 12 calves exposed to Map. Measurements were made at 39 weeks postinfection using PPDa, PPDb, and PPDj tuberculins. (b) Mean differences in skin thickness of 6 calves exposed to Maa. Measurements were made at 64 weeks postinfection using PPDa, PPDb, and PPDj tuberculins. (c) Mean differences in skin thickness of 12 calves exposed to Maa. Measurements were made after 7 weeks postinfection using PPDa, PPDb, and PPDj tuberculins.
3.2. Experiment 2—Maa Infection
The purpose of experiment 2 was to expose 6 calves to a known dose of Maa and to measure the IFN-γ released in vitro by antigen stimulated PBMCs to study CMI responses. The Maa group calves' mean IFN-γ response was different to the Map group's. Initially, there was a rapid response of released IFN-γ to PPDa, b, and j tuberculins peaking at weeks 4 and 5 (Figure 1(b)). It then decreased to a low level towards the end of the experiment. There was a high degree of correlation between the tuberculins with a consistent bias in the responses with PPDa having the greatest response and PPDb the lowest. This was also confirmed with the results of the SICCT tests (Figure 3(b)) which show that no calf would have been misclassified and that PPDa induced the greatest delayed-type hypersensitivity (DTH) reaction compared to PPDj and PPDb.
3.3. Experiment 3—Truncated Maa Infection
The purpose of repeating the Maa infection in experiment 3 was to measure the IFN-γ response immediately after exposure up to and including the IFN-γ peak (Figure 1(c)). The responses to PPDa, b, and j were muted compared to experiment 2 but this could reflect a different group of calves being used. However, response patterns were consistent showing a rapid IFN-γ response between weeks 4 and 5. Again the mean response bias was highest with PPDa then PPDj and then PPDb. However, the SICCT responses were more pronounced compared to experiments 1 and 2. Most calves showed a stronger response to PPDa compared to PPDj or PPDb. This is consistent with the SICCT being performed at the peak IFN-γ response phase. As with the previous 2 experiments, no calf's infection status was misclassified using the SICCT test (Figure 3(c)).
3.4. Mycobacterium bovis Differentiation
ESAT-6 [30, 31], an M. tuberculosis complex-specific antigen, was tested at most time points in all three experiments to examine its differentiation potential and to ensure that the PPDb response seen (Figures 1(a), 1(b), and 1(c)) was not due to prior exposure to M. bovis (data not shown). No animal showed any consistent response in any of the three experiments indicating that the PPDb responses were due to cross reactivity from exposure to either Map or Maa organisms and not from bTB infection. This supports the use of ESAT-6 as a more specific antigen for use in bTB diagnostics.
4. Discussion
The series of experiments described above demonstrates the development of infection models to define the immune responses following exposure to two nontuberculous species of mycobacteria, Map and Maa [3, 29]. Both of the species are considered to confound specificity of bTB diagnosis in cattle [21, 23]. Using IFN-γ release assays we demonstrated the development of cell-mediated immune responses to three tuberculin reagents, PPDa, PPDb, and PPDj. The IFN-γ responses to these tuberculins had a slower onset in the group exposed to Map compared to Maa, and responses remained elevated for a longer period of time postexposure. The subsequent, reduced peaks of the Maa group could be explained by the organism being reexposed to the animals' immune system, thus eliciting a more effective CMI response resulting in the organism being cleared; however, this remains uncertain from these results.
It is known that in calves infected with Map there may be a slow progressive infection which takes years to develop into a clinical state [5–7]. As Map infection progresses, the bovine immune response is increasingly exposed to bacterial antigens resulting in the development of humoral and cell-mediated immune responses [32]. In comparison, infection or exposure to Maa would appear to provoke a transient immune response which in our model resulted in a peak of IFN-γ released at approximately 5 weeks postinfection and thereafter declined. In comparison, the IFN-γ response to Map peaked much later and remained high at 39 weeks postinfection.
All IFN-γ PPD responses were significantly correlated to each other (P < .001). However, the strongest IFN-γ correlation among individual calves in the Map infection model was between PPDj and PPDa with the weakest between PPDa and PPDb. These correlations are consistent with those seen in a naturally infected herd (data not shown). The IFN-γ responses of individual calves indicate that if tested for bTB using cut-offs currently used with the Northern Ireland IFN-γ program, the majority would have been negative; however, there are some time points which would have classed the calves as positive. Therefore, this has potentially important implications for current diagnostic policies, for example, large-scale screening of animals in a Map-infected region is likely to lead to a proportion of animals incorrectly identified as M. bovis infected. However, all animals, in each experiment, would have been correctly identified as bTB negative using current skin test cut-offs. Most animals displayed a skin test reaction bias towards PPDa then PPDj and PPDb with the truncated Maa experiment resulting in the highest responses. This is probably due to the test being performed at the peak CMI response.
A recognised aspect of the IFN-γ test using tuberculins is the significant number of animals identified as M. bovis positive without any other evidence for infection [33]. While a significant proportion of these are likely to be truly infected or exposed, it is possible that some may be infected with Map. Therefore, a large-scale field study is warranted to identify if Johne's infected animals is one factor that leads to the apparent reduced specificity of the M. bovis IFN-γ test, as suggested in this study. This work suggests that the use of better defined antigens could be a useful adjunct to disentangle Map infected from M. bovis-infected animals, for example, through the use of ESAT-6.
5. Conclusions
This study illustrates the very high degree of cross reactivity between PPDa, b, and j tuberculins and also differences in the kinetics of the CMI responses after exposure to Maa and Map. Diagnostic comparative tests are essential where animals have been exposed to M. avium complex organisms to avoid misclassification of M. bovis infection status. This is particularly important if the animals are tested soon after exposure to Maa. The Map experimental model highlighted the potential for misidentification as M. bovis positive in Map infected cattle using PPDa and PPDb. Therefore, additional specific antigens may have to be incorporated into future diagnostics, for example, ESAT-6. Given that PPDa induces a skin test response in Maa-and Map-infected cattle, careful consideration should be given to the analysis and interpretation of tests in field cattle to avoid misdiagnosis of Map infection. Therefore, efforts should be made to develop comparative tests to identify Map-exposed animals from M. bovis-and Maa-exposed animals using mycobacterial species-specific diagnostics.
Acknowledgments
The authors thank Adam McGready and other members of the VSD TB Immunology group for assistance in carrying out the immunological assays, Dr. Silin for undertaking bacteriology, and the VSD Animal Care Staff. The work was funded as part of the EU ParaTB Tools Project (FOOD-CT-2006-023106) with additional funding from Agrisearch (Project VSD-D-42-08) and the Department of Agriculture and Rural Development (Project 0538).
References
- 1.Stevenson K, Alvarez J, Bakker D, et al. Occurrence of Mycobacterium avium subspecies paratuberculosis across host species and European countries with evidence for transmission between wildlife and domestic ruminants. BMC Microbiology. 2009;9, article no. 212:1–13. doi: 10.1186/1471-2180-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittington RJ, Windsor PA. In utero infection of cattle with Mycobacterium avium subsp. paratuberculosis: a critical review and meta-analysis. Veterinary Journal. 2009;179(1):60–69. doi: 10.1016/j.tvjl.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Begg DJ, Whittington RJ. Experimental animal infection models for Johne’s disease, an infectious enteropathy caused by Mycobacterium avium subsp. paratuberculosis. Veterinary Journal. 2008;176(2):129–145. doi: 10.1016/j.tvjl.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Chiodini RJ, Van Kruiningen HJ, Merkal RS. Ruminant paratuberculosis (Johne’s disease): the current status and future prospects. The Cornell Veterinarian. 1984;74(3):218–262. [PubMed] [Google Scholar]
- 5.Doyle T. Susceptibility to Johne’s disease in relation to age. The Veterinary Record. 1953;65(23):363–365. [Google Scholar]
- 6.Payne J, Rankin J. A comparison of the pathogenesis of eperimental Johne’s disease in calves and cows. Research in Veterinary Science. 1961;2:175–179. [Google Scholar]
- 7.Windsor PA, Whittington RJ. Evidence for age susceptibility of cattle to Johne’s disease. Veterinary Journal. 2010;184(1):37–44. doi: 10.1016/j.tvjl.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Clarke CJ. The pathology and pathogenesis of paratuberculosis in ruminants and other species. Journal of Comparative Pathology. 1997;116(3):217–261. doi: 10.1016/s0021-9975(97)80001-1. [DOI] [PubMed] [Google Scholar]
- 9.Benedictus G, Dijkhuizen AA, Stelwagen J. Economic losses due to paratuberculosis in dairy cattle. Veterinary Record. 1987;121(7):142–146. doi: 10.1136/vr.121.7.142. [DOI] [PubMed] [Google Scholar]
- 10.Stott AW, Jones GM, Humphry RW, Gunn GJ. Financial incentive to control paratuberculosis (Johne’s disease) on dairy farms in the United Kingdom. Veterinary Record. 2005;156(26):825–831. doi: 10.1136/vr.156.26.825. [DOI] [PubMed] [Google Scholar]
- 11.Köhler H, Burkert B, Pavlik I, et al. Evaluation of five ELISA test kits for the measurement of antibodies against Mycobacterium avium subspecies paratuberculosis in bovine serum. Berliner und Munchener Tierarztliche Wochenschrift. 2008;121(5–6):203–210. [PubMed] [Google Scholar]
- 12.McKenna SLB, Keefe GP, Barkema HW, Sockett DC. Evaluation of three ELISAs for Mycobacterium avium subsp. paratuberculosis using tissue and fecal culture as comparison standards. Veterinary Microbiology. 2005;110(1–2):105–111. doi: 10.1016/j.vetmic.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen SS, Toft N. Ante mortem diagnosis of paratuberculosis: a review of accuracies of ELISA, interferon-γ assay and faecal culture techniques. Veterinary Microbiology. 2008;129(3–4):217–235. doi: 10.1016/j.vetmic.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Bannantine JP, Zhang Q, et al. The complete genome sequence of Mycobacterium avium subspecies paratuberculosis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(35):12344–12349. doi: 10.1073/pnas.0505662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bannantine JP, Bayles DO, Waters WR, Palmer MV, Stabel JR, Paustian ML. Early antibody response against Mycobacterium avium subspecies paratuberculosis antigens in subclinical cattle. Proteome Science. 2008;6, article no. 5:1–12. doi: 10.1186/1477-5956-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Juan L, Álvarez J, Romero B, et al. Comparison of four different culture media for isolation and growth of type II and type I/III Mycobacterium avium subsp. paratuberculosis strains isolated from cattle and goats. Applied and Environmental Microbiology. 2006;72(9):5927–5932. doi: 10.1128/AEM.00451-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stabel JR. Production of γ-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. Journal of Veterinary Diagnostic Investigation. 1996;8(3):345–350. doi: 10.1177/104063879600800311. [DOI] [PubMed] [Google Scholar]
- 18.OIE, World Organisation for Animal Health. Manual of Standards for Diagnostic Tests and Vaccines. 4th edition. Paris, France: World Organisation for Animal Health, O.I.d.E. (OIE), Office International des Epizooties; 2000. [Google Scholar]
- 19.Gwozdz JM, Thompson KG, Murray A, Reichel MP, Manktelow BW, West DM. Comparison of three serological tests and an interferon-γ assay for the diagnosis of paratuberculosis in experimentally infected sheep. Australian Veterinary Journal. 2000;78(11):779–783. doi: 10.1111/j.1751-0813.2000.tb10452.x. [DOI] [PubMed] [Google Scholar]
- 20.Huda A, Jungersen G, Lind P. Longitudinal study of interferon-gamma, serum antibody and milk antibody responses in cattle infected with Mycobacterium avium subsp. paratuberculosis. Veterinary Microbiology. 2004;104(1–2):43–53. doi: 10.1016/j.vetmic.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Álvarez J, de Juan L, Bezos J, et al. Effect of paratuberculosis on the diagnosis of bovine tuberculosis in a cattle herd with a mixed infection using interferon-gamma detection assay. Veterinary Microbiology. 2009;135(3–4):389–393. doi: 10.1016/j.vetmic.2008.09.060. [DOI] [PubMed] [Google Scholar]
- 22.Pollock JM, Andersen P. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. Journal of Infectious Diseases. 1997;175(5):1251–1254. doi: 10.1086/593686. [DOI] [PubMed] [Google Scholar]
- 23.Aagaard C, Govaerts M, Meikle V, et al. Detection of bovine tuberculosis in herds with different disease prevalence and influence of paratuberculosis infection on PPDB and ESAT-6/CFP10 specificity. Preventive Veterinary Medicine. 2010;96(3–4):161–169. doi: 10.1016/j.prevetmed.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen SS, Toft N. A review of prevalences of paratuberculosis in farmed animals in Europe. Preventive Veterinary Medicine. 2009;88(1):1–14. doi: 10.1016/j.prevetmed.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 25.SB4022: An Integrated Strategy to Determine the Herd Level Prevalence of Johne’s Disease in the UK Dairy Herd. DEFRA; 2009. [Google Scholar]
- 26.Schiller I, Vordermeier HM, Waters WR, et al. Comparison of tuberculin activity using the interferon-γ assay for the diagnosis of bovine tuberculosis. Veterinary Record. 2010;167(9):322–326. doi: 10.1136/vr.c3403. [DOI] [PubMed] [Google Scholar]
- 27.Bannantine JP, Baechler E, Zhang Q, Li L, Kapur V. Genome scale comparison of Mycobacterium avium subsp. paratuberculosis with Mycobacterium avium subsp. avium reveals potential diagnostic sequences. Journal of Clinical Microbiology. 2002;40(4):1303–1310. doi: 10.1128/JCM.40.4.1303-1310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweeney RW, Uzonna J, Whitlock RH, Habecker PL, Chilton P, Scott P. Tissue predilection sites and effect of dose on Mycobacterium avium subs. paratuberculosis organism recovery in a short-term bovine experimental oral infection model. Research in Veterinary Science. 2006;80(3):253–259. doi: 10.1016/j.rvsc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Hines ME, Stabel JR, Sweeney RW, et al. Experimental challenge models for Johne’s disease: a review and proposed international guidelines. Veterinary Microbiology. 2007;122(3–4):197–222. doi: 10.1016/j.vetmic.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Buddle BM, Ryan TJ, Pollock JM, Andersen P, de Lisle GW. Use of ESAT-6 in the interferon-γ test for diagnosis of bovine tuberculosis following skin testing. Veterinary Microbiology. 2001;80(1):37–46. doi: 10.1016/s0378-1135(00)00375-8. [DOI] [PubMed] [Google Scholar]
- 31.van Pinxteren LAH, Ravn P, Agger EM, Pollock J, Andersen P. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clinical and Diagnostic Laboratory Immunology. 2000;7(2):155–160. doi: 10.1128/cdli.7.2.155-160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stabel JR. Host responses to Mycobacterium avium subsp. paratuberculosis: a complex arsenal. Animal Health Research Reviews. 2007;7(1–2):61–70. doi: 10.1017/S1466252307001168. [DOI] [PubMed] [Google Scholar]
- 33.Welsh MD, McNair J, McDowell SWJ, et al. The Northern Ireland interferon-gamma (IFN-g) testing programme. Cattle Practice. 2008;16(2):136–139. [Google Scholar]


