Abstract
The cingulate cortex frequently shows gray matter loss with age as well as gender differences in structure and function, but little is known about whether individual cingulate Brodmann areas show gender-specific patterns of age-related volume decline. This study examined age-related changes, gender differences, and the interaction of age and gender in the relative volume of cingulate gray matter in areas 25, 24, 31, 23, and 29, over seven decades of adulthood. Participants included healthy, age-matched men and women, aged 20–87 (n = 70). Main findings were: (1) The whole cingulate showed significant age-related volume declines (averaging 5.54% decline between decades, 20s–80s). Each of the five cingulate areas also showed a significant decline with age, and individual areas showed different patterns of decline across the decades: Smaller volume with age was most evident in area 31, followed by 25 and 24. (2) Women had relatively larger cingulate gray matter volume than men overall and in area 24. (3) Men and women showed different patterns of age-related volume decline in area 31, at midlife and late in life. By delineating normal gender differences and age-related morphometric changes in the cingulate cortex over seven decades of adulthood, this study improves the baseline for comparison with structural irregularities in the cingulate cortex associated with psychopathology. The Brodmann area-based approach also facilitates comparisons across studies that aim to draw inferences between age- and gender-related structural differences in the cingulate gyrus and corresponding differences in cingulate function.
Keywords: Cingulate cortex, aging, gender differences, MRI, gray matter, morphometry
1. Introduction
The cingulate cortex comprises a number of structurally and functionally distinct areas (Devinsky et al., 1995; Vann et al., 2009; Vogt et al., 1992; Vogt et al., 1995) that mediate aspects of attention, emotional regulation, and the integration of cognitive and emotional processes, among other functions (Bush et al., 2000; Critchley, 2005; Devinsky et al., 1995; Vogt and Laureys, 2005). In studies of healthy aging, the cingulate has often shown vulnerability to gray matter loss (Alexander et al., 2006; Bergfield et al., 2010; Good et al., 2001b; Jernigan et al., 2001; Raji et al., 2009; Resnick et al., 2003; Salat et al., 2009; Sowell et al., 2003; Takahashi et al., 2010), although some studies have found relative sparing (Fjell et al., 2009; Grieve et al., 2005; Raz et al., 1997; Raz et al., 2004). Studies have typically found more significant gray matter loss with age in the anterior cingulate cortex (ACC; Alexander et al., 2006; Bergfield et al., 2010; Good et al., 2001b; Salat et al., 2009; Sowell et al., 2003; but also see Raz et al., 1997; Raz et al., 2004), while the posterior cingulate cortex (PCC) has more often shown relative structural preservation (Kalpouzos et al., 2009; Smith et al., 2007). Given that the cingulate contributes to higher-order cognitive processes and appears susceptible to age-related gray matter loss, its atrophy potentially contributes to important aspects of cognitive decline with age (Hazlett et al., 2010; Vaidya et al., 2007).
One of the few studies to examine age effects on cingulate subregions in healthy adults analyzed gray matter volume and cerebral blood flow (CBF) in the ACC's dorsal, rostral, and subgenual components (Vaidya et al., 2007). Older age correlated most significantly with smaller volume and lower CBF in the rostral ACC (approximately areas 24a and 24b), and volume decline only partially mediated age effects on CBF. Because of the ACC's role in emotional regulation, these findings suggest that improving vascular health in the elderly may help to reduce their depressive symptoms (Vaidya et al., 2007). Our prior examination of aging effects on glucose metabolism yielded similar results; normal controls' metabolism decreased with age in area 24 and not in the PCC (Buchsbaum and Hazlett, 1997).
A concurrent, but largely separate, line of research from the aging studies has shown the cingulate to be site of structural and functional gender differences. Women typically have proportionally larger cingulate gray matter volume than men (Chen et al., 2007; Cosgrove et al., 2007; Goldstein et al., 2010; Good et al., 2001a; Paus et al., 1996). Most reports of more specific morphological gender differences have focused on the ACC (e.g., Brun et al., 2009; Kovalev et al., 2003; Pujol et al., 2002; Yücel et al., 2001). Functional gender differences have also been reported more frequently in the ACC than the PCC—often related to the ACC's role in emotion (Wager et al., 2003) but also in cognitive domains (Butler et al., 2007; Hazlett et al., 2010). Though the PCC has less often shown functional gender differences, some have been found during emotional responding (Proverbio et al., 2009) and emotional memory (Canli et al., 2002).
In light of the evidence that the cingulate shows age- and gender-related differences, it is notable that relatively few studies examining both age and gender effects in this region have found age by gender interactions. In the analyses that did report sex differences in the effects of age in this region, men showed more age-related gray matter loss in the cingulate gyrus (Takahashi et al., 2010; Thambisetty et al., 2010) and the cingulate sulcus (Kochunov et al., 2005) than women. However, most similar studies found no age by gender interaction (Alexander et al., 2006; Bergfield et al., 2010; Good et al., 2001b; Lemaitre et al., 2005; Smith et al., 2007; Sowell et al., 2007). Inconsistent findings across studies may be due to differences in their characterizations of “ACC” and “PCC,” respectively (Nielsen et al., 2005). Also of note, these studies—with the exception of Sowell et al. (2007)—like a number of other investigations of age effects that included the cingulate cortex (e.g., Grieve et al., 2005; Raji et al., 2009; Resnick et al., 2003; Salat et al., 2009; Takahashi et al., 2010), used voxel-based morphometry to examine many brain regions and structures for focal areas of age-related change. Together, these factors make it difficult to compare findings and track patterns of age-related gray matter loss in individual cingulate areas.
Consequently, little is known about whether age effects on individual cingulate regions unfold during different time frames in men and women. The current study examined age-related changes, gender differences, and their interaction in cingulate gray matter volume in a large sample (n = 70). We examined five cingulate regions, approximating Brodmann areas 25, 24, 31, 23, 29. This approach facilitates volumetric comparisons of individual areas and allows for averaging of relative volume across areas or hemispheres based on neuroanatomical and theoretical assumptions.
This study extends our previously published FDG-PET studies of this same sample, which showed age-related declines in frontal lobe metabolism and verbal memory performance (Hazlett et al., 1998), and age-related gender differences in relative glucose metabolism that were specific to the cingulate gyrus; they did not characterize other frontal lobe regions or the temporal, parietal, or occipital lobes (Hazlett et al., 2010). These earlier FDG-PET findings drove the hypotheses of the current morphometric analyses. In particular, the latter study, which analyzed relative glucose metabolism in the gray matter of 39 cortical areas during a verbal memory task, found the strongest correlations with age in areas 25 and 24; the largest gender differences in areas 24 and 29; and a significant gender-specific correlation between relative glucose metabolism and memory performance in area 24. Based on these results, we hypothesized (1) the ACC (areas 25 and 24) would show the most robust age-related volume decline; (2) area 24 would show the most pronounced gender differences in volume; and (3) men and women would show different patterns of cingulate gray matter loss with age.
2. Results
2.1 Whole brain volume
Men (1,246,677 ± 78,681 mm3) had larger whole brain volume than women (1,126,012 ± 109,366 mm3), F(1,56) = 30.79, p < 0.0005. Whole brain volume did not show a significant main effect of Decade (F(6,56) = 1.74, p = 0.128) or a significant Decade by Gender interaction (F(6,56) = 0.41, p = 0.868).
2.2 Age-related volume decline in cingulate Brodmann areas
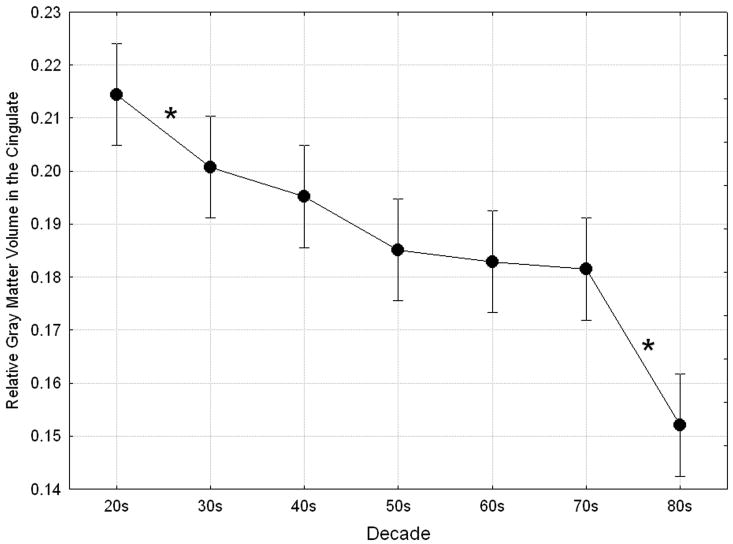
There was a significant overall effect of Decade in the cingulate cortex (averaged across gender, Brodmann area, and hemisphere) reflecting a decrease in volume from age 20 to 87 (Main effect of Decade, F(6, 56) = 16.48, p < 0.0005; Fig. 1). Planned post hoc comparisons of cingulate volume between successive decades (20s vs. 30s, 30s vs. 40s, etc.) showed significant volume decreases between the 20s and 30s (p = 0.048, 6.32% difference) and between the 70s and 80s (p < 0.0005, 15.95% difference). Overall, the relative volume of cingulate gray matter declined an average of 5.54% between decades from the 20s to the 80s. Correlational analyses showed that the linear rate of age-related gray matter loss was significant (averaged across hemispheres and Brodmann areas; r = -0.70, p < 0.0005) and in the left (r = -0.71, p < 0.0005) and right (r = -0.65, p < 0.0005) hemispheres individually.
Figure 1.
Aging effects in the cingulate cortex. Age-related gray matter loss showed a significant main effect of Decade, F(6, 56) = 16.48, p < 0.0005, Wilks. Asterisks indicate significant declines in gray matter volume between pairs of successive decades.
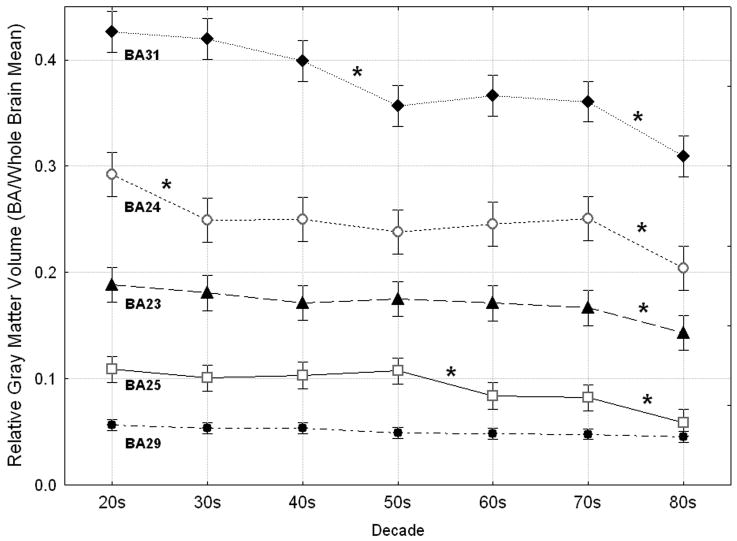
The five Brodmann areas also showed different patterns of age-related decline in gray matter volume (averaged across gender and hemisphere), resulting in a significant Decade by Cingulate area interaction (Decade × Cingulate area, F(24, 186) = 5.45, p < 0.0005, Fig. 2). A simple effects analysis determined which pairs of successive decades of age were associated with significant gray matter loss in each area. The linearity of age-related change was confirmed using age as a continuous variable in a regression analysis of gray matter volume in each area.
Figure 2.
Differential effects of age on cingulate Brodmann areas. The five cingulate areas showed different patterns of age-related gray matter decline (Decade × Cingulate area, F(24, 186) = 5.45, p < 0.0005, Wilks). Asterisks indicate significant declines in gray matter volume between pairs of successive decades (post hoc Fischer's LSD, df = 6, all p values ≤ 0.049).
2.2.1 Area 25
Area 25 showed a significant effect of decade on relative gray matter volume (F(6, 56) = 8.74, p < 0.0005; Fig. 2). Post hoc tests of the volume differences between successive decades revealed two significant declines, the first at midlife and the second in old age. Participants in their 60s had significantly less gray matter in area 25 than those in their 50s (p = 0.010, 21.78% difference), and participants in their 80s had significantly less gray matter in this area than those in their 70s (p = 0.009, 28.64% difference). Correlational analyses also demonstrated a significant linear rate of age-related volume decline in area 25 averaged across hemispheres (r = -0.55, p < 0.0005), as well as in the left (r = -0.54, p < 0.0005) and right (r= -0.53, p < 0.0005) hemispheres individually.
2.2.2 Area 24
Area 24 also showed a significant effect of Decade on volume (F(6, 56) = 6.30, p < 0.0005; Fig. 2). Gray matter volume in BA 24 showed significant declines between successive decades in early adulthood and in old age. Participants in their 30s had significantly smaller volume in area 24 than participants in their 20s (p = 0.005, 14.67% difference), and participants in their 80s had significantly smaller volume in this area than those in their 70s (p = 0.002, 18.61% difference). Correlational analyses showed a significant linear rate of volume decline in area 24 averaged across hemispheres (r = -0.45, p < 0.0005), as well as in the left (r = -0.46, p < 0.0005) and right (r = -0.39, p = 0.001) hemispheres individually.
2.2.3 Area 31
Among the five cingulate regions, area 31 showed the most significant effect of Decade on relative volume of gray matter (F(6, 56) = 18.50, p < 0.0005; Fig. 2). In this area, volume declined significantly at midlife and in old age. Participants in their 50s had significantly less gray matter in area 31 than those in their 40s (p < 0.0005, 10.53% difference), and participants in their 80s had significantly less gray matter than those in their 70s (p = 0.003, 14.26% difference). Correlational analyses indicated a significant linear rate of decline in gray matter in area 31 averaged across hemispheres (r = -0.74, p < 0.0005), and in each hemisphere (left: r = -0.74, p < 0.0005; right: r = -0.67, p < 0.0005).
2.2.4 Area 23
The gray matter volume of area 23 showed a significant effect of Decade (F(6, 56) = 2.97, p = 0.014; Fig. 2). This area a showed a significant volume difference between successive decades only late in life. Participants in their 80s had significantly smaller volume in area 23 than those in their 70s (p = 0.049, 14.06% difference). Correlational analyses demonstrated a significant linear rate of decline in gray matter in area 23 averaged across hemispheres (r = -0.42, p < 0.0005), and in the left hemisphere (r = -0.46, p < 0.0005). The correlation between age and volume in right area 23 did not remain significant after Bonferroni correction (r = -0.32, p = 0.007).
2.2.5 Area 29
The volume of area 29 also showed a significant effect of Decade (F(6, 56) = 2.51, p = 0.032; Fig. 2), though its rate of decline was consistently gradual and none of the volume differences between pairs of successive decades reached significance. Correlational analyses demonstrated a significant linear rate of gray matter decline in area 29 averaged across hemispheres (r = -0.42, p <0.0005), as well as in the right hemisphere (r = -0.44, p < 0.0005). The correlation between age and volume in the left hemisphere did not remain significant after Bonferroni correction (r = -0.33, p = 0.006).
2.3 Gender differences in volume of cingulate Brodmann areas
Women had relatively larger volume of gray matter in the cingulate cortex compared to men (main effect of Gender, F(1, 56) = 13.91 p < 0.0005, 6.97% difference; mean relative volume expressed as mean gray matter volume for the area/whole brain mean), women: 0.194 ± 0.024; men: 0.181 ± 0.023. Women had larger volume in area 24 (p < 0.0005, 13.14% difference) and there were no gender differences in areas 25, 31, 23, and 29 (Gender × Cingulate area, F(4, 53) = 3.37, p = 0.016).
Correlations between age and relative gray matter volume were also computed for each gender separately and subjected to Bonferroni correction. In these separate analyses of each gender, the age–volume relationship in area 25 bilaterally was significant both for men (left: r = -0.50, p = 0.002; right: r = -0.61, p < 0.0005) and for women (left: r = -0.59, p < 0.0005; right: r = -0.50, p = 0.002). The age–volume correlation was also significant in area 31 bilaterally, both for men (left: r = -0.70, p < 0.0005; right: r = -0.70, p < 0.0005) and for women (left: r = -0.79, p < 0.0005; right: r = -0.65, p < 0.0005).
Only in women, age and volume were significantly correlated in area 24 bilaterally (left: r = -0.62, p < 0.0005; right: r = -0.53, p < 0.0005). Only in men, age–volume correlations were significant in left area 23 (r = -0.50, p = 0.002) and in right area 29 (r = -0.49, p = 0.003). Fisher's Z tests indicated that gender differences in the age–volume correlations did not reach significance in any region.
2.4 Decade by Gender interaction in volume of cingulate Brodmann areas
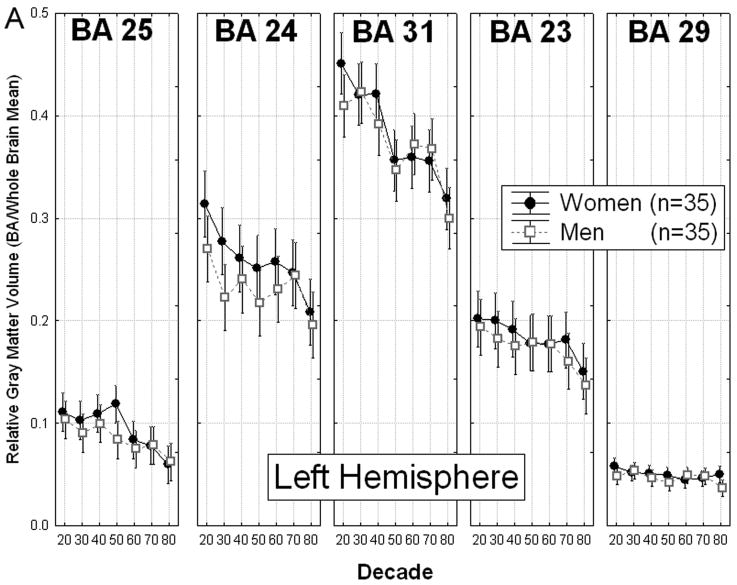
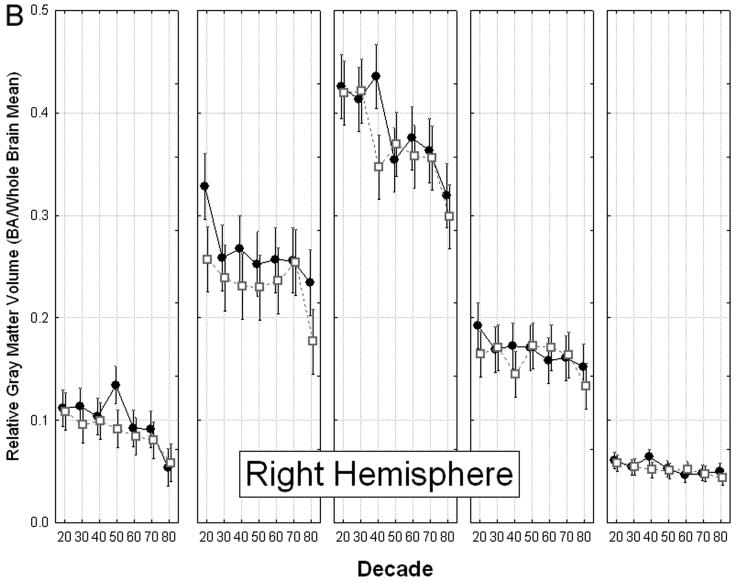
Changes in cingulate gray matter volume in men and women across the seven decades showed a complex pattern (Decade × Gender × Cingulate area × Hemisphere interaction, F(24, 186) = 1.59, p = 0.047, Fig. 3), which was interpreted by means of a simple effects analysis.
Figure 3.
Gender differences in age-related volume loss in cingulate Brodmann areas. A: Left hemisphere. B: Right hemisphere. The relative volume of cingulate BAs showed a complex pattern of aging effects and gender differences, with a significant Decade × Gender × Cingulate area × Hemisphere interaction, F(24, 186) = 1.59, p = 0.047, Wilks. As shown, the largest gender differences were in right area 24 and the smallest differences were in area 29 bilaterally. Greatest decline with age was observed in area 31, and the smallest decline was in area 29.
Analysis of simple effects indicated that only area 31 showed patterns of age-related change that differed significantly between men and women (Decade × Gender × Hemisphere interaction, F(6,56) = 2.51, p = 0.032). Gender differences in this region's pattern of volume decline were apparent at midlife and late in life.
Although both genders showed a significant reduction in volume in area 31 bilaterally between the 30s and the 50s, the decrease occurred during different decades of age for men than for women. In men, the volume of right area 31 was significantly smaller among participants in their 40s than among those in their 30s (p = 0.008, 17.53% difference). In contrast, women showed no significant decline in area 31 volume between the 30s and 40s. They did, however, show a decline of similar magnitude in area 31 bilaterally between the 40s and 50s (left: p = 0.020, 15.34% difference; right: p = 0.004, 18.56% difference). Thus, volume of right area 31 was significantly smaller among men in their 40s than among age-matched women (p = 0.002 25.30% difference). However, due to women's significant bilateral volume decline in this area between the 40s and 50s, this gender difference did not characterize subsequent decades (50s, 60s, 70s).
Men and women also differed in the pattern of volume loss in area 31 in old age. Men in their 80s showed significantly smaller volume in area 31 bilaterally than those in their 70s (left, p = 0.016, 18.33% difference; right, p = 0.038, 16.10% difference). Women in these same decades of age showed a volume decrease that did not reach significance.
3. Discussion
Analyses of age-related decline and gender differences in the gray matter volume of five cingulate Brodmann areas revealed distinctive patterns of change in individual areas and significant morphological gender differences. The main findings are: (1) Each of the five cingulate areas showed a significant age-related decline in relative gray matter volume, and they showed different patterns of decline across the decades. Smaller volume with age was most evident in area 31, followed by areas 25 and 24. This effect was notably weaker, although still statistically significant, in areas 23 and 29. (2) Women had relatively larger gray matter volume than men in the cingulate cortex overall and in area 24. (3) Men and women showed different patterns of age-related decline in gray matter volume in area 31, at midlife and late in life.
3.1 Age-related volume decline in cingulate Brodmann areas
This morphometric analysis of cingulate gray matter documented different patterns of age-related volume decline in individual cingulate areas. The finding of more significant age-related decline in areas 24 and 25 (ACC) than in areas 23 and 29 of the PCC accords with prior observations that ACC gray matter appears more vulnerable to age-related volume decline (Alexander et al., 2006; Bergfield et al., 2010; Good et al., 2001b; Salat et al., 2009; Sowell et al., 2003; but see Raz et al., 1997; Raz et al., 2004) than PCC gray matter (Kalpouzos et al., 2009; Smith et al., 2007), and that the ACC shows age-related declines in blood flow (Schultz et al., 1999; Vaidya et al., 2007). More generally, this pattern of findings supports a refined frontal aging hypothesis (Tisserand and Jolles, 2003), suggesting that particular frontal lobe structures are more vulnerable to effects of age than other brain areas are.
Age-related changes in the ACC have been associated with declines in various types of cognitive performance. Relative to younger adults, older people show differences in ACC activity associated with poorer attentional control on the Stroop task (Milham et al., 2002), declines in verbal fluency (Pardo et al., 2007) and route encoding (Meulenbroek et al., 2004), as well as slower (Keightley et al., 2007) and less accurate (Gunning-Dixon et al., 2003) processing of negative facial expressions, among other functions. Among the oldest participants, we observed a more significant effect of decade in area 24 than in any other cingulate area that we examined, consistent with Vaidya and colleagues' (2007) finding that gray matter volume in subregions 24a and 24b declined more significantly with age than other ACC subregions did. Area 24 hyperactivity during a Stroop-like task has also been shown to distinguish elderly subjects with late-life depression and comorbid anxiety from those with pure late-life depression, suggesting a key role for area 24 in dysfunctional threat-monitoring late in life (Andreescu et al., 2009), the time frame in which its volume also declines significantly.
Area 24 was also the only area to show marked age-related volume loss between participants in their 20s and those in their 30s. This finding in a rigorously screened, healthy cohort is interesting in light of the relative prevalence in early adulthood (Kessler et al., 2005; Lenzenweger et al., 2007) of psychiatric diagnoses that have also been associated with area 24 abnormalities, such as major depression (Mayberg et al., 1997; Yücel et al., 2008), bipolar disorder (Fountoulakis et al., 2008), borderline personality disorder (Hazlett et al., 2005), restrictive anorexia nervosa (Joos et al., 2010) and schizophrenia (Mitelman et al., 2005).
More generally, the pattern of greater volume decline in the ACC than the PCC in these healthy volunteers aligns with the evidence that relative preservation of PCC gray matter may distinguish healthy older adults from those with either mild cognitive impairment (MCI; for many a prodromal stage of Alzheimer's disease) or early-stage Alzheimer's disease (Kalpouzos et al., 2009; McDonald et al., 2009). Although normal aging in late middle age has been longitudinally associated with PCC metabolic decline (Caselli et al., 2008), other studies have correlated Alzheimer's disease and MCI with area 29 hypoactivity (Desgranges et al., 2002) and gray matter atrophy (Pengas et al., 2010) that are not considered characteristic of healthy aging.
Because the literature consistently describes greater age-related decline in the ACC than the PCC in healthy adults, the finding that area 31 showed the greatest reduction in volume with age was unexpected. However, to our knowledge, most studies reporting relative PCC preservation with age have not specifically examined volumetric change in area 31. Instead, reports of normal aging effects typically either describe preservation in the PCC at large (e.g., Smith et al., 2007; Sowell et al., 2003) or report values from individual PCC regions other than area 31 (e.g., areas 23 and 30 in Bergfield et al., 2010; Jones et al., 2006; Kalpouzos et al., 2009). Likewise, comparisons of normal PCC volume decline with age and the effects of Alzheimer's disease and MCI generally have not included area 31 (e.g., areas 23 and 29/30 in Jones et al., 2006; Pengas et al., 2010). Our finding is also consistent with functional imaging studies showing that the PCC (Caselli et al., 2008) and area 31 in particular (Grady et al., 2006; Lustig et al., 2003; Sambataro et al., 2010) are vulnerable to the effects of normal aging.
As described in postmortem analyses, area 31 includes the thickest layer IV of any cingulate area and the highest neurofilament protein immunoreactivity (Vogt et al., 2001). Because layer IV is a primary termination for specific thalamocortical projections, and because the thalamus shows a linear age-related decrease in volume between the third and eighth decades (Sullivan et al., 2004), an age-related loss of thalamocortical synapses in layer IV may contribute to the magnitude of area 31 volume loss. Current histology-based literature suggests that cortical volume loss in normal aging is unlikely to originate from neuronal death; careful postmortem studies have found relatively comparable neuronal counts between older and younger subjects (Morrison and Hof, 1997). Instead, neuronal shrinkage and reduction in dendritic arborization are more likely to account for cortical thinning (Hof and Morrison, 2004). Area 31 stands out as the only component of the posteromedial cortex that has efferent or afferent (or both) connections with all regions of the cingulate gyrus, superior and inferior parietal lobules, the frontal pole, and entorhinal cortex (Parvizi et al., 2006). Together with area 23, it has the highest functional connectivity density of any brain region (Tomasi and Volkow, 2010). To the extent that cortical atrophy with aging reflects dendritic reduction and loss of synapses, the high synaptic density of area 31 could make it particularly vulnerable to such loss.
Although caution is warranted when inferring change from cross-sectional data (Kraemer et al., 2000), the finding that cingulate gray matter undergoes the most pervasive declines at midlife (between the 40s and 50s in area 31, and between the 50s and 60s in area 25) and late in life (between the 70s and 80s in areas 25, 24, 31, and 23) aligns with other reports of the age spans associated with significant cortical volume loss. Westlye et al. (2010) reported that the cingulate showed significant reductions in gray matter signal intensity in middle age—making it one of the earliest cortical structures to show age-related deterioration—and that elderly participants showed the most widespread effects of age on cortical gray matter.
3.2 Gender differences in volume of cingulate Brodmann areas
Consistent with previous reports, women in this cohort had relatively larger cingulate gray matter volume than men (e.g., Chen et al., 2007; Cosgrove et al., 2007; Good et al., 2001a; Paus et al., 1996), and this volume difference was localized to the ACC (Brun et al., 2009; Pujol et al., 2002). Other reports of specific morphological gender differences in the ACC include greater fissuration in the left ACC in men (Yücel et al., 2001), and greater asymmetry in the textural features of posterior cingulate in men (Kovalev et al., 2003).
Area 24, which showed gender differences in volume in this cohort, has also shown functional gender differences, frequently in studies of emotional processes. In a meta-analysis of 65 functional neuroimaging studies of the effects of emotional valence, women showed significantly greater peak density of activation than men in area 24 (Wager et al., 2003). In the current cohort, the correlation between age and relative gray matter volume in area 24 was significant in women bilaterally but not significant in men. Women also showed a greater age-related decline than men in area 24 between the 20s and 30s, although the gender difference did not reach significance.
Several of the aforementioned disorders associated with area 24 irregularities—major depression (Kessler et al., 2005), bipolar II disorder (Arnold, 2003), borderline personality disorder (Lenzenweger et al., 2007), and restrictive anorexia nervosa (Hudson et al., 2007)—are more prevalent among women than men. Although the other previously mentioned disorders characterized by area 24 abnormalities—bipolar I disorder and schizophrenia—do not share this gender difference in prevalence, women are less likely than men to be diagnosed with either disorder before reaching early adulthood (Sherazi et al., 2006).
The current findings, along with reports of maturation of cingulate gray matter continuing into young adulthood (e.g., Westlye et al., 2010), suggest that future studies examining ACC morphology and morphometry should carefully account for both the age and gender of participants as potential confounds, and that participants' ages (or the breadth of their age range) likely affect measurements of the ACC throughout adulthood.
3.3 Decade by Gender interaction in volume of cingulate Brodmann areas
In the current study, only area 31 showed a gender difference in age-related volume loss. In this area, men and women differed in patterns of change at midlife (30s through 50s) and late in life (between the 70s and 80s). To our knowledge, prior studies reporting gender differences in age-related cingulate volume loss have not examined individual PCC regions. However, one study describing a significant gender difference in area 31 gray matter volume was an examination of regional gray matter volumes in a large sample (n = 411) of healthy, middle-aged adults (age 44–48; Chen et al., 2007). Among the regions examined in this cohort, gray matter volume in women most significantly exceeded volume in men in dorsal posterior cingulate (i.e., area 31), which showed a larger gender difference than any other cingulate region. The current finding that only men showed a significant age-related decline in gray matter volume in area 31 between the 30s and 40s, and that only women showed an age-related decline of similar magnitude in area 31 between the 40s and 50s, suggests that the gender difference in area 31 described by Chen and colleagues (2007) may be specific to a circumscribed midlife age range.
3.4 Limitations
A number of limitations in the present study ought to be kept in mind, including: (1) The stereotaxic Perry atlas (Perry et al., 1991) has limitations that are both analogous and complementary to those of significance probability mapping. Both methods combine subjects and are limited by the quality of brain normalization. Our study is limited by the accuracy of the Perry atlas, lack of statistical information on its variability across subjects, and imperfections in applying the atlas with segmentation to individual brains, as discussed elsewhere (Mitelman et al., 2003), particularly in regions such as the cingulate gyrus that exhibit marked variability among individuals. (2) Gray matter segmentation is also subject to variations, such that threshold error could influence measurements of gray matter volume. Due to age-related thinning of the cortex or widening of sulci, fewer voxels might be identified as gray matter and included in older participants' volume measures. (3) Our cross-sectional design requires more caution than a longitudinal design would in inferring decline (Kraemer et al., 2000). However, given that our study examined volumetric differences across 70 years, logistical concerns with participant attrition and maintenance of consistent scanner mechanics would make a longitudinal design unfeasible. Furthermore, even a 5-year interval between scans would reduce statistical power in inferring age-related decline. (4) Participants' high level of education may limit the generalizability of these findings, as education level has sometimes (Coffey et al., 1999), though not consistently (Raz et al., 2005), been associated with reduced cortical atrophy in old age. (5) Morphometric differences in mid and late life, either between genders or among women, may also be affected by gonadal hormone levels. We did not, however, collect endocrine data such as menopausal status or use of hormonal replacement therapy. Because estrogen replacement therapy has shown some neuroprotective effects (see Sherwin and Henry, 2008 for a review), including potentially slowing age-related gray matter loss in the anterior and posterior cingulate (Boccardi et al., 2006), hormone replacement could either increase or diminish age-related gender differences in cingulate gray matter volume. Our finding of nonsignificant volume increases in areas 25 and 24 in middle-aged women may relate to neuroprotective effects of hormone replacement therapy, though this is speculative. Future studies of gender differences in brain morphometry that include menopausal or postmenopausal women should take these considerations into account. In addition, future work with a larger number of participants will be useful for replicating our findings and extending them to other structural imaging techniques, including diffusion tensor, to better understand gender and aging effects in the cingulate gyrus.
4. Conclusion
This study represents an important step toward delineating age-related change and gender differences in the cingulate over the healthy adult lifespan. Animal work and postmortem studies are refining cingulate parcellation in terms of its cytoarchitecture (Vogt, 2009), and functional imaging continues to advance the understanding of individual cingulate regions' contributions to normal and pathological brain function. In this context, more precisely documenting normal age-related morphometric changes and gender differences, including patterns of change in individual areas, can provide an important link between the anatomical findings and their implications for psychopathology, functional gender differences, and cognitive and affective changes with age.
5. Experimental procedure
5.1. Participants
The study included 70 healthy adults (age range = 20–87 years) recruited through advertisements in the community (for more details on the sample, see Hazlett et al., 1998). The group included five men and five women within each of seven consecutive decades of age from the 20s through the 80s (women: mean age = 54.4 ± 20.4 years; men: mean age = 54.5 ± 20.1 years; mean age for women and men within each decade did not significantly differ). All participants were right-handed, spoke English as the primary language, and had at minimum a high school education. They all scored within the normal range for intelligence on the Wechsler Adult Intelligence Scale–Revised (Wechsler, 1981) and on neuropsychological testing. They also underwent medical and psychiatric examinations and a structured psychiatric interview (Comprehensive Assessment of Symptoms and History; Andreasen et al., 1992), and all were compensated for their participation in the study. After the study protocol was explained, participants provided written informed consent, which was approved by the Institutional Review Board of Mount Sinai School of Medicine. Exclusion criteria included neurologic disorders, history of head injury with loss of consciousness greater than 5 minutes or with neurocognitive sequelae, mental retardation, medical illness associated with significant neurocognitive impairment, history of treatment with psychoactive medication, any history of substance abuse or dependence, psychiatric illness, or family history (first-degree relatives) of major psychiatric illness. A positive urine test for drugs of abuse on the day of the scan was also considered exclusionary.
5.2. MRI imaging
Each participant received a T1-weighted axial MRI scan with a 1.5 T Signa-5× system. The acquisition parameters were: repetition time = 24 ms, echo time = 5 ms, flip angle = 40°, slice thickness = 1.2 mm, pixel matrix = 256×256, field of view = 23 cm, total slices = 128. MRI scans were re-sectioned to standard Talairach-Tournoux position (Talairach and Tournoux, 1988).
5.3. Image analysis
An analysis of the areas of the cingulate cortex, as they were defined by Brodmann, was conducted on coronal MRI slices (Hazlett et al., 1998; Mitelman et al., 2005) using the Perry coronal atlas of the brain, a digitized version of a histologically based atlas (Perry et al., 1991). The Perry atlas comprises 33 equally spaced coronal brain sections with maps of Brodmann's areas, based on microscopic examination of one postmortem brain (Fig. 4). This Brodmann approximation method for gray/white matter segmentation has been validated. (For a detailed description of the methodology, see Hazlett et al., 1998; Mitelman et al., 2003, 2005.) We obtained volume data from 39 Brodmann areas identified by the Perry atlas. The current study reports relative volume of gray matter in five cingulate cortex regions: areas 25, 24 (ACC); 31, 23 (PCC); and 29 (PCC, retrosplenial).
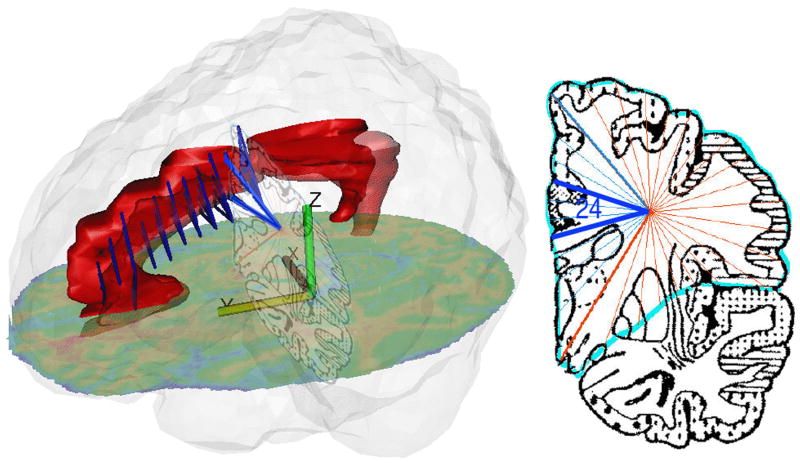
Figure 4.
Assessment of gray matter volume of Brodmann area 24. Left: Cingulate (hand-traced on MRI) is shown as red volume. Perry atlas slices 6 to 16 are shown in perspective as blue triangles, from sector point at frontal lobe center to Brodmann area 24. Right: Perry atlas slice 15 with area 24 marked. Perspective slightly distorted to provide clearer view of Perry atlas slice.
5.4. Statistical methods
We tested our hypotheses about relative gray matter volume in five areas of the cingulate gyrus using a mixed-model multivariate analysis of variance (MANOVA). The MANOVA included Decade (20s vs. 30s vs. 40s vs. 50s vs. 60s vs. 70s vs. 80s) and Gender (men vs. women) as between-group factors. Cingulate region and hemisphere served as repeated measures. The dependent variables for these MANOVAs were expressed as relative volume of each of our regions of interest (ROI/whole brain volume). Whole brain volume was calculated by adding gray and white matter volume for all 39 Brodmann areas measured. Our prior work (e.g., Mitelman et al., 2003) shows that relative and absolute volume analyses produce similar findings, and that relative volume measures are more conservative. However, we also examined whether whole brain volume showed any effects of Decade, Gender, or a Decade by Gender interaction with a factorial ANOVA.
This method of multivariate analysis allowed us to examine regional aging effects in both hemispheres of each of the five Brodmann areas and whether these aging effects differ between men and women. For all multivariate ANOVAs, the multivariate F (Wilks Lambda) from Statistica (StatSoft, 2003) is reported to adjust probabilities for repeated-measure effects with more than two levels. Fisher's Least Significant Difference (LSD) tests and simple-effects were used to follow-up significant between-group interaction effects.
On a more exploratory basis, we computed age regression slopes for all five cingulate areas for the left and right hemispheres and averaged across hemispheres. We present the age regression slopes for areas that showed statistically significant age effects after Bonferroni correction for 10 comparisons (five areas in two genders; p < 0.005). This approach is used as an analogue of significance probability mapping, which is often used in group contrasts. For comparison purposes, these associations were also calculated for men and women separately; Fisher's Z tests were used to determine whether men and women significantly differed in regional rates of age-associated volume loss.
Research Highlights.
Volume loss with age was most evident in area 31, followed by 25 and 24.
Women had larger cingulate gray matter volume than men overall and in area 24.
Volume loss with age showed sex differences in area 31 at midlife and late in life.
Acknowledgments
The authors thank Dr. Richard Mohs for helping to recruit and screen the sample and Dr. Lina Shihabuddin for conducting physical exams of the participants. We thank Dr. Cheuk Tang for instrumentation physics support, and Richard Azueta, Christina Luu, and Yuliya Zelmanova for technical assistance.
Role of the Funding Source: The collection of the MRI data was supported by a grant from the Charles A. Dana Foundation to Drs. Mohs and Buchsbaum. This research was supported in part by a NIMH grant to Dr. Hazlett (MH073911) and the VISN 3 Mental Illness Research, Education, and Clinical Center, Department of Veterans Affairs.
Footnotes
Conflicts of Interest: None.
Contributors: Ms. Mann is a graduate student in the General Psychology Masters Program at New York University who has worked in Dr. Hazlett's laboratory for the past year. Under Dr. Hazlett's direct supervision, she conducted the statistical analyses, drafted the manuscript, and prepared Figures 1 through 3. Dr. Hazlett conceived the idea for this study and co-edited the manuscript. She and Dr. Haznedar were also involved in the recruitment, screening, and collection of MRI data for this sample. Drs. Byne, Buchsbaum, Cohen, Haznedar, Hof, Mitsis, and Siever and Ms. Goldstein read drafts of the manuscript and provided comments. Dr. Byne wrote a section of the paper pertaining to area 31 and its cytology. Dr. Cohen provided statistical consultation. Dr. King-Wai Chu provided image-processing support. He and Dr. Buchsbaum prepared Figure 4.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, Chen K, Merkley TL, Reiman EM, Caselli RJ, Aschenbrenner M, Santerre-Lemmon L, Lewis DJ, Pietrini P, Teipel SJ, Hampel H, Rapoport SI, Moeller JR. Regional network of magnetic resonance imaging gray matter volume in healthy aging. NeuroReport. 2006;17:951–956. doi: 10.1097/01.wnr.0000220135.16844.b6. [DOI] [PubMed] [Google Scholar]
- Andreasen N, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH): An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Butters M, Lenze EJ, Venkatraman VK, Nable M, Reynolds CF, 3rd, Aizenstein HJ. fMRI activation in late-life anxious depression: a potential biomarker. International Journal of Geriatric Psychiatry. 2009;24:820–828. doi: 10.1002/gps.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold LM. Gender differences in bipolar disorder. The Psychiatric Clinics of North America. 2003;26:595–620. doi: 10.1016/s0193-953x(03)00036-4. [DOI] [PubMed] [Google Scholar]
- Bergfield KL, Hanson KD, Chen K, Teipel SJ, Hampel H, Rapoport SI, Moeller JR, Alexander GE. Age-related networks of regional covariance in MRI gray matter: reproducible multivariate patterns in healthy aging. NeuroImage. 2010;49:1750–1759. doi: 10.1016/j.neuroimage.2009.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi M, Ghidoni R, Govoni S, Testa C, Benussi L, Bonetti M, Binetti G, Frisoni GB. Effects of hormone therapy on brain morphology of healthy postmenopausal women: a Voxel-based morphometry study. Menopause. 2006;13:584–591. doi: 10.1097/01.gme.0000196811.88505.10. [DOI] [PubMed] [Google Scholar]
- Brun CC, Lepore N, Luders E, Chou YY, Madsen SK, Toga AW, Thompson PM. Sex differences in brain structure in auditory and cingulate regions. NeuroReport. 2009;20:930–935. doi: 10.1097/wnr.0b013e32832c5e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Hazlett EA. Functional brain imaging and aging in schizophrenia. Schizophr Res. 1997;27:129–141. doi: 10.1016/S0920-9964(97)00076-5. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Butler T, Imperato-McGinley J, Pan H, Voyer D, Cunningham-Bussel AC, Chang L, Zhu YS, Cordero JJ, Stern E, Silbersweig D. Sex specificity of ventral anterior cingulate cortex suppression during a cognitive task. Human Brain Mapping. 2007;28:1206–1212. doi: 10.1002/hbm.20340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Chen K, Lee W, Alexander GE, Reiman EM. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol. 2008;65:1231–1236. doi: 10.1001/archneurol.2008.1. [DOI] [PubMed] [Google Scholar]
- Chen X, Sachdev PS, Wen W, Anstey KJ. Sex differences in regional gray matter in healthy individuals aged 44-48 years: a voxel-based morphometric study. NeuroImage. 2007;36:691–699. doi: 10.1016/j.neuroimage.2007.03.063. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology. 1999;53:189–196. doi: 10.1212/wnl.53.1.189. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of comparative neurology. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Desgranges B, Baron JC, Lalevee C, Giffard B, Viader F, de La Sayette V, Eustache F. The neural substrates of episodic memory impairment in Alzheimer's disease as revealed by FDG-PET: relationship to degree of deterioration. Brain. 2002;125:1116–1124. doi: 10.1093/brain/awf097. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell M, Vogt B. Contributions of anterior cingulate cortex to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN, Giannakopoulos P, Kovari E, Bouras C. Assessing the role of cingulate cortex in bipolar disorder: neuropathological, structural and functional imaging data. Brain Res Rev. 2008;59:9–21. doi: 10.1016/j.brainresrev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Frey BN, Skelin I, Sakai Y, Nishikawa M, Diksic M. Gender differences in alpha-[(11)C]MTrp brain trapping, an index of serotonin synthesis, in medication-free individuals with major depressive disorder: a positron emission tomography study. Psychiatry Res. 2010;183:157–166. doi: 10.1016/j.pscychresns.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. The Journal of Neuroscience. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C, Johnsrude I, Ashburner J, Henson R, Friston K, Frackowiak S. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001a;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001b;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. Journal of Cognitive Neuroscience. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Human Brain Mapping. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, Chan RM, Loughead JW, Alsop DC, Maldjian J, Gur RE. Age-related differences in brain activation during emotional face processing. Neurobiol Aging. 2003;24:285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Hazlett E, Buchsbaum M, Mohs R, Spiegel-Cohen J, Wei TC, Azueta R, Haznedar M, Singer M, Shihabuddin L, Luu-Hisa C. Age-Related Shift in Brain Region Allocation During Successful Memory Performance. Neurobiol Aging. 1998;19:437–445. doi: 10.1016/s0197-4580(98)00075-x. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Byne W, Brickman AM, Mitsis EM, Newmark R, Haznedar MM, Knatz DT, Chen AD, Buchsbaum MS. Effects of sex and normal aging on regional brain activation during verbal memory performance. Neurobiol Aging. 2010;31:826–838. doi: 10.1016/j.neurobiolaging.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, New AS, Newmark R, Haznedar MM, Lo JN, Speiser LJ, Chen AD, Mitropoulou V, Minzenberg M, Siever LJ, Buchsbaum MS. Reduced Anterior and Posterior Cingulate Gray Matter in Borderline Personality Disorder. Biol Psychiatry. 2005;58:614–623. doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends in Neurosciences. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jones BF, Barnes J, Uylings HB, Fox NC, Frost C, Witter MP, Scheltens P. Differential regional atrophy of the cingulate gyrus in Alzheimer disease: a volumetric MRI study. Cereb Cortex. 2006;16:1701–1708. doi: 10.1093/cercor/bhj105. [DOI] [PubMed] [Google Scholar]
- Joos A, Kloppel S, Hartmann A, Glauche V, Tuscher O, Perlov E, Saum B, Freyer T, Zeeck A, Tebartz van Elst L. Voxel-based morphometry in eating disorders: correlation of psychopathology with grey matter volume. Psychiatry Res. 2010;182:146–151. doi: 10.1016/j.pscychresns.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Chetelat G, Baron JC, Landeau B, Mevel K, Godeau C, Barre L, Constans JM, Viader F, Eustache F, Desgranges B. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. 2009;30:112–124. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Chiew KS, Winocur G, Grady CL. Age-related differences in brain activity underlying identification of emotional expressions in faces. Soc Cogn Affect Neurosci. 2007;2:292–302. doi: 10.1093/scan/nsm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Mangin JF, Coyle T, Lancaster J, Thompson P, Riviere D, Cointepas Y, Regis J, Schlosser A, Royall DR, Zilles K, Mazziotta J, Toga A, Fox PT. Age-related morphology trends of cortical sulci. Human Brain Mapping. 2005;26:210–220. doi: 10.1002/hbm.20198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalev VA, Kruggel F, von Cramon DY. Gender and age effects in structural brain asymmetry as measured by MRI texture analysis. NeuroImage. 2003;19:895–905. doi: 10.1016/s1053-8119(03)00140-x. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? The American Journal of Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Grassiot B, Alperovitch A, Tzourio C, Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. NeuroImage. 2005;26:900–911. doi: 10.1016/j.neuroimage.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;62:553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg H, Brannan S, Mahurin R, Jerabek P, Brickman J, Tekell J, Silva J, McGinnis S, Glass T, Martin C. Cingulate function in depression: a potential predictor of treatment response. NeuroReport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- McDonald CR, McEvoy LK, Gharapetian L, Fennema-Notestine C, Hagler DJ, Jr, Holland D, Koyama A, Brewer JB, Dale AM. Regional rates of neocortical atrophy from normal aging to early Alzheimerdisease. Neurology. 2009;73:457–465. doi: 10.1212/WNL.0b013e3181b16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenbroek O, Petersson KM, Voermans N, Weber B, Fernandez G. Age differences in neural correlates of route encoding and route recognition. NeuroImage. 2004;22:1503–1514. doi: 10.1016/j.neuroimage.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain and Cognition. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. MRI assessment of gray and white matter distribution in Brodmann's areas of the cortex in patients with schizophrenia with good and poor outcomes. Am J Psychiatry. 2003;160:2154–2168. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophr Res. 2005;72:91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Nielsen FA, Balslev D, Hansen LK. Mining the posterior cingulate: Segregation between memory and pain components. NeuroImage. 2005;27:520–532. doi: 10.1016/j.neuroimage.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Lee JT, Sheikh SA, Surerus-Johnson C, Shah H, Munch KR, Carlis JV, Lewis SM, Kuskowski MA, Dysken MW. Where the brain grows old: decline in anterior cingulate and medial prefrontal function with normal aging. NeuroImage. 2007;35:1231–1237. doi: 10.1016/j.neuroimage.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Otaky N, Caramanos Z, MacDonald D, Zijdenbos A, D'Avirro D, Gutmans D, Holmes C, Tomaiuolo F, Evans AC. In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: hemispheric asymmetries, gender differences and probability maps. The Journal of Comparative Neurology. 1996;376:664–673. doi: 10.1002/(SICI)1096-9861(19961223)376:4<664::AID-CNE12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Pengas G, Hodges JR, Watson P, Nestor PJ. Focal posterior cingulate atrophy in incipient Alzheimer's disease. Neurobiol Aging. 2010;31:25–33. doi: 10.1016/j.neurobiolaging.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Perry R, Oakley A, Perry E. Coronal brain map and dissection guide: Localization of Brodman areas in coronal sections 1991 [Google Scholar]
- Proverbio AM, Adorni R, Zani A, Trestianu L. Sex differences in the brain response to affective scenes with or without humans. Neuropsychologia. 2009;47:2374–2388. doi: 10.1016/j.neuropsychologia.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Pujol J, Lopez A, Deus J, Cardoner N, Vallejo J, Capdevila A, Paus T. Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. NeuroImage. 2002;15:847–855. doi: 10.1006/nimg.2001.1004. [DOI] [PubMed] [Google Scholar]
- Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT. Age, Alzheimer disease, and brain structure. Neurology. 2009;73:1899–1905. doi: 10.1212/WNL.0b013e3181c3f293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. The Journal of Neuroscience. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Lee SY, van der Kouwe AJ, Greve DN, Fischl B, Rosas HD. Age-associated alterations in cortical gray and white matter signal intensity and gray to white matter contrast. NeuroImage. 2009;48:21–28. doi: 10.1016/j.neuroimage.2009.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: Impact on working memory performance. Neurobiol Aging. 2010;31:839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SK, O'Leary DS, Boles Ponto LL, Watkins GL, Hichwa RD, Andreasen NC. Age-related changes in regional cerebral blood flow among young to mid-life adults. NeuroReport. 1999;10:2493–2496. doi: 10.1097/00001756-199908200-00011. [DOI] [PubMed] [Google Scholar]
- Sherazi R, McKeon P, McDonough M, Daly I, Kennedy N. What's new? The clinical epidemiology of bipolar I disorder. Harvard Review of Psychiatry. 2006;14:273–284. doi: 10.1080/10673220601070047. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28:1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- StatSoft I. Statistica. Tulsa, OK: 2003. www.statsoft.com. [Google Scholar]
- Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging. 2004;25:185–192. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Ishii K, Kakigi T, Yokoyama K. Gender and age differences in normal adult human brain: Voxel-based morphometric study. Human Brain Mapping. 2010 doi: 10.1002/hbm.21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co planar Stereotaxic Atlas of the Human Brain. Thieme; Stuttgart: 1988. [Google Scholar]
- Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM. Longitudinal changes in cortical thickness associated with normal aging. NeuroImage. 2010;52:1215–1223. doi: 10.1016/j.neuroimage.2010.04.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive aging. Cortex. 2003;39:1107–1128. doi: 10.1016/s0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional connectivity density mapping. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya JG, Paradiso S, Boles Ponto LL, McCormick LM, Robinson RG. Aging, grey matter, and blood flow in the anterior cingulate cortex. NeuroImage. 2007;37:1346–1353. doi: 10.1016/j.neuroimage.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nature Reviews Neuroscience. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Regions and subregions of the cingulate cortex. In: Vogt BA, editor. Cingulate Neurobiology and Disease. Oxford University Press; New York: 2009. pp. 5–26. [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt LJ, Perl DP, Hof PR. Cytology of human caudomedial cingulate, retrosplenial, and caudal parahippocampal cortices. J Comp Neurol. 2001;438:353–376. doi: 10.1002/cne.1320. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R manual. Wechsler Adult Intelligence Scale–Revised. The Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Differentiating maturational and aging-related changes of the cerebral cortex by use of thickness and signal intensity. NeuroImage. 2010;52:172–185. doi: 10.1016/j.neuroimage.2010.03.056. [DOI] [PubMed] [Google Scholar]
- Yücel K, McKinnon MC, Chahal R, Taylor VH, Macdonald K, Joffe R, MacQueen GM. Anterior cingulate volumes in never-treated patients with major depressive disorder. Neuropsychopharmacology. 2008;33:3157–3163. doi: 10.1038/npp.2008.40. [DOI] [PubMed] [Google Scholar]
- Yücel M, Stuart GW, Maruff P, Velakoulis D, Crowe SF, Savage G, Pantelis C. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cereb Cortex. 2001;11:17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]