Abstract
Cryptococcus remains an important opportunistic infection in HIV patients despite considerable declines in prevalence during the HAART era. This is particularly apparent in sub-Saharan Africa, where Cryptococcus continues to cause significant mortality and morbidity. This review discusses the microbiology, epidemiology, pathogenesis, and clinical presentation of cryptococcal infections in HIV patients. Additionally, a detailed approach to the management of cryptococcosis is provided.
Keywords: Cryptococcus, HIV, diagnosis, treatment
Introduction
Cryptococcus neoformans is an encapsulated yeast that remains an important pathogen, particularly among patients with the human immunodeficiency virus (HIV). A rare disease prior to the HIV epidemic, Cryptococcus is now among the leading causes of meningitis in sub-Saharan Africa.1, 2 Despite the advent of highly active antiretroviral therapy (HAART), Cryptococcus is a significant disease among HIV-infected persons. For example, the 10-week mortality in HIV patients in developed countries remains high, ranging from 10–25%.3,4 In resource limiting settings, mortality is even higher, with rates of 37–43%, even when amphotericin B has been used.5, 6 In a Zambian study of 130 AIDS patients without access to antiretroviral therapy who were treated with fluconazole (200 mg), the 6-month mortality was 100%, with a median survival of only 19 days.7
This review discusses the epidemiology and pathophysiology of cryptococcosis, as well as its most notable clinical manifestations. Additionally, a summary of the approach to treatment for Cryptococcus, as described in recent guidelines, is provided8.
Microbiology and Epidemiology of Cryptococcal Infections in HIV Patients
The asexual yeast Cryptococcus neoformans had been classified as four serotypes based on the capsular polysaccharide, glucuronoxylomannan (GXM).1 Capsular types A through D correspond to the variants C. neoformans var. grubii (A), C. neoformans var gattii (B and C), and C. neoformans var. neoformans (D). Recently, C. gattii has been classified as a separate species, as detailed analysis has shown it to be genetically distinct from C. neoformans.9
Cryptococcus is distributed worldwide and exists in high concentrations in bird guano, particularly pigeons and chickens.10,11 However, human infection usually occurs without a history of direct contact with birds.1 C. gattii, on the contrary, generally occurs in the tropics and subtropics and is found in decaying vegetation, particularly from the red river gum (i.e., eucalyptus) trees.12 An outbreak of C. gattii infections that occurred on Vancouver Island from 1999–2004 was linked to the importation of eucalyptus trees from Australia.13,14
Cryptococcus is a well-recognized opportunistic infection among those with deficits in cell-mediated immunity, such as HIV patients and other immunocompromised populations including patients with organ transplantation and rheumatologic conditions requiring immunosuppressive agents.15,16 In HIV patients, it is classified as an AIDS-defining condition.17 Despite the reduction in the number of cases among HIV patients since the advent of HAART in the developed world,18 approximately one million cases of cryptococcosis occur worldwide each year,19 with the largest burden in Africa. In the U.S., cases continue to be seen, especially among HIV patients diagnosed late in the disease course and among persons receiving immunosuppressive agents.
Pathogenesis and Clinical Manifestations
Cryptococcus is a basidiomycetous yeast that survives environmentally in the sexual form, producing hyphae with terminal basidiospores (chains of unbudded yeast). These basidiospores may break off and become aerosolized,1 and at 3 microns in size, are small enough to deposit in the alveoli.20 In the majority of hosts, infection is asymptomatic. However, in the patient with severe cell-mediated immunodeficiency, the organism may enter the circulation and survive in vivo in the haploid, asexual state leading to disseminated disease. Characteristics of Cryptococcus that permit its survival within the host include a polysaccharide capsule, which allows the organism to escape phagocytosis.21,22 Further, the phenol oxidase enzyme uses catecholamines as substrate to produce melanin, which accumulates in the cell wall. It is the use of catecholamines that may provide a predilection for involvement of the central nervous system (CNS).23
Clinical manifestations of Cryptococcus most commonly involve the lungs and CNS, although several other sites may be involved. The site of infection appears to vary by the serotype of Cryptococcus and the immunocompetence of the host. For example, Cryptococcus gattii typically causes a pulmonary infection in immunocompetent hosts, but may also cause disease among HIV-infected persons. Pulmonary disease usually presents as an acute pneumonia or as non-calcified granulomas, which are often difficult to detect radiographically. Some patients develop a prolonged cough or dyspnea due to chronic pneumonia.
Cryptococcus typically causes opportunistic disease in patients with deficits in cell-mediated immunity.3,15,16,24 Although incidence and mortality have decreased in the era of HAART, cryptococcal meningitis remains an important cause of morbidity and mortality in the AIDS population, especially in the developing world.25 Cryptococcal meningitis generally presents with a headache of insidious onset and several weeks duration. Fevers usually do not occur until late in the disease course, and nuchal rigidity is often absent.1 In AIDS patients with cryptococcal meningitis, extraneuronal involvement is quite common, as high as 50% in one case series.26 Common sites of infection include the lungs, bone marrow, skin, and genitourinary tract.27 Cutaneous dissemination may be seen in about 10% of cases (typically described as molluscum-appearing skin lesions) and as osseous involvement in approximately 5%.1 While cryptococcal meningitis generally occurs in immunocompromised patients, it can be seen in immunocompetent individuals as well.28, 29
Diagnosis
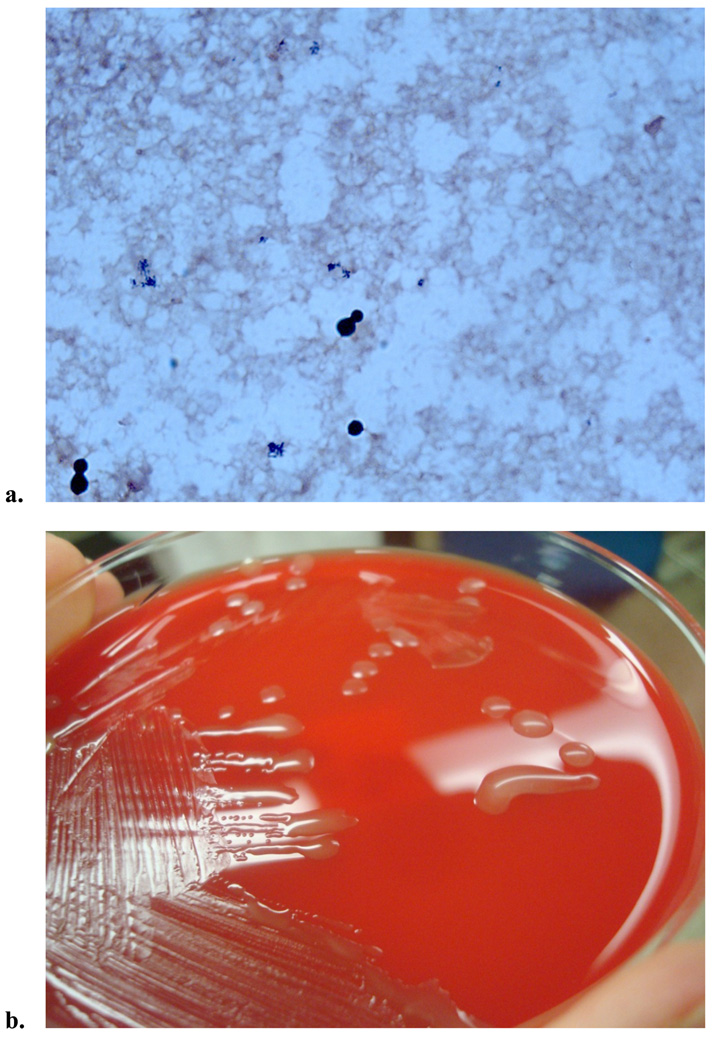
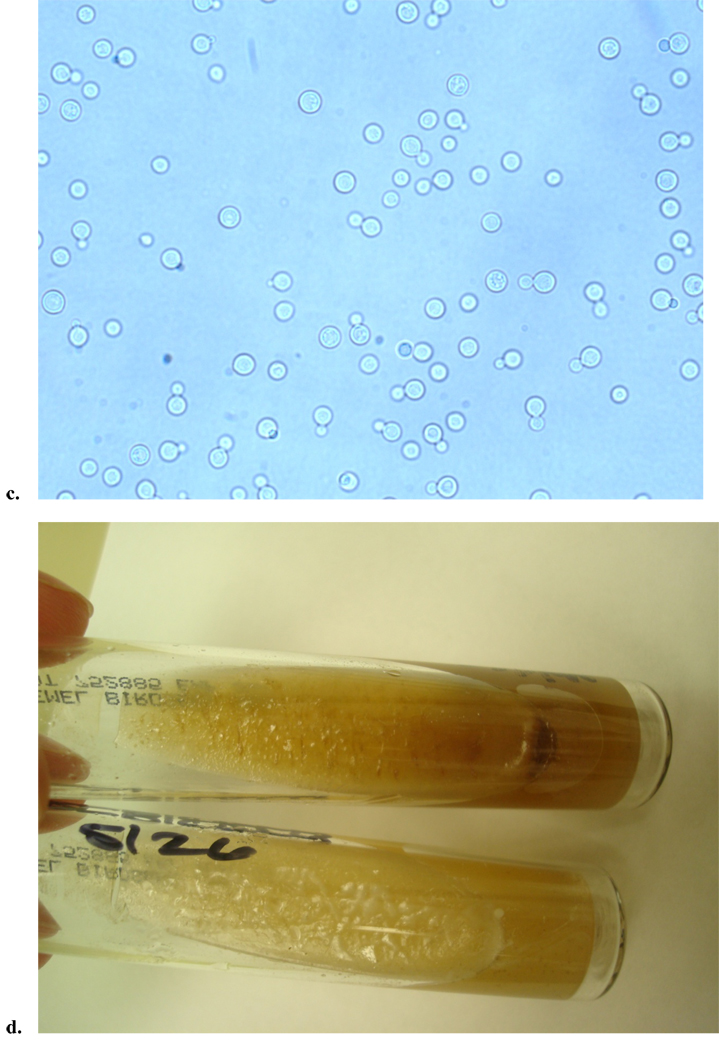
The diagnosis of cryptococcosis is most often made by the latex agglutination test for capsular polysaccharide antigen. This antigen can be obtained from either cerebrospinal fluid (CSF) or serum, and when present in CSF, is over 90% sensitive and specific for the diagnosis of cryptococcal meningitis.30 False positives can occur though, particularly in the presence of a positive rheumatoid factor.31 In resource limited settings, the latex agglutination antigen test is often not available, thus methods of detection of the organism include blood cultures (Figure 1), CSF culture, and India ink smear. However, India ink stains are less sensitive than the capsular antigen.1 In one study of HIV-negative adults with cryptococcosis, India ink was 51% sensitive and CSF culture 89% sensitive among the 157 patients with meningitis. In contrast, the antigen test had a sensitivity of 97% and 87% from the CNS and blood, respectively.32 In a study involving both HIV positive and negative patients, the India ink stain was positive in 80% (48/60) of patients with cryptococcal meningitis.33 If isolated from culture, Cryptococcus appears as singular, narrow-based budding yeast that is urease negative and can be distinguished by its preferential growth on birdseed agar (Figure 1).
Figure 1.
Clinical images from a patient with AIDS who presented with cryptococcal fungemia. a. Gram stain from the positive blood culture showed narrow based budding yeast. b. The yeast grew on fungal media (Sabouraud dextrose agar), but also grew on routine media, chocolate agar and sheep’s blood agar (shown here), displaying cream colored, smooth, mucoid colonies. c. Wet mount was performed, which exhibited round celled yeast, with narrow budding single daughter cell, consistent with Cryptococcus. d. C. neoformans was confirmed by both biochemical testing and the brown colored colony growth on birdseed agar, as C. neoformans selectively absorbs melanin from this media (top is patient’s sample, and bottom is negative control growing Candida albicans).
The diagnosis of cryptococcal meningitis is established by lumbar puncture which usually shows elevated opening pressure, high protein, and elevated white cell count. Indicators of more severe disease on CSF sampling include elevated opening pressure, low glucose, leukocyte count less than 20 cells per mm3, elevated cryptococcal antigen titer, and presence of the organism by India ink stain.34
Treatment
The treatment for meningitis or other forms of invasive cryptococcal disease is divided into a two week induction phase, followed by an 8 week consolidation phase, and then a prolonged maintenance phase thereafter.35, 36 An important distinction is made in the guidelines to differentiate treatment of CNS versus non-CNS disease;8, 35 therefore, a lumbar puncture should be performed among immunocompromised patients with cryptococcal disease to rule out meningitis.8
The recommended initial management of cryptococcal meningitis in patients with HIV consists of the rapidly fungicidal regimen of amphotericin B (0.7 to 1 mg/kg/day) plus flucytosine (100 mg/kg/day; Table 1).8, 35 Clinical outcomes were improved in AIDS patients with cryptococcal meningitis in South Africa when the induction phase consisted of the combination of flucytosine and amphotericin B, versus amphotericin B alone.36 Studies have shown that amphotericin B (0.7 mg/kg/day) plus flucytosine (100 mg/kg/day) was found to be more rapidly fungicidal and have lower risk of mycological failure at two weeks than amphotericin B alone or in combination with fluconazole.37, 38
Table 1.
Recommended Therapy for Cryptococcal Meningitis, Disseminated Disease, or Fungemia in HIV patients.
| Phase | Primary therapy |
Alternative regimens | |||
|---|---|---|---|---|---|
| Induction | AmBd1 or lipid formulation AmB2 PLUS Flucytosine3 for 2 weeks | AmBd1 or LFAmB2 for 4 to 6 weeks | AmBd1 PLUS fluconazole 800 mg daily for 14 days | Fluconazole ≥800 mg (1200 mg preferred) daily plus flucytosine3 for 6 weeks | Fluconazole 800–2000 mg daily as monotherapy for at least 10 weeks |
| Consolidation | Fluconazole 400 mg daily for minimum of 8 weeks | None | Fluconazole 400 mg daily | None | None* |
| Maintenance4 (suppression) | Fluconazole 200 mg daily OR Itraconazole 400 mg daily OR AmBd 1 mg/kg/week5 | Same as primary | Same as primary | Same as primary | Same as primary |
Adapted from Perfect JR, et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the IDSA. Clin Infect Dis. 2010; 50:291–322 (reference 8).
Amphotericin B deoxycholate (AmBd) 0.7 to 1 mg/kg IV daily.
Lipid Formulations of amphotericin (LFAmB): Liposomal AmB 3–4 mg/kg daily or AmB lipid complex (ABLC) 5 mg/kg IV daily.
Flucytosine 100 mg/kg daily, divided four times a day, oral route preferred.
Maintenance therapy to be continued for at least 12 months. Can then consider stopping if receiving HAART with a CD4>100 cells/mm3 and undetectable or low HIV viral load for at least three months.
Reserved for patients not able to tolerate azoles.
Already receiving fluconazole
Regarding the appropriate dose of amphotericin B, a study compared two different doses (0.7 mg/kg/day versus 1 mg/kg/day) in a group of HIV patients with cryptococcal meningitis in South Africa;39 both groups were also treated with flucytosine for 14 days. The higher dose amphotericin B was more rapidly fungicidal. While there was no difference in mortality in this study, it has been shown that more rapid clearance of infection correlates with improved clinical outcomes. 40, 41
Lipid formulations of amphotericin are considered as alternate therapies to amphotericin B; these agents are useful in the setting of renal impairment and are generally associated with fewer adverse events compared to amphotericin B. Although there has been no proven benefit in clinical outcomes with liposomal amphotericin, a trend toward earlier fungicidal clearance has been shown.42, 43 Of note, these formulations are considerably more expensive and are generally unavailable in resource limited settings. For any amphotericin product, it is important to provide intravenous hydration before and after dosages as a renal-protective measure, and to monitor electrolytes and kidney function closely. 8 Typically patients receiving amphotericin require administration of supplemental potassium, and sometimes magnesium and phosphorus as well.
Improvement in outcomes for patients with cryptococcal meningitis depends not only upon the choice of initial antifungal therapy, but also appropriate management of elevated intracranial pressure. An increased opening pressure is a poor prognostic indicator in cryptococcal meningitis34,44 and is associated with higher CNS fungal burden.45 Therefore, opening pressures greater than 25 cm of CSF should be treated with serial (e.g., daily) lumbar punctures until the pressure normalizes to less than 20 cm of CSF.8, 35, 44 One approach is to remove a CSF volume that halves opening pressure (typically 20–30 ml), especially in settings of extremely high pressures8, 35, 46 For recurrent symptoms, repeat lumbar drainage should be performed. While CNS mass lesions or cryptococcomas are uncommon, imaging with CT scan or MRI should be performed prior to lumbar puncture to avoid the risk of herniation.8 Placement of temporary lumbar drain may be necessary if opening pressure cannot be otherwise controlled, particularly if neurologic sequelae are persistent.35, 47
Even in the absence of neurologic symptoms, a lumbar puncture should be performed among immunocompromised patients with cryptococcal disease to exclude underlying meningitis. Even in the absence of meningitis, immunocompromised patients with severe or disseminated disease (i.e., involvement in more than one organ system or a positive blood culture for Cryptococcus) should be managed with the same antifungal regimen as those with meningitis.8
In resource poor settings, some recommendations are difficult to adhere to, since amphotericin B, and particularly its lipid formulations, may not be readily available.42 While data are limited regarding the most effective initial therapy in settings where there is no immediate access to these medications, a recent study showed that fluconazole at high doses (1200 mg daily) leads to a more rapid decrease in CSF antigen titer than traditional dosing of 800 mg daily.48 Furthermore, a randomized controlled trial in Malawi showed that an oral induction regimen of fluconazole (1200 mg daily) plus flucytosine (100 mg/kg daily) resulted in earlier fungicidal clearance and a trend toward decreased mortality when compared to induction with 1200 mg fluconazole alone.41 This approach is an alternative recommendation in current guidelines,8 and particularly useful in Africa where fluconazole is now widely available, although access to flucytosine remains limited.41 It’s important to recognize that in cases where fluconazole alone is utilized, resistance has been reported (especially in Africa) and may result in symptomatic relapse.49
If access to flucytosine is limited, the induction regimen of amphotericin B plus fluconazole is preferred.8 A recently published phase II study showed a trend toward better long-term outcomes of induction therapy with amphotericin B plus 800 mg fluconazole daily compared to amphotericin B alone.50
When amphotericin B formulation plus flucytosine can be used, the induction therapy should be continued for two weeks, after which time a repeat lumbar puncture should be performed to evaluate for CSF sterilization. Consolidation therapy with fluconazole 400 mg daily for 8–10 weeks should be initiated, but if the CSF culture is positive, consideration should be made for reinstituting the two week induction regimen depending upon the clinical status of the patient (Table 1).8 Of note, growth within the CSF typically occurs within one to two weeks. Extension of induction therapy is also recommended among patients who remain comatose, are clinically deteriorating, or have persistent elevated intracranial pressures and a poor clinical response.8
In the HIV-positive patient with meningitis, lifelong maintenance therapy is often necessary. Following induction and consolidation therapy (Table 1), lifelong suppressive treatment with fluconazole (200 mg daily) is recommended once CSF sterilization has occurred.8, 35 Itraconazole (400 mg daily) can be used as well, but has been shown to be inferior to fluconazole,51 so is therefore recommended only in cases where fluconazole cannot be given.8 If immune reconstitution occurs due to initiation of a successful HAART regimen, the CD4+ T lymphocyte count remains greater than 100 cells/mm3 for at least three months and the HIV viral load is low/undetectable, consideration of discontinuation of therapy can be made.
Of note, a minimum of 12 months of antifungal therapy should be administered before discontinuation. Some specialists prefer to document sterilization of CSF with repeat lumbar puncture in this situation, although this practice is not essential.35 One prospective randomized study of HIV patients with cryptococcal meningitis evaluated whether maintenance therapy could be stopped after HAART initiation and subsequent immune reconstitution. After 48 weeks of follow-up, there were no cases of recurrent meningitis in those patients whose maintenance therapy had been stopped.52 However, if the CD4 count falls below 100 cells/mm3 during follow-up, maintenance therapy with fluconazole should be reinstituted.8
Despite the important role of antigen testing in the diagnosis of cryptococcal disease, both the serum and CSF antigens have a limited role in monitoring response to therapy.53,54 While the serum titer decreases over time in most patients, it does not differentiate well between clinical response and persistent disease.53 However, monitoring the CSF antigen level may be useful during acute infection, in that an unchanging or increasing titer has been correlated with clinical and microbiologic failure.54
There are several notable drug interactions between the antifungal agents used to treat cryptococcosis and antiretrovirals. Nevirapine clearance is decreased two-fold by fluconazole; to compensate for this in one clinical trial, those who were prescribed nevirapine had their dosage of fluconazole for consolidation therapy decreased from 800 mg to 400 mg daily.41 Fluconazole also increases the area under the curve for zidovudine, so monitoring for zidovudine toxicity is important when these drugs are co-administered. The combination of zidovudine and flucytosine administration carries the combined potential bone marrow toxicities of both agents, so close monitoring is warranted. Flucytosine levels should be monitored if this test is available,35 but adverse effects have been relatively low in clinical trials performed in developing countries without drug level monitoring.36, 41
Finally, it should be noted that in HIV patients with cryptococcal meningitis who initiate antiretroviral therapy are at particularly high risk for the immune reconstitution inflammatory syndrome (IRIS), manifested by meningeal symptoms and elevated opening pressure.55 In one study, up to 30% of patients developed IRIS when HAART was initiated within one month of diagnosis with cryptococcal meningitis.56 It is important to monitor for signs (e.g., confusion, papilledema, clonus) of increased intracranial pressure (ICP) and to manage it with serial lumbar punctures, especially if the ICP is high (>25 cm of CSF).35 It is essential to determine if CNS symptoms are due to IRIS versus disease progression or relapse; results of repeat cultures and trends in antigen titers can be helpful.56 Management of IRIS cases includes continuation or introduction of antifungal therapy and in severe cases, consideration for corticosteroids. Recent guidelines suggest that initiation of HAART should occur 2–10 weeks after commencement of antifungal therapy in an effort to reduce the occurrence of IRIS,8 with some favoring waiting closer to the 10 week time point. Overall, a history of cryptococcal infection is an important consideration before HAART initiation.
In addition to IRIS that occurs during the treatment of cryptococcal disease, “unmasking” IRIS can also occur in which symptoms due to Cryptococcus appear after HAART is initiated. In a retrospective study in South Africa, stored plasma from 7% of patients who had initiated HAART were positive for cryptococcal antigen. In the subset of patients with CD4 count less than 100 cells/mm3, a cryptococcal antigen titer of 1:8 was 100% sensitive and 96% specific for predicting the development of cryptococcal meningitis during the first year of HAART, suggesting a potential role for a preemptive treatment strategy in this highly endemic setting to reduce the risk of the development of both meningitis and IRIS; further studies are needed.57 Primary prophylaxis among HIV patients in the U.S. is not currently recommended.8
Alternate therapies such as mannitol or acetazolamide for elevated ICP in the setting of meningoencephalities are not currently recommended.8 Corticosteroids may be considered in select settings, including IRIS with CNS inflammation and increased intracranial pressure, cryptococcomas with mass effect and edema, and acute respiratory distress syndrome (ARDS).
For patients who develop pulmonary cryptococcosis with mild to moderate illness (no diffuse pulmonary infiltrates, ARDS, or dissemination,) fluconazole 400 mg for 6–12 months is recommended (Table 2), although there have been no randomized trials to date that have determined the optimal treatment in HIV-infected persons.8 Patients with disease manifesting as a severe pulmonary illness, such as ARDS, should be initially managed with amphotericin B (0.7 to 1 mg/kg/d) plus flucytosine, as in meningitis.8 Therapy for non-CNS disease can generally be discontinued after one year of therapy if the CD4 count is >100 cells/mm3 and the titer is ≤1:512 and/or is stable.
Table 2.
Treatment Approach for Pulmonary Cryptococcosis in HIV Patients
| Host factors | Medication | Duration | Comments |
|---|---|---|---|
| Mild-moderate symptoms | Fluconazole 400 mg daily | Lifelong therapy with fluconazole | In HIV patients, consideration can be made to stop therapy if CD4 count rises above 100 cells/mm3. |
| Severe symptoms | Treat as CNS disease |
Adapted from Perfect JR, et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the IDSA. Clin Infect Dis. 2010; 50:291–322 (reference 8).
Conclusion
In summary, cryptococcal disease remains an important cause of morbidity and mortality, particularly in HIV patients in resource poor environments. Cryptococcal infection should be considered on the differential diagnosis of immunocompromised patients presenting with meningitis, pneumonia, or molluscum-like skin lesions. Updated treatment guidelines have recently been published to assist in the management of cryptococcal disease.8 Although still a significant cause of morbidity and mortality in the HIV patient, great strides have been made to reduce this burden through adherence to treatment guidelines and the availability of HAART.
Footnotes
The authors have no commercial or other association that might pose a conflict of interest in this work.
The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the DoD or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
This work is original and has not been published elsewhere.
References
- 1.Hajjeh RA, Brandt ME, Pinner RW. Emergence of cryptococcal disease: Epidemiologic perspectives 100 years after its discovery. Epidemiol Rev. 1995;17:303–320. doi: 10.1093/oxfordjournals.epirev.a036195. [DOI] [PubMed] [Google Scholar]
- 2.Bekondi C, Bernede C, Passone N, et al. Primary and opportunistic pathogens associated with meningitis in adults in Bangui, Central African Republic, in relation to human immunodeficiency virus serostatus. Int J Infect Dis. 2006;10:387–395. doi: 10.1016/j.ijid.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS. 2007;21:2119–2129. doi: 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- 4.Lotholary O, Poizat G, Zeller V, Neuville S, Boibieux A, Alvarez M, et al. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006;20:2183–2191. doi: 10.1097/01.aids.0000252060.80704.68. [DOI] [PubMed] [Google Scholar]
- 5.Imwidthaya P, Poungvarin N. Cryptococcosis in AIDS. Postgrad Med J. 2000;76:85–88. doi: 10.1136/pmj.76.892.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker LG, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naïve or –experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45:76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 7.Mwaba P, Mwansa J, Chintu C, Pobee J, Scarborough M, Portsmouth S, et al. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad Med J. 2001;77:769–773. doi: 10.1136/pmj.77.914.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Disease Society of America. Clin Infect Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bovers M, Hagen F, Boekhout T. Diversity of the Cryptococcus neoformans-Cryptococcus gattii species complex. Rev Iberoam Micol. 2008;25:S4–S12. doi: 10.1016/s1130-1406(08)70019-6. [DOI] [PubMed] [Google Scholar]
- 10.Emmons CW. Saprophytic sources of Cryptococcus neoformans associated with the pigeon (Columba livia) Am J Hyg. 1955;62:227–332. doi: 10.1093/oxfordjournals.aje.a119775. [DOI] [PubMed] [Google Scholar]
- 11.Ajello L. Occurrence of Cryptococcus neoformans in soils. Am J Hyg. 1958;67:72–77. doi: 10.1093/oxfordjournals.aje.a119921. [DOI] [PubMed] [Google Scholar]
- 12.Ellis DH, Pfeiffer TJ. Natural habitat of Cryptococcus neoformans var. gattii. J Clin Microbiol. 1990;28:1642–1644. doi: 10.1128/jcm.28.7.1642-1644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindberg J, Hagen F, Laursen A, Stenderup J, Boekhout T. Cryptococcus gattii risk for tourists visiting Vancouver Island, Canada. Emerg Infect Dis. 2007;13:178–179. doi: 10.3201/eid1301.060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fyfe M, MacDougall L, Romney M, et al. Cryptococcus gattii infections on Vancouver Island, British Columbia, Canada: Emergence of a tropical fungus in a temperate environment. Canada Communicable Disease Report. 2008;34:1–12. [PubMed] [Google Scholar]
- 15.Singh N, Dromer F, Perfect J, Lortholary O. Cryptococcosis in solid organ transplant recipients: current state of the science. Clin Infect Dis. 2008;47:1321–1327. doi: 10.1086/592690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hage CA, Wood KL, Winer-Muram HT, Wilson SJ, Sarosi G, Knox KS. Pulmonary cryptococcosis after initiation of anti-tumor necrosis factor-alpha therapy. Chest. 2003;124:2395–2397. doi: 10.1378/chest.124.6.2395. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 1993 Revised system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992;41(R-17) [PubMed] [Google Scholar]
- 18.Mirza S, Phelan M, Rimland D, et al. The changing epidemiology of cryptococcosis: An update from population-based active surveillance in 2 large population areas, 1992–2000. Clin Infect Dis. 2003;36:789–794. doi: 10.1086/368091. [DOI] [PubMed] [Google Scholar]
- 19.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 20.Ellis DH, Pfeiffer TJ. The ecology of Cryptococcus neoformans. Eur J Epidemiol. 1992;8:321–325. doi: 10.1007/BF00158562. [DOI] [PubMed] [Google Scholar]
- 21.Patterson TF, Andriole VT. Current concepts in cryptococcosis. Eur J Clin Microbiol Infect Dis. 1989;8:457–465. doi: 10.1007/BF01964060. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharjee AK, Kwon-Chung KJ, Glaudemans CPJ. The major capsular polysaccharide of Cryptococcus neoformans serotype B. Carbohydr Res. 1992;233:271–272. doi: 10.1016/s0008-6215(00)90942-x. [DOI] [PubMed] [Google Scholar]
- 23.Perfect JR, Wong B, Chang YC, Kwon-Chung KJ, Williamson PR. Cryptococcus neoformans: virulence and host defences. Med Mycol. 1998;36 Suppl. 1:79–86. [PubMed] [Google Scholar]
- 24.Jarvis J, Dromer F, Harrison T, Lortholary O. Managing Cryptococcus in the immunocompromised host. Curr Opin Infect Dis. 2008;21:596–603. doi: 10.1097/QCO.0b013e3283177f6c. [DOI] [PubMed] [Google Scholar]
- 25.Lortholary O, Poizat G, Zeller V, et al. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006;20:2183–2191. doi: 10.1097/01.aids.0000252060.80704.68. [DOI] [PubMed] [Google Scholar]
- 26.Eng RHK, Bishburg E, Smith SM, Kapila R. Cryptococcal infections in patients with acquired immune deficiency syndrome. Am J Med. 1986;81:19–23. doi: 10.1016/0002-9343(86)90176-2. [DOI] [PubMed] [Google Scholar]
- 27.Dismukes WE. Cryptococcal meningitis in patients with AIDS. J Infect Dis. 1988;157:624–628. doi: 10.1093/infdis/157.4.624. [DOI] [PubMed] [Google Scholar]
- 28.Wilbur L, Heyborne R. Transient loss of consciousness caused by cryptococcal meningitis in an immunocompetent patient: a case report. Cases J. 2009;2:60. doi: 10.1186/1757-1626-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuanjie Z, Jianghan C, Julin G, Hai W. Cryptococcus meningitis in an immunocompetent 8 year old girl. Mycoses. 2008 doi: 10.1111/j.1439-0507.2008.01625.x. [DOI] [PubMed] [Google Scholar]
- 30.Satischandra P, Mathew T, Gadre G, Nagarathna S, Chandramukhi A, Mahadevan A, Shankar SK. Cryptococcal meningitis: Clinical, diagnostic and therapeutic overviews. Neurol India. 2007;55:226–232. doi: 10.4103/0028-3886.35683. [DOI] [PubMed] [Google Scholar]
- 31.Bennett JE, Bailey JW. Control for rheumatoid factor in the latex test for cryptococcosis. Am J Clin Pathol. 1971;56:360–365. doi: 10.1093/ajcp/56.3.360. [DOI] [PubMed] [Google Scholar]
- 32.Pappas P, Perfect JR, Cloud GA, Larsen RA, Pankey GA, Lancaster DJ, Henderson H, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33:690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 33.Khanna N, Chandramuki A, Desai A, Ravi V. Cryptococcal infections of the central nervous system: an analysis of predisposing factors, laboratory findings and outcomes in patients from South India with special reference to HIV infection. J Med Microb. 1996;45:376–379. doi: 10.1099/00222615-45-5-376. [DOI] [PubMed] [Google Scholar]
- 34.Diamond RD, Bennett JE. Prognostic factors in cryptococcal meningitis: a study of 111 cases. Ann Intern Med. 1974;80:176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Centers for Disease Control and Prevention (CDC); National Institutes of Health; HIV Medicine Association of the Infectious Diseases Society of America. MMWR. 2009 Apr;58(RR-4):48–49. [PubMed]
- 36.Van der Horst CM, Saag MS, Cloud GA, Hamill RJ, Graybill JR, Sobel JD, et al. Treatment of cryptococcal meningitis associated with the acquired immune deficiency syndrome. New Eng J Med. 1997;337:15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 37.Brouwer A, Rajanuwong A, Chierakul W, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomized trial. Lancet. 2004;363:1764–1767. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]
- 38.Dromer F, Bernede-Bauduin C, Guillemot D, Lortholary O. Major role for amphotericin B-flucytosine combination in severe cryptococcosis. PLoS ONE. 2008;3:e2870. doi: 10.1371/journal.pone.0002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bicanic T, Wood R, Meintjes G, et al. High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: a randomized trial. Clin Infect Dis. 2008;47:123–130. doi: 10.1086/588792. [DOI] [PubMed] [Google Scholar]
- 40.Bicanic T, Muzoora C, Brouwer A, Meintjes G, Longley N, Taseera K, Rebe K, et al. Independent association between rate of clearance of infection and outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis. 2009;49:702–709. doi: 10.1086/604716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nussbaum JC, Jackson A, Namarika D, Phulusa J, Kenala J, Kanyemba C, Jarvis JN, et al. Combination flucytosine and high-dose fluconazole compared with fluconazole monotherapy for the treatment of cryptococcal meningitis: a randomized trial in Malawi. doi: 10.1086/649861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen SC. Cryptococcosis in Australasia and the treatment of cryptococcal and other fungal infections with liposomal amphotericin B. J Antimicrob Chemother. 2002;49:57–61. doi: 10.1093/jac/49.suppl_1.57. [DOI] [PubMed] [Google Scholar]
- 43.Leenders AC, Reiss P, Portegeis P, Clezy K, Hop WCJ, Hoy J, et al. Liposomal amphotericin B (Ambisome) compared with amphotericin B followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meningitis. AIDS. 1997;11:1463–1471. doi: 10.1097/00002030-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Graybill JR, Sobel J, Saag M The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. Clin Infect Dis. 2000;30 doi: 10.1086/313603. 47-5. [DOI] [PubMed] [Google Scholar]
- 45.Bicanic T, Brouwer AE, Meintjes G, et al. Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS. 2009;23:701–706. doi: 10.1097/QAD.0b013e32832605fe. [DOI] [PubMed] [Google Scholar]; Clin Infect Dis. 2010;50:338–344. doi: 10.1086/649861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fessler RD, Sobel J, Guyot L, Crane L, Vazquez J, Szuba MJ, Diaz FG. Management of elevated intracranial pressure in patients with cryptococcal meningitis. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:137–142. doi: 10.1097/00042560-199802010-00006. [DOI] [PubMed] [Google Scholar]
- 47.Macsween KF, Bicanic T, Brouwer AE, Marsh H, Macallan DC, Harrison TS. Lumbar drainage for control of raised cerebrospinal fluid pressure in cryptococcal meningitis: case report and review. J Infect. 2005;51:e221–e224. doi: 10.1016/j.jinf.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Longley N, Muzoora C, Taseera K, et al. Dose response effect of high dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. 2008;47:1556–1561. doi: 10.1086/593194. [DOI] [PubMed] [Google Scholar]
- 49.Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin Infect Dis. 2006;43:1069–1073. doi: 10.1086/507895. [DOI] [PubMed] [Google Scholar]
- 50.Pappas P, Chetchotisakd P, Larsen R, et al. A phase II randomized trial of amphotericin B alone or combined with fluconazole in the treatment of HIV-associated cryptococcal meningitis. Clin Infect Dis. 2009;48:1775–1783. doi: 10.1086/599112. [DOI] [PubMed] [Google Scholar]
- 51.Saag MS, Cloud GA, Graybill R National Institute of Allergy and Infectious Diseases Mycoses Study Group. A comparison of itraconazole versus fluconazole as maintenance therapy for AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1999;28:291–296. doi: 10.1086/515110. [DOI] [PubMed] [Google Scholar]
- 52.Vibhagool A, Sungkanuparph S, Mootsikapun P, et al. Discontinuation of secondary prophylaxis for cryptococcal meningitis in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy: a prospective, multicenter, randomized study. Clin Infect Dis. 2003;36:1329–1331. doi: 10.1086/374849. [DOI] [PubMed] [Google Scholar]
- 53.Aberg JA, Watson J, Segal M, Chang LW. Clinical utility of monitoring serum cryptococcal antigen (sCRAG) titers in patients with AIDS-realted cryptococcal disease. HIV Clin Trials. 2000;1:1–6. doi: 10.1310/NQXR-ULMG-MM1B-3T2B. [DOI] [PubMed] [Google Scholar]
- 54.Powderly WG, Cloud GA, Dismukes WE, Saag Ms. Measurement of cryptococcal antigen in serum and cerebrospinal fluid: value in the management of AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1994;18:789–792. doi: 10.1093/clinids/18.5.789. [DOI] [PubMed] [Google Scholar]
- 55.Woods ML, 2nd, MacGinley R, Eisen DP, Allworth AM. HIV combination therapy: partial immune restitution unmasking latent cryptococcal infection. AIDS. 1998;12:1491–1494. doi: 10.1097/00002030-199812000-00011. [DOI] [PubMed] [Google Scholar]
- 56.Shelburne SA, Darcourt J, White C, Jr, et al. The role of immune reconstitution inflammatory syndrome in AIDS related Cryptococcal neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:1049–1052. doi: 10.1086/428618. [DOI] [PubMed] [Google Scholar]
- 57.Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48:856–862. doi: 10.1086/597262. [DOI] [PMC free article] [PubMed] [Google Scholar]