Abstract
Leishmania infantum is a protozoan parasite causing severe vector-borne visceral diseases both in humans and dogs. The latter are the most important natural reservoir and therefore should be the main target of control measures. The real efficacy of seropositive dogs culling as a direct control method is still debated, and the new sensitivity of large part of population considers ethically unacceptable this kind of approach. Treatment of infectious dogs with one of the available therapeutic protocols is recommendable as it allows to reduce parasite burdens and therefore the possibility of transmission of Leishmania infantum to vectors. Vaccination has been proven to be a very effective control tool, but the absence of a commonly recognized diagnostic method able to distinguish vaccinate from seropositive individuals is still an important limit. Concerning indirect control methods, a number of studies have demonstrated the efficacy of topical insecticides treatment (collars, spot-on, and sprays) in reducing incidence and prevalence of L. infantum. Also, the reduction of the odds of seroconversion in humans in endemic areas has been reported after the application of indirect control measures on dogs. The contemporary use of direct and indirect methods is even more effective in reducing seroprevalence in dogs.
1. Introduction
Leishmania infantum is a protozoa, obligatory intracellular parasite of mammalian macrophages. This parasite is transmitted by phlebotomine sandflies causing severe vector-born visceral infectious diseases: zoonotic visceral leishmaniosis (ZVL) in human and canine leishmaniosis (CanL) in dogs. Infected dogs, both owned and not, can be an important threat to public health, because L. infantum has the domestic dog as principal reservoir. To date, ZVL is endemic to large areas of Europe, Asia, and Africa, particularly in the countries of the Mediterranean Basin [1, 2]. In Latin America ZVL is linked to L. chagasi, now considered as a synonymous of L. infantum and therefore included in the same species [3–5].
During the past years, the importance of ZVL public health concerns has increased, considering the increase in risk factors associated with environmental changes, human migration, and, mostly, the presence of Leishmania infantum-infected dogs in previous nonendemic areas. In fact, the emergence of cases of ZVL in new territories is usually preceded by an increase in the prevalence of CanL. An effective control of CanL is therefore crucial for the prevention of ZVL in humans: in particular, vector control through the employment of repellent drugs seems to be an efficient tool for the reduction of seroprevalence [5–9]. Culling of infected dogs is also used in some areas, but animal welfare concerns could arise from this issue, and its real usefulness is debated: epidemiological studies reported evidences of the inefficacy of ZVL control programs that provide for the elimination of infected dogs [10–12]. Treatment of infected dogs reduces or eliminates clinical signs, but it is not usually parasitological curative [13] and treated dogs could still be capable of transmitting the parasite [14]. Recently, a canine vaccine has been licensed in some countries and has been proved to be an efficient preventive tool in order to diminish both the number of human and canine cases [1, 12]. However, the use of this vaccine is still debated in many countries, considering its possible effect on the serological condition of healthy vaccinated individuals and, therefore, on the efficacy of serological control campaigns [15, 16].
Here, after a wide description of ZVL and CanL clinical and epidemiological aspects in humans and dogs, we consider what is the state of the art about CanL control methods, particularly regarding their efficacy and feasibility for large scale-control plans.
2. Leishmania infantum in Humans: Zoonotic Visceral Leishmaniasis (ZVL)
Human leishmaniosis is a parasitic disease caused by intracellular protozoan of the genus Leishmania. These parasites infect many mammalian species, and the transmission from one species to another happens through arthropods vectors, mostly phlebotomine sandflies. Among 15 species of Leishmania, 13 have a zoonotic nature and cause severe diseases transmitted from mammalian reservoirs to humans. There are two main forms of zoonotic leishmaniosis: the zoonotic visceral leishmaniosis (ZVL) and the zoonotic cutaneous leishmaniosis (ZCL).
ZCL is less severe, usually not fatal and self-healing, but responsible for a large number of cases, mostly in Latin America and Middle East [17]. It is caused by many different species of the genus Leishmania, including dermotropic L. infantum, but it is mostly due to L. major and other species hosted by rodents and many tropical wild animals (armadillos, sloths, and procavias). Clinical forms of ZCL in humans usually consist of localized noduloulcerative lesions; rarely these forms evolve to more severe and diffuse cutaneous leishmaniosis, with multiple nodules over large areas of skins.
ZVL is much more severe than ZCL, and it is caused by the viscerotropic L. infantum. Viscerotropic parasites addressed to the genus Leishmania have been classified into four species, precisely, L. donovani, L. archibaldi, L. infantum, and L. chagasi. The last one is now considered a New World synonymous of L. infantum [4]. In fact, L. infantum is thought to have been imported into the Americas by the dogs of European settlers during the colonization [18]. In humans, all these parasites cause visceral leishmaniosis, an acute, life-threatening disease during which the agents multiply in the macrophages of the reticuloendothelial system, resulting in generalized symptoms like fever, splenomegaly, and pancytopenia. L. donovani and L. archibaldi cause anthroponotic visceral leishmaniosis (AVL) that is only transmitted from human to human via sandfly bites and affects people of all ages while ZVL from L. infantum affects mainly young children and is transmitted from dogs. The only domestic and primary reservoir of this zoonotic disease is the dog [19], in which L. infantum causes the canine leishmaniosis (CanL). ZVL is a disease re-emerging in the whole Mediterranean Basin [19]: about 200 cases are yearly reported in Italy, usually in the same areas where CanL is endemic [20]. Worldwide reports show that sixty-six Old World and twenty-two New World countries are endemic for human leishmaniosis. The estimate yearly incidence of ZVL is 500,000 cases [21, 22]. About 90% of ZVL occur in rural and suburban areas of five countries (Bangladesh, India, Nepal, Sudan, and Brazil); however, this non uniform distribution is due to the evidence that official data are probably underestimated, because it was obtained through incomplete, passive case detection [17]. The clinic evolution of ZVL can greatly vary according to the immune status of the patient: in fact, it usually has a chronic course, but in people with reduced immune response (e.g., HIV-affected people) fulminant disease has been described [23]. In the classic, chronic evolution, early symptoms include abdominal pain, weight loss, weakness, and typically remittent febrile episodes, with two spikes per day. Later, lymphnodes swelling and liver and/or spleen enlargement may occur. Typically, there is hypergammaglobulinemia with circulating immune complexes and nephritis. Patients in the final stages of the disease show thrombocytopenia, bleeding, haematuria, and consequent anemia. Death may occur following hemorrhage and complications due to reduction of the immune response.
3. Canine Leismaniasis (CanL): Epidemiology, Pathogenesis, and Clinical Signs
Canine leishmaniosis (CanL) is a chronic visceral-cutaneous syndrome, which clinical signs could noticeably change from a patient to another: it may be manifested as a subclinical infection, often self-limiting [24] or can be a nonself-limiting and severe illness [25]. The transmission of L. infantum from domestic dogs to humans by the bite of previously infected sandflies was first demonstrated in 1930 [26, 27]. Since then, many studies confirmed the role of the domestic dog as the main reservoir of ZVL [17]. In fact, dogs have a high prevalence of both infection and infectiousness, have long-lasting infections, and are common in the peridomestic environment where usually the transmission to human occurs [5]. During the past years the scientific interest in CanL has grown considering the progressive increasing of the global incidence of ZVL and its occurrence in areas previously not involved, possibly as a consequence of global climate changes [17, 28]. Moreover, it is possible that the zoonotic potential of CanL has been enhanced, considering the more and more abundant presence of immunocompromised humans, particularly in developing countries [29–32]. However, even if dogs are recognized as the most important peridomestic reservoir of L. infantum infection to humans, only one study has shown that the owner of an infected dog could be at risk of transmission of the infection from his pet [33]. In fact, the presence of an infected dog in a household does not seem to increase the risk of infection for the members of the family in endemic areas [25], as the infected sandfly can transmit L. infantum in a range of 2.5 km [34]. Pathogenesis of CanL is common to others forms of leishmaniosis. In fact, all protozoan of the genus Leishmania are parasites that need two hosts to complete its life cycle: a phlebotomine sandfly vector and a mammal. The arthropod harbors the flagellated extracellular promastigote while the nonflagellated, intracellular amastigote develops in the mammal. The metacyclic promastigote (e.g., the final promastigote form) is transmitted to a new mammal host, that can belong to the same species of the previous extraphlebotomine host, or to another receptive species. The transmission occurs when an infected female sandfly feed on the mammal, inoculating promastigotes via the proboscis. Male sandflies are not effective vectors as they are not hematophagous [35]. In dogs, the subsequent course of infection is linked to the host immune response and to the degree of persistence and multiplication of the parasite [36]. As it has already been shown in humans [37], the presence or absence of T-cell response is important for the developing of clinical signs: analyses of circulating lymphocites in symptomatic dogs have shown low levels of CD4+ and CD8+ T-cells and a decrease in CD21+ B-cells and CD14+ monocytes, and changes related to bone marrow parasite density [38]. The absence of an adequate T-cell response leads to insufficient control of the parasite and consequent appearance of clinical signs, high Leishmania-specific antibody levels, and high parasite burdens in skin, bone marrow, spleen, liver, and lymphnodes [14, 39]. Asymptomatic dogs, in which Leishmania infection is manifested as a subclinical, self-limiting disease, are characterized by the presence of protective immunity that is CD4+ T-cells-mediated [11, 40, 41]. On the contrary, symptomatic dogs are characterized by disease susceptibility associated to the production of a marked humoral non-protective immune response and a reduced cell-mediated immunity [36, 42]. Therefore, CanL can range from a mild dermatitis associated with specific cellular immunity and low humoral response to a severe disease characterized by immune complex deposition due to a massive humoral, non-protective response and renal damage consequent to the frequent development of glomerulonephritis and tubulointerstitial nephritis [43, 44].
Clinical signs of CanL show after a period of incubation from two to eight months [45], and up to fifteen months [46]. One author reported the development of clinical signs in dogs living in a nonendemic country after 5–7 years they had visited an endemic area [47]. Clinical signs vary widely from a patient to another due to the diversity of immune responses and the variety of organs affected, resulting in several clinical findings [36, 42, 48]. Any organ could be potentially involved; however, some signs are more frequent than others. The main groups of clinical signs of CanL are described below.
3.1. Cutaneous Lesions
Cutaneous lesions are the most common clinical manifestations of CanL, they are reported in more than 50% of cases [36], even 90% according to several authors [36, 47], and an extensive range of dermatological entities have been described and classified in groups (reported percentages are from a study of Ferrer on 43 dogs): (1) alopecia and peeling (nonpruritic exfoliative dermatitis) generalized or localized over face, ears, and limbs (60%); (2) ulcerative dermatitis with ulcers in the skin of the limbs and joints (23%); (3) focal or multifocal nodular dermatitis (11%); (4) pustular dermatitis and general exanthema (6%). Papular dermatitis has also been described [44] and therefore considered as the fifth group of main dermatological entities associated to CanL [25]. Other less frequent cutaneous manifestations are depigmentation, digital, and nasal hyperkeratosis, while pyodermitis by Staphylococcus spp. is a frequent complication [25, 49]. Onychogryphosis has been reported as present in 75% of cases in a study by Semiao-Santos et al. [50]. Perionyxis and onychorrhexis have also been reported [47, 50].
3.2. Lymphadenopathy and Splenomegaly
Lymphadenopathy (increasing of size and consistency of lymph nodes) is a very common clinical sign, usually appearing relatively early during the course of the disease. The presence of this clinical sign has been reported in the 93.5% of cases [36, 50]. The general increase of limph nodes size facilitate palpating of superficial ones, in particular, popliteal, prescapular, and submaxillar [36]. Considerable splenomegaly have also been described [25, 47].
3.3. Ocular Manifestations
Ocular manifestations are quite frequent during CanL, being reported from 16 to 80% of cases [25]. The most frequent eye lesions are conjunctivitis, with mucous to mucopurulent conjunctivitis reported in 18.5% of cases [50]. These are characterized by a yellowish secretion that adhere to the lacrimal margins while the mucosa is usually pale, due to anemia [36]. Similarly, blepharitis (exfoliative, ulcerative, or nodular), anterior uveitis and keratoconjunctivitis have also been reported [51]. Keratoconjunctivitis can also progress to ulceration and blindness [52]. Uncommon are ocular consequences of systemic hypertension (e.g., retinal detachment), reported in 5.7% of hypertensive dogs with CanL [25, 53].
3.4. Renal Disease
Renal disease may be the only clinical manifestation of CanL [25]. Glomerulonephritis and tubulointerstitial nephritis due to the deposition of immune complex are the most common pathological findings in leishmaniotic dogs that develop chronic renal failure [43], which is the principal cause of death in CanL [25, 36]. However, renal disease associated to this pathology does not always reach these severe developments: membranoproliferative glomerulonephritis is usually associated with chronic renal failure while dogs without clinical evidence of renal disease more frequently reveal mesangioproliferative lesions [54].
3.5. Other Clinical Signs
CanL is a systemic disease that could involve many organs, so a variety of other clinical signs are possible, often making difficult differential diagnosis to other diseases. Weight loss, locomotor abnormalities, apathy and anorexia are still quite frequent, being reported in 15–30% of cases; less frequent are polydypsia, poliphagia, polyuria, epistaxis, vomiting, and diarrhea [36, 50]. In particular, cachexia and muscular atrophy are common findings in dogs at the final stages of the disease, when locomotor abnormalities are also frequent. These could be associated to arthritis, osteomyelitis, and arthrosynovitis in uncommon clinical forms of leishmaniosis [47, 55]. Other uncommon clinical forms include neurological disease and polymyositis [56], as well as various autoimmune and cardiovascular disorders (pericarditis, vasculitis) [25].
4. Canine Leismaniasis (CanL): Laboratory Diagnosis
Accurate and early diagnosis of CanL is crucial to identify infectious dogs and organize control strategies. In order to diagnosis CanL, clinical, anatomopathological, and laboratory findings should be evaluated together. However, control programs are usually based mostly on mass screening, therefore laboratory techniques will be primarily taken into consideration: a large part of the CanL reservoir is composed by infected clinically healthy dogs. As concerns clinical signs and anatomopathological findings in CanL patients, also useful for the diagnosis in infected dogs with clinically evident disease, we recommend to refer to the previous chapter.
Different methods of laboratory diagnosis are currently available, and they can be divided between (1) parasite isolation (direct and indirect) and (2) immunodiagnostic techniques for the detection of cellular and humoral responses [36]. The detection of the parasite is the main conclusive diagnosis and it is usually carried out directly through the demonstration of amastigotes in smears or after culture of samples (aspirates) obtained from cutaneous lesions, bone marrow, lymphnodes, and spleen [25]. The most important indirect method of detection of the parasite is the polymerase chain reaction (PCR), characterized by high sensitivity and specificity. It can be carried out from samples of blood, skin, lymphnodes, conjunctiva, and bone marrow [36]. Obtaining blood could be the most simple and noninvasive procedure in order to perform PCR, but the parasite load is often lower than in other tissues. Therefore, the application of parasite detection techniques is often difficult when a high number of animals have to be sampled for screening purposes. In this cases, immunodiagnostic techniques are of great utility, in order to detect anti-Leishmania circulating antibodies (mainly IgG): immunofluorescent antibody test (IFAT), enzyme linked immunosorbent assay (ELISA), and direct agglutination test (DAT) are the most used methods. To date the IFAT test, the most specific and sensitive among the serological techniques mentioned above, is reasonably considered the “gold standard” for mass screening of dogs [57, 58]. In fact, infectiousness is positively associated with high antiparasite antibody response and low CD4+ T-cell count [11, 40, 41], therefore high antibody levels are considered conclusive of a diagnosis of CanL [25, 38]. However, false-positive results due to serological cross-reactivity with other pathogens (e.g., Trypanosoma cruzi) have been described [59] and low sensitivity in detecting asymptomatic dogs has also been reported [60]. The cutoff titre to distinguish positive and negative results ranges from 1 : 40 to 1 : 160 according to different laboratories [61].
5. Leishmania infantum Vectors: Sandfly and Non-Sandfly Transmission Routes
Leishmania infantum is transmitted from the canine reservoir to other dogs or humans via sandflies bites. There is an exclusive association between sandfly species and Leishmania species due to specific enzyme and ligands present in the gut of these insects: only specific Leishmania spp. are allowed to attach to gut's wall and replicate without being excreted [62]. Therefore, the transmission of each Leishmania species occurs only in that areas where the specific vectors species are present. In particular, female sandflies of the genus Phlebotomus (Europe, Asia, and Africa) and Lutzomya (Latin America) are the vectors of L. infantum [19, 34, 35]. In the countries of the Mediterranean Basin the most important vector species is Phlebotomus perniciosus [63–65]. Other proven sandfly vectors present in the Mediterranean Basin and in the Middle East are P. ariasi, P. neglectus, P. perfiliewi, P. langeroni, and P. tobbi [5, 19, 35]. In northern Africa also P. sergenti and P. arabicus have been reported as proven vectors of L. infantum [25]. These arthropods are present both in tropical and temperate areas, but in the last ones they are usually active only during warm months, from spring to autumn, at temperatures between 15° and 28°C [25, 35]. The importance of sandfly transmission routes in the epidemiology of ZVL and CanL is demonstrated by the spatial and temporal overlapping of clinical cases and the presence of incriminated vector species [5]. For example, new cases of autochthonous ZVL in humans have been reported in areas of Northern Italy previously considered free [20], in consequence to new CanL outbreaks [66–68] and the proven northerly expansion of two specific vector species P. perniciosus and P. neglectus [28]. This situation has been attributed, just as new northward cases of CanL, to climate changes [17]. A recent European Union-funded project “Climate Change and Adaptation Strategies for Human Health in Europe” (cCASHh) concluded that so far there are no evidences that sandfly distributions in Europe have altered after recent climate changes, but it also stated that a considerable potential for climate-driven changes could be in Leishmaniosis distribution in the future [69, 70]. Moreover, the EDEN project (Emerging Diseases in a Changing European Environment) has highlighted the potential of leishmaniosis to expand to new territories with its vector as environments change, and various researches carried out in the framework of this project have come to the conclusion that significant changes have occurred in the geographic distribution of CanL and VZL cases and of flebotomine vectors, involving areas previously considered as nonendemic [71–74].
Non-sandfly transmission routes should be also considered. In fact, symptomatic CanL is characterized by widespread dissemination not only in blood but also in other body fluids, like semen or saliva, therefore biting [5], blood transfusions [75], and used needles [76, 77] have been suggested to be non-sandfly transmission routes, as well as sexual contact [78], congenital transmission [79, 80] and mechanical dog-to-dog transmission via ticks [81]. However, non-sandfly transmission seems to play only a marginal role in the epidemiology of leishmaniosis [25].
6. Leishmania infantum Dog Reservoir: Methods of Control
The primary objective of the control of CanL is to reduce the cases of ZVL in humans through the reduction of the prevalence in dogs. Different methods of control are available for the control of the L. infantum canine reservoir. Control methods could be divided into two different groups: (1) methods aimed at infectious dogs (direct control methods) and (2) methods aimed at vectors (indirect control methods). As concern the first group, a big issue is to identify what dogs should be subjected to control methods. Infectious dogs should be recognized, and this is not an easy work. According to the guidelines by Solano-Gallego et al. [25], dogs in CanL endemic regions could be divided into sick (animals with clinical leishmaniosis) and healthy. Moreover, healthy dogs encompasses both noninfected and infected clinically healthy animals. Only part of infected clinically healthy dogs could develop disease (potentially “presymptomatic” dogs): in fact, there are CanL-infected dogs that could be considered “resistant” to clinical leishmaniosis that will never develop clinical signs. There is a debate about the degree of infectiousness of infected clinically healthy and infected sick dogs, also linked to the usefulness or not of therapeutic treatment on dogs for the control of ZVL in humans. Moreover, it is debated if only sick dogs are worth subjecting to direct control methods (e.g., clinical treatment or culling) in order to limit the transmission of L. infantum. In fact, infectiousness would increase with clinical severity, but the infectiousness of infected clinically healthy dogs would also be sufficiently high that direct control needs to be targeted to all serologically positive dogs [5]. However, according to a longitudinal study by Courtenay et al. [11], dogs infected but “resistant” to clinical leishmaniosis would contribute very little to transmission. So, direct control methods targeted to infectious dogs (presymptomatic and symptomatic dogs) could be effective if a reliable test were available. Although of undoubted utility in mass screening, IFAT is not a sensitive and specific test to detect infectiousness, neither are skin biopsies, in fact, studies comparing infectiousness and parasite loads in the skin or lymphnodes have not shown the presence of a correlation [82]. The use of quantitative PCR for the assessment of skin parasite load could be more informative [5], but to date the detection of anti-Leishmania circulating antibodies with a serological titre ≥ 1 : 160 is the preferred screening method to identify dogs with a very high chance to be infectious and, therefore, worth to be subjected to direct control methods [19]. It is obvious that all dogs (infectious and noninfectious) should be subjected to indirect control methods. Hereinafter, direct and indirect control methods are described, including their efficacy, advantages, and disadvantages.
6.1. Direct Control Methods
6.1.1. Culling
The removal of infectious, serologically positive dogs through euthanasia could seem the most obvious direct control method of the Leishmania infantum reservoir. In fact, this is a debated issue, as different studies tried to demonstrate the efficacy of this tool, without reaching definitive evidences [83, 84]. The new sensitivity of a large part of population, particularly in most developed countries, considers this kind of approach ethically unacceptable [7, 85]. For this reason, more and more countries are choosing to follow only no-kill strategies for the control of free-roaming dogs and, therefore, for the control of the canine reservoir of ZVL. However, the main evidence against the dog culling approach to control leishmaniosis is the failure of large programs of systematic elimination of seropositive dogs carried on during past years that did not result in any reduction in the number of human cases [86]. A high proportion of dogs should be euthanized to achieve effective reduction in disease transmission [87], and this is often a too ambitious objective, mostly in undeveloped countries with limited economic resources. Moreover, the diagnosis of infectious dogs can be very difficult: sometimes serological diagnosis produces false negatives and often there is a long interval before the effective elimination of infectious dogs [88]. Finally, the removal of dogs only subjected to serological diagnosis can have counterproductive effects: infected dogs, when removed, would be replaced by susceptible dog that could become infectious in a short time [11].
6.1.2. Treatment
Treatment of symptomatic or asymptomatic dogs with high levels of anti-Leishmania circulating antibodies is the only way to reduce the infectiousness of these subjects in countries where culling is not allowed. Infectious dogs should always be treated, not only for ethical reasons, but also for public health reasons. In fact, there is a correlation between infectiousness of dogs and parasite burden: the reduction of parasite loads after treatment would reduce the possibility of transmission of Leishmania infantum to vectors and, therefore, to other dogs or humans [89, 90]. Response to treatment vary from a patient to another and according to the protocol used, but particularly for the first choice drugs it is normally quick: in few days weight increases, cutaneous lesions reduce, blood parameters return to normality, and there is general improvement of the dog's condition [36].
To date, there are different protocols of treatment for leishmaniosis in dogs, using drugs from different categories. The “classic” and still considered “first choice” treatment is based on the use of pentavalent antimonial compounds. In particular, meglumine antimoniate (Glucantime), is the most used commercial formulation, usually combined with allopurinol, according to several different protocols. Considering the possibility of significant side effects, many different protocols have been suggested, varying considerably regarding the dosage, dose interval, and duration of treatment. Possible adverse effects may be arthralgias, myalgias, gastrointestinal problems, alterations in hepatic function and nephrotoxicity [36]. The last one is the most severe effect, and occurs mostly in dogs with reduced glomerular filtration rate [25]. The preferred route for the administration of antimonial compounds is subcutaneous [25]. Intramuscular or intravenous administration has also been suggested [36], even though they could be cause of muscular fibrosis, abscess formation [13], and thrombophlebitis [47]. Allopurinol is always administered orally. Allopurinol has synergic effect in CanL therapy, therefore it is used in many different protocols. It is an inhibitor of xanthine oxidase, that results in the inhibition of the synthesis of parasite proteins [91]. It has scarce side effects (xanthine urolithiasis) and therefore can be used for long periods [92, 93]. The protocol based on the combined use of antimonials and allopurinol considered as “first line treatment” by the guidelines from Solano-Gallego et al. [25] and Oliva et al. [94] is: meglumine antimoniate (MA) 75–100 mg/kg/SID S.C. for 4–8 weeks + allopurinol (A) 10 mg/kg/BID P.O. for six months or more [95–98]. Another effective protocol based on the use of the same drugs is: MA 50–75 mg/kg/BID S.C. for 1-2 months + A 10–20 mg/kg/die P.O. for 1–8 months [19, 99]. During treatment clinicopathological parameters should be monitored, including complete CBC, biochemical profile, serum protein electrophoresis, and urinalysis, after the first month of analysis and then at least every 3-4 months [25]. In order to limit side effects, modulation of doses according to kidney and liver functions has also been used [9]: MA 75 mg/kg/BID S.C. + A 20 mg/kg/SID P.O. were used in dogs with GOT, GPT, BUN and creatinine inside normal range; MA 50 mg/kg/SID S.C. and A 20 mg/kg/SID P.O. were used in dogs with liver or renal failure. In the same study, the moment of stopping antimonial treatment was decided on the basis of results from serum protein electrophoresis, enduring the treatment until the albumin/globulin was ≥0.80.
The second choice “classic” treatment for the therapy of CanL is amphotericin B: it is a very powerful anti-Leishmania drug [100], but it is preferably administered by slow intravenous infusion [25, 101], although also subcutaneous administration has been suggested [102]. The speed of intravenous infusion is closely related to the occurrence of side effects like trembling, fever, nausea, and vomiting [103]. Another important disadvantage of amphotericin B is its severe nephrotoxicity, resulting in decreasing of the rate of glomerular filtration with consequent increasing in levels of serum creatinine and urea nitrogen [36]. The recommended protocol foresees the administration of doses from 0.1 to 1 mg/kg/SID or twice per week (lower doses when administration is daily) I.V. for two months [101, 102]. The development of formulations of amphotericin B in lipid carriers has been performed to diminish toxicity [104], but these products have high costs. Moreover, these are the first line drugs for the therapy of human leishmaniosis, so their use in dogs is not recommended to avoid drug parasite resistance [25]. Experimentally, liposomal amphotericin B has been used in dogs at the dose of 3 mg/kg/SID I.V. for five consecutive days, intravenously: results were good, but clinical signs usually reappeared after 4–6 months [105].
Recently, miltefosine has been successfully used in association with allopurinol for the treatment of CanL [6, 106]. It is an antineoplastic agent that has proved to be efficient against Leishmania [107], now registered for the use in dogs as Milteforan. The main advantages of this drug are the oral administration and the low toxicity, even if some gastrointestinal side effects (vomiting, diarrhea) have been reported, mostly at high doses, so that administration with food is recommended [108, 109]. Another possible side effect could be the early development of drug resistance due to the long half-life of this agent [110]. The protocol successfully used [6] foresees the administration of miltefosine 2 mg/kg/SID P.O. for four weeks and allopurinol 10 mg/kg/BID P.O. for six months.
6.1.3. Vaccine and Immunotherapy
The use of an anti-Leishmania dog vaccine could be the most practical and efficient tool for the control and, possibly, the eradication of the disease in endemic areas [3, 87]. In the last years, many studies have been carried on several immunoprophylactic vaccine candidates, in order to find an efficient tool able to directly prevent leishmanian infections [111–117]. To date, only two vaccines have been licensed, both in Brazil, precisely the Leishmune (Fort Dodge Animal Health) and the Leish-Tec (Hertape Calier Saùde Animal). The second one consists of adenovirus expressing a Leishmania donovani A2 antigen, but only partial results on field trials testing its efficacy have been published so far [14, 118, 119]. Leishmune vaccine has already shown its efficacy in various field studies [15, 113, 120, 121]. It is a FML-saponin vaccine that protect 98% of vaccinated dogs [15], blocking the transmission of the disease to sandfly vectors. This tool acts on a crucial moment of the life cycle of Leishmania infantum, with important epidemiological effects: in fact, it was demonstrated that vaccinated dogs do not present the parasite in blood, skin and lymph nodes [120], not exposing the parasite to sandflies. In some areas of Brazil, the anti-Leishmania vaccination of healthy dogs has been used as a preventive tool systematically used in association to regular culling of serologically positive dogs, with encouraging effects: from 2004 to 2006 the vaccination of 5.7% of the healthy dogs led to a decrease of 25% of cases of CanL in dogs and of 61% of cases of ZVL in humans [12]. Anti-Leishmania vaccination is debated because of the possible effects on the serological response of vaccinated, healthy dogs: after vaccination they could present seroconversion, therefore being indistinguishable from infectious dogs [15, 16]. So, serological control campaigns, employing either ELISA or IFAT serological screening tests, would be no more useful, because more than 70% of vaccinated dogs would result positive [12, 16]. Recently, it has been reported that the use of a specific anti-Leishmania ELISA test, suggested by the Brazilian Ministry of Health, would be able to detect only 1.3% of positivity among uninfected vaccinees [12], therefore not interfering with regular serological control campaigns.
The anti-Leishmania vaccine has also demonstrated its efficacy as immunotherapic agent, used alone or in association with allopurinol and amphotericin B (chemoimmunotherapy) for the treatment of symptomatic dogs [1]. For these purposes, a vaccine formulation, different from the one registered for prophylactic purposes, was used, with positive effects both considering immunotherapy (reduction of symptomatic cases from 100% to 38%) and chemoimmunotherapy (reduction of symptomatic cases from 100% to 12%). In particular, the combination chemotherapy-vaccine (chemoimmunotherapy) resulted very effective in eliminating also latent infections, given that 80% of patients showed PCR negative lymphnodes after eight months of treatment, versus only 33% of patients treated with immunotherapy alone.
6.2. Indirect Control Methods
6.2.1. Repellent Tools
During the last decade, a number of studies have been carried out demonstrating the efficacy of topical insecticides treatment of dogs exposed to sandfly bites in order to control the canine reservoir of Leishmania infantum [5, 6, 25]. It is now widely accepted that the prevention of sandfly bites on dogs is efficient in reducing not only the incidence of CanL [7, 122], but also the risk of human infections [33, 123]. There are three different types of topical preventive tools, namely, collars, spot-on and spray formulations, all based on synthetic pyrethroids at various concentrations: permethrin 50%-imidacloprid 10% (spot-on), permethrin 65% (spot-on), permethrin 1.9%-pyriproxyfen 0.02% (spray), and deltamethrin 4% (collar). These tools are efficient through two actions. (1) antifeeding effect: the presence of the insecticide on the skin has a strong repellent effect, so the sandfly has only a fleeting contact with the dog, that is sufficient to disorientate and irritate the insect, resulting in absence or reduction of the blood feeding; (2) insecticide effect: usually the sandfly, even if disorientated and incapable of feeding, remains on the skin for quite long time, absorbing a dose sufficient to achieve toxic effects [35, 124]. Therefore, these topical insecticides not only manage to prevent sandfly bites and, consequently, L. infantum transmission, but also can reduce the number of infectious phlebotomus by direct elimination of them. Deltamethrin-impregnated collars have antifeeding and lethality effects for six months [6], while spot-on and spray formulations have shorter effects, so they have to be applied at least once a month [7]. On the contrary, because of the slow release of the insecticide, collars achieve full protective activity only 1 week after application while spot-on formulations need 24–48 hours, and sprays are immediately active [123]. Collars have demonstrated to have a protective effect from 72% to more than 90% according to different studies [6, 36, 122]; moreover, they have shown to have a mortality effect ranging from 30% to 67% [36]. Spot-on formulations have demonstrated to be more effective, with a protection rate ranging from 88.9% to 97.7% and even to 100%, according to the intervals between one application and another (once or twice a month) [7, 124, 125]. On the contrary, the insecticide effect of spot-on formulations seems to not differ very much from the one attributed to collars, ranging from 23% to 67% [123]. The only available spray formulation has shown a repellent efficacy of 71.4% but an insecticide effect just of 7.2%, after 21 days [126, 127]. Field studies have been performed for collars and spot-on formulations, in order to evaluate the impact of mass use on the incidence of leishmaniosis, reporting reductions from 50% to 53% in tropical or subtropical countries [128, 129] and from 86% (collars) to over 90% (spot-on) in temperate countries [7, 9, 122, 130]. Moreover, in an Iranian study based on the application of deltamethrin impregnated collars a reduction of 43% of the odds of seroconversion in children was reported [33], highlighting the indirect effects of these tools on human visceral leishmaniosis. These are important achievements, but even more important results could be obtained in the future through these tools. In fact, so far these control methods have been used only in reduced and controlled areas: the application of topical insecticides in wider areas, involving entire communities and a consistent percentage of the dog population (free-roaming and not) could have a greater effect on the incidence of both CanL and ZVL.
6.2.2. Environmental Control
The efficacy of environmental insecticides in order to control the sandfly population is debated, considering high costs, difficulties in application, pollution concerns, and the possibility of resistances. There is a great variety of species of vectors, and in some cases the presence of resistance was reported, in particular for Phlebotomus papatasi (resistance to DDT, permethrin, and lambdachyalothrin) and Phlebotomus argentipes (permethrin and deltamethrin) [131]. However, other studies have reported that sandfly control by insecticides should be a very effective control method: the increase of sandfly mortality reduces the number of vectors, directly influencing the disease transmission [87]. Synthetic pyrethroids are at present the most used anti-sandfly insecticides, even if their short-lived residual activity compared to DDT could be a limitation and a reason of the low efficacy sometimes reported [132]. The effectiveness of control campaigns based on insecticide spraying for the reduction of ZVL cases was reported in Italy [133], India, Greece, Israel [36] and in China [84]. In particular, the efficacy of insecticide spraying was reported to be more evident in the intra- or peridomiciliary environment, rather than reduce the overall sandfly population [134], as this kind of control is effective on adult insects only, because it is not known were immature forms live [133]. Moreover, spraying in areas like forests, burrows, caves, and other places where sandfly larvae and pupae are suspected to live is technically very difficult [36], and could have effects on other insects species, with consequent environmental damages. It has been suggested to concentrate spraying in the peridomiciliary environment to the inside walls of houses, doors, windows, kennels, hen houses, and drains [36] in order to maximize its effect against indoor-biting vectors. Recently, the use of insecticide-treated nets has been suggested as a preventive tool for ZVL in humans [135, 136]. The reduction of microhabitats favorable to sandflies has also been reported as a step to be taken in order to achieve effective prevention [25]. However, the use of repellent tools on dogs remains the most effective antivectorial method to control the L. infantum canine reservoir, and the use of environmental control in association to spot-on formulation should be particularly efficient in areas with large and concentrate dog populations (i.e., dog shelters).
6.3. Contemporary Use of Direct and Indirect Methods
A number of studies have shown the efficacy of single control methods of the Leishmania infantum dog reservoir, inducing the ministry, health organizations, and groups of scientists to suggest the contemporary use of different tools, particularly preventive and therapeutic tools, in order to enhance their effects [19, 35, 36, 133]. However, these guidelines reported the absence of field studies or mathematically predictive studies on the effect of this “holistic” approach on the prevalence and the incidence of leishmaniosis [19]. It has been hypothesized that reinfection in endemic areas is likely [25], due to the persisting action of sandflies also on subjects treated with therapeutic protocols [137]. Recently, a field study of the authors [9] reported long term effects (4-year period) of therapeutic treatment associated with the application of spot-on preventive tools. The retrospective study was carried on in a dog shelter sited in an endemic area in Central Italy, reporting a significant, progressive reduction of prevalence and incidence of serologic reactivity to L. infantum with a very low rate of relapses (only 8% after 4 years in a pool of 67 subjects that were already seropositive at the beginning of the study). A total of 656 dogs sheltered in a municipal dog kennel were included in the study. Antimonial treatment was administered to animals assigned to one of the following three classes: (1) serological titre ≥ 1 : 160, (2) serological titre = 1 : 80, albumins/globulins ratio < 0,80, and (3) serological titre = 1 : 80 titre, albumins/globulins ratio ≥ 0,80, and presence of clinical signs of CanL. The therapeutic protocol was based on the administration of meglumine antimoniate and allopurinol. Doses were individually adjusted according to kidney and liver functions (meglumine antimoniate 75 mg/kg/BID + allopurinol 20 mg/kg/SID in dogs with normal functions, meglumine antimoniate 50 mg/kg/SID + allopurinol 20 mg/kg/SID in dog with kidney or liver failure). Antimonial treatment endured until the serum protein electrophoresis gave an albumins/globulins ratio ≥ 0.80; however, it was never longer than 12 months or shorter than 2 months. Allopurinol administration continued until complete serum protein electrophoresis normalization. Since the second year of the study a spot-on preventive treatment was applied once a month to all dogs that were in the shelter, using imidacloprid 10%-permethrin 50% (Advantix; Bayer; Germany) at a dose of 1 mL/10 kg. A progressive reduction of prevalence and incidence of seroreactivity to L. infantum was observed. In particular, during the four-year period seroprevalence was lowered from 10.8% to 4% (χ2 = 22.02; P = 0.000, significant); while incidence was reduced from 8.92% to 1.63% (χ2 = 52.226; P = 0.000, significant Figure 1). It should be highlighted that the introduction of the association of preventive and curative treatment was followed by a significant reduction in prevalence from the second to the third year of study (reduction of prevalence from 10.63 to 6.41; χ2 = 6.90; P = 0.009), which contrasts with what had been observed from the first to the second year, probably as a consequence of the absence of preventive treatment (reduction of prevalence from 10.79 to 10.63; χ2 = 0.08; P = 0.928, not significant). The very low rate of relapses could be considered as the key of this success. In fact, previous studies using antimonial treatment without preventing tools had reported seroreversion rates of 32% [138], 74.3% [99], 39.6% [139], and 86% [140]; these are values much more higher than the 8% reported in the research above described [9]. However, more studies are needed in order to confirm the role of preventive tools in reducing the rate of relapses and reinfections in endemic areas.
Figure 1.
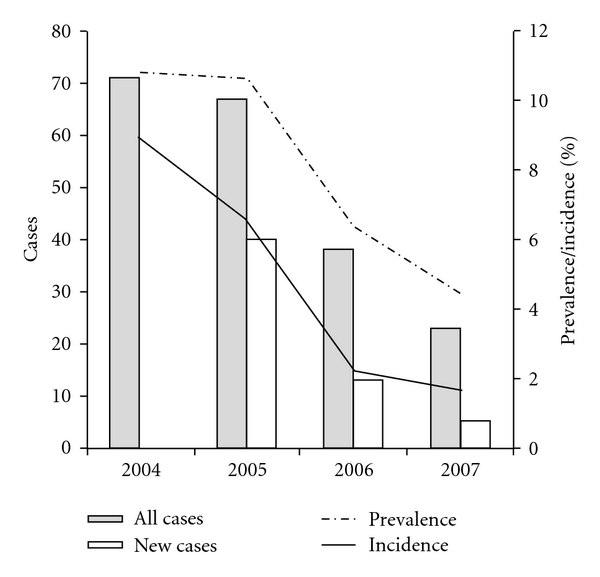
Evolution of prevalence and incidence of serological reactivity versus Leishmania infantum in two dog shelters during a 4-year period of contemporary use of direct (treatment with meglumine antimoniate and allopurinol) and indirect (spot-on repellent formulation imidacloprid 10%-permethrin 50%) control methods [9].
7. Conclusions
The domestic dog is the most important natural reservoir of Leishmania infantum and, therefore, it is crucial in the transmission of both canine leishmaniosis and zoonotic visceral leishmaniosis. A number of control methods of the L. infantum canine reservoir have been experimented, but at present the use of preventive tools directly applied on dogs seems to be the most successful one. Culling of infectious animals is now widely considered unacceptable from an ethical point of view: more and more countries are producing new “no-kill” laws with which control programs must necessarily deal. Moreover, it has been reported that this kind of approach often fails in achieving good results concerning its effects on reducing both the number of human and canine cases, particularly if applied without the contemporary use of repellent tools [86]. Treatment of symptomatic dogs could seem too costly to afford for many countries but, according to animal welfare laws and the new “humane” sensitivity of a large part of the population, the absence of therapies could be intended as maltreatment and therefore unacceptable [141]. The absence of treatments can also be a risk for public health, considering the correlation between infectiousness of dogs and parasite burden [89, 90]. The contemporary use of more than one control method has been repeatedly suggested in the past in order to enforce their efficacy [19, 25] and, in particular, the use of repellent tools can integrate the use of therapeutic treatments improving their effects and, therefore, their usefulness and affordability. Moreover, the community-wide use of repellent tools (spot-on or impregnated collars) could be a valid alternative or support to control programs based on dog euthanasia, both considered for their proven efficacy and limited costs. The anti-Leishmania vaccination has been successfully used [12] and it could be a valuable tool in order to implement efficient surveillance programs to control the L. infantum canine reservoir [3]. However, until a certain and commonly recognized diagnostic method will not be available to distinguish vaccinated from seropositive individuals, the use of this tool on large-scale-control programs should not be suggested yet. At present, repellent tools associated with the treatment of infectious dogs seems to be the most desirable and widely accepted approach in order to control the L. infantum canine reservoir.
References
- 1.Borja-Cabrera GP, Santos FN, Santos FB, et al. Immunotherapy with the saponin enriched-Leishmune® vaccine versus immunochemotherapy in dogs with natural canine visceral leishmaniasis. Vaccine. 2010;28(3):597–603. doi: 10.1016/j.vaccine.2009.09.071. [DOI] [PubMed] [Google Scholar]
- 2.Manzillo VF, Paparcone R, Cappiello S, De Santo R, Bianciardi P, Oliva G. Resolution of tongue lesions caused by Leishmania infantum in a dog treated with the association miltefosine-allopurinol. Parasites and Vectors. 2009;2(supplement 1, article S6) doi: 10.1186/1756-3305-2-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gradoni L. An update on antileishmanial vaccine candidates and prospects for a canine Leishmania vaccine. Veterinary Parasitology. 2001;100(1-2):87–103. doi: 10.1016/s0304-4017(01)00486-1. [DOI] [PubMed] [Google Scholar]
- 4.Mauricio IL, Stothard JR, Miles MA. The strange case of Leishmania chagasi. Parasitology Today. 2000;16(5):188–189. doi: 10.1016/s0169-4758(00)01637-9. [DOI] [PubMed] [Google Scholar]
- 5.Quinnell RJ, Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology. 2009;136(14):1915–1934. doi: 10.1017/S0031182009991156. [DOI] [PubMed] [Google Scholar]
- 6.Foglia Manzillo V, Oliva G, Pagano A, Manna L, Maroli M, Gradoni L. Deltamethrin-impregnated collars for the control of canine leishmaniasis: evaluation of the protective effect and influence on the clinical outcome of Leishmania infection in kennelled stray dogs. Veterinary Parasitology. 2006;142(1-2):142–145. doi: 10.1016/j.vetpar.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 7.Otranto D, Paradies P, Lia RP, et al. Efficacy of a combination of 10% imidacloprid/50% permethrin for the prevention of leishmaniasis in kennelled dogs in an endemic area. Veterinary Parasitology. 2007;144(3-4):270–278. doi: 10.1016/j.vetpar.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Otranto D, Testini G, Dantas-Torres F, et al. Diagnosis of canine vector-borne diseases in young dogs: a longitudinal study. Journal of Clinical Microbiology. 2010;48(9):3316–3324. doi: 10.1128/JCM.00379-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podaliri Vulpiani M, Iannetti L, Di Mattia T, Dalla Villa P. Leishmania infantum in a Central Italy dog shelter: retrospective study of serologic reactivity during a 4-year period in a confined dog population subjected to preventive and therapeutic treatment. Veterinary Parasitology. 2009;160(3-4):190–197. doi: 10.1016/j.vetpar.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Ashford DA, David JR, Freire M, et al. Studies on control of visceral leishmaniasis: impact of dog control on canine and human visceral leishmaniasis in Jacobina, bahia, Brazil. The American Journal of Tropical Medicine and Hygiene. 1998;59(1):53–57. doi: 10.4269/ajtmh.1998.59.53. [DOI] [PubMed] [Google Scholar]
- 11.Courtenay O, Quinnell RJ, Garcez LM, Shaw JJ, Dye C. Infectiousness in a cohort of Brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. Journal of Infectious Diseases. 2002;186(9):1314–1320. doi: 10.1086/344312. [DOI] [PubMed] [Google Scholar]
- 12.Palatnik-de-Sousa CB, Silva-Antunes I, Morgado ADA, Menz I, Palatnik M, Lavor C. Decrease of the incidence of human and canine visceral leishmaniasis after dog vaccination with Leishmune® in Brazilian endemic areas. Vaccine. 2009;27(27):3505–3512. doi: 10.1016/j.vaccine.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 13.Baneth G, Shaw SE. Chemotherapy of canine leishmaniosis. Veterinary Parasitology. 2002;106(4):315–324. doi: 10.1016/s0304-4017(02)00115-2. [DOI] [PubMed] [Google Scholar]
- 14.Reis AB, Giunchetti RC, Carrillo E, Martins-Filho OA, Moreno J. Immunity to Leishmania and the rational search for vaccines against canine leishmaniasis. Trends in Parasitology. 2010;26(7):341–349. doi: 10.1016/j.pt.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Borja-Cabrera GP, Santos FN, Bauer FS, et al. Immunogenicity assay of the Leishmune® vaccine against canine visceral leishmaniasis in Brazil. Vaccine. 2008;26(39):4991–4997. doi: 10.1016/j.vaccine.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Oliveira Mendes C, Paraguai De Souza E, Borja-Cabrera GP, et al. IgG1/IgG2 antibody dichotomy in sera of vaccinated or naturally infected dogs with visceral leishmaniosis. Vaccine. 2003;21(19-20):2589–2597. doi: 10.1016/s0264-410x(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 17.Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. International Journal for Parasitology. 2005;35(11-12):1169–1180. doi: 10.1016/j.ijpara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Tuon FF, Amato Neto V, Sabbaga Amato V. Leishmania: origin, evolution and future since the Precambrian. FEMS Immunology and Medical Microbiology. 2008;54(2):158–166. doi: 10.1111/j.1574-695X.2008.00455.x. [DOI] [PubMed] [Google Scholar]
- 19.Gradoni L, Gramiccia M, Khoury C, Maroli M. Rapporti ISTISAN 04/12. Rome, Italy: Istituto Superiore di Sanità, Dipartimento di Malattie Infettive, Parassitarie ed Immunomediate; 2004. Linee guida per il controllo del serbatoio canino della leishmaniosi viscerale zoonotica in Italia. [Google Scholar]
- 20.Mondino E, Bottari G, Caponcelli PL, Manfrinato C, Poggio A. Un caso autoctono di leishmaniosi viscerale in una regione alpina italiana. Giornale Italiano di Malattie Infettive. 2001;7(1, supplement 1, article S125) [Google Scholar]
- 21.Desjeux P. Leishmaniasis: public health aspects and control. Clinics in Dermatology. 1996;14(5):417–423. doi: 10.1016/0738-081x(96)00057-0. [DOI] [PubMed] [Google Scholar]
- 22.Tesh RB. Control of zoonotic visceral leishmaniasis: is it time to change strategies? The American Journal of Tropical Medicine and Hygiene. 1995;52(3):287–292. doi: 10.4269/ajtmh.1995.52.287. [DOI] [PubMed] [Google Scholar]
- 23.Neuber H. Leishmaniasis. Journal der Deutschen Dermatologischen Gesellschaft. 2008;6:4754–765. doi: 10.1111/j.1610-0387.2008.06809.x. [DOI] [PubMed] [Google Scholar]
- 24.Bottero E, Poggi M, Viglione M. Lesioni papulari indotte da Leishmania spp in 8 cani giovani. Veterinaria. 2006;1:33–36. [Google Scholar]
- 25.Solano-Gallego L, Koutinas A, Miró G, et al. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Veterinary Parasitology. 2009;165(1-2):1–18. doi: 10.1016/j.vetpar.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Adler S, Theodor O. Investigation on mediterranean kala-azar. VI—canine visceral Leishmaniasis. Proceedings of the Royal Society of London. 1932;110:402–412. [Google Scholar]
- 27.Parrot L, Donatien A, Lestoquard F. Sur le développement de la leishmaniose canine visceral chez Phlebotomus major var. perniciosus Newstead. Bulletin de la Societe de Pathologie Exotique. 1930;23:724–726. [Google Scholar]
- 28.Maroli M, Rossi L, Baldelli R, et al. The northward spread of leishmaniasis in Italy: evidence from retrospective and ongoing studies on the canine reservoir and phlebotomine vectors. Tropical Medicine and International Health. 2008;13(2):256–264. doi: 10.1111/j.1365-3156.2007.01998.x. [DOI] [PubMed] [Google Scholar]
- 29.De la Rosa R, Pineda JA, Delgado J, et al. Incidence of and risk factors for symptomatic visceral leishmaniasis among human immunodeficiency virus type 1-infected patients from Spain in the era of highly active antiretroviral therapy. Journal of Clinical Microbiology. 2002;40(3):762–767. doi: 10.1128/JCM.40.3.762-767.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gradoni L, Scalone A, Gramiccia M, Troiani M. Epidemiological surveillance of leishmaniasis in HIV-1-infected individuals in Italy. AIDS. 1996;10(7):785–791. doi: 10.1097/00002030-199606001-00014. [DOI] [PubMed] [Google Scholar]
- 31.Paradies P, Capelli G, Cafarchia C, De Caprariis D, Sasanelli M, Otranto D. Incidences of canine leishmaniasis in an endemic area of southern Italy. Journal of Veterinary Medicine Series B: Infectious Diseases and Veterinary Public Health. 2006;53(6):295–298. doi: 10.1111/j.1439-0450.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- 32.Paredes R, Muñoz J, Domingo P, Gurgui M, Clotet B. Leishmaniasis in HIV infection. Journal of Postgraduate Medicine. 2003;49(1):39–49. doi: 10.4103/0022-3859.929. [DOI] [PubMed] [Google Scholar]
- 33.Mazloumi Gavgani AS, Hodjati MH, Mohite H, Davies CR. Effect of insecticide-impregnated dog collars on incidence of zoonotic visceral leishmaniasis in Iranian children: a matched-cluster randomised trial. The Lancet. 2002;360(9330):374–379. doi: 10.1016/s0140-6736(02)09609-5. [DOI] [PubMed] [Google Scholar]
- 34.Sharma U, Singh S. Insect vectors of Leishmania: distribution, physiology and their control. Journal of Vector Borne Diseases. 2008;45(4):255–272. [PubMed] [Google Scholar]
- 35.Killick-Kendrick R. The biology and control of Phlebotomine sand flies. Clinics in Dermatology. 1999;17(3):279–289. doi: 10.1016/s0738-081x(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 36.Alvar J, Cañavate C, Molina R, Moreno J, Nieto J. Canine leishmaniasis. Advances in Parasitology. 2004;57:1–88. doi: 10.1016/S0065-308X(04)57001-X. [DOI] [PubMed] [Google Scholar]
- 37.Molina R, Lohse JM, Pulido F, Laguna F, López-Vélez R, Alvar J. Infection of sand flies by humans coinfected with Leishmania infantum and human immunodeficiency virus. The American Journal of Tropical Medicine and Hygiene. 1999;60(1):51–53. doi: 10.4269/ajtmh.1999.60.51. [DOI] [PubMed] [Google Scholar]
- 38.Reis AB, Teixeira-Carvalho A, Giunchetti RC, et al. Phenotypic features of circulating leucocytes as immunological markers for clinical status and bone marrow parasite density in dogs naturally infected by Leishmania chagasi. Clinical and Experimental Immunology. 2006;146(2):303–311. doi: 10.1111/j.1365-2249.2006.03206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reis AB, Martins-Filho OA, Teixeira-Carvalho A, et al. Systemic and compartmentalized immune response in canine visceral leishmaniasis. Veterinary Immunology and Immunopathology. 2009;128(1–3):87–95. doi: 10.1016/j.vetimm.2008.10.307. [DOI] [PubMed] [Google Scholar]
- 40.da Costa-Val AP, Cavalcanti RR, de Figueiredo Gontijo N, et al. Canine visceral leishmaniasis: relationships between clinical status, humoral immune response, haematology and Lutzomyia (Lutzomyia) longipalpis infectivity. Veterinary Journal. 2007;174(3):636–643. doi: 10.1016/j.tvjl.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guarga JL, Moreno J, Lucientes J, et al. Canine leishmaniasis transmission: higher infectivity amongst naturally infected dogs to sand flies is associated with lower proportions of T helper cells. Research in Veterinary Science. 2000;69(3):249–253. doi: 10.1053/rvsc.2000.0419. [DOI] [PubMed] [Google Scholar]
- 42.Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. Canine leishmaniosis—new concepts and insights on an expanding zoonosis: part one. Trends in Parasitology. 2008;24(7):324–330. doi: 10.1016/j.pt.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Costa FAL, Goto H, Saldanha LCB, et al. Histopathologic patterns of nephropathy in naturally acquired canine visceral leishmaniasis. Veterinary Pathology. 2003;40(6):677–684. doi: 10.1354/vp.40-6-677. [DOI] [PubMed] [Google Scholar]
- 44.Ordeix L, Solano-Gallego L, Fondevila D, Ferrer L, Fondati A. Papular dermatitis due to Leishmania spp. infection in dogs with parasite-specific cellular immune responses. Veterinary Dermatology. 2005;16(3):187–191. doi: 10.1111/j.1365-3164.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 45.Gaeta GB, Gradoni L, Gramiccia M, et al. Leishmaniosi viscerale in Italia. Epidemiologia, clinica, terapia. Recenti Progressi di Medicina. 1994;85:340–347. [PubMed] [Google Scholar]
- 46.Rioux JA, Killick-Kendrick R, Leaney AJ, Turner DP, Bailly M, Young CJ. Ecologie des leishmanioses dans le sud de la France. 11. La leishmaniose viscerale canine: succes de la transmission experimentale chien–phlebotome–chien par la piqure de Phlebotomus ariasi Tonnoir 1921. Annales de Parasitologie Humaine et Comparee. 1979;54:401–407. [PubMed] [Google Scholar]
- 47.Slappendel RJ. Canine leishmaniasis. A review based on 95 cases in The Netherlands. Veterinary Quarterly. 1988;10(1):1–16. doi: 10.1080/01652176.1988.9694140. [DOI] [PubMed] [Google Scholar]
- 48.Ciaramella P, Oliva G, De Luna R, et al. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. The Veterinary Record. 1997;141(21):539–543. doi: 10.1136/vr.141.21.539. [DOI] [PubMed] [Google Scholar]
- 49.Blavier A, Keroack S, Denerolle P, et al. Atypical forms of canine Leishmaniosis. Veterinary Journal. 2001;162(2):108–120. doi: 10.1053/tvjl.2000.0556. [DOI] [PubMed] [Google Scholar]
- 50.Semiao-Santos SJ, El Harith A, Ferreira E, Pires CA, Sousa C, Gusmao R. Evora district as a new focus for canine leishmaniasis in Portugal. Parasitology Research. 1995;81(3):235–239. doi: 10.1007/BF00937115. [DOI] [PubMed] [Google Scholar]
- 51.Koutinas AF, Polizopoulou ZS, Saridomichelakis MN, Argyriadis D, Fytianou A, Plevraki KG. Clinical considerations on canine visceral leishmaniasis in Greece: a retrospective study of 158 cases (1989–1996) Journal of the American Animal Hospital Association. 1999;35(5):376–383. doi: 10.5326/15473317-35-5-376. [DOI] [PubMed] [Google Scholar]
- 52.Swenson CL, Silverman J, Stromberg PC, et al. Visceral leishmaniasis in an English Foxhound from an Ohio research colony. Journal of the American Veterinary Medical Association. 1988;193(9):1089–1092. [PubMed] [Google Scholar]
- 53.Cortadellas O, del Palacio MJF, Bayón A, Albert A, Talavera J. Systemic hypertension in dogs with leishmaniasis: prevalence and clinical consequences. Journal of Veterinary Internal Medicine. 2006;20(4):941–947. doi: 10.1892/0891-6640(2006)20[941:shidwl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 54.Plevraki K, Koutinas AF, Kaldrymidou H, et al. Effects of allopurinol treatment on the progression of chronic nephritis in canine leishmaniosis (Leishmania infantum) Journal of Veterinary Internal Medicine. 2006;20(2):228–233. doi: 10.1892/0891-6640(2006)20[228:eoatot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 55.Rallis T, Day MJ, Saridomichelakis MN, et al. Chronic hepatitis associated with canine leishmaniosis (Leishmania infantum): a clinicopathological study of 26 cases. Journal of Comparative Pathology. 2005;132(2-3):145–152. doi: 10.1016/j.jcpa.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Vamvakidis CD, Koutinas AF, Kanakoudis G, Georgiadis G, Saridomichelakis M. Masticatory and skeletal muscle myositis in canine leishmaniasis (Leishmania infantum) The Veterinary Record. 2000;146(24):698–703. doi: 10.1136/vr.146.24.698. [DOI] [PubMed] [Google Scholar]
- 57.Passantino A. Medico-legal considerations of canine leishmaniosis in Italy: an overview of an emerging disease with reference to the buying and selling of dogs. OIE Revue Scientifique et Technique. 2006;25(3):1111–1123. [PubMed] [Google Scholar]
- 58.OIE (World Organization for Animal Health) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2008. Leishmaniosis. Diagnostic techniques; pp. 240–250. [Google Scholar]
- 59.Ferreira EDC, de Lana M, Carneiro M, et al. Comparison of serological assays for the diagnosis of canine visceral leishmaniasis in animals presenting different clinical manifestations. Veterinary Parasitology. 2007;146(3-4):235–241. doi: 10.1016/j.vetpar.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 60.Mettler M, Grimm F, Capelli G, Camp H, Deplazes P. Evaluation of enzyme-linked immunosorbent assays, an immunofluorescent- antibody test, and two rapid tests (immunochromatographic-dipstick and gel tests) for serological diagnosis of symptomatic and asymptomatic Leishmania infections in dogs. Journal of Clinical Microbiology. 2005;43(11):5515–5519. doi: 10.1128/JCM.43.11.5515-5519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferroglio E, Vitale F. Diagnosis of leishmaniosis: between old doubts and new uncertainties. Veterinary Research Communications. 2006;30(1):35–38. [Google Scholar]
- 62.Volf P, Hostomska J, Rohousova I. Molecular crosstalks in Leishmania-sandfly-host relationships. Parasite. 2008;15(3):237–243. doi: 10.1051/parasite/2008153237. [DOI] [PubMed] [Google Scholar]
- 63.Bettini S, Gradoni L. Canine leishmaniasis in the Mediterranean area and its implication for human leishmaniasis. Insect Science and Its Application. 1986;7:241–245. [Google Scholar]
- 64.Maroli M, Gramiccia M, Gradoni L, Ready PD, Smith DF, Aquino C. Natural infections of phlebotomine sandflies with Trypanosomatidae in central and south Italy. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988;82(2):227–228. doi: 10.1016/0035-9203(88)90421-x. [DOI] [PubMed] [Google Scholar]
- 65.Maroli M, Gramiccia M, Gradoni L, Troiani M, Ascione R. Natural infection of Phlebotomus perniciosus with MON 72 zymodeme of Leishmania infantum in the Campania region of Italy. Acta Tropica. 1994;57(4):333–335. doi: 10.1016/0001-706x(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 66.Ferroglio E, Maroli M, Castaldo S, et al. Survey of phlebotomine sandflies in North-West Italy. Parassitologia. 2002;44(supplement 1):p. 68. [Google Scholar]
- 67.Maroli M, Khoury C, Bianchi R, Ferroglio E, Natale A. Recent findings of Phlebotomus neglectus Tonnoir, 1921 in Italy and its western limit of distribution. Parassitologia. 2002;44(1-2):103–109. [PubMed] [Google Scholar]
- 68.Poglayen G, Marangon S, Manca MG, et al. A new outbreak of canine leishmaniosis in the North-East of Italy. Acta Parasitologica Turcica. 1997;21:p. 143. [Google Scholar]
- 69.European Union. The cCASHh Project: climate change and adaptation strategies for human health in Europe. 2011, http://ec.europa.eu/research/environment/pdf/env_health_projects/climate_change/cl-ccashh.pdf.
- 70. Ready PD. Leishmaniasis emergence in Europe. Euro Surveillance, 2010 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19505. [PubMed]
- 71.European Union. The EDEN project. Emerging diseases in a changing European environment. In: Annual Meeting 2008; January 2008; Brno, Czech Republic. [Google Scholar]
- 72.Martín-Sánchez J, Barón SD, Morales-Yuste M, Díaz V, Morillas-Márquez F. Leishmaniasis in southern Spain: changes over the past two decades and risk factors. In: EDEN International Conference; May 2010; Montpellier, France. [Google Scholar]
- 73.Ertabaklar H, Balcioglu C, Sakru N, Ozensoy Toz S, Ozbel Y. First epidemiological and entomological studies of canine leishmaniasis in Kırklareli province, northwestern part of Turkey. In: EDEN International Conference; May 2010; Montpellier, France. [Google Scholar]
- 74.Brazil RP, Brazil-Rocha U, Brazil BG. mpact of climatic changes and habitat degradation on Phlebotominae (Diptera: Psychodidae) distribution and leishmaniasis dispersion in Brazil. 2011, http://international-conference2010.eden-fp6project.net/var/eden_colloque/storage/fckeditor/file/Poster%20session_web.pdf.
- 75.Owens SD, Oakley DA, Marryott K, et al. Transmission of visceral leishmaniasis through blood transfusions from infected English Foxhounds to anemic dogs. Journal of the American Veterinary Medical Association. 2001;219(8):1076–1083. doi: 10.2460/javma.2001.219.1076. [DOI] [PubMed] [Google Scholar]
- 76.Alvar J, Aparicio P, Aseffa A, et al. The relationship between leishmaniasis and AIDS: the second 10 years. Clinical Microbiology Reviews. 2008;21(2):334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cruz I, Morales MA, Noguer I, Rodríguez A, Alvar J. Leishmania in discarded syringes from intravenous drug users. The Lancet. 2002;359(9312):1124–1125. doi: 10.1016/s0140-6736(02)08160-6. [DOI] [PubMed] [Google Scholar]
- 78.Silva FL, Oliveira RG, Silva TMA, Xavier MN, Nascimento EF, Santos RL. Venereal transmission of canine visceral leishmaniasis. Veterinary Parasitology. 2009;160(1-2):55–59. doi: 10.1016/j.vetpar.2008.10.079. [DOI] [PubMed] [Google Scholar]
- 79.Masucci M, De Majo M, Contarino RB, Borruto G, Vitale F, Pennisi MG. Canine leishmaniasis in the newborn puppy. Veterinary Research Communications. 2003;27(1):771–774. doi: 10.1023/b:verc.0000014268.61966.69. [DOI] [PubMed] [Google Scholar]
- 80.Rosypal AC, Troy GC, Zajac AM, Frank G, Lindsay DS. Transplacental transmission of a North American isolate of Leishmania infantum in an experimentally infected beagle. The Journal of Parasitology. 2005;91(4):970–972. doi: 10.1645/GE-483R.1. [DOI] [PubMed] [Google Scholar]
- 81.Zanatta Coutinho MT, Bueno LL, Sterzik A, et al. Participation of Rhipicephalus sanguineus (Acari: Ixodidae) in the epidemiology of canine visceral leishmaniasis. Veterinary Parasitology. 2005;128(1-2):149–155. doi: 10.1016/j.vetpar.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 82.Travi BL, Tabares CJ, Cadena H, Ferro C, Osorio Y. Canine visceral leishmaniasis in colombia: relationship between clinical and parasitologic status and infectivity for sand flies. The American Journal of Tropical Medicine and Hygiene. 2001;64(3-4):119–124. doi: 10.4269/ajtmh.2001.64.119. [DOI] [PubMed] [Google Scholar]
- 83.Gradoni L, Gramiccia M, Mancianti F, Pieri S. Studies on canine leishmaniasis control. 2. Effectiveness of control measures against canine leishmaniasis in the Isle of Elba, Italy. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988;82(4):568–571. doi: 10.1016/0035-9203(88)90511-1. [DOI] [PubMed] [Google Scholar]
- 84.Shao QF. Surveillance of kala-azar following preliminary eradication. Chinese Journal of Entomology. 1982;3(1):35–37. [PubMed] [Google Scholar]
- 85.Moreno J, Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends in Parasitology. 2002;18(9):399–405. doi: 10.1016/s1471-4922(02)02347-4. [DOI] [PubMed] [Google Scholar]
- 86.Palatnik-de-Sousa CB, dos Santos WR, França-Silva JC, et al. Impact of canine control on the epidemiology of canine and human visceral leishmaniasis in Brazil. The American Journal of Tropical Medicine and Hygiene. 2001;65(5):510–517. doi: 10.4269/ajtmh.2001.65.510. [DOI] [PubMed] [Google Scholar]
- 87.Dye C. The logic of visceral leishmaniasis control. The American Journal of Tropical Medicine and Hygiene. 1996;55(2):125–130. doi: 10.4269/ajtmh.1996.55.125. [DOI] [PubMed] [Google Scholar]
- 88.Braga MDM, Coelho ICB, Pompeu MML, et al. Canine kala-azar control: aftermath comparison of a fast deletion program of serum-reactive dogs by immuno-enzimatic essay with another of late deletion program of serum-reactive dogs by indirect immunofluorescence of filter paper eluate. Revista da Sociedade Brasileira de Medicina Tropical. 1998;31:419–424. doi: 10.1590/s0037-86821998000500001. [DOI] [PubMed] [Google Scholar]
- 89.Gradoni L, Maroli M, Gramiccia M, Mancianti F. Leishmania infantum infection rates in Phlebotomus perniciosus fed on naturally infected dogs under antimonial treatment. Medical and Veterinary Entomology. 1987;1(4):339–342. doi: 10.1111/j.1365-2915.1987.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 90.Ribeiro RR, Moura EP, Pimentel VM, et al. Reduced tissue parasitic load and infectivity to sand flies in dogs naturally infected by Leishmania (Leishmania) chagasi following treatment with a liposome formulation of meglumine antimoniate. Antimicrobial Agents and Chemotherapy. 2008;52(7):2564–2572. doi: 10.1128/AAC.00223-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Looker DL, Marr JJ, Berens RL. Mechanisms of action of pyrazolopyrimidines in Leishmania donovani. The Journal of Biological Chemistry. 1986;261(20):9412–9415. [PubMed] [Google Scholar]
- 92.Cavaliero T, Arnold P, Mathis A, Glaus T, Hofmann-Lehmann R, Deplazes P. Clinical, serologic, and parasitologic follow-up after long-term allopurinol therapy of dogs naturally infected with Leishmania infantum. Journal of Veterinary Internal Medicine. 1999;13(4):330–334. doi: 10.1892/0891-6640(1999)013<0330:csapfu>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 93.Pennisi MG, Reale S, Lo Giudice S, et al. Real-time PCR in dogs treated for leishmaniasis with allopurinol. Veterinary Research Communications. 2005;29(supplement 2):301–303. doi: 10.1007/s11259-005-0067-4. [DOI] [PubMed] [Google Scholar]
- 94.Oliva G, Roura X, Crotti A, et al. Guidelines for treatment of leishmaniasis in dogs. Journal of the American Veterinary Medical Association. 2010;236(11):1192–1198. doi: 10.2460/javma.236.11.1192. [DOI] [PubMed] [Google Scholar]
- 95.Denerolle P, Bourdoiseau G. Combination allopurinol and antimony treatment versus antimony alone and allopurinol alone in the treatment of canine leishmaniasis (96 cases) Journal of Veterinary Internal Medicine. 1999;13(5):413–415. doi: 10.1892/0891-6640(1999)013<0413:caaatv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 96.Ikeda-Garcia FA, Lopes RS, Marques FJ, et al. Clinical and parasitological evaluation of dogs naturally infected by Leishmania (Leishmania) chagasi submitted to treatment with meglumine antimoniate. Veterinary Parasitology. 2007;143(3-4):254–259. doi: 10.1016/j.vetpar.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 97.Manna L, Reale S, Vitale F, Picillo E, Pavone LM, Gravino AE. Real-time PCR assay in Leishmania-infected dogs treated with meglumine antimoniate and allopurinol. Veterinary Journal. 2008;177(2):279–282. doi: 10.1016/j.tvjl.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 98.Oliva G, Gradoni L, Cortese L, et al. Comparative efficacy of meglumine antimoniate and aminosidine sulphate, alone or in combination, in canine leishmaniasis. Annals of Tropical Medicine and Parasitology. 1998;92(2):165–171. doi: 10.1080/00034989860003. [DOI] [PubMed] [Google Scholar]
- 99.Slappendel RJ, Teske E. The effect of intravenous or subcutaneous administration of meglumine antimonate (glucantime) in dogs with leishmaniasis. A randomized clinical trial. Veterinary Quarterly. 1997;19(1):10–13. doi: 10.1080/01652176.1997.9694729. [DOI] [PubMed] [Google Scholar]
- 100.Berman JD. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clinical Infectious Diseases. 1997;24(4):684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 101.Lamothe J. Activity of amphotericin B in lipid emulsion in the initial treatment of canine leishmaniasis. The Journal of Small Animal Practice. 2001;42(4):170–175. doi: 10.1111/j.1748-5827.2001.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 102.Malik R, Craig AJ, Wigney I, Martin P, Love DN. Combination chemotherapy of canine and feline cryptococcosis using subcutaneously administered amphotericin. Australian Veterinary Journal. 1996;73(4):124–128. doi: 10.1111/j.1751-0813.1996.tb10003.x. [DOI] [PubMed] [Google Scholar]
- 103.Rubin SI, Krawiec DR, Gelberg H, Shanks RD. Nephrotoxicity of Amphotericin B in dogs: a comparison of two methods of administration. Canadian Journal of Veterinary Research. 1989;53(1):23–28. [PMC free article] [PubMed] [Google Scholar]
- 104.Hiemenz JW, Walsh TJ. Lipid formulations of amphptericin B: recent progress and future directions. Clinical Infectious Diseases. 1996;22(supplement 2):133–144. doi: 10.1093/clinids/22.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 105.Oliva G, Gradoni L, Ciaramella P, et al. Activity of liposomal amphotericin B (AmBisome) in dogs naturally infected with Leishmania infantum. Journal of Antimicrobial Chemotherapy. 1995;36(6):1013–1019. doi: 10.1093/jac/36.6.1013. [DOI] [PubMed] [Google Scholar]
- 106.Vischer C, Grousson D, Medaille C. Preliminary safety study of the combination therapy of Mitelfosina and allopurinol in dogs. In: Proceedings of the 32nd World Small Animal Veterinary Conference; August 2007; Sydney, Australia. [Google Scholar]
- 107.Kuhlencord A, Maniera T, Eibl H, Unger C. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrobial Agents and Chemotherapy. 1992;36(8):1630–1634. doi: 10.1128/aac.36.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Manna L, Vitale F, Reale S, et al. Study of efficacy of miltefosine and allopurinol in dogs with leishmaniosis. Veterinary Journal. 2009;182(3):441–445. doi: 10.1016/j.tvjl.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 109.Sundar S, Makharia A, More DK, et al. Short-course of oral miltefosine treatment of visceral leishmaniasis. Clinical Infectious Diseases. 2000;31:1110–1113. doi: 10.1086/318122. [DOI] [PubMed] [Google Scholar]
- 110.Janvier F, Morillon M, Olliaro P. Clinical sensitivity and resistance to various therapeutic agents. Médecine Tropicale. 2008;68(1):89–101. [PubMed] [Google Scholar]
- 111.Borja-Cabrera GP, Correia Pontes NN, da Silva VO, et al. Long lasting protection against canine kala-azar using the FML-QuilA saponin vaccine in an endemic area of Brazil (São Gonçalo do Amarante) Vaccine. 2002;20(27-28):3277–3284. doi: 10.1016/s0264-410x(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 112.Carson C, Antoniou M, Ruiz-Argüello MB, et al. A prime/boost DNA/Modified vaccinia virus Ankara vaccine expressing recombinant Leishmania DNA encoding TRYP is safe and immunogenic in outbred dogs, the reservoir of zoonotic visceral leishmaniasis. Vaccine. 2009;27(7):1080–1086. doi: 10.1016/j.vaccine.2008.11.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.da Silva VO, Borja-Cabrera GP, Correia Pontes NN, et al. A phase III trial of efficacy of the FML-vaccine against canine kala-azar in an endemic area of Brazil (São Gonçalo do Amaranto, RN) Vaccine. 2000;19(9-10):1082–1092. doi: 10.1016/s0264-410x(00)00339-x. [DOI] [PubMed] [Google Scholar]
- 114.Fujiwara RT, Vale AM, França da Silva JC, et al. Immunogenicity in dogs of three recombinant antigens (TSA, LeIF and LmSTI1) potential vaccine candidates for canine visceral leishmaniasis. Veterinary Research. 2005;36(5-6):827–838. doi: 10.1051/vetres:2005033. [DOI] [PubMed] [Google Scholar]
- 115.Lemesre J-L, Holzmuller P, Gonçalves RB, et al. Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of France: double-blind randomised efficacy field trial. Vaccine. 2007;25(21):4223–4234. doi: 10.1016/j.vaccine.2007.02.083. [DOI] [PubMed] [Google Scholar]
- 116.Mohebali M, Khamesipour A, Mobedi I, Zarei Z, Hashemi-Fesharki R. Double-blind randomized efficacy field trial of alum precipitated autoclaved Leishmania major vaccine mixed with BCG against canine visceral leishmaniasis in Meshkin-Shahr district, I.R. Iran. Vaccine. 2004;22(29-30):4097–4100. doi: 10.1016/j.vaccine.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 117.Rodríguez-Cortés A, Ojeda A, López-Fuertes L, et al. Vaccination with plasmid DNA encoding KMPII, TRYP, LACK and GP63 does not protect dogs against Leishmania infantum experimental challenge. Vaccine. 2007;25(46):7962–7971. doi: 10.1016/j.vaccine.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 118.Fernandes AP, Costa MMS, Coelho EAF, et al. Protective immunity against challenge with Leishmania (Leishmania) chagasi in beagle dogs vaccinated with recombinant A2 protein. Vaccine. 2008;26(46):5888–5895. doi: 10.1016/j.vaccine.2008.05.095. [DOI] [PubMed] [Google Scholar]
- 119.Dantas-Torres F. Canine leishmaniosis in South America. Parasites and Vectors. 2009;2(supplement 1, article S1) doi: 10.1186/1756-3305-2-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nogueira FS, Moreira MAB, Borja-Cabrera GP, et al. Leishmune® vaccine blocks the transmission of canine visceral leishmaniasis: absence of Leishmania parasites in blood, skin and lymph nodes of vaccinated exposed dogs. Vaccine. 2005;23(40):4805–4810. doi: 10.1016/j.vaccine.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 121.Saraiva EM, Barbosa ADF, Santos FN, et al. The FML-vaccine (Leishmune®) against canine visceral leishmaniasis: a transmission blocking vaccine. Vaccine. 2006;24(13):2423–2431. doi: 10.1016/j.vaccine.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 122.Ferroglio E, Poggi M, Trisciuoglio A. Evaluation of 65% permethrin spot-on and deltamethrin-impregnated collars for canine Leishmania infantum infection prevention. Zoonoses and Public Health. 2008;55(3):145–148. doi: 10.1111/j.1863-2378.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- 123.Maroli M, Gradoni L, Oliva G, et al. Guidelines for prevention of leishmaniasis in dogs. Journal of the American Veterinary Medical Association. 2010;236(11):1200–1206. doi: 10.2460/javma.236.11.1200. [DOI] [PubMed] [Google Scholar]
- 124.Mencke N, Volf P, Volfova V, Stanneck D. Repellent efficacy of a combination containing imidacloprid and permethrin against sand flies (Phlebotomus papatasi) in dogs. Parasitology Research. 2003;90(3):S108–S111. doi: 10.1007/s00436-003-0905-7. [DOI] [PubMed] [Google Scholar]
- 125.Miró G, Gálvez R, Mateo M, Montoya A, Descalzo MA, Molina R. Evaluation of the efficacy of a topically administered combination of imidacloprid and permethrin against Phlebotomus perniciosus in dog. Veterinary Parasitology. 2007;143(3-4):375–379. doi: 10.1016/j.vetpar.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 126.Mercier P, Jasmin P, Sanquer A. Prevention of sand fly attack by topical application of a permethrin/pyriproxyfen combination on dogs. Veterinary Therapeutics: Research in Applied Veterinary Medicine. 2003;4(3):309–316. [PubMed] [Google Scholar]
- 127.Molina R, Miró G, Gálvez R, Nieto J, Descalzo MA. Evaluation of a spray of permethrin and pyriproxyfen for the protection of dogs against Phlebotomus perniciosus. The Veterinary Record. 2006;159(7):206–209. doi: 10.1136/vr.159.7.206. [DOI] [PubMed] [Google Scholar]
- 128.Mazloumi Gavgani AS, Mohite H, Edrissian GH, Mohebali M, Davies CR. Domestic dog ownership in Iran is a risk factor for human infection with Leishmania infantum. The American Journal of Tropical Medicine and Hygiene. 2002;67(5):511–515. doi: 10.4269/ajtmh.2002.67.511. [DOI] [PubMed] [Google Scholar]
- 129.Giffoni JH, de Almeida CE, dos Santos SO, Ortega VS, de Barros AT. Evaluation of 65% permethrin spot-on for prevention of canine visceral leishmaniasis: effect on disease prevalence and the vectors (Diptera: Psychodidae) in a hyperendemic area. Veterinary Therapeutics: Research in Applied Veterinary Medicine. 2002;3(4):485–492. [PubMed] [Google Scholar]
- 130.Maroli M, Mizzoni V, Siragusa C, D’Orazi A, Gradoni L. Evidence for an impact on the incidence of canine leishmaniasis by the mass use of deltamethrin-impregnated dog collars in southern Italy. Medical and Veterinary Entomology. 2001;15(4):358–363. doi: 10.1046/j.0269-283x.2001.00321.x. [DOI] [PubMed] [Google Scholar]
- 131.Amalraj DD, Sivagnaname N, Srinivasan R. Susceptibility of Phlebotomus argentipes and P. papatasi (Diptera : Psychodidae) to insecticides. Journal of Communicable Diseases. 1999;31(3):177–180. [PubMed] [Google Scholar]
- 132.Oliveira Filho AM, Melo MT. Vectors control importance on leishmaniasis transmission. Memorias do Instituto Oswaldo Cruz. 1994;89(3):451–456. doi: 10.1590/s0074-02761994000300029. [DOI] [PubMed] [Google Scholar]
- 133.Alexander B, Maroli M. Control of phlebotomine sandflies. Medical and Veterinary Entomology. 2003;17(1):1–18. doi: 10.1046/j.1365-2915.2003.00420.x. [DOI] [PubMed] [Google Scholar]
- 134.Chaniotis BN, Parsons RE, Harlan HJ, Correa MA. A pilot study to control phlebotomine sand flies (Diptera: Psychodidae) in a neotropical rain forest. Journal of Medical Entomology. 1982;19(1):1–5. doi: 10.1093/jmedent/19.1.1. [DOI] [PubMed] [Google Scholar]
- 135.Ostyn B, Vanlerberghe V, Picado A, et al. Vector control by insecticide-treated nets in the fight against visceral leishmaniasis in the Indian subcontinent, what is the evidence? Tropical Medicine and International Health. 2008;13(8):1073–1085. doi: 10.1111/j.1365-3156.2008.02110.x. [DOI] [PubMed] [Google Scholar]
- 136.Ritmeijer K, Davies C, Van Zorge R, et al. Evaluation of a mass distribution programme for fine-mesh impregnated bednets against visceral leishmaniasis in eastern Sudan. Tropical Medicine and International Health. 2007;12(3):404–414. doi: 10.1111/j.1365-3156.2006.01807.x. [DOI] [PubMed] [Google Scholar]
- 137.Miranda S, Martorell S, Costa M, Ferrer L, Ramis A. Characterization of circulating lymphocyte subpopulations in canine leishmaniasis throughout treatment with antimonials and allopurinol. Veterinary Parasitology. 2007;144(3-4):251–260. doi: 10.1016/j.vetpar.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 138.Denerolle P. Leishmaniose canine : difficultés du diagnostic et du traitement (125 cas) Pratique Médicale et Chirurgicale de l’Animal de Compagnie. 1996;31(2):137–145. [Google Scholar]
- 139.Amusategui I. Tratamiento de la leishmaniosis canina: valoracion, caracterizacion y comparacion de la respuesta a distintos protocolos a base de antimoniato de meglumina (asociado o no a alopurinol) Madrid, Spain: Departamento de Patologıa Animal II, Facultad de Veterinaria, Universidad Complutense de Madrid; 1998. Doctoral thesis. [Google Scholar]
- 140.Ginel PJ, Lucena R, López R, Molleda JM. Use of allopurinol for maintenance of remission in dogs with leishmaniasis. The Journal of Small Animal Practice. 1998;39(6):271–274. doi: 10.1111/j.1748-5827.1998.tb03649.x. [DOI] [PubMed] [Google Scholar]
- 141.Pezza F. La protezione ed il benessere animale. In: Pezza F, editor. Guida All’esercizio Professionale del Medico Veterinario Dipendente e Libero Professionista, Aggiornamento 2005. Torino, Italy: C.G. Edizioni Medico Scientifiche; 2005. pp. 519–612. [Google Scholar]


