Abstract
Lipoarabinomannan (LAM) is a major glycolipidic antigen on the mycobacterial envelope. The aim of this study was to characterize the humoral immune response induced by immunization with a LAM extract in bovines and to evaluate the role of the generated antibodies in the in vitro infection of macrophages with Mycobacterium avium subsp. paratuberculosis (MAP). Sera from fourteen calves immunized with LAM extract or PBS emulsified in Freund's Incomplete Adjuvant and from five paratuberculosis-infected bovines were studied. LAM-immunized calves developed specific antibodies with IgG1 as the predominant isotype. Serum immunoglobulins were isolated and their effect was examined in MAP ingestion and viability assays using a bovine macrophage cell line. Our results show that the antibodies generated by LAM immunization significantly increase MAP ingestion and reduce its intracellular viability, suggesting an active role in this model.
1. Introduction
Paratuberculosis is a chronic granulomatous enteric disease affecting ruminants. The causative agent, Mycobacterium avium subsp. paratuberculosis (MAP), enters orally, crosses the intestinal barrier, and is phagocytized by macrophages within the lamina propria. These cells serve as the intracellular site in which MAP survives and multiplies [1, 2]. Several studies have been carried out to evaluate the MAP-macrophage interaction, due to its importance in paratuberculosis pathogenesis [3]. It has been proved that various receptors are involved in endocytosis of mycobacteria [4, 5] and that different routes of entry can alter the intracellular fate of pathogens. For example, ligation to receptors for the Fc portion of the immunoglobulins (FcR) is generally accompanied by activation of the respiratory burst [6], and maturation of phagolysosomes [7], whereas uptake mediated by complement receptors occurs in the absence of pro-inflammatory signals [8].
Generally, the humoral immune response against mycobacterial infections has been considered nonprotective. However, evidence for an active role of B cells and antibodies in some intracellular infections has been accumulated during the last years [9–15]. As regards paratuberculosis, it is accepted that the humoral immune response appears late in the infection and probably associated with the progression of disease from a subclinical to a clinical stage [16]. However, few works have suggested that antibodies could enhance some immune mechanism against MAP. A recent report has evaluated the effect of immune serum on the MAP macrophage interaction suggesting an active role of antibodies [17]. In addition, our group has previously reported that purified specific antibodies against MAP could enhance the MAP-macrophage interaction in vitro and improve the activation of the nuclear factor NF-κB in infected cells [18].
Lipoarabinomannan (LAM) is the main glycolipidic antigen on the mycobacteria envelope and has a molecular weight of approximately 40 kDa. Its structure presents similarities among pathogenic mycobacteria and differences in relation to LAM of nonpathogenic members of the genus [19, 20]. The role of LAM in mycobacterial pathogenesis has been studied by different research groups [21, 22]. Antibodies against LAM have been shown to be beneficial in passive protection experiments in murine tuberculosis models [10]. As regards paratuberculosis in bovines, the serological response against this compound has been extensively studied in order to improve diagnosis. However, little is known about the role of LAM-specific antibodies in this infection and, to our knowledge, there are no published reports on this topic.
The aim of this work was to characterize the humoral immune response induced by immunization with a LAM extract in bovines and to evaluate the role of the generated antibodies in the in vitro infection of macrophages with MAP.
2. Materials and Methods
2.1. LAM Extract
Mycobacterium avium subsp. avium (MAA) was grown to log phase in Dorset-Herley medium, heat-inactivated and kindly provided by Dr. A. Bernardelli (Servicio Nacional de Sanidad Animal, Argentina). The bacterial pellet was centrifuged and resuspended in PBS (NaH2PO4 3 mM, Na2HPO4 7.5 mM, NaCl 145 mM, pH 7.2–7.4) for further sonication. LAM was extracted from 5.2 g of total bacteria according to the method previously described elsewhere [23] and adapted to our laboratory conditions [24]. Carbohydrate concentration was determined by the phenol-sulphuric acid method [25] using glucose as standard. Protein concentration was determined by the Bradford method [26] using bovine serum albumin as standard. From these data, the percentage of protein removal achieved was estimated as total protein amount in the LAM extract × 100/initial total protein amount. The LAM extract was characterized by SDS-PAGE, stained with Bio-Rad Silver Stain (Bio-Rad Laboratories Inc., Hercules, CA, USA) modified for carbohydrate detection [27]. Electrophoresis was performed in a Mini-Protean II electrophoresis cell (Bio-Rad) on 12% polyacrylamide gels, following the manufacturer's instructions. Samples containing 5 μg carbohydrate/lane were twofold diluted in sample buffer and heat-treated (85°C, 5 min) before running (2 h at 96 mV). An ELISA was carried out using anti-LAM of Mycobacterium tuberculosis monoclonal antibody (mab CS-35) and purified M. tuberculosis LAM as pattern (both reagents were kindly provided by Dr. J. Belisle, Colorado State University, Fort Collins, CO, USA). Flat-bottomed 96-well polystyrene plates (Greiner Microlon, Greiner Bio-One North America Inc., Monroe, NC, USA) were coated with LAM extract or LAM pattern at 25 μg carbohydrate/well. An HRP-conjugated antimouse IgG (KPL, Kirkegaard & Perry Laboratories Inc., Gaithsburg, MD, USA) was used at a dilution of 1 : 500. Plates were developed using ortho-phenylendiamine dihydrochloride (OPD, Sigma-Aldrich Corp., St. Louis, MO, USA) in citrate-phosphate buffer (Sigma-Aldrich). The reaction was stopped after 10 min by the addition of 50 μL/well of 1 M sulphuric acid, and plates were read in an OpsysMR spectrophotometer (Dynex Technologies, Chantilly, VA, USA). Results are expressed in ELISA Units (EUs), estimated as the mean optical density value obtained at 490 nm (OD) for each sample × 100/OD for the negative control. In this case, an irrelevant mab was tested as negative control.
2.2. Animals and Samples
A total of fourteen five-month-old Holstein calves from tuberculosis- and paratuberculosis-free herds from the Pampas region of Argentina were kept under field conditions during all the experimental period. Calves were randomly assigned into the LAM group (n = 9), which subcutaneously received 2 mg of LAM extract dissolved in 1 mL of PBS and emulsified in 1 mL of Freund's Incomplete Adjuvant (FIA, Sigma-Aldrich), or the normal control group (NC group, n = 5), which were mock-immunized with 1 mL of PBS emulsified in 1 mL of FIA. The first immunization was received on day 0 and the booster 35 days later. Blood samples were taken on days 0 and 65. This experiment was performed under the approval and supervision of the Institutional Committee for the care and use of experimental animals of Facultad de Ciencias Veterinarias of Universidad de Buenos Aires, Argentina.
Serum samples from five naturally infected bovines with clinical signs of paratuberculosis were included in the current study as the infected control group (IC group). The diagnosis was confirmed by fecal culture and amplification of the IS900 fragment from isolated colonies by PCR [28].
2.3. Evaluation of Humoral Immune Response against LAM Extract
2.3.1. ELISA
Plates were coated (4°C, 2 days) with LAM extract (25 μg carbohydrate/well in PBS), washed three times with rinsing buffer (0.05% Tween 20 in PBS) and blocked with blocking buffer (0.05% Tween 20 and 10% skimmed milk in PBS). All subsequent incubations were performed at 37°C for 1 h and after each one, plates were washed three times with rinsing buffer. For comparisons of specific antibody levels, EUs of serum samples diluted 1 : 100 in blocking buffer were measured. An HRP-conjugated goat antibovine IgG (KPL) was added in a 1 : 1000 dilution. For specific isotype evaluation, HRP-conjugated sheep anti-bovine IgM, IgG1, and IgG2 antibodies (Behtyl Laboratories Inc., Montgomery, TX, USA) diluted 1 : 300 and rabbit antibovine IgG3 antibody [18] diluted 1 : 500 followed by HRP-conjugated goat antirabbit IgG (KPL) diluted 1 : 1000 were used. Plates were developed as described above. Results are expressed in EUs, using normal control sera as negative.
2.3.2. Immunoblot
To characterize the specificity of the generated antibodies, immunoblots were performed using LAM extract as antigen. Electrophoretic transfer onto nitrocellulose membranes (Trans-blot transfer medium, Bio-Rad) was carried out in a Trans-Blot SD cell (Bio-Rad) following the manufacturer's instructions. Membranes were incubated in blocking buffer and then with bovine sera diluted 1 : 250. Subsequently, HRP-conjugated goat anti-bovine IgG (KPL) was added in a 1 : 1000 dilution. The reaction was developed using 0.5 mg/mL DAB (HRP Color Development Reagent 3,3′-diaminobenzidine, Bio-Rad) and 1 μL/mL H2O2 100 vol. in TBS buffer (20 mM Tris, 500 mM NaCl, pH 7.5) for 10 min. All incubations were performed at 37°C for 1 h, and each step was followed by three washes in rinsing buffer. In order to confirm specificity against nonprotein molecules, a proteolytic treatment of LAM extract with 0.2 mg/mL proteinase K (Amresco Inc., Solon, OH, USA) was performed before running the SDS-PAGE, as described by Reichel et al. [29]. All sera were analyzed by immunoblot against digested and undigested LAM extract. As a digestion control, 1 mg/mL ovalbumin (OVA, Sigma-Aldrich) solution was incubated with proteinase K in the same conditions as the LAM extract. Digested and undigested OVA were evaluated with a hyperimmune bovine serum against OVA.
2.4. Precipitation and Purification of Antibodies
Serum antibodies of two animals from LAM-immunized, infected, and normal control groups were isolated and purified for further use in functional assays. Sera were heat-inactivated at 56°C for 30 min. Ammonium sulfate precipitation (44% saturation) was performed to obtain precipitated immunoglobulins (Igs). Then, purification by protein G affinity chromatography (protein G-agarose, Exalpha Biologicals, Shirley, MA, USA) was carried out, and purified Igs were obtained. In order to check the purity of the Igs fractions, SDS-PAGE and electrophoresis were conducted. The concentration of Igs was estimated by the Bradford method, considering the percentage of gamma-globulins detected by densitometric analysis of electrophoresis. The Igs fractions were filtered by 0.22 μm and stored at –70°C until use.
2.5. Functional Evaluation of Antibodies
These experiments were performed using the SV40-transformed bovine peritoneal macrophage cell line (Bomac) [30]. Bomac cells were cultured in RPMI-1640 medium (GIBCO, Invitrogen Corp., Carlsband, CA, USA) supplemented with 50 μg/mL gentamicin (Sigma-Aldrich) and 5% foetal calf serum (Invitrogen) at 37°C and 5% CO2. The K-10 MAP reference strain [31], generously provided by Dr. F. Paolicchi (Instituto Nacional de Tecnología Agropecuaria, Argentina), was grown at 37°C in Middlebrook 7H9 broth (Difco, BD biosciences, Franklin Lakes, NJ, USA) containing 10% albumin-dextrose-sodium chloride, 0.05% Tween 80 (Sigma-Aldrich), and 2 μg/mL mycobactin J (Allied Monitor Inc., Fayette, MO, USA). Titration was performed by serial dilution and seeding onto 7H9 agar plates. Stock was centrifuged and frozen at −70°C in 15% glycerol medium. Before use, MAP was unfrozen and cultured overnight at 37°C, then centrifuged, disaggregated by passages through a 25-gauge needle, and resuspended in RPMI medium to a final concentration of 109 Colony Forming Units (CFU)/mL. Multiplicity of infection was set at 10 : 1 (bacteria : cell), and antibodies were used at a final concentration of 100 μg/mL.
2.5.1. Ingestion Assay
Bomac cells (1 × 106 viable cells/mL) were seeded onto 20 mm × 20 mm sterile coverslips, allowed to adhere for 2 h and incubated overnight in RPMI medium. Bacteria were opsonized with precipitated Igs (100 μg/mL) from LAM-immunized, infected or normal control bovines, at 37°C for 1 h in a shaker. Immediately prior to inoculation of monolayers, the bacterial suspension was disaggregated as described above. MAP-macrophages interaction was allowed for 45 min. Cells were washed with cold PBS, fixed with 0.37% formaldehyde, and stained with Ziehl-Nielsen. A minimum of 100 cells/coverslip were counted in immersion fields (1000x) by light microscopy. The percentage of phagocytic cells (%PhC) and the mean number of internalized MAP detected in each cell (iMAP) were recorded. The phagocytic index (PI) was calculated as % PhC × iMAP [32].
2.5.2. Intracellular Viability Assay
To evaluate the viability of ingested MAP, Bomac cells (1 × 106 viable cells/mL) were seeded onto 24-well tissue culture plates and incubated at 37°C overnight in 5% CO2. Bacteria were opsonized and disaggregated as described above. Then, inoculated into Bomac cultures, in duplicate. After 2 h, monolayers were washed with PBS and one of each duplicate well was lysed with 0.2% sodium dodecylsulfate for initial CFU counting. The other duplicate well was incubated with RPMI medium containing 0.1 mg/mL gentamicin for 2 h in order to avoid contamination [33], then replacing it with antibiotic-free medium. Infected macrophages were cultured for 72 h and then lysed for final CFU counting. Lysates were serially plated on 7H9 agar and cultured at 37°C for 5 weeks until the CFU were counted. Results are expressed as percent change in viability (final CFU/initial CFU × 100). Additionally, an FcR-blocking assay was conducted as described by Manca et al. [34]. Bomac cells were preincubated for 1 h with 100 μg/mL of protein G-purified Igs from a normal control bovine that had been heat-aggregated.
2.6. Statistical Analysis
Data were analyzed for statistical significance using STATISTIX 8.0 software. ANOVA followed by Tukey's test was used except for isotype analysis. In that case, Kruskal-Wallis test followed by pairwise comparisons was run. The level of significance was set at P value <.05.
3. Results
3.1. Characterization of LAM Extract
We obtained an extract containing 105 mg of total carbohydrate. Protein presence was largely reduced (46.8 mg of protein initially versus 0.4 mg of residual protein in the LAM extract), thus showing a percentage of protein removal of 99.2%. SDS-PAGE results demonstrated that the LAM extract was mostly composed of a carbohydrate mixture, as revealed by the presence of many bands when modified silver stain was performed. Indeed, the predominant component migrated similar to M. tuberculosis LAM (Figure 2(a), lane 1 and 2). As expected, sera from the MAP-infected control group recognized our LAM extract of MAA (Figure 2(a), lane 3). In the mab CS35-ELISA, the result obtained for our LAM extract was 493.5 ± 35.1 EU, whereas for purified tuberculosis LAM it was 657.8 ± 10.6 EU. These results show cross reactions between LAM of pathogenic mycobacteria.
Figure 2.
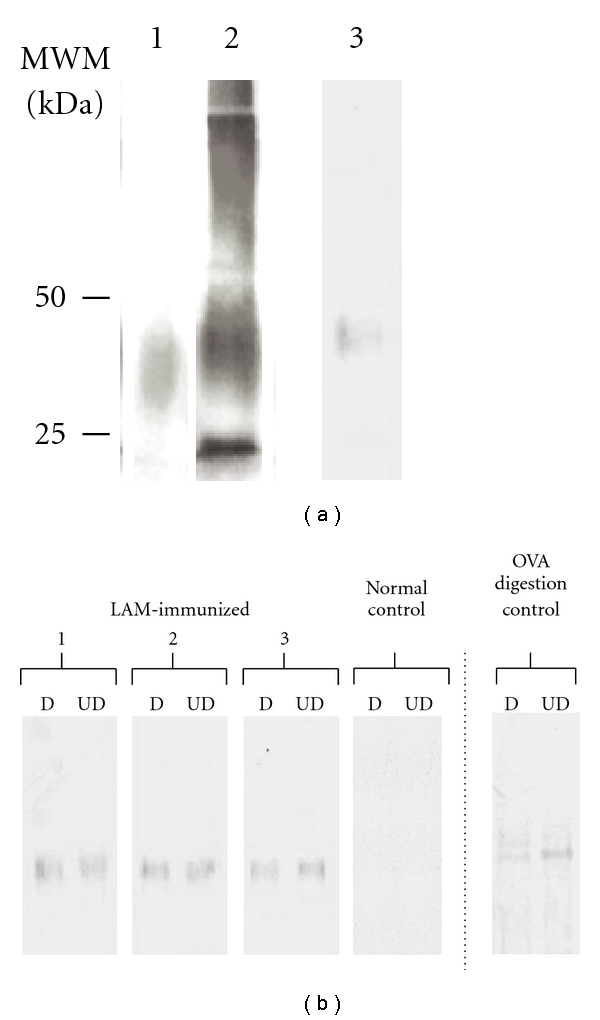
(a) Characterization of LAM extract by SDS-PAGE (lane 1: purified LAM of M. tuberculosis, lane 2: LAM extract, both silver-stained for carbohydrate detection) and immunoblot. (lane 3: LAM extract reactivity assessed against one representative serum from the infected control group). (b) Immunoblot of bovine sera reactivity against LAM extract. Left side of the dotted line: LAM extract was run as antigen. D and UD indicate protein-digested or undigested antigen. 1, 2 and 3 show results of three representative sera from LAM-immunized group. Normal control shows results representative for sera in that group. Right side of the dotted line: ovalbumin (OVA) was run as antigen and reactivity assessed against serum of an OVA-immunized bovine, as a positive control of protein digestion. MWM: molecular weight markers.
3.2. Humoral Immune Response against LAM Extract
The reactivity of sera against LAM extract was assessed and the level of specific antibodies and the isotypic profile were determined. All the calves in the LAM group generated a specific humoral immune response, with antibody levels at 1 : 100 serum dilution between 180 and 790 EU. Comparable levels were detected in the infected control group (range between 180 and 723 EU). As regards the isotype analysis, the presence of specific IgM, IgG1, IgG2, and IgG3 was evaluated in sera from LAM-immunized, infected, and normal control bovines. We could not detect specific IgM (LAM group 106.8 ± 5.5 EU, IC group 97.9 ± 15.8 EU, NC group 101.6 ± 8.1 EU). The results obtained for specific IgG1, IgG2, and IgG3 are shown in Figure 1. The humoral immune response of LAM-immunized bovines was mostly predominated by IgG1 with minor presence of IgG2 and IgG3, although statistically significant differences were found for the three of them when comparing with the normal control group (P < .05). In the infected control group, a similar trend was detected, with IgG1 as the only isotype with levels significantly higher than in the normal control group.
Figure 1.
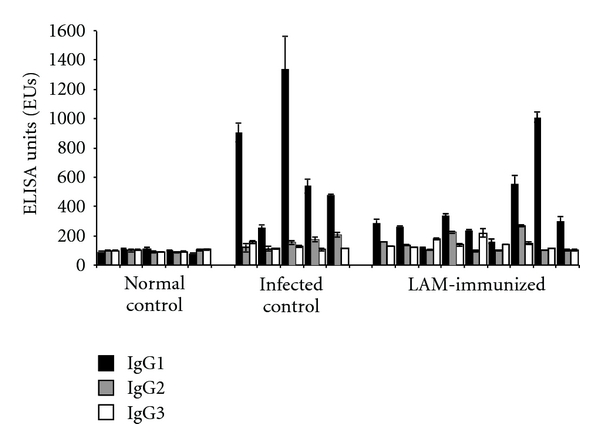
Relative levels of LAM extract-specific IgG isotypes expressed as ELISA Units (EUs) ± standard deviation of two independent measurements for each animal at 1 : 100 dilution of sera.
Sera were also tested against the LAM extract by immunoblot assay (Figure 2(b)). For all LAM-immunized calves, only one band of molecular weight between 25 and 50 kDa was detected. This band remained at equal strength after proteolytic digestion of the extract, demonstrating the nonprotein nature of the antigen involved (Figure 2(b), D versus UD lanes).
3.3. Functional Effects of Antibodies
The precipitated and purified Igs obtained (Figure 3(b), boxes) were used in functional assays.
Figure 3.
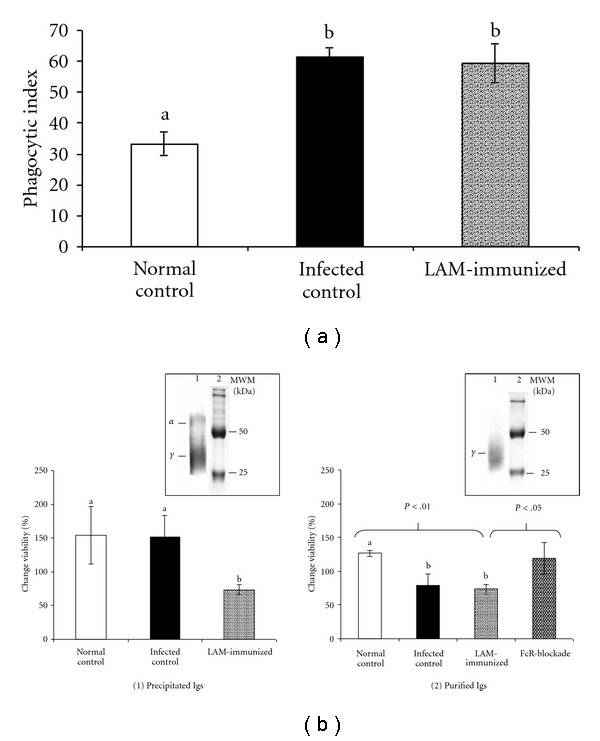
Functional effects of antibodies: (a) on MAP ingestion. Results are expressed as mean phagocytic index for each treatment ± standard deviation; (b) on MAP intracellular viability. Results are expressed as % change in MAP viability 72 h after the ingestion by Bomac cells. Boxes are representative of electrophoretic (lane 1) and SDS-PAGE (lane 2) pattern of precipitated and purified Igs. α and γ indicate alpha- and gamma- globulins bands. MWM: molecular weight markers. Error bars indicate standard deviation of three independent assays and different letters or numbers over bars represent significant differences between groups.
Effect on MAP Ingestion —
As shown in Figure 3(a), opsonization of MAP with specific antibodies increased the ingestion of bacteria. The antibodies from both LAM-immunized and infected control bovines showed a phagocytic index significantly higher (P < .01) than the one obtained when opsonizing MAP with normal control antibodies (phagocytic index of 59.4, 61.5, and 33.2, resp.).
Effect on MAP Intracellular Viability —
When assays were conducted by opsonizing MAP with precipitated antibodies, LAM group Igs significantly reduced (P < .05) the percentage of MAP viability, as compared with that obtained for normal and infected control Igs opsonization (Figure 3(b)(1)).
When MAP was opsonized with purified Igs, comparable and more repeatable results were obtained for LAM-immunized and normal control groups (P < .01). For the infected control group, the result was similar to that of the treatment with LAM group Igs. Preincubation of macrophages with aggregated Igs (FcR blockade) resulted in a significant increase of MAP viability as compared with the effect of antibodies from LAM group without previous incubation with aggregated Igs (P < .05) (Figure 3(b)(2)). This result suggests that the effect observed for the opsonization with LAM group Igs could be FcR-mediated.
4. Discussion
In the present work, we examined the immune response induced in cattle by immunization with a LAM extract and the effect of the generated antibodies in the MAP-macrophage interaction.
We used MAA for LAM extraction instead of MAP due to the antigenic homology between both [29] and the faster growth in culture of the former. Besides, our ELISA and immunoblot results support cross reactions among mycobacterial glycolipids previously described [19, 20, 29] and show that the method of LAM extraction applied preserved its antigenicity. To our knowledge, this is the first report of bovine immunization using a mycobacterial LAM extract. With the immunization protocol used, we were able to induce high levels of specific antibodies with increases of all IgG isotypes and the predominance of IgG1 in most of the studied calves. IgG1 was also detected as the predominant isotype in the infected control group. Similar responsiveness against LAM was previously described in bovines with clinical paratuberculosis [35]. Taking into account that this isotype represents the most relevant immunoglobulin in mammary gland secretions of bovines [36], the presence of local specific IgG1 might be relevant for the passive protection of newborn calves.
The influence of opsonization with antibodies on MAP phagocytosis and intracellular viability has been previously evaluated [17, 18, 33, 37, 38]. However, published reports have been generally based on assays where total serum was examined as a source of antibodies. While many other serum components are known to possess opsonic activity and could affect intracellular viability of bacteria, we purified Igs for functional evaluation. The purification methodology applied was successful. However, a heavy band was observed in SDS-PAGE, probably corresponding to the presence of incompletely reduced Igs molecules, as suggested by the reactivity with an antibovine IgG antibody (data not shown).
We found that the phagocytic level of Bomac cells increased almost twofold when MAP was opsonized with antibodies from either LAM-immunized or infected bovines. MAP-phagocytosis enhancement by hyperimmune sera has been previously reported in Bomac cells [18] and bovine blood monocyte-derived macrophages (BMDMs) [17, 37]. The phagocytic levels detected herein were comparable to those published by Woo et al. for Bomac cells [38], although our indexes were lower than those described for BMDM when ingesting MAP [17, 37]. Therein, the kinetics of MAP uptake was evaluated and a significant increase in the ingestion level was detected after 60 to 120 min of incubation [17, 37]. Taking these observations into account, it is possible that the lower phagocytic index detected herein could be related to the shorter MAP-macrophages incubation time or to the use of Bomac cell line instead of BMDM.
Controversial results in viability assays opsonizing MAP with whole sera from healthy and infected bovines were published [17, 37, 38]. In our model, opsonization with precipitated Igs from normal and infected bovines shows a beneficial effect on MAP viability. However, we found different results when precipitated and purified Igs from infected bovines were used for opsonization. The presence of other opsonins in precipitated Ig fractions, such as collectins, C-Reactive protein, or fibronectin, could explain the difference found [39–41]. Noteworthy, our results show that antibodies induced by LAM immunization could significantly reduce the intracellular viability of MAP.
These results raise questions about the biological relevance of LAM antibodies in paratuberculosis. We here found that purified antibodies present in sera from bovines immunized with LAM of MAA could reduce MAP intracellular viability as well as antibodies from MAP clinically infected cattle. However, in the natural infection, the antibodies appear late and probably associated with the progression of disease. It remains to be established whether specific antibodies present at the moment of the infection could modify the course of paratuberculosis.
Our findings provide new data about basic aspects of the role of antibodies in MAP-macrophage infection in vitro. More studies, using other MAP strains, especially field isolates, and macrophages derived directly from bovines, must be conducted in order to better approach to the role of antibodies in the natural MAP-macrophage interaction that takes place in the host.
Acknowledgments
The authors thank VMD Laura Bass and VMD Lucas Goldman for their helpful technical assistance. This work was supported by UBACyT v038 (2004–2007) and v023 (2008–2010).
References
- 1.Bendixen PH, Bloch B, Jorgensen JB. Lack of intracellular degradation of Mycobacterium paratuberculosis by bovine macrophages infected in vitro and in vivo: light microscopic and electron microscopic observations. American Journal of Veterinary Research. 1981;42(1):109–113. [PubMed] [Google Scholar]
- 2.Clarke CJ. The pathology and pathogenesis of paratuberculosis in ruminants and other species. Journal of Comparative Pathology. 1997;116(3):217–261. doi: 10.1016/s0021-9975(97)80001-1. [DOI] [PubMed] [Google Scholar]
- 3.Weiss DJ, Souza CD. Review paper: modulation of mononuclear phagocyte function by Mycobacterium avium subsp. paratuberculosis. Veterinary Pathology. 2008;45(6):829–841. doi: 10.1354/vp.45-6-829. [DOI] [PubMed] [Google Scholar]
- 4.Souza CD, Evanson OA, Sreevatsan S, Weiss DJ. Cell membrane receptors on bovine mononuclear phagocytes involved in phagocytosis of Mycobacterium avium subsp. paratuberculosis. American Journal of Veterinary Research. 2007;68(9):975–980. doi: 10.2460/ajvr.68.9.975. [DOI] [PubMed] [Google Scholar]
- 5.Sohal JS, Singh SV, Tyagi P, et al. Immunology of mycobacterial infections: With special reference to Mycobacterium avium subspecies paratuberculosis. Immunobiology. 2008;213(7):585–598. doi: 10.1016/j.imbio.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto K, Johnston RB., Jr. Dissociation of phagocytosis from stimulation of the oxidative metabolic burst in macrophages. Journal of Experimental Medicine. 1984;159(2):405–416. doi: 10.1084/jem.159.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trivedi V, Zhang SC, Castoreno AB, et al. Immunoglobulin G signaling activates lysosome/phagosome docking. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(48):18226–18232. doi: 10.1073/pnas.0609182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlesinger LS. Mycobacterium tuberculosis and the complement system. Trends in Microbiology. 1998;6(2):47–49. doi: 10.1016/S0966-842X(97)01203-1. [DOI] [PubMed] [Google Scholar]
- 9.Igietseme JU, Eko FO, He Q, Black CM. Antibody regulation of T-cell immunity: implications for vaccine strategies against intracellular pathogens. Expert Review of Vaccines. 2004;3(1):23–34. doi: 10.1586/14760584.3.1.23. [DOI] [PubMed] [Google Scholar]
- 10.Glatman-Freedman A. The role of antibody-mediated immunity in defense against Mycobacterium tuberculosis: advances toward a novel vaccine strategy. Tuberculosis (Edinb) 2006;86(3-4):191–197. doi: 10.1016/j.tube.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann SHE. The contribution of immunology to the rational design of novel antibacterial vaccines. Nature Reviews Microbiology. 2007;5(7):491–504. doi: 10.1038/nrmicro1688. [DOI] [PubMed] [Google Scholar]
- 12.Källenius G, Pawlowski A, Hamasur B, Svenson SB. Mycobacterial glycoconjugates as vaccine candidates against tuberculosis. Trends in Microbiology. 2008;16(10):456–462. doi: 10.1016/j.tim.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 13.López Y, Yero D, Falero-Diaz G, et al. Induction of a protective response with an IgA monoclonal antibody against Mycobacterium tuberculosis 16 kDa protein in a model of progressive pulmonary infection. International Journal of Medical Microbiology. 2009;299(6):447–452. doi: 10.1016/j.ijmm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Maglione PJ, Chan J. How B cells shape the immune response against Mycobacterium tuberculosis. European Journal of Immunology. 2009;39(3):676–686. doi: 10.1002/eji.200839148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abebe F, Bjune G. The protective role of antibody responses during Mycobacterium tuberculosis infection. Clinical and Experimental Immunology. 2009;157(2):235–243. doi: 10.1111/j.1365-2249.2009.03967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stabel JR. Transitions in immune responses to Mycobacterium paratuberculosis. Veterinary Microbiology. 2000;77(3-4):465–473. doi: 10.1016/s0378-1135(00)00331-x. [DOI] [PubMed] [Google Scholar]
- 17.Hostetter JM, Kagan R, Steadham E. Opsonization effects on Mycobacterium avium subsp. paratuberculosis-macrophage interactions. Clinical and Diagnostic Laboratory Immunology. 2005;12(6):793–796. doi: 10.1128/CDLI.12.6.793-796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mundo SL, Fontanals AM, García M, et al. Bovine IgG1 antibodies against Mycobacterium avium subsp. paratuberculosis protein p34-cx improve association of bacteria and macrophages. Veterinary Research. 2008;39(1):6 pages. doi: 10.1051/vetres:2007043. [DOI] [PubMed] [Google Scholar]
- 19.Nigou J, Gilleron M, Puzo G. Lipoarabinomannans: from structure to biosynthesis. Biochimie. 2003;85(1-2):153–166. doi: 10.1016/s0300-9084(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 20.Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Molecular Microbiology. 2004;53(2):391–403. doi: 10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- 21.Welin A, Winberg ME, Abdalla H, et al. Incorporation of Mycobacterium tuberculosis lipoarabinomannan into macrophage membrane rafts is a prerequisite for the phagosomal maturation block. Infection and Immunity. 2008;76(7):2882–2887. doi: 10.1128/IAI.01549-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweet L, Singh PP, Azad AK, Rajaram MV, Schlesinger LS, Schorey JS. Mannose receptor-dependent delay in phagosome maturation by Mycobacterium avium glycopeptidolipids. Infection and Immunity. 2010;78(1):518–526. doi: 10.1128/IAI.00257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamasur B, Källenius G, Svenson SB. A new rapid and simple method for large-scale purification of mycobacterial lipoarabinomannan. FEMS Immunology and Medical Microbiology. 1999;24(1):11–17. doi: 10.1111/j.1574-695X.1999.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 24.Jolly A, Colavecchia S, Jar A, Fernandez E, Mundo S. Lipoarabinomanano (LAM) de Mycobacterium sp.: respuesta inmune inducida en terneros. Investigacion Veterinaria. 2006;8:103–109. [Google Scholar]
- 25.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28(3):350–356. [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 27.Fomsgaard A, Freudenberg MA, Galanos C. Modification of the silver staining technique to detect lipopolysaccharide in polyacrylamide gels. Journal of Clinical Microbiology. 1990;28(12):2627–2631. doi: 10.1128/jcm.28.12.2627-2631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins DM, Gabric DM, De Lisle GW. Identification of a repetitive DNA sequence specific to Mycobacterium paratuberculosis. FEMS Microbiology Letters. 1989;60(2):175–178. doi: 10.1016/0378-1097(89)90503-x. [DOI] [PubMed] [Google Scholar]
- 29.Reichel MP, Kittelberger R, Penrose ME, et al. Comparison of serological tests and faecal culture for the detection of Mycobacterium avium subsp. paratuberculosis infection in cattle and analysis of the antigens involved. Veterinary Microbiology. 1999;66(2):135–150. doi: 10.1016/s0378-1135(98)00311-3. [DOI] [PubMed] [Google Scholar]
- 30.Stabel JR, Stabel TJ. Immortalization and characterization of bovine peritoneal macrophages transfected with SV40 plasmid DNA. Veterinary Immunology and Immunopathology. 1995;45(3-4):211–220. doi: 10.1016/0165-2427(94)05348-v. [DOI] [PubMed] [Google Scholar]
- 31.Foley-Thomas EM, Whipple DL, Bermudez LE, Barletta RG. Phage infection, transfection and transformation of Mycobacterium avium complex and Mycobacterium paratuberculosis. Microbiology. 1995;141(5):1173–1181. doi: 10.1099/13500872-141-5-1173. [DOI] [PubMed] [Google Scholar]
- 32.Campbell PA, Canono BP, Drevets DA. Measurement of bacterial ingestion and killing by macrophages. In: Coligan JE, Bierer BE, Margulies DH, Shevach EM, Strober W, Coico R, editors. Current Protocols of Immunology. chapter 14, unit 14.6. John Wiley & Sons; 1999. [DOI] [PubMed] [Google Scholar]
- 33.Hostetter J, Steadham E, Haynes J, Bailey T, Cheville N. Phagosomal maturation and intracellular survival of Mycobacterium avium subspecies paratuberculosis in J774 cells. Comparative Immunology, Microbiology and Infectious Diseases. 2003;26(4):269–283. doi: 10.1016/S0147-9571(02)00070-X. [DOI] [PubMed] [Google Scholar]
- 34.Manca F, Fenoglio D, Pira GL, Kunkl A, Celada F. Effect of antigen/antibody ratio on macrophage uptake, processing, and presentation to T cells of antigen complexed with polyclonal antibodies. Journal of Experimental Medicine. 1991;173(1):37–48. doi: 10.1084/jem.173.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koets AD, Rutten V, De Boer M, Bakker D, Valentin-Weigand P, Van Eden W. Differential changes in heat shock protein-, lipoarabinomannan-, and purified protein derivative-specific immunoglobulin G1 and G2 isotype responses during bovine Mycobacterium avium subsp. paratuberculosis infection. Infection and Immunity. 2001;69(3):1492–1498. doi: 10.1128/IAI.69.3.1492-1498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler JE, Kehrli ME., Jr. Immunoglobulins and immunocytes in the mammary glands and its secretions. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGee JR, Mayer L, editors. Mucosal immunology. 3rd edition. chapter 103. Vol. 3. Elsevier Academic Press; 2005. pp. 1776–1784. [Google Scholar]
- 37.Zurbrick BG, Czuprynski CJ. Ingestion and intracellular growth of Mycobacterium paratuberculosis within bovine blood monocytes and monocyte-derived macrophages. Infection and Immunity. 1987;55(7):1588–1593. doi: 10.1128/iai.55.7.1588-1593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo SR, Sotos J, Hart A, Barletta RG, Czuprynski CJ. Bovine monocytes and a macrophage cell line differ in their ability to phagocytose and support the intracellular survival of Mycobacterium avium subsp. paratuberculosis. Veterinary Immunology and Immunopathology. 2006;110(1-2):109–120. doi: 10.1016/j.vetimm.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Bermudez LE, Young LS, Enkel H. Interaction of Mycobacterium avium complex with human macrophages: Roles of membrane receptors and serum proteins. Infection and Immunity. 1991;59(5):1697–1702. doi: 10.1128/iai.59.5.1697-1702.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palaniyar N. Antibody equivalent molecules of the innate immune system: Parallels between innate and adaptive immune proteins. Innate Immunity. 2010;16(3):131–137. doi: 10.1177/1753425910370498. [DOI] [PubMed] [Google Scholar]
- 41.Dec M, Wernicki A. Conglutinin, CL-43 and CL-46—three bovine collectins. Polish Journal of Veterinary Sciences. 2006;9(4):265–275. [PubMed] [Google Scholar]


