Abstract
Most cells can dynamically shift their relative reliance on glycolytic versus oxidative metabolism in response to nutrient availability, during development, and in disease. Studies in model systems have shown that re-directing energy metabolism from respiration to glycolysis can suppress oxidative damage and cell death in ischemic injury. At present we have a limited set of drugs that safely toggle energy metabolism in humans. Here, we introduce a quantitative, nutrient sensitized screening strategy that can identify such compounds based on their ability to selectively impair growth and viability of cells grown in galactose versus glucose. We identify several FDA approved agents never before linked to energy metabolism, including meclizine, which blunts cellular respiration via a mechanism distinct from canonical inhibitors. We further show that meclizine pretreatment confers cardioprotection and neuroprotection against ischemia-reperfusion injury in murine models. Nutrient-sensitized screening may offer a useful framework for understanding gene function and drug action within the context of energy metabolism.
Virtually all cells exhibit metabolic flexibility and are capable of shifting their relative reliance on glycolysis versus mitochondrial respiration. Such shifts can occur at different timescales via a variety of mechanisms allowing cells to cope with prevailing nutrient availability or energetic demands. There is mounting evidence that targeting this shift may hold therapeutic potential. For example, many cancer cells rely on aerobic glycolysis (termed the Warburg effect)1 and a recent study has shown that pharmacologically shifting their metabolism towards respiration can retard tumor growth2. Conversely, studies in animal models have shown that inhibition of mitochondrial respiration can prevent the pathological consequences of ischemia-reperfusion injury in myocardial infarction and stroke3-7.
These observations motivate the search for agents that can safely induce shifts in cellular energy metabolism in humans. Promising work in this area has focused on hypoxia inducible factor (HIF)8, a well-studied transcriptional regulator of genes involved in the cellular adaptation to hypoxia9,10. HIF inhibitors and activators have been identified through both academic and pharmaceutical drug screens and have been shown to exhibit preclinical efficacy in cancer11 and in ischemic disease12. Other approaches to treat ischemic injury include induced hypothermia, which has been met with mixed results13. New classes of agents that shift energy metabolism may yet provide important therapeutic value in a variety of human diseases.
Here, we utilize a nutrient-sensitized screening strategy to identify drugs that toggle cellular energy metabolism based on their selective effect on cell growth and viability in glucose versus galactose media. Nutrient sensitized screening is based on the evidence that mammalian cells redirect their energy metabolism in response to the available sugar source14. Culturing cells in galactose as the sole sugar source forces mammalian cells to rely on mitochondrial oxidative phosphorylation (OXPHOS) and is a strategy previously used to diagnose human mitochondrial disorders or drug toxicity15,16. By screening our chemical library for drugs that selectively inhibit cell growth and proliferation in galactose relative to glucose, we identify a number of FDA approved compounds that redirect oxidative metabolism to glycolysis. We pursue the mechanism and therapeutic potential of one drug, meclizine, which is available without prescription, crosses the blood brain barrier, and has never been linked to energy metabolism.
RESULTS
A metabolic-state dependent growth and viability assay
Consistent with previous studies focused on other cell types14,17, we find that human skin fibroblasts grown in glucose derive ATP from both aerobic glycolysis and mitochondrial glutamine oxidation (Fig. 1a, c). However, when these cells are grown in galactose they exhibit a 5-6 fold decrease in the extracellular acidification rate (ECAR)18, reflecting decreased glycolysis, and a 2-fold increase in the oxygen consumption rate (OCR), consistent with a switch to glutamine oxidation14 (Fig. 1b, c). Moreover, cells grown in galactose maximize mitochondrial ATP production by using a larger fraction of respiration for ATP synthesis (Supplementary Fig. 1 online).
Figure 1. Metabolic plasticity of human fibroblasts.

(a-b) Schematic representation of cellular energy metabolism pathways. (a) Cells grown in glucose rich media derive ATP from glycolysis as well as from glutamine-driven respiration. (b) Replacing glucose with galactose forces cells to generate ATP almost exclusively from glutamine-driven oxidative metabolism14. (TCA = Tricarboxylic Acid; ETC = Electron Transport Chain)
(c) Measurement of extracellular acidification rate (ECAR), a proxy for the rate of glycolysis, and oxygen consumption rate (OCR), a proxy for mitochondrial respiration, of fibroblasts grown in 10 mM glucose or 10 mM galactose containing media for three days. Data are expressed as mean ± SD (n=5).
The metabolic flexibility of fibroblasts allows us to search for compounds that retard growth or are lethal to cells only in a given metabolic state. In a pilot experiment, we confirmed nutrient-dependent sensitivity of fibroblasts to known inhibitors of OXPHOS (Supplementary Fig. 2 online). In order to screen a library of chemicals, we designed a high throughput microscopy-based growth assay to identify compounds that differentially affect growth and viability in galactose vs. glucose (Fig 2a, Supplementary Fig. 3a online). Because the proliferation rates are higher for cells grown in glucose relative to galactose (Supplementary Fig. 3b online), we consider the normalized cell number in each of the two nutrient conditions. By measuring growth and survival over a three-day period, we were able to increase our power to discover compounds with even subtle effects on energy metabolism.
Figure 2. A nutrient sensitized screen to discover agents that shift energy metabolism.
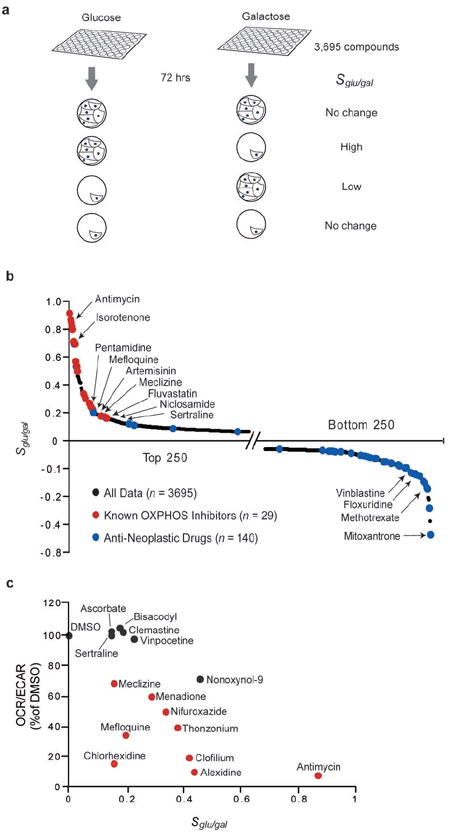
(a) Schematic of the drug screen. MCH58 cells grown in 96 well plates in glucose or galactose containing media are exposed to a chemical library of 3695 compounds for 72 hours. The logarithm of the normalized cell number in glucose versus galactose serves as a summary statistic (Sglu/gal) for each compound.
(b) Results from a nutrient sensitized screen. Sglu/gal is plotted for top and bottom 250 compounds. Known oxidative phosphorylation (OXPHOS) inhibitors are highlighted in red and anti-neoplastic drugs are highlighted in blue.
(c) Secondary assays to evaluate compounds with modest yet positive Sglu/gal scores. The OCR/ECAR ratio of selected compounds is plotted against the compounds’ corresponding Sglu/gal score from panel (b). OCR and ECAR measurements were made on MCH58 cells grown in glucose and are normalized to cell viability. Compounds indicated by red symbols exhibited a statistically significant decrease in the OCR/ECAR ratio based on at least three independent replicates (P<0.05; two-sided t-test).
A small molecule screen for agents that shift energy metabolism
We screened a library of 3,695 chemical compounds in duplicate. The library has been previously described19 and consists of two commercially available compound collections that span nearly half of all FDA-approved drugs as well as other bioactives and natural products. We jointly analyzed the glucose and galactose results to assign each drug a score, Sglu/gal, defined as the log ratio of normalized cell number in glucose divided by normalized cell number in galactose. The full table of results is provided in Supplementary Table 1 online. Positive Sglu/gal scores indicate drugs that are selectively lethal or growth inhibitory in galactose such as inhibitors of OXPHOS. Negative Sglu/gal scores may arise from inhibition of glycolysis or from inhibition of proliferation, since fibroblasts cultured in glucose divide more rapidly (Supplementary Fig. 3b online). For most drugs Sglu/gal is close to zero, indicating similar effects on growth and viability in glucose and galactose (Fig. 2b). Reassuringly, the upper tail of the Sglu/gal distribution (Fig. 2b) is highly enriched for known respiratory chain and OXPHOS inhibitors: the top 25 compounds include 20 compounds previously known to disrupt respiration by directly interrupting or uncoupling electron transport from ATP synthesis (Supplementary Table 2 online). Conversely, the lower tail is enriched for known anti-neoplastic agents (Fig. 2b): 14 of the 25 lowest Sglu/gal scores correspond to known chemotherapeutic agents that are likely selectively toxic to cells rapidly proliferating in glucose (Supplementary Table 3 online).
We next asked if any clinically used drugs exhibit high Sglu/gal scores. Amongst the top 2% of the Sglu/gal distribution (83 compounds) we identified 25 agents that have been used clinically (Supplementary Table 4 online). Previous reports provide evidence that nine out of these 25 drugs (papaverine, phenformin, artemisinin, pentamidine, clomiphene, pimozide, niclosamide, fluvastatin, carvedilol) can directly inhibit or uncouple the mitochondrial respiratory chain (Supplementary Table 4 online). This list includes two anti-malarial drugs (mefloquine and artemisinin), the latter of which has been reported to require mitochondrial respiration in the parasite for its action20. The remaining 16 clinically used agents cover a broad range of indications and diverse mechanisms of action and, to our knowledge, have never been linked to energy metabolism. We were particularly interested in identifying compounds that induce subtle metabolic shifts, since they may represent particularly safe drugs with which to manipulate energy metabolism. To this end, we focused on commercially available drugs exhibiting low to intermediate, positive Sglu/gal scores (0.45 to 0.15). We carried out secondary assays of OCR, ECAR and cell viability and confirmed that 8 of these agents induce statistically significant metabolic shifts (Fig. 2c). Of these eight clinically used drugs, we were particularly interested in meclizine, which has been approved for the treatment of nausea and vertigo for decades, is available over the counter, has a favorable safety profile, and likely penetrates the blood-brain barrier given its efficacy in disorders of the central nervous system.21
Meclizine blunts respiration in a manner distinct from classic inhibitors
In secondary assays we replicated our screening result and confirmed that galactose grown cells are more sensitive to increasing doses of meclizine (Fig. 3a). In agreement with our secondary screening assay (Fig. 2c), treatment with meclizine reduced the oxygen consumption rate (OCR) in a dose-dependent manner in cells cultured in glucose-rich media (Fig. 3b). Meclizine-induced reduction in OCR and concomitant increase in the extracellular acidification rate (ECAR) occurred in all cell types tested, including immortalized mouse striatal cells, human embryonic kidney cells, and HeLa cells (Fig. 3c-d and Supplementary Fig. 4 online). Although meclizine is classified as a histamine receptor (H1) antagonist and a weak muscarinic acetylcholine receptor antagonist22, the other 64 annotated H1 receptor antagonists and 33 annotated anti-muscarinic antagonists in our chemical library did not exhibit elevated Sglu/gal scores (anti-cholinergic P = 0.26, anti-H1 P = 0.77; Mann-Whitney rank sum test). We tested two classic anti-histamines – pyrilamine and pheniramine as well as two well characterized anti-muscarinic agents – atropine and scopolamine for their ability to inhibit OCR. Unlike meclizine these agents did not inhibit cellular OCR (Supplementary Fig. 5 online). These results suggest that meclizine’s effect on energy metabolism occurs via a mechanism not involving cholinergic or histamine receptors.
Figure 3. Effects of meclizine on cellular energy metabolism.

(a) Cell viability of MCH58 fibroblasts cells cultured in glucose or galactose media with varying doses of meclizine for three days. Data are expressed as mean ± SD (n = 5).
(b) OCR in MCH58 fibroblasts cells cultured in glucose media with varying doses of meclizine for 200 min. Data are expressed as mean ± SD (n = 3). (*P<0.05; **P<0.005; two-sided t-test).
(c,d) OCR (c) and ECAR (d) in multiple cell types cultured in glucose media with 50 μM meclizine or DMSO for 200 min. Data are expressed as mean ± SD (n ≥ 3). (* P<0.05; two-sided t-test).
(e) Time course of meclizine (50 μM) mediated OCR reduction over DMSO baseline compared to other inhibitors of OXPHOS (1 μM each) in 293 cells. Data are expressed as mean ± SD (n ≥ 3).
(f) HIF-1α and HIF-2α detection by Western blot analysis of protein extract from HeLa cells after 6 hrs treatment with 0.1 % DMSO, 100 μM deferoxamine (DFO) or 50 μM meclizine. The complete immunoblot is provided as Supplementary Fig. 7b.
Meclizine’s suppression of cellular oxygen consumption occurs with much slower kinetics than canonical inhibitors of OXPHOS that directly target the respiratory chain or ATPase (Fig. 3e). The slow kinetics suggests that it takes time for meclizine to accumulate in mitochondria or alternatively, that it might act indirectly. To distinguish between these alternatives, we studied the effect of meclizine on isolated mitochondria. Using glutamate/malate, pyruvate/malate, or succinate as fuel substrates, we found no effect of meclizine on respiration of isolated mitochondria (Fig. 4a-c). Meclizine did not have a qualitative impact on membrane potential or redox potential during respiratory state transitions of isolated mitochondria (Fig. 4d,e). Furthermore, meclizine treatment had no effect on mitochondrial morphology, membrane potential, mitochondrial (mt) DNA copy number or the expression of mtRNAs in intact cells (Supplementary Fig. 6 online). Collectively, these observations demonstrate that unlike classic inhibitors or uncouplers such as rotenone, antimycin, oligomycin or carbonyl cyanide m-chlorophenyl hydrazone (CCCP), meclizine does not itself directly inhibit/uncouple the OXPHOS machinery in isolated mitochondria, and that it does not reduce mitochondrial biogenesis in intact cells. Instead it may act via novel signaling or transcriptional mechanisms.
Figure 4. Effect of meclizine on bioenergetics of isolated mitochondria.

(a-c) Acute effect of meclizine on oxygen consumption in isolated mitochondria. Traces are representative of five independent measurements.
(d) Acute effect of meclizine on mitochondrial membrane potential measured with tetramethyl rhodamine methyl ester (TMRM, 546±7 nm excitation, 590±4 nm emission) in isolated mitochondria. Traces are representative of five independent measurements.
(e) Acute effect of meclizine on mitochondrial NADH (370±7 nm excitation, 440±4 nm emission) in isolated mitochondria. Traces are representative of five independent measurements. Mitochondria (Mito), glutamate and malate (G/M), Succinate (S), Pyruvate/Malate (P/M), meclizine (Mec) or DMSO, ADP, and carbonyl cyanide m-chlorophenyl hydrazone (CCCP) were added at indicated time points.
Activation of hypoxia inducible factor 1α (HIF1-α) or 2α (HIF2-α) is known to induce transcriptional rewiring of energy metabolism from respiration to glycolysis23. However, unlike with deferoxamine (DFO), a known inducer of the HIF pathway, we did not observe HIF1-α or HIF2-α stabilization following meclizine treatment (Fig. 3f and Supplementary Fig. 7a,b online). Moreover, the kinetics of meclizine’s OCR inhibition argue against a transcriptional mechanism, as meclizine showed inhibition within two hours, whereas OCR inhibition mediated by DFO only became apparent after 12 hours (Supplementary Fig. 7c online). In addition, we examined the effect of meclizine treatment on HIF-responsive genes using a HIF response element/luciferase reporter construct and recorded no induction of luciferase activity following 6 hour treatment (Supplementary Fig. 7d online).
Collectively these studies suggest that meclizine inhibits cellular respiration indirectly, in a HIF-independent manner that does not involve histaminergic or muscarinic receptor signaling.
Meclizine confers protection against ischemic injury
Previous studies have clearly demonstrated that brief, nonlethal episodes of ischemia can confer prophylaxis against subsequent stroke3,7 or myocardial infarction4,5, and studies in model systems have shown that chemical inhibition of mitochondrial respiration can mimic this protection, a process coined “chemical preconditioning”6. Having shown that meclizine, an over-the-counter drug that crosses the blood brain barrier can gently silence respiration, we sought to determine whether this drug is cardioprotective and neuroprotective in cellular and animal models.
First, we tested meclizine in an adult rat ventricular cardiomyocyte model of simulated ischemia-reperfusion (SIR) injury (Fig. 5a). A 20 minute meclizine pre-incubation followed by washout prior to ischemia elicited a dose-dependent protection of cardiomyocytes against SIR-induced death whereas other anti-histamines (pyrilamine and pheniramine) and anti-muscarinic agents (scopolamine and atropine) did not provide protection (Fig. 5b). These results are consistent with the hypothesis that mild OXPHOS inhibition is cytoprotective in ischemic injury. As with the other cell types, meclizine inhibited oxygen consumption in cardiomyocytes in a dose dependent manner (Fig. 5c and Supplementary Fig. 8a,b online) but not in isolated cardiac mitochondria (Supplementary Fig. 8c,d online). Next, we tested if meclizine protects isolated perfused rat hearts from ischemia-reperfusion injury in an ex vivo model of ischemic injury. Meclizine preserved heart pump function following the ischemic event (Fig. 5d) and significantly reduced the infarct area of Langendorff perfused rat hearts subjected to 25 minutes of global ischemia (Fig. 5e).
Figure 5. Meclizine is cardioprotective in cellular and ex vivo models of cardiac ischemia.
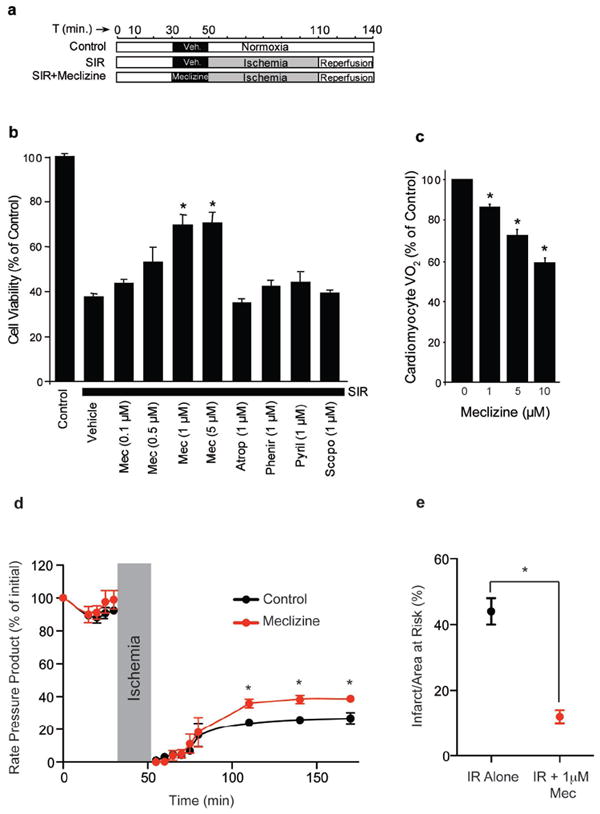
(a) Protocol for the simulated ischemia-reperfusion (SIR) model.
(b) Viability of adult rat cardiomyocytes subjected to SIR, in the presence of indicated concentrations of meclizine (Mec), Atropine (Atrop), Pheniramine (Phenir), Pyrilamine (Pyril) and Scopolamine (Scopo).
(c) Respiration of cardiomyocytes following exposure to indicated concentrations of meclizine.
(d-e) Langendorff perfused rat hearts were subjected to 25 minutes of global ischemia followed by 2 hours of reperfusion. Meclizine treatment comprised infusion of 1μM (Mec) from a port above the aortic cannula for 20 minutes, followed by a 1 minute washout prior to ischemia. (d) Rate pressure product (heart rate × left ventricular developed pressure) is expressed as a % of the initial value throughout the ischemia-reperfusion (IR) protocol. (e) Following IR, hearts were stained with 2,3,5-Triphenyltetrazolium chloride (TTC) and infarct size was quantified. All data are means ± SEM from 4-6 individual experiments. (*P<0.05, ANOVA in panel d, Student’s t-test in panel e).
Chemical preconditioning has also been shown to be protective in the animal models of cerebral ischemia6,12,24-26. To determine whether meclizine might similarly be useful in this context, we first established safety and pharmacokinetic parameters for an intraperitoneal dosing regimen (see Methods). We found that mice tolerate daily IP injections of 100 mg/kg meclizine without any weight loss or behavioral changes even after four consecutive days. Six hours after a single IP dose the plasma concentration is in the 3-5 uM range, a concentration sufficient to blunt cellular respiration of primary mouse neurons (Supplementary Fig. 9 online). We then tested whether meclizine is protective in cerebral ischemia by pre-treating mice with two intraperitoneal injections of 100 mg/kg meclizine, or an equal volume of vehicle at 17 and 3 hours prior to a one-hour transient middle cerebral artery occlusion (Fig. 6a). We found that total infarct volume was significantly reduced by 23% in meclizine treated animals (Fig. 6c). In addition, meclizine significantly reduced the area of infarction in brain slices with the greatest area of infarct (Fig. 6d,e). The in vivo protective effect of meclizine is likely independent of its anti-histamine or anti-muscarinic property as treatment with pyrilamine or scopolamine did not decrease infarct volume (Fig. 6c) or reduce the area of infarction in brain slices (Fig. 6d,e). Furthermore, meclizine pre-treated animals tended towards having preserved neurological function compared to controls (P=0.07, Kruskal-Wallis non-parametric ANOVA).The cerebral blood flow deficit (Fig. 6b) and the amount of postoperative weight loss did not differ between the groups.
Figure 6. Meclizine is neuroprotective in a mouse model of stroke.

(a) Protocol for the murine model of stroke. Male C57BL/6 mice were treated with two intraperitoneal injections of 100 mg/kg meclizine, 20 mg/kg pyrilamine and 0.5 mg/kg scopolamine or vehicle at 17 and 3 hours prior to 1 hour transient middle cerebral artery occlusion followed by 24 hours of reperfusion.
(b) Cerebral blood flow (CBF) measured at baseline and after common carotid and middle cerebral artery occlusion (CCAO and MCAO, respectively) upon treatment with meclizine, scopolamine, pyrilamine or vehicle. Data represent mean ± SD.
(c) Infarct volume measured on TTC-stained 1 mm thick coronal slices obtained from mice treated with meclizine, scopolamine, pyrilamine or vehicle. Data points refer to independent experiments, and the solid line represents their mean. (*P<0.05 vs. vehicle and scopolamine, P<0.01 vs. pyrilamine; one-way ANOVA followed by Tukey’s multiple comparison test)
(d) Representative images of TTC-stained 1 mm thick coronal brain sections (slice 1-10).
(e) Infarct area in the rostrocaudal extent of the brain (slice 1-10) upon treatment with meclizine, scopolamine, pyrilamine or vehicle. Data points represent the mean area of infarction in individual slice levels ± SD in mm2 (n=14 for vehicle, n=8 for meclizine, n=8 for pyrilamine, n=5 for scopolamine, *P<0.05).
DISCUSSION
Recent studies have shown that changes in cellular energy metabolism can accompany a range of human diseases, and that targeting energy metabolism may hold therapeutic potential. However, at present we lack an arsenal of clinically safe and useful agents that target energy metabolism. In this study, we have introduced a facile, nutrient-sensitized screening strategy aimed at identifying small molecules that shift cellular energy metabolism from respiration to glycolysis. We have identified several FDA-approved drugs that exhibit such activity and now hold therapeutic potential for a spectrum of human diseases. Focusing on one specific hit from our screen, meclizine, we have demonstrated that it suppresses OXPHOS via a mechanism distinct from classic inhibitors or uncouplers, and that it confers protection against cardiac and cerebral ischemic injury.
A large body of literature demonstrates that agents that blunt respiration can offer prophylaxis against cell death following ischemia and reperfusion in the heart4,5,27 or brain3,6,26. This effect is thought to occur via suppression of oxidative injury and cell-death and may be related to protection conferred by ischemic preconditioning, though the precise molecular mechanism is not known. Notably, redirecting energy metabolism towards glycolysis has been shown to minimize oxidative damage and suppress apoptosis28-30. Interestingly, switching to an anaerobic metabolism appears to be a natural adaptation to reduced oxygen availability31, and activation of the HIF pathway provides one such strategy for redirecting energy metabolism towards glycolysis9,32. Recent studies utilizing genetic and chemical approaches of activating the HIF pathway have shown promising results in various models of ischemia-reperfusion injury. For example, myofibers of prolyl hydroxylase 1 (Phd1) knockout mice were shown to be resistant to acute ischemia because of reduced generation of oxidative stress33. In preclinical studies, PHD inhibitors have been shown to confer protection in models of myocardial infarction34, stroke35 and renal ischemia36. However, HIF regulates the expression of a plethora of genes, and unwanted side effects have remained a concern37, suggesting that it might be useful to expand the arsenal of agents that shift energy metabolism.
Our screen has identified a new metabolic activity for meclizine, an over the counter drug that has been in use in the United States for more than 40 years for treatment of nausea and vertigo. In the current study, we found that 1 μM meclizine provided cytoprotection in vitro and ex vivo models of cardiac ischemia-reperfusion injury (Fig. 5). In addition, we showed that meclizine significantly reduced infarct volume in an in vivo model of cerebral ischemia (Fig. 6). The utility of pre-treatment paradigms described in this study arises in clinical settings in which ischemic insults can be anticipated. Examples of such situations include patients undergoing high-risk surgical procedures as well as in the large cohort of patients that suffer from diseases of recurrent ischemia such as unstable angina or recurrent transient ischemic attacks38. Interestingly, currently approved doses of meclizine are predicted to approach plasma concentrations that we now show confer cardioprotection. Post marketing surveillance data supports the safe nonprescription use of meclizine, and published studies in animals including nonhuman primates have shown that higher doses can be tolerated39,40. However, because the potency of meclizine in blunting respiration appears to vary across cell types (Fig. 3c, 5c and Supplementary Fig. 9 online), preclinical studies of efficacy and toxicity are required to rigorously determine optimal dosing and safety regimens before evaluating the therapeutic potential of meclizine in humans.
Our detailed studies on the effects of meclizine on cellular energy metabolism clearly show that it silences mitochondrial respiration in a manner distinct from other drugs of known mechanism of action and without activating the HIF pathway (Fig. 3f and Supplementary Fig. 7 online). In contrast to canonical inhibitors, meclizine does not directly target the OXPHOS machinery in isolated mitochondria (Fig. 4) and can be titrated over a broad range of concentrations to achieve inhibition of cellular OCR by 10 to 60% (Fig. 3b). Our data suggest that meclizine acts independent of the muscarinic or histamine receptors, as drugs affecting these two receptors did not inhibit OCR (Supplementary Fig. 5 online) and they do not confer neuroprotection or cytoprotection in our models (Fig.5 and Fig. 6). At present we do not know the precise molecular target of meclizine responsible for this effect on energy metabolism, but one possibility is a metabolic target outside the mitochondrion whose subsequent impact is to re-route metabolism away from respiration. Alternatively, it is possible that meclizine may undergo a bio-transformation into a product that directly targets the OXPHOS machinery. We cannot exclude the possibility that meclizine, like many clinically used drugs, hits multiple cellular targets to impact cellular energetics and confer cytoprotection.
Nutrient-sensitized screening, as we have presented it, builds on previous studies that that shown that many cultured cells generate their ATP from either glycolysis or glutamine oxidation14,17. However, the strategy may not necessarily work in other cell types, e.g., cells with less metabolic flexibility, cells that do not have pathways for glutamine oxidation, or postmitotic cells in which a growth assay is not possible. Another limitation of our approach is that the compounds that emerge from the screen may act not just on energy related pathways, but potentially other properties influenced by the switch in nutrients. For example, we have noted that cells grown in glucose tend to proliferate more quickly, and for this reason, drugs from the right side of the tail (Fig. 2b) could either be blunting glycolytic metabolism or impacting rapid proliferation. Hence secondary assays are still required to confirm the energetic consequences of a drug identified by our screening assay.
Our screen contained only a few thousand compounds and has already shown high sensitivity for identifying drugs that target cellular pathways of energy metabolism. As we have shown here, compounds emerging from the upper tail of the distribution (Fig. 2b and Supplementary Table 2,4 online) could serve as valuable lead compounds for prophylaxis against heart attack, stroke, or more broadly, a wide variety of diseases involving oxidative damage, while the opposite tail includes dozens of compounds already used as chemotherapeutic agents, perhaps due to their selective toxicity in more rapidly proliferating cells. We anticipate that this strategy can be extended to other nutrients – such as fatty acids or ketone bodies. The nutrient sensitized assay can also be employed to screen a much larger library of compounds or even genome-wide RNAi perturbations to systematically understand drug action and gene function within the broader context of cellular energy homeostasis.
Supplementary Material
Acknowledgments
We thank E. Shoubridge for the MCH58 cell line; M. MacDonald for immortalized striatal cells; R. Xavier for the HRE luciferase construct; S. Norton and B. Wagner of the Broad Institute for assistance in compound arraying; J. Evans of the Whitehead Institute for assistance with high throughput microscopy; C. Belcher-Timme for technical assistance; T. Kitami for assistance with mitochondrial imaging; M. Mehta for assistance with meclizine toxicity data; S. Calvo, A. Chess, R. Gould, E. Lander, A. Ting, S. Vafai and members of Mootha lab for valuable discussions and comments. This work was supported by fellowships or grants from the United Mitochondrial Disease Foundation (VMG); Howard Hughes Medical Institute (SAS and VKM); National Institutes of Health (RO1 HL-071158 to PSB); Deane Institute for Integrative Research in Stroke and Atrial Fibrillation (CA); American Heart Association (#0815770D to APW); the Burroughs Wellcome Fund (VKM); the Center for Integration of Medical and Innovative Technology (VKM); and the American Diabetes Association/Smith Family Foundation (VKM).
Footnotes
AUTHOR CONTRIBUTIONS: V.M.G. and V.K.M. conceived the project; V.M.G., S.A.S., J.H.L., W.C., F. P., C.B.C and A.W. performed experiments; V.M.G., S.A.S., J.H.L., R.N., F.P., C.A., P.S.B. and V.K.M. performed statistical and data analysis; V.M.G., S.A.S., and V.K.M. wrote the paper.
Competing Financial Interest: VKM, VMG, and SAS are listed as inventors on a patent application filed by the Massachusetts General Hospital.
Note: Supplementary information is available on the Nature Biotechnology website.
All experiments were done in accordance with the national and institutional guidelines for animal welfare, adhering to protocols approved by the institutional subcommittee on research animal care.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet S, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Huber R, Spiegel T, Buchner M, Riepe MW. Graded reoxygenation with chemical inhibition of oxidative phosphorylation improves posthypoxic recovery in murine hippocampal slices. J Neurosci Res. 2004;75:441–449. doi: 10.1002/jnr.10868. [DOI] [PubMed] [Google Scholar]
- 4.Burwell LS, Nadtochiy SM, Brookes PS. Cardioprotection by metabolic shut-down and gradual wake-up. J Mol Cell Cardiol. 2009;46:804–810. doi: 10.1016/j.yjmcc.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- 6.Riepe MW, et al. Increased Hypoxic Tolerance by Chemical Inhibition of Oxidative Phosphorylation: “Chemical Preconditioning”. J Cereb Blood Flow Metab. 1997;17:257–264. doi: 10.1097/00004647-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996;27:327–331. doi: 10.1161/01.str.27.2.327. [DOI] [PubMed] [Google Scholar]
- 8.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda R, et al. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2009 Nov 30; doi: 10.1038/onc.2009.441. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraisl P, Aragones J, Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov. 2009;8:139–152. doi: 10.1038/nrd2761. [DOI] [PubMed] [Google Scholar]
- 13.Hoesch RE, Geocadin RG. Therapeutic hypothermia for global and focal ischemic brain injury--a cool way to improve neurologic outcomes. Neurologist. 2007;13:331–342. doi: 10.1097/NRL.0b013e318154bb79. [DOI] [PubMed] [Google Scholar]
- 14.Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–2676. [PubMed] [Google Scholar]
- 15.Robinson BH, Petrova-Benedict R, Buncic JR, Wallace DC. Nonviability of cells with oxidative defects in galactose medium: a screening test for affected patient fibroblasts. Biochem Med Metab Biol. 1992;48:122–126. doi: 10.1016/0885-4505(92)90056-5. [DOI] [PubMed] [Google Scholar]
- 16.Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y. Circumventing the Crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci. 2007;97:539–547. doi: 10.1093/toxsci/kfm052. [DOI] [PubMed] [Google Scholar]
- 17.DeBerardinis RJ, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu M, et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 19.Wagner BK, et al. Large-scale chemical dissection of mitochondrial function. Nat Biotechnol. 2008;26:343–351. doi: 10.1038/nbt1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE. Current perspectives on the mechanism of action of artemisinins. Int J Parasitol. 2006;36:1427–1441. doi: 10.1016/j.ijpara.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 21.The Food and Drug Administration. Antiemetic drug products for over-the-counter human use; final monograph. Federal Register. 1987;52:15866–15893. [Google Scholar]
- 22.Brunton LL, Lazo JS, Parker KL. Goodman And Gilman’s The Pharmacological Basis of Therapeutics. 11. The Mcgraw-Hill Companies; 2006. [Google Scholar]
- 23.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 25.Sugino T, Nozaki K, Takagi Y, Hashimoto N. 3-Nitropropionic acid induces ischemic tolerance in gerbil hippocampus in vivo. Neurosci Lett. 1999;259:9–12. doi: 10.1016/s0304-3940(98)00875-1. [DOI] [PubMed] [Google Scholar]
- 26.Ratan RR, et al. Translation of ischemic preconditioning to the patient: prolyl hydroxylase inhibition and hypoxia inducible factor-1 as novel targets for stroke therapy. Stroke. 2004;35:2687–2689. doi: 10.1161/01.STR.0000143216.85349.9e. [DOI] [PubMed] [Google Scholar]
- 27.Lesnefsky EJ, et al. Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- 28.Jeong DW, Kim TS, Cho IT, Kim IY. Modification of glycolysis affects cell sensitivity to apoptosis induced by oxidative stress and mediated by mitochondria. Biochem Biophys Res Commun. 2004;313:984–991. doi: 10.1016/j.bbrc.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 29.Hunter AJ, Hendrikse AS, Renan MJ. Can radiation-induced apoptosis be modulated by inhibitors of energy metabolism? Int J Radiat Biol. 2007;83:105–114. doi: 10.1080/09553000601121157. [DOI] [PubMed] [Google Scholar]
- 30.Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol. 2008;10:1477–1483. doi: 10.1038/ncb1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez JM, Folkow LP, Blix AS. Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu Rev Physiol. 2007;69:113–143. doi: 10.1146/annurev.physiol.69.031905.163111. [DOI] [PubMed] [Google Scholar]
- 32.Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem. 2008;283:28106–28114. doi: 10.1074/jbc.M803508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aragones J, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- 34.Philipp S, et al. Stabilization of hypoxia inducible factor rather than modulation of collagen metabolism improves cardiac function after acute myocardial infarction in rats. Eur J Heart Fail. 2006;8:347–354. doi: 10.1016/j.ejheart.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Siddiq A, et al. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem. 2005;280:41732–41743. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernhardt WM, et al. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol. 2006;17:1970–1978. doi: 10.1681/ASN.2005121302. [DOI] [PubMed] [Google Scholar]
- 37.Brahimi-Horn MC, Pouyssegur J. Harnessing the hypoxia-inducible factor in cancer and ischemic disease. Biochem Pharmacol. 2007;73:450–457. doi: 10.1016/j.bcp.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giurgea M, Puigdevall J. Experimental teratology with Meclozine. Med Pharmacol. 1966;15:375–388. doi: 10.1159/000135891. [DOI] [PubMed] [Google Scholar]
- 40.Lione A, Scialli AR. The developmental toxicity of the H1 histamine antagonists. Reprod Toxicol. 1996;10:247–255. doi: 10.1016/0890-6238(96)00053-6. [DOI] [PubMed] [Google Scholar]
- 41.Carpenter AE, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biology. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mootha VK, Arai AE, Balaban RS. Maximum oxidative phosphorylation capacity of the mammalian heart. Am J Physiol. 1997;272:H769–775. doi: 10.1152/ajpheart.1997.272.2.H769. [DOI] [PubMed] [Google Scholar]
- 43.Wojtovich AP, Brookes PS. The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels. Basic Res Cardiol. 2009;104:121–129. doi: 10.1007/s00395-009-0001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wojtovich AP, Brookes PS. The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: implications for ischemic preconditioning. Biochim Biophys Acta. 2008;1777:882–889. doi: 10.1016/j.bbabio.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nadtochiy SM, Tompkins AJ, Brookes PS. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: implications for pathology and cardioprotection. Biochem J. 2006;395:611–618. doi: 10.1042/BJ20051927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyazaki S, Imaizumi M, Onodera K. Effects of thioperamide, a histamine H3-receptor antagonist, on a scopolamine-induced learning deficit using an elevated plus-maze test in mice. Life Sci. 1995;57:2137–2144. doi: 10.1016/0024-3205(95)02206-x. [DOI] [PubMed] [Google Scholar]
- 47.Toyota H, et al. Behavioral characterization of mice lacking histamine H(3) receptors. Mol Pharmacol. 2002;62:389–397. doi: 10.1124/mol.62.2.389. [DOI] [PubMed] [Google Scholar]
- 48.Baughman JM, et al. A computational screen for regulators of oxidative phosphorylation implicates SLIRP in mitochondrial RNA homeostasis. PLoS Genet. 2009;5:e1000590. doi: 10.1371/journal.pgen.1000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


