Abstract
Nonalcoholic fatty liver disease (NAFLD) is associated with obesity, insulin resistance, and impaired glucose tolerance. We investigated whether metformin or changes in metabolic measurements (weight, fasting plasma glucose (FPG), or fasting insulin (FI)) improved serum alanine aminotransferase (ALT) activity, as a marker for NAFLD, in the Diabetes Prevention Program (DPP). From 1996 to 1999, 2,153 participants without marked elevations of serum ALT at baseline were randomized (1,081 to placebo, 1,072 to metformin) and treated for an average of 3.2 years. ALT increased during the first 2 years of the study, and was slightly but significantly lower in the participants randomized to metformin. In regression models adjusted for sex, baseline age, FPG, and FI, these differences remained significant, but disappeared after adjustment for weight, FPG, and FI changes at each examination. The 3-year cumulative incidence for development of abnormal ALT concentrations was not significantly different ((mean ± s.e.) 21.4 ± 1.4% and 24.6 ± 1.4%, P = 0.11) in the metformin vs. placebo groups but was lower in individuals in both groups that lost more weight by the end of year 1 (metformin: 19.4 ± 2.4% vs. 27.5 ± 3.7%, for highest vs. lowest quartile of weight loss; placebo: 18.7 ± 3.4% vs. 28.8 ± 2.6%). Over 3 years of follow-up in persons at high risk for development of diabetes, serum ALT was consistently lower in those treated with metformin compared with placebo. This effect was mediated by weight loss, indicating that the effects of metformin therapy on ALT is via its effects on weight.
INTRODUCTION
Unexplained modest elevations in serum aminotransferase activities are common in the general population, and the majority of these are thought to be due to nonalcoholic fatty liver disease (NAFLD). NAFLD is closely linked to higher body weight, BMI, waist circumference, and the associated metabolic features such as elevated fasting insulin (FI) and triglyceride concentrations and low- and high-density lipoprotein levels (1–3). NAFLD varies considerably in severity. In some patients liver biopsy shows bland steatosis without apparent injury; in others there is inflammation and hepatocyte necrosis that is referred to as nonalcoholic steatohepatitis (NASH). NASH is also associated with hepatic fibrosis, which can be progressive, leading to cirrhosis, end-stage liver disease, and hepatocellular carcinoma (4). Currently, there is no definitive therapy for NAFLD. Although weight loss is recommended (5), the effect of weight loss achieved through lifestyle changes on hepatic histology remains unproven (6). Several small trials have suggested that insulin sensitizing agents such as metformin and the thiazolidinediones can improve the biochemical and histologic features of NASH (7–9).
The Diabetes Prevention Program (DPP) was a large, randomized clinical trial that compared the effects of a rigorous program of diet and exercise with treatment with either metformin or placebo in overweight or obese adults with elevated fasting glucose and impaired glucose tolerance. The intensive lifestyle intervention reduced the risk of diabetes by 58% whereas metformin therapy reduced the risk by 31% as compared with placebo treatment (10). Metformin also had a modest impact on body weight and waist circumference. Participants who received metformin or placebo (but not those in the lifestyle arm) had routine measurements of alanine aminotransferase (ALT). The current analysis focuses on the impact of metformin treatment on serum ALT concentrations and the effect of changes in weight, fasting glucose, and FI.
METHODS AND PROCEDURES
Brief description of DPP
Detailed methods for the DPP were previously described (11). The trial was conducted at 27 sites around the United States. Criteria for enrollment were age of at least 25 years, BMI of >24 kg/m2 (>22 kg/m2 for Asian Americans), a fasting glucose of 5.27–6.94 mmol/l (<6.94 mmol/l in the American Indian centers) and a 2 h glucose of 7.77–11.04 mmol/l after a 2 h glucose tolerance test. Participants were ineligible if they had a history of excessive alcohol intake, defined as any one of the following: (i) average consumption of three or more alcoholic beverages daily; (ii) consumption of seven or more alcoholic beverages within a 24-h period in the past 12 months; (iii) clinical assessment of alcohol dependence based on two or more positive responses to the CAGE questionnaire; or on other evidence available to the clinic staff. Participants were randomly assigned to lifestyle intervention, metformin (850 mg twice daily) or placebo (twice daily) and treated for an average of 3.2 years (maximum 4.5 years) until main study results were announced and treatments unmasked. Because serum enzyme concentrations were measured only at baseline in the lifestyle group, this analysis was restricted to the metformin and placebo groups only. The primary outcome for participants in DPP was development of diabetes according to the 1997 American Diabetes Association criteria (12). Participants underwent semiannual measurements of fasting glucose and body weight. A 75 g oral glucose tolerance test was performed yearly, along with measurements of FI concentration and hemoglobin A1c. Glucose concentrations were performed enzymatically using the Hitachi 917. Insulin concentrations were measured by a double antibody radioimmunoassay. Assessment of alcohol use at baseline was obtained using the modified version of the Block food frequency questionnaire (13). Compliance with study medication was recorded every 3 months, and use of other medicines was recorded semiannually.
Measurements of aminotransferase
Participants were excluded from DPP if they had markedly elevated ALT or aspartate aminotransferase (AST) concentrations at baseline as defined by age and sex: for age <47 years, ALT >46 U/l for women and >118 U/l for men; for age >47 years, ALT >58 U/l for both men and women; for AST ≥66 U/l per criteria established by the DPP central laboratory. For safety reasons, serum AST and ALT values were measured at baseline, 3 months and then every 6 months using the Hitachi 917 chemistry autoanalyzer only in those randomly assigned to the metformin or placebo groups. Follow-up ALT and AST were not measured in the intensive lifestyle group. Both assays were evaluated for drift over the course of the study. Mean AST concentrations increased in 1999 by ~4 U/l regardless of study visit (baseline, 3, 6 months etc.) consistent with assay drift. A similar change in ALT concentrations was not found. For these reasons, this analysis was limited to ALT.
Statistical analysis
Pearson correlations were calculated for baseline measurements of ALT. To approximate a normal distribution, ALT levels were log transformed. Geometric means for ALT values were calculated for each treatment group and compared by analysis of variance. Regression models controlled for covariates and repeated measures were used to compare ALT levels over the study period in the placebo and metformin groups with least square means tests. This was repeated in a sub-group reporting no alcohol use at baseline. Models were controlled for age, sex, time in study and then for repeated measures including fasting plasma glucose (FPG), FI, change in weight, medicine use, and compliance with study medication. Because FI was measured only annually, the previous value was carried forward for calculations at 6-month visits. Examinations were counted whether or not the participant had developed diabetes. Those who missed examinations for any reason, including death or withdrawal of consent, were included in the analysis until their last examination.
We computed incidence rates of development of abnormal ALT defined as concentrations above the sex-specific cut points at the 95th percentile based on National Health and Nutrition Examination Survey data. For men the 95% cut point for ALT was 46 U/l; for women the cut point was 35 U/l. Cumulative incidence of development of elevated ALT values were analyzed by quartiles of change in weight, FPG, and FI concentrations from baseline to year 1. Each quartile was then divided by treatment group, so changes in weight, FPG, or FI were similar between quartiles, although number of individuals by quartile differed by treatment as expected given the effect of metformin (e.g., more individuals on metformin lost weight compared to placebo). Log rank test was used to compare cumulative incidence after 3 years over the quartiles. Cox proportional hazards regression was used to examine the effects of changes in all variables at year one with development of abnormal ALT.
RESULTS
Baseline measurements for the metformin and placebo groups are shown in (Table 1). There were no significant differences between the two groups. Average serum ALT was 17 U/l and levels were above the National Health and Nutrition Examination Survey 95 percentile, sex-specific cut points in 7%. Correlations adjusted for sex between baseline ALT values and other clinical and laboratory characteristics are shown in Table 2. Higher ALT levels were associated with higher body weight, BMI, FPG, and insulin (all associations had an estimated correlation coefficient r ≥ 0.08, P < 0.001).
Table 1.
Baseline characteristics of Diabetes Prevention Program study participants: metformin vs. placebo groups
| Metformin | Placebo | |
|---|---|---|
| N (male/female) | 1,073 (363/710) | 1,082 (335/747) |
| Age (years) | 50.9 ± 10.3 | 50.3 ± 10.4 |
| BMI (kg/m2) | 33.9 ± 6.6 | 34.2 ± 6.7 |
| FPG (mmol/l) | 5.91 ± 0.47 | 5.92 ± 0.47 |
| 2hrPG (mmol/l) | 9.16 ± 0.95 | 9.13 ± 0.95 |
| Fasting insulin (pmol/l) | 187.5 ± 119.4 | 185.4 ± 118.8 |
| ALT(U/l)a | 17 (12–26) | 17 (12–25) |
Data expressed as means ± s.d. except where indicated.
ALT, alanine aminotransferase; FPG, fasting plasma glucose; 2hrPG, 2-hour plasma glucose.
Median (interquartile range).
Table 2.
Correlations between ALT and baseline variables
FPG, fasting plasma glucose; 2hrPG, 2-hour plasma glucose.
ALT log transformed to approximate normality.
Adjusted for sex.
P < 0.05.
Changes in ALT in placebo and metformin groups
Figure 1 displays the geometric mean values for ALT in the metformin- and placebo-treated groups over the first 48 months of the study. ALT rose over the study period, largely during the first 2 years. This increase was greater in the placebo than the metformin groups, with the difference in mean ALT becoming manifest by 6 months. The geometric mean over the entire time period was lower in the metformin group than in the placebo group (ALT 19.3 U/l vs. 20.1 U/l P = 0.004).
Figure 1.
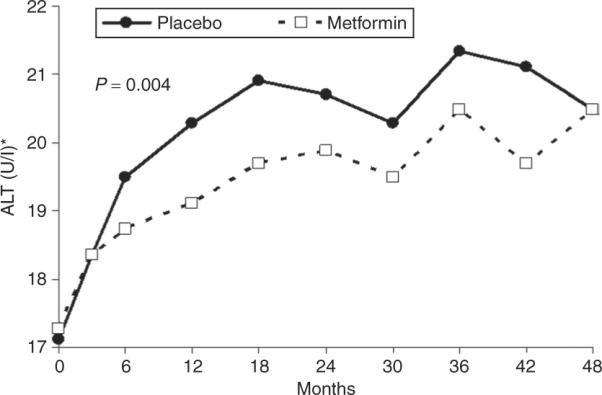
Mean ALT concentrations over time in study. ALT presented as geometric means. Overall difference in ALT values compared by treatment group using repeated-measures analysis of variance.
The differences in mean ALT values between the metformin and placebo groups remained significant when controlled for the baseline characteristics correlated with ALT levels: age, sex, weight, and FPG and insulin concentrations. However, adjustment for changes in weight, fasting glucose, and FI during the study abolished these differences (Figure 2). Additional adjustments at each visit for compliance with study medication, or use of statins or niacin did not change the results (data not shown). In the subset of participants reporting no alcohol use at baseline, ALT values over time remained significantly lower in the metformin than the placebo group (geometric mean 19.6 U/l vs. 20.4 U/l, P <0.05), and similar to the full cohort, this difference was diminished and not significant after adjustment for fasting glucose and insulin concentrations and weight loss at each visit.
Figure 2.
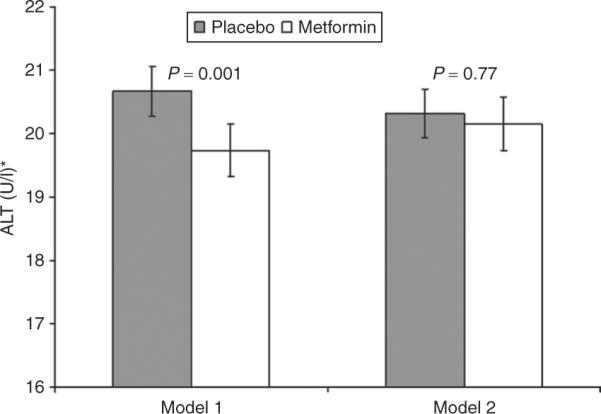
Mean ALT over time in study, adjusted for covariates. Means over study time calculated using repeated-measures regression models adjusted for baseline age, sex, weight, fasting plasma glucose, and fasting insulin and time in study (model 1), with further adjustments for change in weight, fasting plasma glucose and insulin concentrations at each visit (model 2). Groups compared by least squared means test. Error bars represent standard errors.
Cumulative incidence for development of abnormal ALT
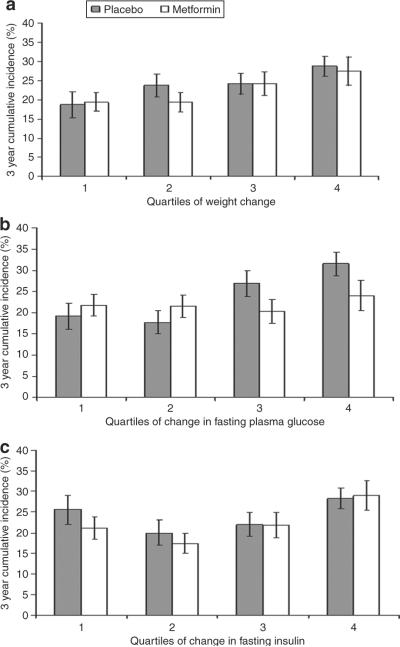
Because changes in weight, glucose, and insulin explained ALT differences between the metformin and placebo groups, and because the eligibility criteria for DPP excluded individuals with elevated ALT concentrations or apparent alcohol abuse, we also examined cumulative incidence of development of abnormal ALT. In individuals with baseline ALT levels below the 95 percentile of sex-specific National Health and Nutrition Examination Survey cutoff, cumulative incidence of abnormal ALT was examined by quartile of change in weight, fasting glucose, and FI concentrations at year one (mean changes for each variable by quartile for are shown in Table 3). The 3-year cumulative incidence of abnormal ALT was not significantly different (21.4 ± 1.4% and 24.6 ± 1.4% in the metformin and placebo groups, P = 0.11). The 3-year cumulative incidence of abnormal ALT was lower in those with greatest weight loss (quartile 1) compared with those who gained weight (quartile 4) over the study for both the placebo and metformin groups (18.7 ± 3.4% vs. 28.8 ± 2.6% for placebo and 19.4 ± 2.4% vs. 27.5 ± 3.7%, for metformin) with a significant trend to favor lower rates with greater weight loss across the quartiles in both groups (P < 0.05)(Figure 3a). Individuals in the placebo group with the greatest decrease in FPG (quartile 1) had significantly lower cumulative incidence of abnormal ALT than those whose glucose increased (quartile 4) (19.2 ± 3.1% vs. 31.5 ± 2.8%, P < 0.001). This was not the case, however, in the metformin group (21.8 ± 2.6% vs. 24.0 ± 3.6%, P = 0.5). For FI, the difference (P = 0.009 for metformin; P = 0.08 for placebo) across the quartiles was driven by the higher cumulative incidence in the quartile in which FI concentrations increased (Figure 3c). In post hoc analysis between the quartiles, in the metformin group there were no significant differences in the cumulative incidence between quartiles 1–3, but significant differences between quartiles 1–3 compared separately with quartile 4 (all P < 0.05). The same trend was seen in the placebo group.
Table 3.
Mean changes in weight, fasting plasma glucose and fasting insulin at end of study year one for each quartile for metformin and placebo groups
| Weight change (kg) | Glucose change (mmol/l) | Fasting insulin change (pmol/l) | |||
|---|---|---|---|---|---|
| Placebo | Metformin | Placebo | Metformin | Placebo | Metformin |
| −8.1 (164) | −7.7 (346) | −0.74 (180) | −0.82 (303) | −125.7 (195) | −118.8 (301) |
| −2.2 (227) | −2.4 (281) | −0.29 (248) | −0.31 (293) | −30.6 (209) | −30.6 (269) |
| +0.2 (289) | +0.05 (226) | +0.02 (258) | +0.02 (248) | +9.7 (263) | +9.0 (229) |
| +3.8 (346) | +3.5 (162) | +0.69 (340) | +0.58 (171) | +104.2 (332) | +91.0 (191) |
Number of individuals in each quartile is given in parenthesis. Quartiles were determined for entire study population with baseline normal ALT (<95% NHANES specific data). Thus mean changes in weight, fasting plasma glucose and fasting insulin are similar for each quartile, but due to the effect of metformin, numbers of individuals per treatment group are different for each quartile. For each weight and glucose quartiles, n = 2,041; for the insulin quartiles n = 1,979 due to missing baseline or 1-year insulin concentrations.
Figure 3.
Three-year cumulative incidence for development of abnormal ALT by quartile of (a) change in weight, (b) change in fasting plasma glucose concentrations, (c) change in fasting insulin concentrations. Bars represent standard errors. Includes individuals with normal ALT at baseline (below 95% sex-specific National Health and Nutrition Examination Survey cutoffs of 46 U/l for men and 35 U/l for women), incidence rates for development of abnormal ALT were calculated by quartiles of change in weight, FPG, and fasting insulin concentrations at year 1 for both placebo and metformin groups. The log rank test was used to compare cumulative incidence over the quartiles.
When change in weight, FPG, and FI were entered in Cox proportional hazard regression models along with treatment group assignment (metformin vs. placebo), only weight loss significantly predicted a lower rate of development of abnormal ALT levels (hazard rate ratio = 0.972; 95% confidence interval = (0.95–0.99).
To analyze further the effect of weight loss on ALT, change in ALT concentrations from baseline to year 1 were plotted against quartile of weight change at year 1 for both placebo and metformin cohorts (Figure 4). ALT reduction was associated with weight loss regardless of assignment to placebo or metformin group.
Figure 4.
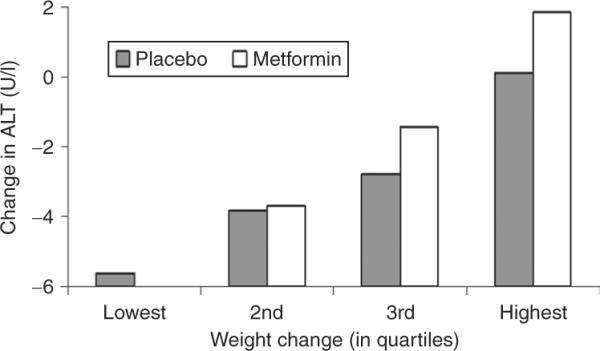
Mean ALT concentration by quartile of change in weight over study. Quartiles by change in weight by end of study, quartile 1 (lowest) representing those with most weight loss.
DISCUSSION
During the DPP, serum ALT concentrations were on average lower in individuals randomly assigned to metformin than in those assigned to placebo, even after adjustment for baseline covariates. However, after further adjustment for changes in weight, and FPG and insulin concentrations, this difference was no longer apparent. Furthermore, in an analysis of ALT concentrations during the course of the study, the rate of onset of abnormal ALT values was lower with lower fasting glucose concentrations in the placebo group, and was lower in both groups in individuals whose FI concentrations did not increase. However, there was a consistent decline in the 4 year cumulative incidence of abnormal ALT levels with greater weight loss in both the placebo and metformin groups. Furthermore, the proportional hazards models with all covariates showed that only weight loss was a significant predictor for development of abnormal ALT. Thus, weight loss appears to be the most effective means to lower or prevent an increase in ALT.
Asymptomatic elevations in aminotransferase concentrations are common, particularly in individuals with risk factors for diabetes including overweight or obesity, or elevations in insulin and glucose levels (1,3,14). In patients with NAFLD, impaired insulin action and central adiposity are common and the degree of insulin resistance and obesity correlate with increased hepatic steatosis (15–17). Serum ALT levels are often used to screen for liver disease, including nonalcoholic fatty liver and NASH. However, patients with normal ALT levels can have fatty liver and the presence of ALT elevations does not reliably separate patients with pure fatty liver from those with NASH with accompanying ongoing inflammation and necrosis (18–20). These studies suggest that in an increased risk for hepatic steatosis is present even in individuals at high risk for diabetes who have normal serum aminotransferase levels, such as those enrolled in the DPP.
Individuals taking metformin had slightly lower ALT concentrations throughout the study (Figure 1), which suggests that metformin may be beneficial in persons at high risk for NAFLD. However, the main effect of metformin was mediated by changes in metabolic variables such as decreased FI concentrations, presumably reflecting decreased insulin resistance, and most consistently by weight loss. ALT was also lower among individuals in the placebo group who lost weight. Figure 4 suggests that mean ALT concentration during the study was more closely tied to weight than to metformin use.
Metformin also lowers aminotransferase levels and decreases liver fat content in both murine models of NAFLD (21) and humans with NASH (8,22). However, many of the published studies in humans to date did not avoid bias or minimize potential confounders by employing randomization. Similar to one pilot study (23), our data suggest that metformin's effect on aminotransferase levels occurs mainly via its effect on weight. Metformin does produce modest weight loss (as was evident in the DPP) (10) likely by producing a mild anorexia, thus decreasing food intake (24,25). Studies of weight loss in individuals with NAFLD suggest that even modest weight loss can produce improvements in markers for NAFLD, namely ALT and imaging markers of liver fat (26–28). Even individuals with NASH by biopsy demonstrate histologic improvement with weight loss (29,30). The findings within the DPP support these smaller studies suggesting that weight loss (either via metformin or other behavioral interventions) can improve or prevent NAFLD.
The proportional hazards model indicated that weight loss was the most important factor in preventing increased ALT. It should be acknowledged though that it is difficult to disentangle the separate effects of weight loss and changes in glucose and insulin concentrations, as the latter two tend to decrease with weight loss. In analyzing these variables separately, lower concentrations of FPG and insulin concentrations were also associated with lower 4 year cumulative incidence of abnormal ALT values. However, the effect of decreasing glucose levels on ALT was less pronounced in the metformin group, and likely blunted by the known effect of metformin on FPG. On the other hand, the cumulative incidence of abnormal ALT values was only higher in those whose FI increased significantly (Figure 3c). That lowering of FI was not more effective in preventing abnormal ALT was surprising, as pioglitazone, an insulin sensitizer, decreases ALT and improves liver histology (7).
Our study has several limitations. First, ALT rose slightly over the study period (Figure 1), but this did not account for the differences seen as study time was included as a covariate in the analysis. In addition, unlike AST in our study, examination of the ALT assay by calendar time did not demonstrate assay drift over the study. Over the study enrollment period of ~3 years, baseline ALT concentrations fell slightly while the 3- and 6-month ALT concentrations rose slightly. This suggests that the reason for the rise in mean ALT levels during the first 2 years of the study was the restrictive eligibility requirements for ALT values; the subsequent rise indicating a regression to the mean following study entry. These restrictive entry criteria also limit the clinical conclusions that can be drawn in terms of treatment of asymptomatic elevated ALT concentrations, as the differences seen in ALT, although statistically significant and based on larger numbers of individuals than in any other treatment study, were small. Subjects enrolled in the DPP were not routinely tested for other causes of liver disease, such as hepatitis B or C or hemochromatosis. However, the reported prevalences of these diseases are only 0.5–2% (31,32). Although the prevalence of hemochromatosis might be different in the DPP study population, a small case–control study which examined markers of body iron stores and development of diabetes found that ferritin concentrations on average were not elevated and none approached those diagnostic for hemochromatosis (33).The other possible confounder was alcohol use, which was determined by self-report. Alcohol use was low in the DPP cohort because heavy drinkers were excluded, and the level of intake (as measured by the Block food frequency questionnaire) remained stable for the duration of the study. Individuals in both the metformin and placebo groups were also strongly cautioned during the study to avoid excessive alcohol intake. Furthermore, when the analysis was restricted to individuals who reported no alcohol use at baseline, a group unlikely therefore to begin using alcohol, the results for ALT were similar. It also must be acknowledged that we did not have histologic or imaging data on these individuals. Hence our results using ALT as a surrogate should be interpreted with caution.
In the DPP, metformin use was associated with a small improvement in ALT levels over time. Weight loss appeared to be the dominant mediator of this effect, and the 4 year cumulative incidence for development of abnormal ALT values was lowest in those who lost the most weight. These results suggest that metformin's effect on liver function tests and hepatic steatosis is mediated primarily via its effect on weight, and that treatments targeting modest weight loss via drug or lifestyle treatment may have a beneficial effect on NAFLD.
ACKNOWLEDGMENTS
The Investigators gratefully acknowledge the commitment and dedication of the participants of the DPP. We thank Dr. Jay Hoofnagle for his advice and editing of the manuscript. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study; collection, management, analysis, and interpretation of the data. The Southwestern American Indian Centers were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources supported data collection at many of the clinical centers. Funding for data collection and participant support was also provided by the Office of Research on Minority Health, the National Institute of Child Health and Human Development, the National Institute on Aging, the Centers for Disease Control and Prevention, the Office of Research on Women's Health, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided medication. This research was also supported, in part, by the intramural research program of the NIDDK. LifeScan Inc., Health O Meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Inc., Merck and Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center. The opinions expressed are those of the investigators and do not necessarily reflect the views of the Indian Health Service or other funding agencies. A complete list of Centers, investigators, and staff can be found in ref. 10.
Footnotes
DISCLOSURE The authors declared no conflict of interest.
REFERENCES
- 1.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 2.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 3.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–79. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 4.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl 1):S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 5.American Gastroenterological Association medical position statement: nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1702–1704. doi: 10.1053/gast.2002.36569. [DOI] [PubMed] [Google Scholar]
- 6.Clark JM. Weight loss as a treatment for nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006;40(Suppl 1):S39–S43. doi: 10.1097/01.mcg.0000168641.31321.fa. [DOI] [PubMed] [Google Scholar]
- 7.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 8.Marchesini G, Brizi M, Bianchi G, et al. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 9.Nair S, Diehl AM, Wiseman M, Farr GH, Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 10.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 13.Block G, Subar AF. Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc. 1992;92:969–977. [PubMed] [Google Scholar]
- 14.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 15.Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 16.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 17.Pagano G, Pacini G, Musso G, et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367–372. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 18.Amarapurkar DN, Patel ND. Clinical spectrum and natural history of non-alcoholic steatohepatitis with normal alanine aminotransferase values. Trop Gastroenterol. 2004;25:130–134. [PubMed] [Google Scholar]
- 19.Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 20.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 21.Lin HZ, Yang SQ, Chuckaree C, et al. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 22.Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 23.Loomba R, Lutchman G, Kleiner, et al. Clinical trial: Pilot study of metformin for the treatment of nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172–182. doi: 10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paolisso G, Amato L, Eccellente R, et al. Effect of metformin on food intake in obese subjects. Eur J Clin Invest. 1998;28:441–446. doi: 10.1046/j.1365-2362.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- 25.Schultes B, Oltmanns KM, Kern W, et al. Modulation of hunger by plasma glucose and metformin. J Clin Endocrinol Metab. 2003;88:1133–1141. doi: 10.1210/jc.2002-021450. [DOI] [PubMed] [Google Scholar]
- 26.Hickman IJ, Jonsson JR, Prins JB, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413–419. doi: 10.1136/gut.2003.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Méndez-Sánchez N, González V, Chávez-Tapia N, Ramos MH, Uribe M. Weight reduction and ursodeoxycholic acid in subjects with nonalcoholic fatty liver disease. A double-blind, placebo-controlled trial. Ann Hepatol. 2004;3:108–112. [PubMed] [Google Scholar]
- 28.Larson-Meyer DE, Newcomer BR, Heilbronn LK, et al. Pennington CALERIE Team. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 2008;16:1355–1362. doi: 10.1038/oby.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barker KB, Palekar NA, Bowers SP, et al. Non-alcoholic steatohepatitis: effect of Roux-en-Y gastric bypass surgery. Am J Gastroenterol. 2006;101:368–373. doi: 10.1111/j.1572-0241.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 30.Huang MA, Greenson JK, Chao C, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 31.Adams PC, Reboussin DM, Barton JC, et al. Hemochromatosis and Iron Overload Screening (HEIRS) Study Research Investigators. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352:1769–1778. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 32.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 33.Rajpathak SN, Wylie-Rosett J, Gunter MJ, et al. Diabetes Prevention Program (DPP) Research Group. Biomarkers of body iron stores and risk of developing type 2 diabetes. Diabetes Obes Metab. 2009;11:472–479. doi: 10.1111/j.1463-1326.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]