—We can not always build the future for our youth, but we can build our youth for the future.
Franklin D. Roosevelt
Non-alcoholic fatty liver disease (NAFLD), first recognized 30 years ago as a significant cause of liver-related morbidity and mortality, is now the most common cause of liver disease (1, 2). The prevalence of hepatic steatosis in the pediatric population is estimated to be 10% and may be as high as 38% among obese children (2). Two-thirds of children with NAFLD and elevated aminotransferase levels have evidence of steatohepatitis (NASH) on liver biopsy and are at risk for progressive liver disease and cirrhosis (3). Longitudinal studies of NAFLD suggest that the disease may progress more rapidly in children than in adults (4).
Given the possible increase in morbidity associated with NAFLD in the pediatric population, it is important to identify those children with hepatic steatosis who are at greatest risk for developing progressive liver disease. Definitive diagnosis of NASH requires a liver biopsy, which is currently reserved for children with hepatic steatosis who have persistently elevated serum aminotransferase levels. Elevated transaminases are a relatively insensitive indicator of NASH in adults with hepatic steatosis (5). Given the obesity epidemic and the high prevalence of fatty liver disease in children, the current practice of performing a liver biopsy only in those children with aminotransferase elevations may lead to under-diagnosis of NASH and under-estimation of the number of children who are at risk of developing end-stage liver disease.
Could genetic testing permit better risk stratification of children with NAFLD? Recently a nonsynonymous sequence variation (rs738409) that substitutes methionine for isoleucine at codon 148 (I148M) in the gene encoding patatin-like phospholipase domain-containing 3 (PNPLA3) was found to be associated reproducibly with both hepatic steatosis and circulating levels of aminotransferases (6, 7). PNPLA3-I148M carriers also have a greater prevalence of pathological features of NASH on liver biopsy (ballooning degeneration, zone 3 persinusoidal fibrosis, Mallory bodies, etc.) (8). The risk allele is not associated with the two major predisposing factors for hepatic steatosis, obesity and insulin resistance (6). In this issue of HEPATOLOGY, Valenti et al. (9) and Santoro et al. (10) have extended these studies to characterize the role of PNPLA3-I148M in pediatric NAFLD.
Valenti et al. (9) examined the association between PNPLA3 genotype and histological features of NASH in 149 children (ages 6–13 years) who had persistently elevated liver function tests. Liver sections were analyzed using the NASH Clinical Research Network (NASH-CRN) scoring system; the risk allele (PNPLA3-I148M) was strongly associated with hepatic steatosis (odds ratio for moderate or severe steatosis: 18.9). The risk implied by this finding is far greater than that reported by NASH-CRN, where the odds ratios in adult carriers were 1.13 for moderate steatosis and 1.26 for severe steatosis (11). The disparate results of these two studies may be due to differences in selection criteria for enrollment. It is also possible that the PNPLA3-I148M variant has a greater impact on triglyceride accumulation in a young, rapidly growing liver.
The authors of this study also observed that children with severe steatosis were much more likely to have NASH (9), a finding consistent with that reported in adults in the NASH-CRN (12). PNPLA3 genotypes showed a step-wise relationship with disease activity (PNPLA3-148II<IM<MM). Features of NASH were rare in children who did not carry the risk variant (PNPLA3-148II) (3%), but were common in heterozygotes (PNPLA3-148IM) (75%) and universal in homozygotes (PNPLA3-148MM) (100%) (9).
Taken together, the data of Valenti et al. (9) suggest that PNPLA3 genotyping may assist in risk stratification of children with steatosis. Individuals who were homozygous for the common variant (PNPLA3-148II) had a very low risk of having liver injury, as measured by histologic grade and stage, despite persistently elevated liver enzymes (ALT > 40 U/L for at least 6 months). Conversely, almost all children who were homozyogous for the risk allele (PNPLA3-148MM) had severe NASH. However, a new study of both adults and children did not support the clinical utility of PNPLA3 genotyping for risk stratification (13). Rotman et al. (13) reported that the risk allele was associated with earlier onset of disease, but not with histological severity in 223 children enrolled in the NASH-CRN. Thus, it is essential that the finding of Valenti et al. (9) be confirmed in independent study populations before being considered for clinical implementation. Moreover, the study conclusions only pertain to children with both steatosis and elevated liver enzymes. Further studies will be required to determine the prognosis of children with severe steatosis who are homozygous for the risk allele and yet do not have elevated liver enzymes.
In the same issue, Santoro et al. (10) examined the effects of the PNPLA3-I148M variant on fuel homeostasis and adipocyte size in an ethnically diverse, obese pediatric population. Although the study by Santoro et al. (10) was smaller (n=85), the association between hepatic triglyceride content and the PNPLA3-I148M variant was detected in their population. Since liver biopsies were performed in just 6 subjects, the relationship between the risk allele and hepatic pathology could not be examined. Consistent with the original reports (6, 14), no association was found between the variant and metabolic indicators of insulin resistance. Specifically, no differences in hepatic glucose production rate or peripheral glucose disposal were detected by hyperinsulinemic euglycemic clamp studies. This finding confirms and further strengthens the mechanistic dissociation between hepatic triglyceride content and insulin resistance. Although hepatic triglyceride content is strongly associated with insulin resistance, the insulin resistance is not a direct consequence of the increase in hepatic triglyceride content.
This study also probed the effect of the variant on indices of adipose tissue metabolism. No genotype-dependent differences were found in body fat content or distribution, or in the rate of lipolysis, as assessed by glycerol turnover. The size of adipocytes measured in 18 subjects revealed a small reduction in median adipocyte size in carriers (~92 vs. ~80 μm: P=0.05). Given the small number of subjects analyzed (just 11 carriers and 7 controls), this finding must be interpreted with caution, especially since the PNPLA3 genotype is not associated with adiposity or body fat distribution.
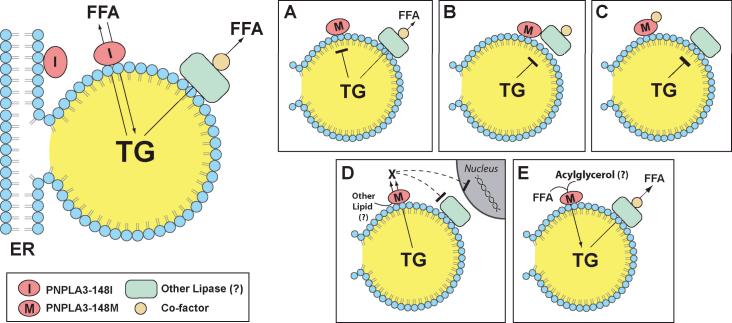
The physiological function of PNPLA3 is enigmatic, and the mechanistic link between the I148M variant and liver disease remains unclear. PNPLA3 is associated with the endoplasmic reticulum and with lipid droplet in hepatoctyes (Figure 1) (15). The enzyme exhibits both triglyceride hydrolase and transacylation activity in vitro (16) so can promote either triglyceride catabolism or anabolism. The substitution of methionine for isoleucine at residue 148 disrupts triglyceride hydrolysis by the enzyme (15), suggesting that PNPLA3-I148M may be is a loss-of-function mutation (Figure 1A). However, ablation of PNPLA3 in two different strains of mice (C57BL/6J and Lepob/ob) yielded no significant increase in hepatic lipid content or serum aminotransferase levels under a variety of dietary conditions (17). Conversely, hepatic overexpression of PNPLA3-I148M in rodents leads to an increase in hepatic triglyceride content, a finding more consistent with a gain-of-function mutation (15). Currently, the full complement of lipase(s) contributing to triglyceride hydolysis in the liver is not known. The mutant protein may interfere with the action of another triglyceride hydrolase, possibly PNPLA2 (adipocyte triglyceride lipase)(18) (Figure 1B). Alternatively, it may sequester a co-factor required for maintenance of triglyceride homeostasis in the liver (Figure 1C). Expression of the mutant enzyme may generate a new signaling molecule that either inhibits lipolysis or promotes deposition of triglycerides (Figure 1D). Finally, the mutant protein may promote formation of triglyceride (Figure 1E) or of a toxic lipid that promotes both steatosis and injury.
Figure 1.
Potential mechanistic links between PNPLA3-I148M and NAFLD. PNPLA3 is associated with both the endoplasmic reticulum (ER) and lipid droplets in hepatocytes and exhibits triglyceride hydrolase and transacylation activity in vitro (top left) (13, (16). In the fed state, the protein may serve to liberate free fatty acids (FFA) from triglyceride (TG) contained within lipid droplets. Alternatively, PNPLA3 could be involved in the conversion of acylglycerols to triglycerides, thereby promoting TG deposition. Substitution of methionine for isoleucine at residue 148 (PNPLA3-I148M) abolishes TG hydrolase activity (15), suggesting that PNPLA3-I148M is a loss-of-function mutation (A). However, overexpression of PNPLA3-I148M in the liver of mice results in steatosis (15). These findings are more consistent with the variant having a gain-of-function. The mutant protein may interfere with the action of another lipase (B), or sequester a necessary co-factor required for maintenance of triglyceride homeostasis in liver (C). Alternatively, expression of the mutant enzyme may generate a new signaling molecule that inhibits lipolysis (D) or promotes triglyceride formation (E).
Elucidating the mechanisms by which the I148M variant confers susceptibility to NAFLD is likely to provide new insights into the pathogenesis and progression of this disorder, from the accumulation of triglyceride in lipid droplets to the development of cirrhosis. A recent study suggests that PNPLA3 may play a role in the development of advanced liver disease, irrespective of the cause. Among alcoholics, the odds ratio of developing cirrhosis is 1.8 and 3.6 for individuals heterozygous and homozygous for the risk variant compared with those who do not carry the risk allele (19). Inasmuch as hepatic steatosis is associated with other forms of liver disease (e.g., hepatitis C, hemochromatosis, drug-induced, etc.), it is likely that this variant contributes to the pathogenesis of these diseases as well. Defining the molecular mechanism by which PNPLA3 confers susceptibility to liver injury will require the identification of the physiological substrate(s) and product(s) of the enzyme, and determination of the effect of the risk allele on its activity.
Finally, the I148M variant is common in Hispanics, a population that also has a high prevalence of hepatic steatosis and cryptogenic cirrhosis (1, 20). Is the high frequency of the PNPLA3-I148M variant in Hispanics simply a result of genetic drift? Or could this variant confer some advantage, perhaps as a component of the so-called “thrifty genome,” by providing a readily utilizable energy source in the liver during periods of food scarcity. Genotyping additional populations from around the world for this sequence variant may answer this question, and provide new insights into a persistent mystery: why do some individuals exposed to liver toxins or insults develop hepatic injury and fibrosis, whereas others do not?
Acknowledgment
We would like to thank Dr. Jay Horton for helpful discussions and the support from the following grants: 5K23DK074396 (JDB), RL1HL092550 and PO1HL20948 (HHH, JCC).
Abbreviations
- PNPLA3
patatin-like phospholipase domain-containing 3
REFERENCES
- 1.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 3.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 4.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538–1544. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 6.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan X, Waterworth D, Perry JR, Lim N, Song K, Chambers JC, Zhang W, et al. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet. 2008;83:520–528. doi: 10.1016/j.ajhg.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sookoian S, Castano GO, Burgueno AL, Gianotti TF, Rosselli MS, Pirola CJ. A nonsynonymous gene variant in the adiponutrin gene is associated with nonalcoholic fatty liver disease severity. J Lipid Res. 2009;50:2111–2116. doi: 10.1194/jlr.P900013-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valenti L, Alisi A, Galmozzi E, Bartuli A, Del Menico B, Alterio A, Dongiovanni P, et al. I148M patatin-like phospholipase domain-containing 3 gene variant and severity of pediatric nonalcoholic fatty liver disease. Hepatology. 2010;30:30. doi: 10.1002/hep.23823. [DOI] [PubMed] [Google Scholar]
- 10.Santoro N, Kursawe R, D' Adamo E, Dykas DJ, Zhang CK, Bale AE, Cali AM, et al. A common variant in the patatin-like phospholipase 3 gene (PNPLA3) is associated with fatty liver disease in obese children and adolescents. Hepatology. 2010;30:30. doi: 10.1002/hep.23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speliotes EK, Butler JL, Palmer CD, Voight BF, Hirschhorn JN. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 2010;18:18. doi: 10.1002/hep.23768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalasani N, Wilson L, Kleiner DE, Cummings OW, Brunt EM, Unalp A. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. J Hepatol. 2008;48:829–834. doi: 10.1016/j.jhep.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantartzis K, Peter A, Machicao F, Machann J, Wagner S, Konigsrainer I, Konigsrainer A, et al. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes. 2009;58:2616–2623. doi: 10.2337/db09-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Chang B, Li L, Chan L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology. doi: 10.1002/hep.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerman R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, et al. Fat mobolization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 19.Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 42:21–23. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 20.Browning JD, Kumar KS, Saboorian MH, Thiele DL. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am J Gastroenterol. 2004;99:292–298. doi: 10.1111/j.1572-0241.2004.04059.x. [DOI] [PubMed] [Google Scholar]