Abstract
Asymptomatic alanine aminotransferase (ALT) elevations have been detected with acetaminophen use at the maximum daily dose of 4 grams/day (g/d) for more than 3 consecutive days in subjects with and without concurrent ethanol use. The purpose of this study is to describe the changes in serum ALT activity in non-drinkers treated with acetaminophen for 10 days.
Methods
Study Objective to describe the change in serum ALT in non-drinkers administered acetaminophen for 10 days. Study Design: Prospective, single arm trial. Setting: Outpatients. Interventions: Acetaminophen 4 g/day starting on day study day 1. Measurements: Serum ALT, total billirubin and international normalized ratio (INR) were measured on study day 0, 4, 7, 9, 11 and 14.
Main Results
Twenty four subjects completed the trial. Median ALT increased from 24 IU/L at day 0 to 39 IU/L by day 7, remained elevated through day 11 (38 IU/L) and began to trend down by day 14 (35 IU/L). The increase in ALT during the study was statistically significant (p= 0.0002). Sixty-six percent of subjects had an ALT above the upper limits of normal at day 11; the largest elevation was 3.8 the upper limits of normal. No subject developed symptoms of liver injury or had an elevation in INR or serum bilirubin.
Conclusions
Daily use of acetaminophen at the daily maximum dose of 4 g/day for 10 days causes asymptomatic ALT elevations in subjects who do not consume ethanol.
Background
Two recent studies have shown that administration of maximal recommended doses of acetaminophen for more than three days causes elevation of the serum alanine aminotransferase (ALT) in some subjects.1,2 The most recent study2 was an outpatient study of subjects who had a history of moderate ethanol consumption (average of 1-3 drinks per day) and who continued to consume ethanol during while taking acetaminophen. The other study by Watkins et al. did not specify the ethanol use pattern of subjects prior to the study, but was conducted on an inpatient research unit, implying that there was no ethanol consumption while taking acetaminophen. While both studies found asymptomatic ALT elevations, the proportion of subjects who experienced ALT elevation and the degree of ALT elevations reported were dramatically higher for the inpatient study. While there are several potential explanations for these results, one potential explanation is that ethanol consumption alters the response to acetaminophen. The objective of this study was to describe the change in serum ALT activity in healthy, non-drinking outpatients who are administered 4 grams of acetaminophen daily for 10 days.
Methods
Design
This report describes the analysis of a secondary outcome of a study designed to describe the course of acetaminophen-cysteine adduct formation during repeated acetaminophen dosing. As our primary outcome was descriptive (rather than comparative), we conducted an open-labeled human volunteer study. Subjects were enrolled between August 2007 and January 2008. This study was approved by the governing institutional review board and all subjects provided informed consent. This study was registered in ClincialTrials.gov (#NCT00616018) and conducted under an investigator initiated IND from the US Food and Drug Administration.
Subjects
Our study subjects were healthy volunteers who reported an average ethanol consumption of less than 1 drink per day for the 30 days preceding enrollment in the study. Exclusion criteria were average of 1 or more alcoholic beverage daily, a history of alcoholism, ingestion of more than 4 g/d of acetaminophen for any of the 4 days preceding study enrollment, serum acetaminophen level greater than 132.4 micromol/L (20 mcg/mL) at baseline, serum ALT or aspartate aminotransferase (AST) greater than 50 IU/L at screening or baseline, pregnancy, clinical intoxication, psychiatric impairment or inability to give informed consent for any reason, a known hypersensitivity to acetaminophen, or enrollment in another trial currently in the preceding 3 months. The first 10 days of the study consisted of a run-in period during which all subjects agreed to abstain from the use of ethanol and acetaminophen. Subjects who ingested ethanol or acetaminophen during the run-in period were also excluded.
Treatment
All subjects were provided with and instructed to take two 500 mg acetaminophen tablets (Extra-Strength Tylenol, McNeil Consumer Healthcare, Ft Washington PA, USA) four times daily for 10 days. Subjects were instructed to take the doses at least 4 hours apart. Missed doses (doses not taken within 2 hours of the scheduled time) were recorded. Study diaries were used to record study drug consumption, daily physical activity and food and beverage intake. We also performed pill counts at each visit. Subjects were given explicit instructions not to take any other acetaminophen containing products as well as ethanol.
Measurements
Prior to dosing, subjects were screened for eligibility by measuring ALT; After the run-in period, baseline testing included viral hepatitis screening (hepatitis A IGM, Hepatitis B surface antigen and core antibody, and Hepatitis C IG), liver panel (AST, ALT, total protein, albumin, total and direct bilirubin, gamma glutamyl transferase), serum ethanol and acetaminophen concentrations, serum pregnancy (female subjects) and INR. Subjects started dosing on Day 1 and took their final dose on day 10. Testing was performed on day 0 4, 7, 9, 11 and 14 and included a liver panel, serum ethanol and acetaminophen concentrations, and INR. Subjects who had an ALT greater than the upper limit of normal (ULN; 36 IU/L female, 40 IU//L male) at day 14 were retested between 3 and 20 days after completing the study to verify that their ALT had returned to normal.
Comparisons
The primary measure for this study was the absolute change in ALT from Day 0 (baseline) through day 11. Secondary measures were the proportion of subjects who developed drug-induced liver injury defined as an ALT or AST more than 3 times the ULN with elevation of the serum bilirubin, and the proportion of subjects who had symptomatic liver injury such as abdominal pain, jaundice or coagulopathy (INR>1.5). As the data were not normally distributed, the change in ALT from baseline to day 11 for each subject was compared using Kruskal-Wallis tests. A p-value of less than 0.05 was considered significant. We calculated 95% confidence intervals for proportions using a binomial approximation.
Results
Subjects
Twenty seven subjects received study drug. Twenty four completed the trial and three were removed for protocol violations (2 were removed for missing study visits on day 4 and 1 was excluded at day 9 due to alcohol consumption). The day 0 ALT values for the 2 subjects for missing their day 4 visits were 24 and 12 IU/L. The peak ALT for the subject excluded at day 9 occurred on day 7 and was 42 IU/L.
For the 24 completed subjects, the mean (SD) age was 38.5 (12.4) years; 29 % were male and 87% were Caucasian. Twenty subjects were taking concomitant prescription medications, 16 were taking non-prescription medications vitamins or supplements and four reported no concomitant medications.
The ALT values for subjects at each determination are shown in Figure 1. There was a significant increase (X2 23.7, 5 DOF, p<0.001) in ALT from baseline that appears evident at day 7. The median (IQR) increased from 24 (17 to 31) IU/L at day 0 to 38 (23 to 75) IU/L at day 11 and then decreased to 35 (21 to 42) IU/L at day 14. There was no change in mean INR or bilirubin during the study. No subject reported abdominal pain or jaundice. In an exploratory analysis, the differences remained statistically significant when subjects were stratified by sex (p=0.03 for females and p=0.001 for males).
Figure 1.
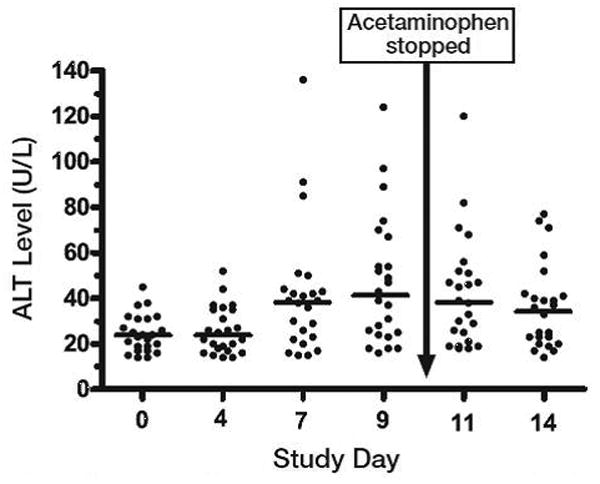
Serum alanine aminotransferase (ALT) levels measured over 14 days in 24 nondrinkers administered acetaminophen 4 g/day for 10 days. Horizontal lines indicate median values; median ALT level on days 7, 9, 11, and 14 were higher than on day 0 (baseline).
Overall, 14 of 24 (58%, 95% CI 39 to 76%) completed subjects had one or more ALT measurements above the upper limit of the reference range. One subject (4%) had an ALT more than three times the ULN for our laboratory on day 7, but no subject had an abnormal elevation of serum bilirubin or symptoms of liver injury. One subject was on warfarin and was excluded from the analysis of coagulopathy; no other subject had an INR above 1.31. All subjects had normal ALT on follow-up testing performed 3-21 days after stopping acetaminophen.
Discussion
We found that a significant proportion of subjects treated with 4g/day of acetaminophen for 10 consecutive days will have elevation of their ALT. The current U.S. labeled recommendation is that acetaminophen should not be taken for more than 10 days without consulting a physician. The degree of elevation varies, with some subjects having peak ALT more than four times their baseline measurement, but most subjects having ALT elevation 1.5 to 2 times their baseline. The elevation is clearly evident within 7 days of starting treatment, and may start to occur within 5 days. These elevations are asymptomatic and not accompanied by signs of drug induced liver injury or liver function impairment.
Although the current study did not include a placebo group, we have no reason to believe that the changes we observed in ALT elevations were not related to acetaminophen. In a previous study of moderate drinkers, 50 placebo-treated subjects had a mean change in ALT of -0.8 IU/L over an 11 day study and only 2 subjects had an ALT reported above the ULN.2 The mean change and rate of elevation we observed in this study are much higher and unlikely due to chance.
The design of this study is similar to our previously conducted study of moderate drinkers.2 The proportion of subjects with ALT elevation in this study at day 11 was higher than the proportions observed in acetaminophen-treated moderate drinkers (20/100). The mean increase in ALT from baseline to day 11 was higher for non-drinkers (17.9 IU/L) than the reported increase for moderate drinkers (8.7IU/L). The highest day 11 ALT in the non-drinkers (120 IU/L) was slightly lower than the highest ALT reported in moderate drinker acetaminophen group (128 IU/L). These differences suggest that moderate alcohol consumption may be protective against ALT elevation. One reason this may occur is that ethanol is an inhibitor of CYP-2E1, the enzyme that catalyzes the formation of the toxic metabolite of acetaminophen.
The rate of ALT elevation in the non-drinkers described in the current study (58%) was similar to the rate reported by Watkins et al in a study describing the safety of several acetaminophen-opioid products when used for 14 days.1 Watkins's study found that 76% of acetaminophen treated subjects had an abnormal ALT at some point during the study. As with our study, no subject had symptoms or other laboratory findings suggesting drug induced liver injury. The proportion of subjects with ALT elevation in the current study is similar that reported in Watkins's study. However, the subjects in Watkins's group had much larger ALT elevations, with 38% of subjects having ALT elevations of more than 3 times the ULN and 8% of subjects having an ALT more than 8 times the ULN. There are some differences in study design that might account for these differences. First, Watkins's subjects had daily ALT measurements while our subjects had measurements every 3 days. It is possible that our sampling schematic missed some transient rises that would have been detected if we sampled more frequently. Second, Watkins's subjects were restricted to a clinical research unit and had standardized diets while our subjects were outpatients. Diet3 and physical activity4 may alter serum ALT measurements. Finally, his subjects were predominately Hispanics, a group that has higher mean ALTs than Caucasians.5
Other studies have reported no change in alcoholic subjects treated with the maximal dose of acetaminophen for up to 5 days. 6-8 The lack of ALT elevation reported in these studies is consistent with our finding that the elevation in ALT was not evident at day 4.
The implication of these asymptomatic ALT elevations remains unclear. We found no evidence of liver dysfunction associated with these changes. Previous analysis of long-term acetaminophen studies suggest that ALT elevations do not progress if treatment is continued.9 Senior has defined low-grade ALT elevations followed by resolution as level 1 liver injury and notes that it is not a reason to discontinue a medication.10 Given the long-term safety of acetaminophen reported in long-term studies, it is likely that the ALT elevations observed in this study would resolve with continued treatment. Future studies should evaluate the course of ALT changes when acetaminophen is continued for a longer period of time.
There are several limitations to this study. The most notable is the lack of a placebo treated group. While we recognize this limitation, we have outlined why we believe it is highly likely that the changes observed in this study are related to acetaminophen. This study was limited to healthy volunteers, and the results may not be generalizable to others with chronic medical conditions or liver disease. Finally, although subjects were instructed to record the time of ingestion for each dose and the use of other medications, we cannot be certain that the subjects were compliant with their study medications or that unmeasured factors may have affected their ALT measurements.
In conclusion, administration of the maximum daily recommended dose of acetaminophen, 4 g/day to healthy non-drinkers for more than 4 consecutive days is associated with asymptomatic ALT elevation in most subjects. ALT elevations are generally between 1.5 and 2 times their pre-treatment measurements are not accompanied by other laboratory findings or symptoms of liver injury and all ALT elevations resolved once acetaminophen administration was stopped.
Acknowledgments
This project was funded by an Investigator-Initiated Grant from McNeil Consumer Products. The sponsor had no role in the design, conduct, analysis or manuscript preparation. The sponsor was allowed to review the manuscript prior to submission, but the final content of the manuscript was determined solely by the authors. The project described was also supported by Award Number K08DA020573 from the National Institute On Drug Abuse to Dr. Heard. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Drug Abuse or the National Institutes of Health
Reference List
- 1.Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, Harris SC. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296:87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 2.Heard K, Green JL, Bailey JE, Bogdan GM, Dart RC. A randomized trial to determine the change in alanine aminotransferase during 10 days of paracetamol (acetaminophen) administration in subjects who consume moderate amounts of alcohol. Aliment Pharmacol Ther. 2007;26:283–90. doi: 10.1111/j.1365-2036.2007.03368.x. [DOI] [PubMed] [Google Scholar]
- 3.Porikos KP, Van Itallie TB. Diet-induced changes in serum transaminase and triglyceride levels in healthy adult men. Role of sucrose and excess calories. Am J Med. 1983;75:624–30. doi: 10.1016/0002-9343(83)90444-8. [DOI] [PubMed] [Google Scholar]
- 4.Sreenivasa Baba C, Alexander G, Kalyani B, Pandey R, Rastogi S, Pandey A, Choudhuri G. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 2006;21:191–8. doi: 10.1111/j.1440-1746.2005.04233.x. [DOI] [PubMed] [Google Scholar]
- 5.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 6.Kuffner EK, Dart RC, Bogdan GM, Hill RE, Casper E, Darton L. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients: a randomized, double-blind, placebo-controlled trial. Arch Intern Med. 2001;161:2247–52. doi: 10.1001/archinte.161.18.2247. [DOI] [PubMed] [Google Scholar]
- 7.Kuffner EK, Green JL, Bogdan GM, Knox PC, Palmer RB, Heard K, Slattery JT, Dart RC. The effect of acetaminophen (four grams a day for three consecutive days) on hepatic tests in alcoholic patients--a multicenter randomized study. BMC Med. 2007;5:13. doi: 10.1186/1741-7015-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartels S, Sivilotti M, Crosby D, Richard J. Are recommended doses of acetaminophen hepatotoxic for recently abstinent alcoholics? A randomized trial. Clin Toxicol (Phila) 2008;46:243–9. doi: 10.1080/15563650701447020. [DOI] [PubMed] [Google Scholar]
- 9.Kuffner EK, Temple AR, Cooper KM, Baggish JS, Parenti DL. Retrospective analysis of transient elevations in alanine aminotransferase during long-term treatment with acetaminophen in osteoarthritis clinical trials. Curr Med Res Opin. 2006;22:2137–48. doi: 10.1185/030079906x148346. [DOI] [PubMed] [Google Scholar]
- 10.Senior JR. Monitoring for hepatotoxicity: what is the predictive value of liver “function” tests? Clin Pharmacol Ther. 2009;85:331–4. doi: 10.1038/clpt.2008.262. [DOI] [PubMed] [Google Scholar]


