Summary
Background
In the 2·8 years of the Diabetes Prevention Program (DPP) randomised clinical trial, diabetes incidence in high-risk adults was reduced by 58% with intensive lifestyle intervention and by 31% with metformin, compared with placebo. We investigated the persistence of these effects in the long term.
Methods
All active DPP participants were eligible for continued follow-up. 2766 of 3150 (88%) enrolled for a median additional follow-up of 5·7 years (IQR 5·5–5·8). 910 participants were from the lifestyle, 924 from the metformin, and 932 were from the original placebo groups. On the basis of the benefits from the intensive lifestyle intervention in the DPP, all three groups were offered group-implemented lifestyle intervention. Metformin treatment was continued in the original metformin group (850 mg twice daily as tolerated), with participants unmasked to assignment, and the original lifestyle intervention group was offered additional lifestyle support. The primary outcome was development of diabetes according to American Diabetes Association criteria. Analysis was by intention-to-treat. This study is registered with ClinicalTrials.gov, number NCT00038727.
Findings
During the 10·0-year (IQR 9·0–10·5) follow-up since randomisation to DPP, the original lifestyle group lost, then partly regained weight. The modest weight loss with metformin was maintained. Diabetes incidence rates during the DPP were 4·8 cases per 100 person-years (95% CI 4·1–5·7) in the intensive lifestyle intervention group, 7·8 (6·8–8·8) in the metformin group, and 11·0 (9·8–12·3) in the placebo group. Diabetes incidence rates in this follow-up study were similar between treatment groups: 5·9 per 100 person-years (5·1–6·8) for lifestyle, 4·9 (4·2–5·7) for metformin, and 5·6 (4·8–6·5) for placebo. Diabetes incidence in the 10 years since DPP randomisation was reduced by 34% (24–42) in the lifestyle group and 18% (7–28) in the metformin group compared with placebo.
Interpretation
During follow-up after DPP, incidences in the former placebo and metformin groups fell to equal those in the former lifestyle group, but the cumulative incidence of diabetes remained lowest in the lifestyle group. Prevention or delay of diabetes with lifestyle intervention or metformin can persist for at least 10 years.
Funding
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Introduction
Prevention of type 2 diabetes mellitus is a major public health challenge because of its large effect on health. Diabetes affected an estimated 171 million people worldwide in 2000, and this number is projected to rise to 366 million by 2030, owing to increases in age, obesity, and urbanisation of the world’s population.1 Diabetes was the world’s fifth leading cause of death in 2000.2 In the Diabetes Prevention Program (DPP), a US multicentre randomised clinical trial, intensive lifestyle intervention or metformin prevented or delayed development of type 2 diabetes in adults at high risk because of raised fasting plasma glucose (5·3–6·9 mmol/L), impaired glucose tolerance (2-h postload glucose 7·8–11·0 mmol/L), and body-mass index of 24 kg/m2 or higher (≥22 kg/m2 in Asian Americans).3,4 Development of diabetes5 was the primary outcome, and cardiovascular disease and its risk factors were secondary outcomes.
The Diabetes Prevention Program Outcomes Study (DPPOS) is a long-term follow-up of the DPP to investigate whether the delay in development of diabetes seen during the DPP can be sustained and to assess long-term effects of the interventions on health. In this first phase of DPPOS, we report the intervention effects on diabetes incidence, weight change, and cardiovascular disease risk factors and their treatment during 10 years of follow-up since DPP randomisation.
Methods
Participants
Recruitment and random assignment of DPP participants and other study methods have been described.3,4,6 We enrolled 3234 participants (68% women, 45% from ethnic and racial minority groups, and 20% aged 60 years or older) between 1996 and 1999. Participants were randomly assigned centrally to one of three interventions: intensive lifestyle (aimed to help participants to achieve and maintain 7% weight loss and 150 min or more per week of moderate-intensity physical activity); metformin 850 mg twice per day; or placebo. The metformin and placebo groups were masked (double blind) to treatment.3,4,6 Masked treatment was discontinued in July, 2001, after we established that lifestyle intervention reduced incidence of diabetes by 58% and metformin by 31% compared with placebo during an average of 2·8 years in the DPP.4 We defined the 13 months from Aug 1, 2001 to August 31, 2002, as the bridge because it bridged the time between the two main protocols—DPP and DPPOS.
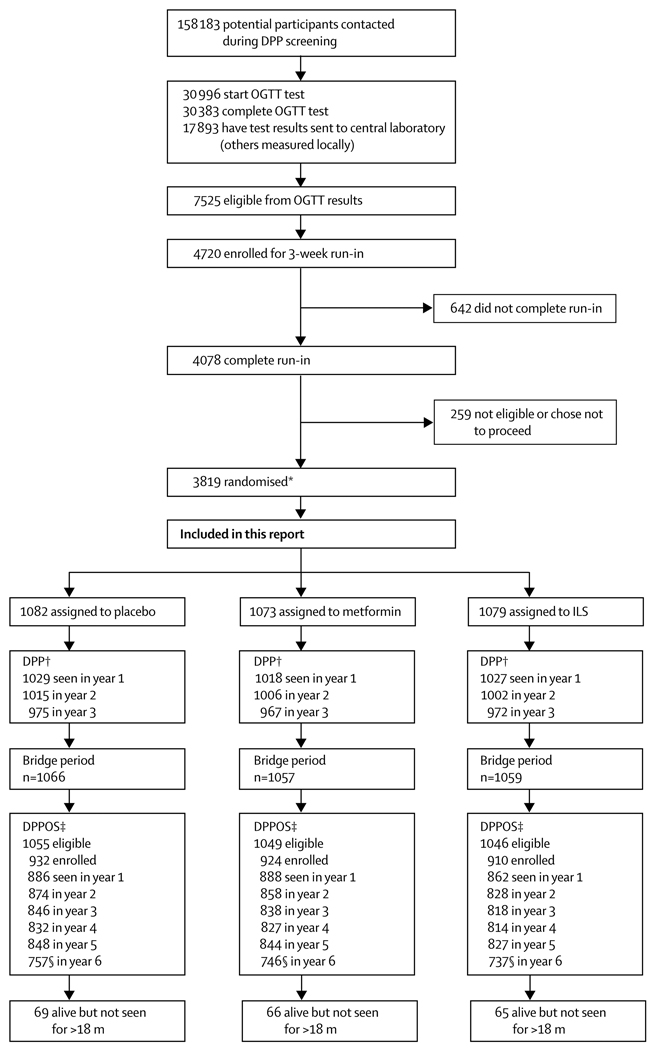
Figure 1 shows recruitment, randomisation, enrolment, and follow-up for the DPPOS. All 3150 surviving DPP participants who had not withdrawn consent were eligible, irrespective of whether they had developed diabetes. Enrolment started in September, 2002, and was largely completed within 1 year, by which time 2665 participants (85%) had enrolled. By Aug 27, 2008, the closing date for this analysis, 2766 (88%) had enrolled. The protocol and informed consent procedures were approved by all responsible institutional review boards. Participants signed written consent forms after discussion of all aspects of the study with study staff.
Figure 1. Trial profile.
Screening and recruitment done in the Diabetes Prevention Program (DPP).3 OGTT=oral glucose tolerance test. ILS=intensive lifestyle intervention. m=month. *Includes 585 randomised to troglitazone before this treatment group was discontinued. †DPP enrolled participants during 3 years ending June, 1999. Participants had varying durations of DPP follow-up, dependent on their year of enrolment. ‡DPP participants who had not died or withdrawn consent as of Sept 1, 2002, were eligible. §Numbers of those examined in year 6 are lower than are those for the other years because the close of data for analysis occurred before all follow-up examinations were scheduled.
Procedures
After participants were informed of the main results from DPP, those in the metformin and placebo groups entered into a 1–2 week drug washout study to identify whether treatment of fasting glucose accounted for the diabetes risk reduction with metformin.7 They were then unmasked to their treatment assignments, and placebo was stopped. All participants, including the original lifestyle group and those who had developed diabetes, were offered a group-administered version of the 16-session lifestyle curriculum as a bridge protocol. The programme, undertaken from Jan 2, 2002 to July 31, 2002,8 was nearly identical in content to the DPP lifestyle intervention, and was delivered, with few exceptions, by the original staff. Individualised problem solving and behaviour-change support were not provided. At least some sessions were attended by the original placebo (57%), metformin (58%), and lifestyle (40%) participants.8
The DPPOS follow-up protocol was started in September, 2002. Lifestyle sessions (HELP) were offered to all participants every 3 months, with provision of educational materials to reinforce the original weight loss and physical activity goals. DPP lifestyle participants were also offered two group classes (BOOST) each comprising four sessions every year to reinvigorate their self-management behaviours for weight loss. Those previously assigned to the metformin group continued to receive metformin 850 mg twice daily, now unmasked, as tolerated, unless the drug was discontinued for safety reasons or participants had developed diabetes with a glycated haemoglobin (HbA1c) of more than 7%, which, according to the protocol, needed management by their own physician. Outcome assessment examinations continued on the same yearly and 6 monthly schedule as in the DPP. We defined the baseline visit for DPPOS as the last yearly visit that occurred between Aug 1, 2001 and Aug 31, 2002, (n=2629). For 115 participants without such a visit, another visit from Aug 1, 2001 to Oct 31, 2003 was used as DPPOS baseline.
The primary outcome, as in the DPP, was development of diabetes according to American Diabetes Association criteria—fasting plasma glucose 7·0 mmol/L or higher measured every 6 months, or 2-h plasma glucose 11·1 mmol/L or higher after a 75 g oral glucose load, measured yearly. A provisional diagnosis by either test needed confirmation with a repeat test.4,5 Weight loss, the main goal of the lifestyle intervention,3 was analysed as a key process variable. Blood pressure, plasma lipids, and medication history were obtained yearly. The primary objectives of follow-up were to assess long-term effects of DPP interventions on the development of diabetes and its complications.
Statistical analysis
Comparisons between groups were done with the χ2 test of independence for qualitative variables, and the ANOVA or t test for quantitative variables apart from triglycerides, which have a highly skewed distribution and for which the non-parametric Kruskal-Wallis or Wilcoxon tests were used. We did three pair-wise comparisons of diabetes incidence in the three intervention groups, each at an α of 0·017—ie, the conventional 0·05 adjusted for three comparisons. The intention-to-treat analysis compared each intervention with placebo on a modified product-limit life-table distribution with a log-rank test statistic. Treatment groups and periods during the study were also compared with incidence per 100 person-years. Cases were new confirmed diagnoses of diabetes. Person-years were the sum of time under follow-up for all participants in a group before diagnosis of diabetes or end of follow-up if diabetes did not develop during the time of interest.
For analyses of changes over time in quantitative measures we used the normal errors longitudinal regression model.9 Interaction between treatment groups and time was assessed first to see if changes over time varied across treatment groups by inclusion of an interaction term in the model. Each analysis of change from the start of DPPOS was adjusted for the study’s baseline value, and each analysis of change from DPP randomisation until the analysis closing date was adjusted for the DPP baseline value. To help with interpretation of the treatment-group comparisons of glycaemia, blood pressure, and lipids over time, we also examined the prevalence of drug use to treat these variables. For the analysis of drug use over time we used generalised estimating equations with repeated measures over time10 to model the percentage of participants taking medicines during the study. Analyses were done with SAS versions 8.1 and 9.1.
Although this follow-up study was not anticipated in the DPP design, we present between-group comparisons of diabetes incidence for the total follow-up from DPP randomisation through the bridge period, plus a median of 5·7 years (IQR 5·5–5·8) of the DPPOS to Aug 27, 2008. Although not independent of the DPP-only and DPPOS-only analyses, these overall analyses were included to describe the cumulative experience of the cohort. This study is registered with ClinicalTrials.gov, number NCT00038727.
Role of the funding source
The sponsor of this study was represented on the Steering Committee and played a part in study design, how the study was done, and publication. The funding agency was not represented on the writing group, although all members of the Steering Committee had input into the report’s contents. All authors in the writing group had access to all data.
Results
Enrolment into this follow-up study from the DPP cohort did not differ significantly by sex or ethnic origin, but was lower in women with a history of gestational diabetes than in those without and higher in participants who had developed diabetes by Sept 1, 2002, than in those without diabetes (table 1). Enrolment was also related to greater age, HbA1c, cholesterol concentrations, and in women, by lower weight and body-mass index (webappendix p 1). Table 2 shows DPPOS baseline characteristics. Median follow-up from randomisation in the DPP to the most recent assessment in the DPPOS was 10·0 years (IQR 9·0–10·5).
Table 1.
Number of enrolled participants, number eligible, and percentage enrolled by sex, ethnic and racial origin, diabetes status, and treatment group
| All groups n/N (%) |
ILS group n/N (%) |
Metformin group n/N (%) |
Placebo group n/N (%) |
|
|---|---|---|---|---|
| Sex | ||||
| Men | 888/1014 (88%) | 291/333 (87%) | 307/353 (87%) | 290/328 (88%) |
| Women | 1878/2136 (88%) | 619/713 (87%) | 617/696 (89%) | 642/727 (88%) |
| With GDM* | 290/344 (84%) | 93/114 (82%) | 97/110 (88%) | 100/120 (83%) |
| No GDM | 1587/1791 (89%) | 526/599 (88%) | 519/585 (89%) | 542/607 (89%) |
| Ethnic and racial origin | ||||
| White | 1506/1712 (88%) | 490/560 (88%) | 515/582 (88%) | 501/570 (88%) |
| African-American | 559/632 (88%) | 176/199 (88%) | 191/219 (87%) | 192/214 (90%) |
| Hispanic | 424/495 (86%) | 138/171 (81%) | 141/160 (88%) | 145/164 (88%) |
| American Indian | 153/169 (91%) | 53/59 (90%) | 46/52 (88%) | 54/58 (93%) |
| Asian American or Pacific Islander | 124/142 (87%) | 53/57 (93%) | 31/36 (86%) | 40/49 (82%) |
| Diabetes† | ||||
| No | 1999/2310 (87%) | 739/855 (86%) | 652/752 (87%) | 608/703 (86%) |
| Yes | 767/840 (91%) | 171/191 (90%) | 272/297 (92%) | 324/352 (92%) |
Data are number of participants enrolled/number eligible (%).
ILS=intensive lifestyle intervention.
Eligible participants are all those randomly assigned to DPP minus those who died or withdrew consent.
GDM (history of gestational diabetes) classification was missing for one woman in the metformin group.
Not diabetic or had diabetes confirmed according to DPP protocol as of Sept 1, 2002. Enrolment was higher in participants with diabetes than in non-diabetic participants, both overall and in the metformin and placebo treatment groups.
Table 2.
Characteristics at DPPOS baseline examination
| All groups (n=2766) |
ILS group (n=910) |
Metformin group (n=924) |
Placebo group (n=932) |
|
|---|---|---|---|---|
| Age (years) | 55·2 (10·3) | 55·3 (11·0) | 55·5 (10·1) | 54·8 (10·0) |
| Fasting plasma glucose (mmol/L) | 6·03 (1·10) | 5·98 (0·98) | 5·94 (1·04) | 6·18 (1·24) |
| HbA1c (%) | 5·95 (0·69) | 5·89 (0·64) | 5·94 (0·63) | 6·01 (0·79) |
| Men (n) | 888 | 291 | 307 | 290 |
| Weight (kg) | 95·6 (20·2) | 93·4 (21·6) | 95·7 (19.6) | 97·7 (19·2) |
| Height (cm) | 175·0 (7·2) | 175·0 (7·4) | 175·1 (7·0) | 174·8 (7·4) |
| Body-mass index (kg/m2) | 31·1 (5·9) | 30·4 (6·3) | 31·1 (5·6) | 31·9 (5·9) |
| Women (n) | 1878 | 619 | 617 | 642 |
| Weight (kg) | 90·3 (21·0) | 89·2 (21·7) | 90·2 (20·7) | 91·4 (20·5) |
| Height (cm) | 162·3 (6·7) | 162·4 (6·9) | 162·6 (6·8) | 162·0 (6·5) |
| Body-mass index (kg/m2) | 34·2 (7·2) | 33·7 (7·3) | 34·1 (7·2) | 34·7 (7·1) |
| Without diabetes (n) | 1999 | 739 | 652 | 608 |
| Fasting plasma glucose (mmol/L) | 5·72 (0·52) | 5·72 (0·51) | 5·66 (0·53) | 5·79 (0·51) |
| 2-h plasma glucose (mmol/L) | 8·11 (1·92) | 7·98 (1·92) | 8·19 (2·02) | 8·20 (1·78) |
| HbA1c (%) | 5·78 (0·46) | 5·75 (0·48) | 5·79 (0·43) | 5·80 (0·46) |
| Systolic blood pressure (mm Hg) | 121·8 (14·7) | 120·6 (14·9) | 122·7 (14·4) | 122·3 (14·7) |
| Diastolic blood pressure (mm Hg) | 75·1 (9·0) | 74·4 (9·0) | 75·9 (8·8) | 75·2 (9·2) |
| Cholesterol (mmol/L) | 5·08 (0·90) | 5·08 (0·91) | 5·07 (0·86) | 5·08 (0·93) |
| HDL cholesterol (mmol/L) | 1·23 (0·33) | 1·25 (0·34) | 1·23 (0·34) | 1·19 (0·31) |
| LDL cholesterol (mmol/L) | 3·11 (0·80) | 3·12 (0·80) | 3·08 (0·75) | 3·12 (0·85) |
| Triglycerides (mmol/L) | 1·38 (0·98–1·98) | 1·31 (0·93–1·89) | 1·41 (1·02–2·00) | 1·43 (1·03–2·01) |
| With diabetes (n) | 767 | 171 | 272 | 324 |
| Fasting plasma glucose (mmol/L) | 6·83 (1·65) | 7·10 (1·56) | 6·61 (1·54) | 6·88 (1·76) |
| HbA1c (%) | 6·38 (0·96) | 6·47 (0·88) | 6·30 (0·85) | 6·40 (1·07) |
| Systolic blood pressure (mm Hg) | 123·8 (14·2) | 123·7 (14·0) | 124·5 (13·8) | 123·3 (14·6) |
| Diastolic blood pressure (mm Hg) | 76·3 (9·3) | 76·8 (9·9) | 75·7 (9·5) | 76·5 (8·9) |
| Cholesterol (mmol/L) | 5·00 (0·88) | 5·05 (0·88) | 4·99 (0·89) | 4·98 (0·88) |
| HDL cholesterol (mmol/L) | 1·14 (0·29) | 1·12 (0·29) | 1·20 (0·30) | 1·11 (0·28) |
| LDL cholesterol (mmol/L) | 3·02 (0·78) | 3·07 (0·76) | 2·98 (0·78) | 3·02 (0·80) |
| Triglycerides (mmol/L) | 1·60 (1·11–2·16) | 1·57 (1·16–2·20) | 1·51 (1·12–2·14) | 1·65 (1·08–2·21) |
Data are mean (SD) apart from triglycerides for which data are median (IQR), or number of participants (n). Data from the study baseline examination were stratified by presence of diabetes as of Sept 1, 2002. At enrolment to the Diabetes Prevention Program Outcomes Study (DPPOS), we identified significant differences between treatment groups for fasting plasma glucose, HbA1c, weight (overall and in men only) and body-mass index (BMI; overall and in each sex). We also noted significant differences between treatment groups in those without diabetes for fasting plasma glucose, systolic and diastolic blood pressure, HDL cholesterol, and triglycerides, and in those with diabetes for fasting plasma glucose and HDL cholesterol. Data were missing for 22 enrolled people who had no DPPOS baseline examination. Data were missing for additional people, in varying numbers, for some of the variables.
ILS=intensive lifestyle intervention. HbA1c=glycosylated haemoglobin.
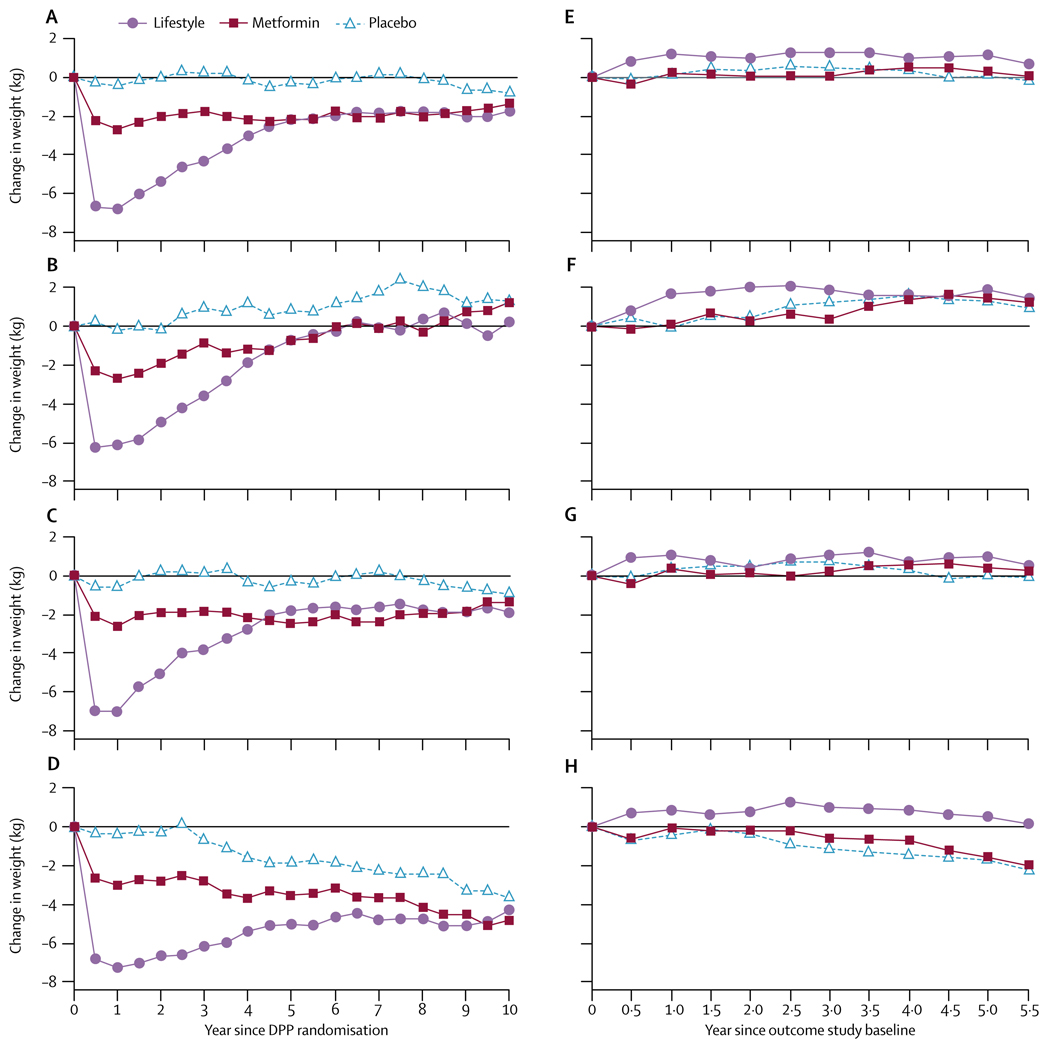
Figure 2 shows weight change by treatment group from DPP randomisation and during DPPOS only. Participants had similar changes in BMI and waist circumference (webappendix pp 3–5). The lifestyle group participants initially lost the most weight (a mean of 7 kg by 1 year), but gradually regained, although still weighing about 2 kg less than they did at randomisation. The metformin group lost a mean of 2·5 kg during DPP and maintained most of that weight loss. The placebo group’s mean weight loss was less than 1 kg from DPP entry. Thus, the groups’ mean weights differed at the start of DPPOS—90·6 kg for lifestyle, 92·0 kg for metformin, and 93·4 kg for placebo (p=0·0158). The lifestyle group subsequently regained about 1 kg, whereas the metformin and placebo groups initially lost and then regained weight back to their respective levels at DPPOS baseline (figure 2). Participants younger than 45 years at randomisation had less sustained weight loss from randomisation throughout the DPPOS than did those aged 45 years and older. Participants in both the metformin and placebo groups who were aged 60–85 years at DPP randomisation lost weight (figure 2). Every age-group in the lifestyle intervention gained weight, on average, during the DPPOS.
Figure 2. Mean weight changes.
Weight changes for originally assigned treatment group since Diabetes Prevention Program (DPP) randomisation for (A) all participants, (B) those aged 25–44 years at randomisation, (C) 45–59 years, and (D) 60 years and older; and since enrolment in the present study for (E) all participants, (F) those aged 25–44 years, (G) 45–59 years, and (H) 60 years and older, including participants irrespective of whether they developed diabetes during follow-up. Webappendix p 2 shows numbers of those in every datapoint.
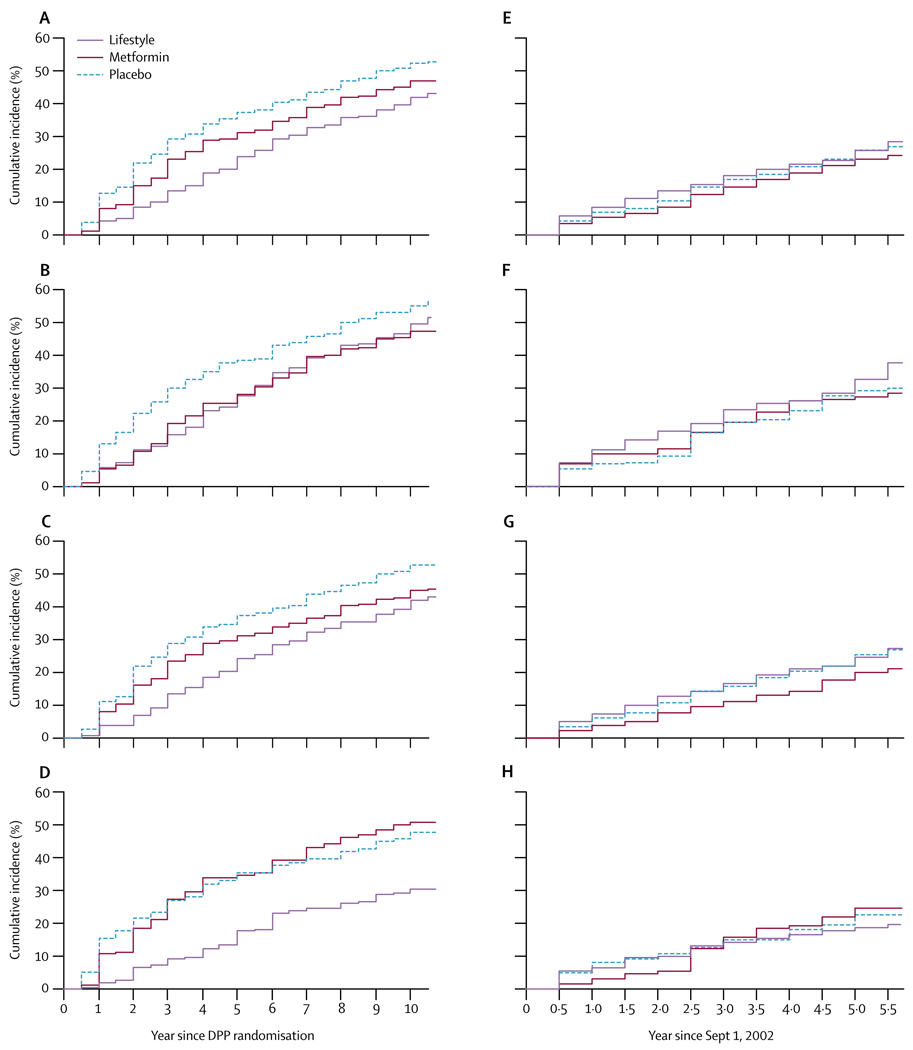
Figure 3 shows the cumulative frequency of diabetes since randomisation to DPP and during the DPPOS only. During this study, cumulative incidence curves for the 1994 participants who remained without diabetes on Sept 1, 2002, (five of the original 1999 had no follow-up) did not continue to separate as they had in DPP, because the accumulation of additional cases of diabetes was similar in the three groups (figure 3).
Figure 3. Cumulative frequency of diabetes.
Development of diabetes since Diabetes Prevention Program (DPP) randomisation for (A) all participants, (B) those aged 25–44 years at randomisation, (C) 45–59 years, (D) and 60 years and older; and since enrolment in the DPPOS (Sept 1, 2002) for (E) all participants, (F) those aged 25–44 years at randomisation, (G) 45–59 years, and (H) 60 years and older. Webappendix p 6 shows numbers of participants remaining at risk of diabetes at every datapoint.
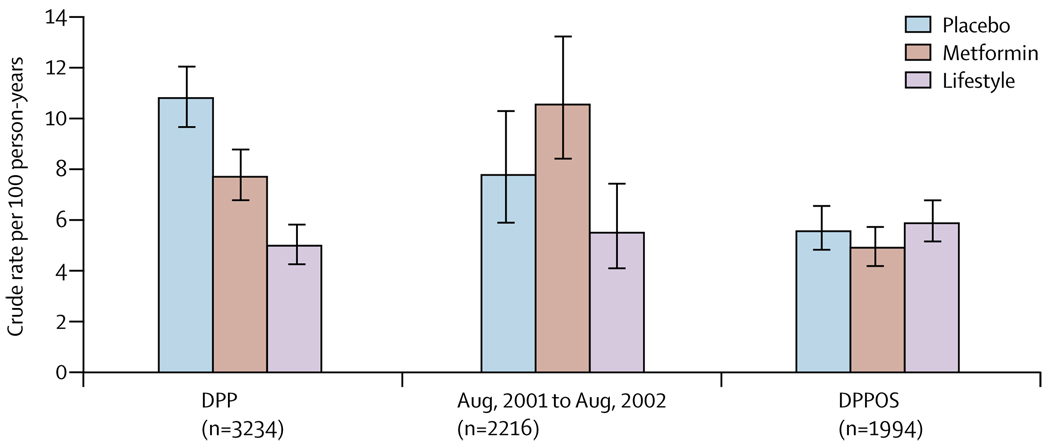
Table 3 and figure 4 show diabetes incidence during the different study periods. The original DPP report covered an average 2·8 years of follow-up4 and when the masked treatment phase of DPP was stopped in July, 2001, the average follow-up had extended to 3·2 years. During DPPOS, diabetes incidence rates did not significantly differ between groups (table 3). Incidence rates were stable in the lifestyle group, but fell in the placebo and metformin groups during the DPPOS. During the combined DPP, bridge, and DPPOS periods, the incidence rate of the lifestyle group was reduced by 34% (95% CI 24–42) and metformin by 18% (7–28) compared with placebo. The lifestyle effect was greatest in participants aged 60–85 years at randomisation (49% rate reduction), in whom metformin had no significant effect (figure 3).
Table 3.
Incidence (cases per 100 person-years) of diabetes during DPP, bridge period, and DPPOS
| ILS group | Metformin group | Placebo group | |
|---|---|---|---|
| DPP (2·8 years) | 4·8 (4·1–5·7) | 7·8 (6·8–8·8) | 11·0 (9·8–12·3) |
| End of masked treatment (3·2 years) | 5·0 (4·3–5·8) | 7·7 (6·8–8·8) | 10·8 (9·7–12·0) |
| Bridge period | 5·5 (4·1–7·5) | 10·6 (8·4–13·2) | 7·8 (5·9–10·3) |
| DPPOS (n=2766) | 5·9 (5·1–6·8) | 4·9 (4·2–5·7) | 5·6 (4·8–6·5) |
| Combined incidence | 5·3 (4·8–5·8) | 6·4 (5·9–7·1) | 7·8 (7·2–8·6) |
Data are incidence rates (95% CI). The bridge period was Aug 1, 2001, to Aug 31, 2002.
ILS=intensive lifestyle intervention. DPP=Diabetes Prevention Program.
Figure 4. Incidence rates of diabetes during the three study phases of DPP, bridge, and DPPOS.
The bars show diabetes incidence rates and the error bars 95% CIs. DPP=Diabetes Prevention Program.
The median delay to onset of diabetes can be estimated from the differences between treatment groups in the time to 40% cumulative incidence of diabetes (40% was used because 50% cumulative incidence had not yet occurred in all groups, figure 3). This point was delayed by about 4 years by lifestyle and 2 years by metformin, compared with placebo. At the most recent yearly examination, 23% in the lifestyle, 19% in the metformin, and 19% of participants in the placebo groups had become normoglycaemic by criteria defined and reported previously (fasting glucose <6·1 mmol/L, 2-h glucose <7·8 mmol/L, and no previous diagnosis of diabetes).4,5 With a definition of normoglycaemia of fasting glucose less than 5·6 mmol/L,11 2-h glucose less than 7·8 mmol/L, and no previous diagnosis of diabetes, the corresponding frequencies were 13%, 11%, and 10%.
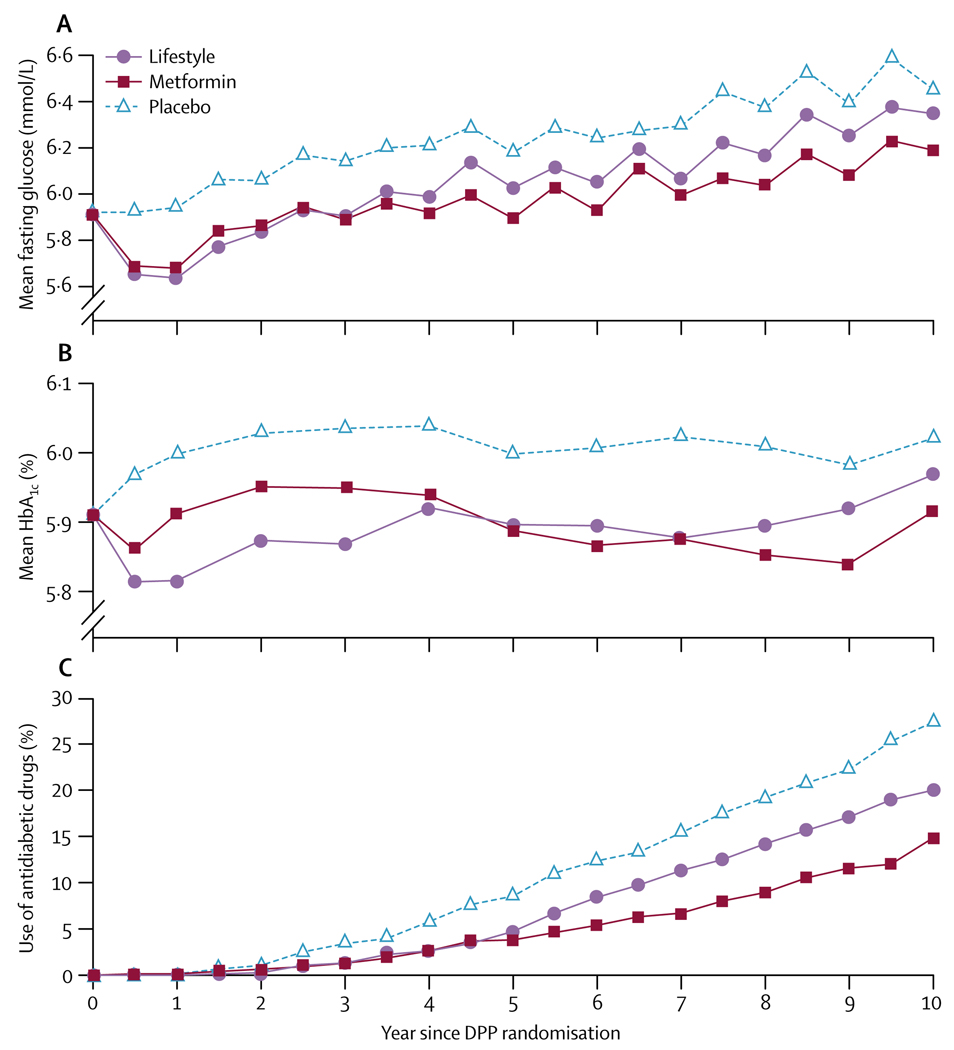
Figure 5 shows differences in fasting glucose, HbA1c, and antidiabetic medicine use over time by treatment group for all participants, irrespective of whether diabetes had developed. HbA1c and fasting glucose concentrations were lower in the metformin and lifestyle groups than in the original placebo group, despite more people in the placebo group using antidiabetic drugs than those in the metformin (excluding study-assigned metformin) or lifestyle groups. Cardiovascular disease risk factors improved in all three treatment groups since randomisation. Averaged over all follow-up, systolic and diastolic blood pressure and triglycerides were lower in the lifestyle than in the other groups, although use of anti-hypertensive drugs was less frequent. Overall, systolic and diastolic blood pressure and triglycerides were lower in the lifestyle than in the metformin and placebo groups, but these differences were not maintained by the end of follow-up (table 4).
Figure 5. Fasting glucose, glycosylated haemoglobin, and antidiabetic drug use.
A=fasting glucose in mmol/L. B=HbA1c (%). C=use of antidiabetic drugs (%). All participants were included irrespective of whether they developed diabetes during follow-up. Study-assigned metformin is excluded from antidiabetic drug use. Information for each data point is shown in webappendix p 7.
Table 4.
Cardiovascular disease risk factors averaged over all follow-up since randomisation to DPP
| ILS group (n= 910) | Metformin group (n=924) | Placebo group (n= 932) | |
|---|---|---|---|
| Antihypertensive drugs | 32·9% (32·2–33·6) | 37·1% (36·4–37·8) | 35·6% (34·9–36·3) |
| Lipid-lowering drugs | 18·4% (17·8–19·0) | 22·6% (22·0–23·2) | 22·7% (22·1–23·3) |
| Systolic/diastolic blood pressure (mm Hg) | 120·8/74·4 (120·2/74·1–121·3/74·8) | 122·4/75·6 (121·9/75·3–122·9/75·9) | 122·3/75·6 (121·8/75·3–122·8/75·9) |
| Serum cholesterol (mmol/L) | 4·92 (4·89–4·95) | 4·93 (4·90–4·96) | 4·97 (4·94–5·00) |
| Geometric serum triglycerides (mmol/L) | 1·37 (1·34–1·39) | 1·45 (1·42–1·47) | 1·45 (1·43–1·48) |
Data are % (95% CI) or mean (95% CI).
DPP=Diabetes Prevention Program. ILS=intensive lifestyle intervention.
During DPPOS, 57% of the non-diabetic metformin participants took 80% or more of the prescribed metformin dose and 70% took metformin in any amount, compared with 1% of non-diabetic participants in the lifestyle group and 3% in the original placebo group who took metformin prescribed outside the study. Attendance at quarterly lifestyle sessions (HELP) averaged 18% for the original lifestyle group, 15% for the metformin group, and 14% for the placebo group (20%, 15%, and 14%, respectively, in non-diabetic participants). In each sex and treatment group, attendance was roughly twice as high in those aged 60–85 years at randomisation than in those aged 25–44 years. Attendance at BOOST sessions for the original lifestyle group averaged 17% (19% for participants without diabetes), and was positively associated with age.
Discussion
We report the first phase of the long-term follow-up (DPPOS) of the DPP cohort. The second phase is due to be completed in 2014. The DPP and other clinical trials12–16 have established the feasibility of interruption of the worsening of hyperglycaemia in overweight people who have raised fasting or postload glycaemia. 10 years after DPP randomisation, cumulative incidence of diabetes remained lower in the lifestyle and metformin groups than in the placebo group, despite changes in treatments after a mean of 3·2 years. Metformin was at least as effective as lifestyle intervention in prevention of rises in fasting glucose and HbA1c (figure 5). This finding became evident during the DPPOS, and could be a result of a greater effect of metformin when combined with lifestyle sessions, or a more sustained effect achieved with metformin than with that of the lifestyle intervention alone. Such conclusions about treatment effects during the DPPOS are tentative because of group differences, including the prevalence of diabetes, at DPPOS enrolment (table 2).
Diabetes incidence during the DPPOS did not differ significantly between the three randomised groups. This finding was not attributable to a rebound effect in the lifestyle group but to a fall in incidence in the placebo and metformin groups that resulted in similar rates as achieved by lifestyle intervention, which changed little throughout follow-up (table 3 and figure 4). The brief drug washout study7 at the end of DPP probably accounted for the transient rise in diabetes incidence in the metformin group during the bridge period. During the washout, an estimated 15 diabetes cases occurred in the metformin group above those expected from the placebo experience. Had these cases not occurred, incidence rates would have been similar in the metformin and placebo groups during the bridge period.
Incidence rates in the former placebo and metformin groups might have fallen during DPPOS because most participants who were susceptible to diabetes developed the disease during the DPP, leaving a reduced number at risk during the present study. However, this hypothesis is not supported by stability over time of diabetes incidence in the lifestyle group, by findings in high-risk Pima Indians, in whom the yearly incidence of diabetes in people with impaired glucose tolerance increased rather than decreased during a 10-year follow-up,17 or by results of the Finnish Diabetes Prevention Study18 in which diabetes incidence in the control group was similar during the clinical trial and follow-up period. Unlike in DPPOS, all intervention in the Finnish trial ended after completion of the original 4 year study. An additional explanation for the reduction in incidence in the former metformin and placebo groups in our study is that participants benefited from the group-implemented lifestyle programme offered to all during the bridge and DPPOS periods. This benefit might have been especially relevant for people aged 60–85 years at randomisation, who lost weight during this time.
In this study, onset of diabetes was delayed by about 4 years by lifestyle intervention and 2 years by metformin compared with placebo. The delay in median time to diabetes diagnosis was previously estimated to be 11 years for the lifestyle and 3 years for the metformin groups on the basis of extrapolation of DPP incidence.19 The present estimated delays are smaller than the original estimates owing largely to the reduction in diabetes incidence in the placebo group. Lifestyle and metformin interventions succeeded in producing long-term (10-year) weight loss. During DPP, weight loss was associated with diabetes prevention.20 As far as we are aware, no other weight-loss study using behavioural or drug interventions has reported this amount of weight loss over such a long period. For example, in the non-surgical treatment group of the Swedish Obese Subjects Study21 of bariatric surgery, a 0·1% weight gain was reported, on average, after 10 years. The extent to which participants’ knowledge of DPP results and the addition of the group-implemented lifestyle intervention contributed to the long-term effects of metformin are unknown. The lifestyle intervention also resulted in improved blood pressure and lipid concentrations despite a reduction in drug treatment prescribed by participants’ personal physicians.
Because diabetes is defined by somewhat arbitrary cut-off points of hyperglycaemia, arrest of progression of hyperglycaemia prevents or delays onset of diabetes. In view of the strong relation between hyperglycaemia and long-term diabetes complications (microvascular and macrovascular disease and neuropathy), one might assume that prevention or delay of onset of diabetes as defined by conventional glycaemic cut-offs would also prevent or delay these clinical manifestations. Both active interventions improved risk factors for cardiovascular disease.22,23 However, diabetes complications were too infrequent for analysis of treatment effects, and the full DPPOS follow-up will be needed to assess whether prevention or delay of diabetes onset will have the same effect on complications.
Our findings that both lifestyle and metformin interventions delayed or prevented diabetes in the DPPOS are consistent with results of other reports of similar interventions with lifestyle or with other antidiabetic drugs.24,25 Continued separation of cumulative incidence of diabetes has been reported in long-term follow-up of two other lifestyle intervention studies.18,26 The Finnish Diabetes Prevention Study used an approach similar to that in the DPP lifestyle group. During the 4-year active intervention, diabetes incidence was reduced by 58%. Although the intervention was not actively delivered during the 3-year follow-up, it remained effective with a 39% reduction in incidence.18 20-year follow-up26 was reported from the Da Qing12 prevention trial in 577 Chinese individuals with impaired glucose tolerance. After 6 years of treatment, cumulative diabetes incidences were high (68% in usual care, 48% in diet, 41% in exercise, and 46% in diet plus exercise groups), with all three interventions being similarly effective. Treatment and regular follow-up were discontinued after 6 years, but cumulative incidence during 20 years, gathered largely from clinical and historical data, remained lower in the three combined treatment groups than in the usual care group.26 However, the change in ascertainment of diabetes over time complicates data interpretation. As far as we know, no long-term follow-up of drug intervention to prevent diabetes has been published except for the DPPOS.
Limitations of the present analyses include the less than complete enrolment in our study and the treatment modifications after the end of DPP. Informing participants and the public of results in 2001, and modifying the protocol to offer core lifestyle sessions to all participants were ethically necessary. For participants in the lifestyle group, decreased intensity of treatment during the bridge and DPPOS might have resulted in lowered adherence at a time when weight regain was taking place. The two groups newly exposed to lifestyle intervention (metformin and placebo) had higher attendance than did the lifestyle group during the bridge period, and they achieved modest weight loss, although less than the lifestyle group did during the DPP.8 Therefore, convergence of diabetes incidence rates in the three treatment groups during the bridge and DPPOS might be a result of either a diminished effect of the lifestyle intervention for the original lifestyle group compared with the intensive lifestyle intervention during the DPP, or the introduction of group-implemented lifestyle intervention to the DPP placebo and metformin groups, or both. Session attendance was positively associated with age, perhaps because of differences in occupational and child-care demands. These factors probably contributed to greater weight loss (figure 2) and protection from diabetes in the older than in the younger participants, particularly in the lifestyle group (figure 3).
Results of the DPP were reported after a mean 2·8 years.4 The present study investigates further diabetes development in about 7 years’ extended follow-up. For perspective, we have summarised the data for the cohort over 10 years. Cumulative data are completely contained within the other separately reported periods, statistically provide no additional information, and do not represent a predesigned uniform intervention over time. Therefore, significance tests or CIs reported for the entire time since randomisation should be interpreted cautiously.
Our results have shown that a reduction in diabetes cumulative incidence by either lifestyle intervention or metformin therapy persists for at least 10 years. Further follow-up will provide crucial data for long-term clinical outcomes, including mortality. In the next phase of the DPPOS, the primary objective is to assess intervention effects on a composite microvascular-neuropathic outcome for diabetic retinopathy, nephropathy, or reduced light touch sensation in the feet. Secondary outcomes include the individual components of the composite primary outcome, cardiovascular disease, further development of diabetes, measures of glycaemia, insulin secretion, insulin sensitivity, cardiovascular disease risk factors, physical activity, nutrition, body-weight, health-related quality of life, and economic assessments. These data are needed because speculation about the long-term benefits on the basis of extrapolation of the DPP and other data by different authors have led to very different conclusions.19,27,28 The long-term reductions in bodyweight and diabetes are encouraging, but further quantification of long-term outcomes is crucial to establish the benefits of diabetes prevention.
Acknowledgments
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) as a cooperative agreement. We thank the participants of the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study for their commitment and dedication; the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health for funding to the clinical and coordinating centres that designed and undertook the study, and collected, managed, analysed and interpreted the data; and the General Clinical Research Center Program and the National Center for Research Resources for support of data collection at many of the clinical centres. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and by the Indian Health Service. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart Lung and Blood Institute, the Office of Women’s Health, the National Center for Minority Health and Human Disease, the Centers for Disease Control and Prevention, and the American Diabetes Association. Lipha (Merck-Sante) provided medicines, and LifeScan donated materials. The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies.
Diabetes Prevention Program Research Group
Principal investigators indicated by an asterisk. Programme coordinators indicated by a dagger. Pennington Biomedical Research Center (Baton Rouge, LA) G A Bray*, A Chatellier†, C Duncan, F L Greenway, E Levy, D H Ryan; University of Chicago (Chicago, IL) K S Polonsky*, J Tobian*, D Ehrmann*, M J Matulik†, B Clark, K Czech, C DeSandre, R Hilbrich, W McNabb, A R Semenske; Jefferson Medical College (Philadelphia, PA) B J Goldstein*, K A Smith†, W Wildman†, C Pepe; University of Miami (Miami, FL) R B Goldberg*, J Calles†, J Ojito†, S Castillo-Florez, H J Florez, A Giannella, O Lara, B Veciana; University of Texas Health Science Center (San Antonio, TX) S M Haffner*, M G Montez†, C Lorenzo, A Martinez; University of Colorado (Denver, CO) R F Hamman*, L Testaverde†, A Bouffard, D Dabelea, T Jenkins, D Lenz, L Perreault, DW Price, S C Steinke; Joslin Diabetes Center (Boston, MA) E S Horton*, C S Poirier†, K Swift†, E Caballero, S D Jackson, L Lambert, K E Lawton, S Ledbury; University of Washington (Seattle, WA) S E Kahn*, B K Montgomery†, W Fujimoto, R H Knopp, E W Lipkin, M Marr, A Murillo, D Trence; University of Tennessee (Memphis, TN) A E Kitabchi*, M E Murphy†, W B Applegate, M Bryer-Ash, S Dagogo-Jack, S L Frieson, H Lambeth, L C Lichtermann, H Otkaei, L M K Rutledge, A R Sherman, C M Smith, J E Soberman, B Williams-Cleaves; Northwestern University’s Feinberg School of Medicine (Chicago, IL) B E Metzger*, M E Molitch*, M K Johnson†, M M Giles, D Larsen, C Niznik, S C Pen, P A Schinleber; Massachusetts General Hospital (Boston, MA) D M Nathan*, C McKitrick†, H Turgeon†, K Abbott, D Altshuler, E Anderson, L Bissett, E Cagliero, K D’Anna, L Delahanty, J C Florez, V Goldman, A Poulos, B Tseng; University of California-San Diego (San Diego, CA) E Barrett-Connor*, M L Carrion-Petersen†, J Horne, D Leos, S Mudaliar, J Smith, K Vejvoda; St. Luke’s-Roosevelt Hospital (New York, NY); F X Pi-Sunyer*, J E Lee,† S T Foo, S Hagamen; Indiana University (Indianapolis, IN) D G Marrero*, S M Kelly†, R T Ackermann, E S Fineberg, A Hadden, M A Jackson, M S Kirkman, K J Mather, P J Roach, M L Wheeler; Medstar Research Institute (Washington, DC) R E Ratner*, V Aroda*, S Shapiro†, C Bavido-Arrage, P Gibbs, Gl Uwaifo, R Wiggins; University of Southern California/UCLA Research Center (Alhambra, CA) M F Saad*, K Watson*, M Botrous†, S Jinagouda†, M Budget, C Conzues, P Magpuri, K Ngo, K Xapthalamous; Washington University (St. Louis, MO) N H White*, S Das†, A Santiago, A L Brown, C Wernimont; Johns Hopkins School of Medicine (Baltimore, MD) C D Saudek*, T Whittington†, J M Clark, A Greene, D Jiggetts, H Mosley, J Reusing, R R Rubin, S Stephen, E Utsey; University of New Mexico (Albuquerque, NM) D S Schade*, K S Adams†, C Hemphill†, P Hyde†, L Butler, J L Canady, K Colleran, Y Gonzales, D A Hernandez-McGinnis, P Katz, C King; Albert Einstein College of Medicine (Bronx, NY) J Crandall*, J O Brown-Friday†, E Adorno, H Duffy, H Martinez, D Pompi, H Shamoon, E A Walker, J Wylie-Rosett; University of Pittsburgh (Pittsburgh, PA) T Orchard*, S Jeffries†, M K Kramer†, M Smith†, A Kriska, J Pettigrew, L Semler, E Venditti, V Weinzierl; University of Hawaii (Honolulu, HI) R F Arakaki*, N K Baker-Ladao†, M K Isonaga†, N E Bermudez, M K Mau; Southwest American Indian Centers (Phoenix, AZ; Shiprock, NM; Zuni, NM) W C Knowler*, N Cooeyate†, M A Hoskin†, C Natewa†, C A Percy†, K J Acton, V L Andre, S Begay, B C Bucca, S Cook, M S Doughty, J Glass, M Glass, R L Hanson, D Hassenpflug, L E Ingraham, K M Kobus, J Krakoff, C Manus, C McCabe, S Michaels, T Morgan, J A Nelson, R J Roy, M Smart, D P Tonemah, C Wilson; George Washington University Biostatistics Center (DPP Coordinating Center Rockville, MD) S Fowler*, T Brenneman†, S Abebe, J Bamdad, J Callaghan, C A Christophi, S L Edelstein, Y Gao, R Gooding, A Gottlieb, N Grover, H Hoffman, K Jablonski, R Katz, P Kolinjivadi, J M Lachin, Y Ma, S Reamer, A Sapozhnikova, H Sherif, M Temprosa; Lifestyle Resource Core E M Venditti*, A M Kriska, L Semler, V Weinzierl; Central Biochemistry Laboratory (Seattle, WA) S Marcovina*, G Strylewicz†, J Albers; Epidemiological Cardiology Research Center- Epicare (Winston-Salem, NC) R J Prineas*, T Alexander, C Campbell, S Hall, S Hensley, Y Li, M Mills, E Soliman, Z Zhang; NIH/NIDDK (Bethesda, MD) J Fradkin, S Garfield, Nutrition Coding Center (Columbia, SC) E Mayer-Davis*, R R Moran†; Quality of Well-Being Center (La Jolla, CA) T Ganiats*, A J Sarkin†. Former members of the Diabetes Prevention Program Research Group, including those who were active in the DPP but not in the DPPOS, are named in reference 4.
Footnotes
Contributors
The writing group members for this report were William C Knowler, Sarah E Fowler, Richard F Hamman, Costas A Christophi, Heather J Hoffman, Anne T Brenneman, Janet O Brown-Friday, Ronald Goldberg, Elizabeth Venditti, and David M Nathan. The writing group takes final responsibility for the paper and is the study guarantor. WCK, SEF, RFH, ATB, JOB-F, RG, EV, DMN, and the full research group designed and undertook the study. All members of the writing group participated in the data analysis, drafted and revised this report, and approved the final version.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Roglic G, Unwin N, Bennett PH, et al. The burden of mortality attributable to diabetes. Diabetes Care. 2005;28:2130–2135. doi: 10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes mellitus. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Prevention Program Research Group. Strategies to identify adults at high risk for type 2 diabetes: the Diabetes Prevention Program. Diabetes Care. 2005;28:150–156. doi: 10.2337/diacare.28.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the Diabetes Prevention Program. Diabetes Care. 2003;26:977–980. doi: 10.2337/diacare.26.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Diabetes Prevention Program Research Group. First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes. Int J Obes. 2008;32:1537–1544. doi: 10.1038/ijo.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown H, Prescott R. Applied mixed models in medicine. New York: John Wiley & Sons Inc; 1999. [Google Scholar]
- 10.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford: Clarendon Press; 1994. [Google Scholar]
- 11.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 12.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 13.Tuomilehto J, Lindström J, Eriksson JG, et al. for the Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 14.Chiasson J-L, Josse RG, Gomis R, et al. for the STOP-NIDDM Trial Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomized trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 15.DREAM (Diabetes Reduction Assessment with ramipril and rosiglitaone medication) Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomized controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran A, Snehalatha C, Mary S, et al. on behalf of the Indian Diabetes Prevention Programme. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 17.Saad MF, Knowler WC, Pettitt DJ, et al. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med. 1988;319:1500–1506. doi: 10.1056/NEJM198812083192302. [DOI] [PubMed] [Google Scholar]
- 18.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 19.The Diabetes Prevention Program Research Group. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med. 2005;142:323–332. doi: 10.7326/0003-4819-142-5-200503010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Diabetes Prevention Program Research Group. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2192–2207. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjöström L, Kindroos A-K, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 22.The Diabetes Prevention Program Research Group. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care. 2005;28:888–894. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Diabetes Prevention Program Research Group. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;4:1566–1572. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crandall JP, Knowler WC, Kahn SE, et al. on behalf of the Diabetes Prevention Program Research Group. The prevention of type 2 diabetes. Nat Clin Pract Endocrinol Metab. 2008;4:382–393. doi: 10.1038/ncpendmet0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334:2997. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371:1783–1789. doi: 10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- 27.Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med. 2005;143:251–264. doi: 10.7326/0003-4819-143-4-200508160-00006. [DOI] [PubMed] [Google Scholar]
- 28.Engelgau MM. Trying to predict the future for people with diabetes: a tough but important task. Ann Intern Med. 2005;143:301–302. doi: 10.7326/0003-4819-143-4-200508160-00011. [DOI] [PubMed] [Google Scholar]