Abstract
This study assessed the intraoperative analgesic effects of intravenous lidocaine administered by a constant rate infusion (CRI) in surgical canine patients. A prospective, blinded, randomized study was designed with 2 treatment groups: A (lidocaine) and B (placebo), involving 41 dogs. All patients were premedicated with acepromazine and buprenorphine, induced with propofol and midazolam; anesthesia was maintained with isoflurane in oxygen. Group A received 2 mg/kg IV lidocaine immediately after induction, followed within 5 min by a CRI at 50 μg/kg/min. Group B received an equivalent volume of saline instead of lidocaine. Changes in heart rate and blood pressure during maintenance were treated by increasing CRI. Fentanyl was used as a supplemental analgesic when intraoperative nociceptive response was not controlled with the maximum dose of lidocaine infusion. There was a significantly lower use of supplemental intraoperative analgesia in the lidocaine than in the placebo group. Group B dogs had almost twice as high a risk of intraoperative nociceptive response as group A dogs.
Résumé
Évaluation d’une perfusion à débit constant de lidocaïne pour une anesthésie équilibrée chez les chiens subissant une chirurgie. Cette étude a évalué les effets analgésiques peropératoire de la lidocaïne intraveineuse administrée par perfusion à débit constant (PDC) chez les patients chirurgicaux canins. Une étude prospective à l’aveugle et aléatoire a été conçue avec 2 groupes de traitement : A (lidocaïne) et B (placebo), ciblant 41 chiens. Tous les patients ont reçu une prémédication avec l’acépromazine et la buprénorphine et une induction au propofol et au midazolam; l’anesthésie a été maintenue avec de l’isoflurane dans de l’oxygène. Le groupe A a reçu 2 mg/kg IV de lidocaïne immédiatement après l’induction, suivie après 5 minutes d’un PDR à 50 μg/kg/min. Le groupe B a reçu un volume équivalent de solution saline au lieu de la lidocaïne. Les changements de la fréquence cardiaque et de la tension artérielle durant le maintien ont été traités en augmentant le PDR. Le fentanyl a été utilisé comme analgésique d’appoint lorsque la réaction nociceptive peropératoire n’était pas contrôlée avec la dose maximum de perfusion de lidocaïne. Il y a eu une utilisation significativement réduite d’analgésie peropératoire d’appoint pour le groupe de lidocaïne par rapport au groupe placebo. Les chiens du groupe B présentaient un risque presque deux fois plus élevé de réaction nociceptive peropératoire que les chiens du groupe A.
(Traduit par Isabelle Vallières)
Introduction
Balanced anesthesia is defined as the concurrent administration of a mixture of small amounts of several anesthetic drugs to decrease the adverse effects of each individual drug. In small animals, balanced anesthesia is mainly used to decrease the requirements of inhalant anesthetics in order to limit the cardiovascular depressant effects that they induce (1).
Lidocaine is an amide local anesthetic and antiarrhythmic agent that has been used for years in canine clinical practice to provide loco-regional analgesia and to treat ventricular dysrhythmias. The use of lidocaine administered IV in clinical practice has gained acceptance due to growing evidence supporting its beneficial effects in a range of clinical situations (2).
Lidocaine is a Na+/Ca++ channel blocker (3) and plays an important role in the control of peri- and post-operative sympathetic response (4). The intravenous use of lidocaine as a supplement to general anesthesia has been reported in humans (5,6), horses (7,8), dogs (9,10) and cats (1). After IV lidocaine infusion, dose-dependent minimum alveolar concentration (MAC) reduction has been reported for inhalant anesthetics such as halothane in ponies (7), enflurane and isoflurane in dogs (9,11), and isoflurane in cats (1). However, the precise mechanism of MAC reduction and pain processing is not clear.
Lidocaine blocks impulses in peripheral nerves due to its action on voltage sensitive sodium channels predominantly (2). Profound effects have been reported in single cells of spinal cord, dorsal horn neurones and in studies of evoked potentials within the spinal cord (12,13). Lidocaine also inhibits the neurons responsible for visceral pain transmission (14,15)
In an experimental pilot trial, lidocaine and morphine were compared as analgesics for intraocular surgery in 12 dogs and the results suggested that intraoperative lidocaine may provide analgesic benefits similar to morphine, causing no clinically significant alterations in blood pressure or heart rate during isoflurane anesthesia (10). In recent studies systemic administration of lidocaine produced no major adverse effects; it was superior to placebo in relieving neuropathic pain (16) and was as effective as other analgesics such as morphine, amitriptyline, and gabapentin, used for this condition in human medicine (17,18).
The objective of this study was to assess the intraoperative analgesic effects of intravenous lidocaine administration by a constant rate infusion (CRI) in clinical surgical patients. Soft tissue and orthopedic procedures are the most common type of clinical cases admitted to a veterinary hospital. Due to this, the present study includes these 2 types of procedure so that the result would be useful for the small animal practitioner.
Materials and methods
Animals and instrumentation
Forty-one dogs (17 males and 24 females, weighing between 3 and 51 kg, and 8 mo to 13 y of age) admitted for orthopedic or soft tissue surgical procedures were included in the study. All the dogs were healthy based on physical examination, complete blood (cell) count (CBC), serum biochemistry, and urinalysis, and were classified as anesthetic risk grade ASA I or II prior to the surgical procedure. Food, but not water, was withheld for at least 12 h before the operation. Owner’s written consent was obtained. The soft tissue surgeries included in the study were mastectomy, splenectomy, perianal hernia, cystotomy, cutaneous nodulectomy, and ovariohysterectomy. Orthopedic procedures included leg amputations, fracture fixation, and cruciate ligament rupture repair.
Patients were randomly assigned to treatment group A (lidocaine) or B (placebo). Staff, anesthesiologist, surgeons (1 soft tissue and 1 orthopedic surgeon) and students working on the case during the surgery were all blinded to the treatment given.
All patients were premedicated with a neuroleptanalgesic combination of acepromazine (Calmo Neosan 5 mg/mL; Pfizer, Terrassa, Barcelona, Spain) at 0.03 mg/kg and buprenorphine (Buprex 0.03 mg/mL; Schering-Plough, Hull, UK) at 0.02 mg/kg administered intramuscularly. Right or left cephalic vein was cannulated using a 20 or 22 G over the needle catheter (Vasocan; Braun, Malaysia) for the administration of the induction agent and lactated Ringer’s solution (500 mL, Braun, Rubí, Barcelona, Spain) at 10 mL/kg body weight (BW) per hour. A second IV catheter was placed for the administration of lidocaine (Lidocaine Iny 2%; Braun), or placebo (SSF 500 mL; Rubi) during general anaesthesia. Between 30 and 40 min later, anesthesia was induced with propofol (Lipuro 1%; Lab Esteve, Zwolle, Holland) at 4 mg/kg IV and midazolam (Dormicum 3 mg/mL; Roche, Basel, Switzerland) at 0.2 mg/kg BW, IV. Anesthesia was maintained with isoflurane (Isoba vet 100%, Schering-Plough) in oxygen. The Ayre T-piece breathing system was used for dogs weighing less than 5 kg at 500 mL/kg BW per minute fresh gas flow (1), The Bain breathing system was used for dogs that weighed between 5 to 20 kg at 300 mL/kg BW per minute, and a rebreathing system was used for dogs heavier than 20 kg at 30 mL/kg BW per minute fresh gas flow.
Isoflurane was constant, maintained at 1.15 ± 0.1 vol% in all cases. Fraction of inspired isoflurane (FI’Iso), end-tidal isoflurane (ETIso), and end-tidal carbon dioxide (ETCO2) concentrations were monitored using an infrared gas analyzer (S/5 Datex-Ohmeda Division, Helsinki, Finland) connected to the endotracheal tube.
Electrocardiogram (lead II), heart rate, SpO2, and esophageal temperature were also monitored (S/5 Datex-Ohmeda Division). Temperature was maintained between 37°C and 39°C by using circulating warm water blankets. The monitor was calibrated before each procedure by using a standard Datex-Ohmeda calibration gas mixture (DOT-34 NRC 300/375 M 1014).
Non-invasive blood pressure measurements were carried out by using an 8 MHz Doppler probe (Ultrasonic Doppler Flow Detector 811-BTS; Parks Medical Electronics, Aloha, Oregon, USA). To achieve this, the cuff was correctly sized at around 40% of the circumference of the extremity and wrapped around the skin surface over the common digital artery at the plantar aspect of the hind or fore paw and a sphygmomanometer (Riester minimus II). Measurements were always taken by the same person and the median of 5 measurements was considered the final blood pressure reading.
A software application (S/5 collect 3.0 Datex-Ohmeda) was used for data collection starting before the loading dose and then every 5 min during maintenance until extubation took place. Blood pressure recording followed the same timing.
Lidocaine infusion
Lidocaine loading dose (2 mg/kg BW, IV) was administrated immediately after induction followed within 5 min by a CRI of 50 μg/kg BW per min using a syringe pump (Perfusor Secura FT; Braun, Netherlands). The infusion in both groups began 15 min prior to the surgical incision. The placebo group received an equivalent volume of saline instead of lidocaine.
Heart rate (HR) and blood pressure (BP) were taken as indicators of nociceptive responses due to surgical stimulation. Changes in these variables above 20% from the previous measurement occurring during maintenance were treated by increasing lidocaine infusion up to the maximum dose of 200 μg/kg BW per min. Whenever the HR, BP, or both was not maintained within clinically acceptable limits based on the weight and age of the dog, intraoperative analgesia supplementation was provided with a 6 μg/kg bolus of fentanyl (Fentanest, 0.05 mg/kg; Kern Pharma, Terrasa, Barcelona, Spain). Meloxicam (Metacam 5 mg/mL; Mérial, Terrassa, Barcelona, Spain) at 0.2 mg/kg SC was given at the end of the procedure for postoperative analgesia in all dogs.
Statistical analyses
To validate the random allocation of the dogs into the 2 groups, Student’s t-test was used for continuous quantitative variables (age, weight, duration of surgical procedure) and Pearson’s Chi-square for qualitative variables (sex, type of surgical procedure). The null hypothesis (H0) was established considering that intraoperative analgesia supplementation had the same effect in both groups (A and B) and no significant differences existed between them. Pearson’s Chi-square test was used for validation.
A Relative Risk analysis was used to analyze how much higher the risk of using intraoperative rescue analgesia (fentanyl) was for group B versus group A. A 95% confidence interval (95% CI) was also calculated. A longitudinal analysis using the Kaplan-Meier test was used to compare between groups when and how often fentanyl had to be administered. Significance of Log Rank statistic was calculated to evaluate differences between survival curves. A P-value < 0.05 was considered significant; PSS 11.0 for Windows, Win Episcope 2.0 and Microsoft Excel 2002 were used for these analyses.
Results
Table 1 shows quantitative and qualitative variables for the dogs in the study. There were no significant differences, confirming that all dogs were randomly allocated to the A or B group.
Table 1.
Continuous quantitative and qualitative variables for dogs in groups A and B
| Group A | Group B | P-value | |
|---|---|---|---|
| Age (years) | 7.22 ± 4.04 | 7.50 ± 3.47 | 0.816 |
| Weight (kg) | 20.60 ± 12.98 | 18.14 ± 11.61 | 0.526 |
| Duration of procedure (min) | 125.75 ± 47.28 | 106.19 ± 48.09 | 0.197 |
| Gender (female/male) | 55.0%/45.0% | 66.7%/33.3% | 0.444 |
| Type of surgical procedure (soft/trauma) | 65.0%/35.0% | 66.7%/33.3% | 0.910 |
Table 2 shows the proportion of patients by surgical procedure that required supplemental intraoperatory analgesia (fentanyl). Significant differences were found when both groups were compared. No significant differences were found when comparing groups according to surgical procedure. Nevertheless, the use of lidocaine (group A) provided a positive analgesic effect in both types of surgical procedure, and the percentage of supplemental intraoperative analgesia was lower in this group. Only 4/20 dogs in group A required supplemental intraoperative analgesia (fentanyl), versus 11/21 dogs in group B. For dogs which did not require fentanyl supplementation in group A, the maximal dose of systemic lidocaine received was 50 μg/kg BW per min in 4 dogs, 100 μg/kg BW per min in 6 dogs, and 150 μg/kg BW per min in 1 dog among those with soft tissue procedures. In dogs undergoing orthopedic procedures the doses were 100 μg/kg BW per min in 1 dog, 150 μg/kg BW per min in 3 dogs and 200 μg/kg BW per min in 1 dog.
Table 2.
Percentage of patients in each group requiring supplemental intraoperatory analgesia
| Group A n (%) | Group B n (%) | P-value | |
|---|---|---|---|
| Global | 4/20 (20.0) | 11/21 (52.4) | 0.031* |
| Soft tissue | 2/13 (15.4) | 6/14 (42.9) | 0.127 |
| Orthopedia | 2/7 (28.6) | 5/7 (71.4) | 0.143 |
Statistically significant.
Calculated relative risk (RR) was 1.907 (95% CI = 1.074, 3.385) indicating that patients of group B had almost twice as high a risk of intraoperative use of fentanyl as patients of group A.
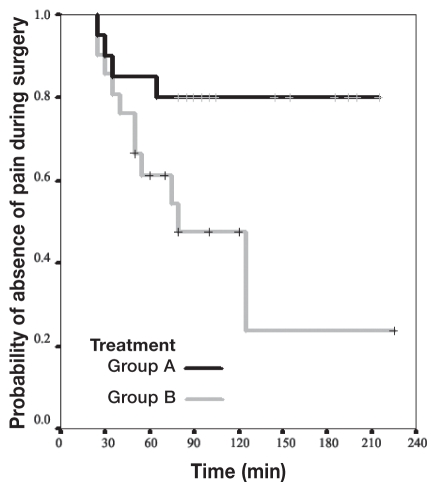
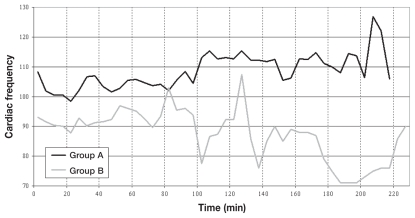
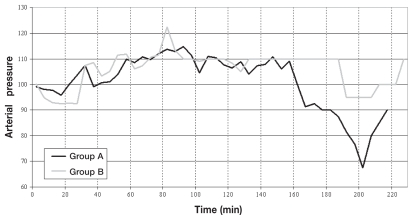
The Kaplan-Meier curves (Figure 1) show significant differences between groups A and B as indicated by Log Rank statistic (P = 0.0221). The changes in HR and BP during maintenance are shown in Figures 2 and 3, respectively.
Figure 1.
Kaplan-Meier curves for survival analysis as probability of no pain during surgery.
Figure 2.
Heart rate trend of patients from groups A and B.
Figure 3.
Blood pressure trend of patients from groups A and B.
Discussion
In experimental studies lidocaine administered intravenously decreased isoflurane end-tidal concentration in a dose-dependent manner in dogs (9,19) and cats (1). This effect has been observed with other inhaled anesthetics, such as halothane. A lidocaine infusion of 15 to 400 μg/kg BW per min resulted in a maximum reduction of halothane MAC of 45% (20). The mechanism for inhaled anesthetic end-tidal concentration reduction with lidocaine is unclear. Whether it is associated with the analgesic or sedating effects of lidocaine is unknown. One pilot study in dogs (10) described the reduction of post-operative ocular pain after the systemic administration of lidocaine. This study suggested that intraoperative lidocaine may provide analgesic benefits similar to morphine for intraocular surgery in dogs.
In this clinical study, sedation, analgesia, and good surgical anesthetic plane were provided to all of the patients, although antinociception assessment is difficult, even for an experienced specialist. Purposeful movements as a response to supramaximal stimulation (surgical procedure) have been described as an inappropriate indicator of lack of intraoperative analgesia (18). In one study, HR and BP variations were considered to be direct physiological signs of intraoperative sympathetic response to nociceptive stimulation (21) and in another study in lambs, HR and BP showed greater sensitivity as indicators of pain than ACTH or cortisol plasma measurements (22).
Lidocaine produces a transient (1 to 2 min) depression in monosynaptic reflexes at doses of ≥ 1 mg/kg BW and intravenous administration of lidocaine produces a selective central blockade of C-fiber-evoked polysynaptic activity associated with stimulation of the sural nerve. There are similarities between the selective C-fiber suppressing actions of systemically administered local anesthetic and the pharmacological actions of narcotic opiates (12).
The analgesic effects of lidocaine are well-known in human medicine. In a controlled study in men undergoing prostatectomy, IV lidocaine administered during surgery and continued for 1 h after surgery, decreased the incidence of post-operative pain, hastened the return of intestinal motility, and shortened hospital stay (23). Lidocaine reduced the sympathetic response to abdominal surgery in humans. The IV infusion of lidocaine during and after major abdominal surgery suppresses extubation-induced hypertension and tachycardia but does not inhibit the general sympathetic response during the first postoperative day. However, lidocaine infusion reduced urinary output of catecholamines during the second postoperative day, suggesting a more rapid decline in the sympathoadrenal response postoperatively in the experimental group (4). Other authors have shown that systemically administered lidocaine reduced hyperalgesia in experimental models such as skin incision (24) or induced pin-prick pain (25).
In veterinary medicine there is limited data about the effect of systemic lidocaine on somatic nociception and therefore it is difficult to establish whether systemic lidocaine provides a true analgesia or only a sedative effect. An electroencephalographic study in ponies undergoing surgery presented evidence for an analgesic effect of lidocaine (26). In the study presented here, HR and BP changes were evaluated as direct physiological signs of intraoperative sympathetic response to nociceptive stimulation; therefore, it appears that systemic lidocaine provided true analgesia and not only a sedative effect.
The lidocaine dose in this study, an initial IV bolus of 2 mg/kg BW followed by a CRI at 50 μg/kg BW per min is known to reduce end-tidal concentration of isoflurane (9). Since lidocaine effects can be observed after only 2 min of an initial IV loading dose and they are maintained for another 10 to 20 min (27), a 5-minute interval was allowed to assess further requirements for analgesia supplementation. If HR and BP showed a 20% increase from the previous measurement, the lidocaine infusion was increased. Our aim was not to determine isoflurane end-tidal concentration reduction but to maintain it at up to 1.15 vol% and see whether lidocaine infusion would play an adjunct role in our clinical model of canine inhalant balanced anesthesia and reduce the use of opioid during surgery.
Fentanyl was used for intraoperative analgesia supplementation in only 20% of the patients in group A versus 52.4% of the patients in group B. Furthermore, the infusion of lidocaine had a better intraoperative nociceptive response to surgery than placebo infusion and the use of fentanyl was reduced. The patients of group B had almost twice as high a risk of intraoperative use of fentanyl than patients of group A. In the cases where fentanyl was used, the first administration in both groups was between 40 and 90 min after infusion was started.
All the patients were hemodynamically stable before the induction of anesthesia and IV crystalloid infusion was run during maintenance. Isoflurane was kept at 1.15 vol%; it was reasonable to expect a minimum cardiovascular instability only altered by nociceptive stimulation that was controlled by the described analgesic management. The cardiovascular system in patients in this study behaved similarly to other nonclinical or experimental studies in which lidocaine CRI between 40 μg/kg BW per min and 200 μg/kg BW per min were administered to dogs, and minimum effects on cardiac index, heart rate, blood pressure, and vascular resistance were described (28,9).
In this study we found that administration of lidocaine infusion at doses between 50 and 200 μg/kg BW per min helped to prevent the sympathetic response to surgical stimulation in clinically healthy dogs thereby reducing the intraoperative use of opioid (fentanyl) and without causing clinically significant hemodynamic instability. We therefore recommend the use of lidocaine infusion as part of a balanced anesthetic technique in the dog to achieve adequate intraoperative analgesia during surgery.
Acknowledgment
The authors are grateful to Dr. Alexander Valverde for assistance with the paper. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Pypendop BH, Ilkiw JE. The effects of intravenous lidocaine administration on the minimun alveolar concentration of isoflurane in cats. Anesth Analg. 2005;100:97–101. doi: 10.1213/01.ANE.0000139350.88158.38. [DOI] [PubMed] [Google Scholar]
- 2.Feary DJ, Mama KR, Wagner AE, et al. Influence of general anesthesia on pharmacokinetics of intravenous lidocaine infusion in horses. Am J Vet Res. 2005;66:574–579. doi: 10.2460/ajvr.2005.66.574. [DOI] [PubMed] [Google Scholar]
- 3.Fozzard HA, Lee PJ, Lipkind GM. Mechanism of local anesthetic drug action on voltage-gated sodium channels. Curr Pharm Des. 2005;11:2671–2686. doi: 10.2174/1381612054546833. [DOI] [PubMed] [Google Scholar]
- 4.Wallin G, Cassuto J, Hogstrom S, et al. Effects of lidocaine infusion on the sympathetic response to abdominal surgery. Anesth Analg. 1987;66:1008–1113. [PubMed] [Google Scholar]
- 5.De Clive-Lowe SG, Desmond J, North J. Intravenous lidocaine anesthesia. Anaesthesia. 1958;13:138–146. doi: 10.1111/j.1365-2044.1958.tb08045.x. [DOI] [PubMed] [Google Scholar]
- 6.Bartlet EE, Hutaserani O. Xylocaine for the relief of postoperative pain. Anesth Analg. 1961;40:296–304. [PubMed] [Google Scholar]
- 7.Doherty TJ, Frazier DL. Effect of intravenous lidocaine on halothane minimum alveolar (CAM) concentration in ponies. Equine Vet J. 1998;30:340–343. doi: 10.1111/j.2042-3306.1998.tb04101.x. [DOI] [PubMed] [Google Scholar]
- 8.Robertson SA, Sanchez LC, Merritt AM, Doherty TJ. Effects of systematic lidocaine on visceral and somatic nociception in conscious horses. Equine Vet J. 2005;27:122–127. doi: 10.2746/0425164054223723. [DOI] [PubMed] [Google Scholar]
- 9.Valverde A, Doherty TJ, Hernández J, et al. Effect of lidocaine on the minimun alveolar concentration of isofluorane in dogs. Vet Anaesth Analg. 2004;31:264–271. doi: 10.1111/j.1467-2995.2004.00165.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith LJ, Bentley E, Shih A, et al. Systemic lidocaine infusion as an analgesic for intraocular surgery in dogs: A pilot study. Vet Anaesth Analg. 2004;31:53–63. doi: 10.1111/j.1467-2995.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 11.Muir WW, Wiese AJ, March PA. Effects of morphine, lidocaine, ketamine, and morphine-lidocaine-ketamine drug combination on minimun alveolar concentration in dogs anesthetized with isoflurane. Am J Vet Res. 2003;64:1155–1160. doi: 10.2460/ajvr.2003.64.1155. [DOI] [PubMed] [Google Scholar]
- 12.Woolf CJ, Wiesenfeld-Hallin Z. The systemic administration of local anaesthetics produces a selective depression of C-afferent fibre evoked activity in the spinal cord. Pain. 1985;23:361–374. doi: 10.1016/0304-3959(85)90006-5. [DOI] [PubMed] [Google Scholar]
- 13.Butterworth J, Cole L, Marlow G. Inhibition of brain cell excitability by lidocaine, QX314, and teradotoxin: A mechanism for analgesia from infused local anesthetics? Acta Anaesthesiol Scand. 1993;37:516–523. doi: 10.1111/j.1399-6576.1993.tb03758.x. [DOI] [PubMed] [Google Scholar]
- 14.Timothy J, Ness MD. Intravenous lidocaine inhibits visceral nociceptive reflexes and spinal neurons in the rat. Anesthesiology. 2000;92:1685–1691. doi: 10.1097/00000542-200006000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Bach FW, Jensen TS, Kastrup J, et al. The effect of intravenous lidocaine on nociceptive processing in diabetic neuropathy. Pain. 1991;46:232–233. doi: 10.1016/0304-3959(90)91047-M. [DOI] [PubMed] [Google Scholar]
- 16.Smith LJ, Shih A, Miletic V, Miletic G. Continual systemic infusion of lidocaine provides analgesia in an animal model of neuropathic pain. Pain. 2002;97:267–273. doi: 10.1016/S0304-3959(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 17.Tremont-Lukats IW, Challapalli V, McNicol ED, et al. Systemic administration of local anesthetics to relieve neuropathic pain: A systematic review and meta-analysis. Anaesth Analg. 2005;101:1738–1749. doi: 10.1213/01.ANE.0000186348.86792.38. [DOI] [PubMed] [Google Scholar]
- 18.Tremont-Lukats IW, Hutson PR, Backonja MM. A randomised, double-masked, placebo-controlled pilot trial of extended IV lidocaine infusion for relief of ongoing neuropathic pain. Clin J Pain. 2006;22:266–271. doi: 10.1097/01.ajp.0000169673.57062.40. [DOI] [PubMed] [Google Scholar]
- 19.Valverde A, Timothy EM, Hernández J, Davies W. Validation of several types of noxious stimuli for use in determining the minimum alveolar concentration for inhalation anesthetics in dogs and rabbits. Am J Vet Res. 2003;64:957–962. doi: 10.2460/ajvr.2003.64.957. [DOI] [PubMed] [Google Scholar]
- 20.Himes RS, DiFazio CA, Burney RG. Effects of lidocaine on the anesthetic requirements for nitrous oxide and halothane. Anesthesiology. 1977;47:437–440. doi: 10.1097/00000542-197711000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Thurman JC, Tanquilli WJ, Benson GJ. Preanesthetics and anesthetic adjuncts. In: Thurmon JC, Tranquilli WJ, Benson GJ, editors. Lumb and Jones Veterinary Anesthesia. 3rd ed. Philadelphia, Pennsylvania: Williams & Wilkins; 1996. pp. 183–203. [Google Scholar]
- 22.Peer A, Mellor DJ, Wintour EM, et al. Blood pressure, heart rate, hormonal and other acute responses to rubber-ring castration and tail docking of lambs. N Z Vet J. 2002;50:56–62. doi: 10.1080/00480169.2002.36251. [DOI] [PubMed] [Google Scholar]
- 23.Groudine SB, Fisher HA, Kaufman RP, et al. Intravenous Lidocaine speeds the return of bowel function decrease postoperative pain and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesth Analg. 1998;86:235–239. doi: 10.1097/00000539-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Kawamata M, Watanabe H, Nishikawa K, et al. Different mechanisms of development and maintenance of experimental incision-induced hyperalgesia in human skin. Anesthesiology. 2002;97:550–559. doi: 10.1097/00000542-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Koppert W, Weigand M, Neumann F, et al. Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg. 2004;98:1050–1055. doi: 10.1213/01.ANE.0000104582.71710.EE. [DOI] [PubMed] [Google Scholar]
- 26.Murrell JC, White KL, Johnson CB, et al. Spontaneous EEG changes in the equine surgical patient: The effect of an intravenous infusion of lidocaine. Proceedings of the 7th World Congress of Veterinary Anaesthesia; Berne, Switzerland. 2000. pp. 114–115. [Google Scholar]
- 27.Wilcke JR, Davis LE, Neff-Davis CA, et al. Pharmacocinetics of lidocaine and its active metabolites in dogs. J Vet Pharmacol Ther. 1983;6:49–57. doi: 10.1111/j.1365-2885.1983.tb00454.x. [DOI] [PubMed] [Google Scholar]
- 28.Nunes de Moraes A, Dyson DH, O’Grady MR, et al. Plasma concentration and cardiovascular influence of lidocaine infusión during isoflurane anestesia in healthy dogs and dogs with subaortic stenosis. Vet Surg. 1998;27:486–497. doi: 10.1111/j.1532-950x.1998.tb00161.x. [DOI] [PubMed] [Google Scholar]