Abstract
This study assessed the effects of a single intracoronary injection of autologous stem cells on the cardiac function of dogs with Chagas cardiomyopathy. Bone-marrow-derived stem cells were delivered into the right and left coronary arteries of 5 mature dogs with mildly compromised cardiac function due to chronic Chagas cardiomyopathy. Blood pressure and electrocardiographic and echocardiographic parameters were recorded at monthly intervals for 6 mo in the 3 dogs that survived. Although no changes were observed in the electrocardiogram and blood pressure, there was a significant increase in peak velocity of aortic flow 3 mo after stem cell transplantation. Pre-ejection period, isovolumic relaxation time, and the Tei index of myocardial performance were reduced significantly 4 mo after the procedure. All significant changes persisted to the end of the study. The results suggest that the transplantation of autologous bone-marrow-derived stem cells into the coronary arteries of dogs with Chagas cardiomyopathy may have a beneficial effect but the small number of dogs studied was a limitation.
Résumé
Fonction cardiaque chez les chiens atteints de cardiomyopathie chronique associée à la maladie de Chagas subissant une transplantation de cellules souches autologues dans les artères coronariennes. Cette étude a évalué les effets d’une seule injection intra-coronarienne de cellules souches autologues sur la fonction cardiaque des chiens atteints de cardiomyopathie associée à la maladie de Chagas. Des cellules souches dérivées de la moelle osseuse ont été injectées dans les artères coronariennes droite et gauche de 5 chiens adultes atteints d’une fonction cardiaque légèrement compromise en raison d’une cardiomyopathie chronique associée à la maladie de Chagas. La tension artérielle, les paramètres électrocardiographiques et écho-cardiographiques ont été enregistrés à des intervalles mensuels pendant 6 mois pour les 3 chiens qui ont survécu. Même si aucun changement n’a été observé dans l’électrocardiogramme et la tension artérielle, il y a eu une hausse significative de la vélocité maximale du débit aortique 3 mois après la transplantation des cellules souches. La phase de pré-éjection, le temps de relâchement isovolumétrique et l’indice Tei du rendement myocardique ont été réduits significativement 4 mois après l’intervention. Tous les changements significatifs ont persisté jusqu’à la fin de l’étude. Les résultats suggèrent que la transplantation de cellules souches autologues dérivées de la moelle osseuse dans les artères coronariennes des chiens atteints de cardiomyopathie associée à la maladie de Chagas peut avoir un effet bénéfique mais le petit nombre de chiens a constitué une limitation.
(Traduit par Isabelle Vallières)
Introduction
Stem cells are cells capable of differentiating into several tissues. Because of their origin, embryonic stem cells are likely to have greater plasticity and increased therapeutic potential; however, ethical considerations restrict their use. Adult stem cells, on the other hand, are not limited by ethical concerns and are found in several tissues, but have lower plasticity than embryonic stem cells (1).
The therapeutic potential of stem cell therapy for repairing damaged myocardium has been investigated for over 10 y, especially in ischemic heart disease. Investigators have suggested a beneficial effect on myocardial function and coronary perfusion (2). However, it is not clear whether these effects are the result of enhanced collateral circulation, a direct effect on myocardial preservation, or differentiation of stem cells into functional myocytes (3). Hematopoietic stem cells have been used in neovascularization experiments (4–6). In patients with compromised cardiac function, the delivery of stem cells into the myocardium has caused an increase in contractility and myocardial perfusion (7–9).
Chagas disease, caused by the parasite Trypanosoma cruzi, results in acute, chronic asymptomatic, and chronic symptomatic phases of disease. The symptomatic stage is characterized by disturbances of the heart rhythm, cardiac dilatation, and heart failure in humans, and by arrhythmias, diffuse myocarditis, cardiomegaly, and heart failure in dogs. The disease is characterized by myocardial inflammation and fibrosis, which may be responsible for the impairment of cardiac function (10,11). In humans with Chagas disease, the intracoronary delivery of hematopoietic stem cells resulted in increased exercise capacity, better quality of life, and improvement of cardiac function parameters (12,13). The purpose of this study was to evaluate changes in cardiac function in dogs with chronic Chagas disease over a 6-month period after a single intracoronary transplantation of autologous stem cells.
Materials and methods
Animals
Five female mature mongrel dogs, with a mean weight of 11.6 kg, were used. The dogs were housed in individual cages, given free access to water, and provided with commercial dog food twice a day. The study was conducted in accordance with guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and was approved by the Commission on the Ethics and Welfare in Animal Experimentation of the College of Agricultural and Veterinarian Sciences, São Paulo State University.
These animals had been positive for Chagas disease for approximately 127 mo at the time of study initiation. The disease was induced by the inoculation of 1000 trypomastigotes of T. cruzi (Colombian strain) per kg body weight intraperitonially. After inoculation, the dogs were maintained in the laboratory for studies on the pathophysiology of chronic Chagas disease; clinical and laboratory parameters were measured monthly. Although several alterations were detected in the echocardiographic and electrocardiographic examinations, there were no clinical signs, and the dogs were known to be in the asymptomatic phase of the disease. At the beginning of this study serologic titer against T. cruzi was at least 1:80 in all animals.
Baseline cardiac function
Alterations in the cardiac function of the dogs included E/A reversal and prolonged isovolumic relaxation time. The comparison of baseline echocardiographic values to normal values in the veterinary literature is shown in Table 1. Heart murmurs were not detected in any of the dogs.
Table 1.
Baseline echocardiographic measurements (mean ± standard deviation) compared to the reference range in the literature for similarly sized healthy dogs
| Baseline value | Reference range | Baseline value | Reference range | ||
|---|---|---|---|---|---|
| LVs | 2.03 ± 0.38 | 2.05 ± 0.49a | PVME | 0.52 ± 0.08 | 0.91 ± 0.15b |
| LVd | 2.99 ± 0.15 | 3.18 ± 0.56a | PVMA | 0.65 ± 0.18 | 0.63 ± 0.13b |
| IVSs | 1.01 ± 0.10 | 1.12 ± 0.17a | PVTE | 0.34 ± 0.15 | 0.86 ± 0.20b |
| IVSd | 0.69 ± 0.07 | 0.75 ± 0.17a | PVTA | 0.56 ± 0.16 | 0.58 ± 0.16b |
| FWs | 1.07 ± 0.12 | 1.10 ± 0.20a | PVME/PVMA | 0.82 ± 0.10 | 1.48 ± 0.31b |
| FWd | 0.85 ± 0.15 | 0.72 ± 0.14a | PVTE/PVTA | 0.60 ± 0.14 | 1.60 ± 0.56b |
| %EF | 61.00 ± 3.08 | 67.00 ± 8.00a | PEP | 69.67 ± 7.02 | 50.31 ± 15.90c |
| %FS | 32.33 ± 9.29 | 36.00 ± 6.00a | LVET | 217.7 ± 12.3 | 216.0 ± 21.6c |
| WSIs | 2.10 ± 0.70 | — | PEP/LVET | 0.32 ± 0.04 | 0.23 ± 0.08c |
| WSId | 4.40 ± 0.20 | — | IVRT | 77.00 ± 13.00 | 43.81 ± 13.01c |
| PVPUL | 0.73 ± 0.15 | 1.20 ± 0.20b | SI | 49.04 ± 8.07 | 67.18 ± 18.74c |
| PVAO | 0.80 ± 0.21 | 1.57 ± 0.33b | CI | 5.52 ± 0.66 | 7.39 ± 3.31c |
| TEI | 0.68 ± 0.02 | 0.43 ± 0.10c |
Acquisition of stem cells
Bone marrow was the source of stem cells. Forty milliliters of marrow was carefully aspirated through the iliac crest of each animal under inhalation anesthesia [1.0 minimum alveolar concentration (MAC) sevoflurane diluted in 100% O2] and local blockade with lidocaine. Separation of mononuclear cells was performed through laboratory procedures described elsewhere (14). The marrow was washed in saline, centrifuged at 400 × g for 10 min, and the supernatant was discarded. Saline and ficoll (Ficoll-Paque Plus; Amersham Biosciences, Uppsala, Sweden) were added to the remainder, which was homogenized and centrifuged at 400 × g for 15 min. The supernatant was removed, and the mononuclear cell phase located between the upper (plasma) and lower (ficoll × red cells) phase was then aspirated and transferred to a new tube, in which nutrient medium (RPMI 1640; Sigma, St. Louis, Missouri, USA) was added to 40 mL. Once again the cells were centrifuged, the supernatant discarded, and the sediment suspended in 15 mL of a 5% solution of autologous canine plasma. After quantification, approximately 100 × 106 cells were aspirated and transferred to another tube, in which 5% autologous canine plasma solution was added to increase the volume to 10 mL. Six milliliters were then aspirated with two 3-mL sterile syringes, which were maintained under refrigeration for approximately 90 min (time needed to prepare the animal for cell delivery into the coronaries) when the suspension was used.
Transplantation of stem cells
The stem cell suspension was delivered into the right and left coronary arteries of the animals. Under general anesthesia (1.0 MAC sevoflurane diluted in 100% oxygen), the Seldinger’s technique was used to insert coronary catheters through the right femoral artery. This procedure has been extensively described elsewhere (15). In brief, the artery was dissected to allow the placement of a dilator catheter, in which a Judkins catheter (Wiseguide Judkins; Boston Scientific Scimed, Maple Grove, USA) was inserted to reach the coronary arteries. A two-way hemostatic valve was immediately attached to the outer end of the Judkins catheter. A Judkins right catheter was used to reach the right coronary artery, followed by a Judkins left catheter to reach its left counterpart.
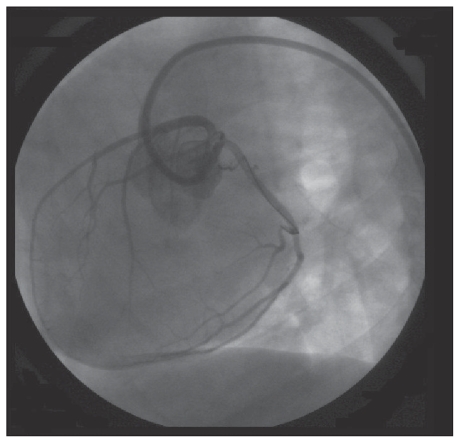
To deliver stem cells directly into the coronary arteries and minimize the risk of the suspension flowing back into the aorta, an angioplasty balloon catheter (Over-the-wire Raptor; Bioxxi, Rio de Janeiro, Brazil) was inserted through the Judkins catheter into the proximal coronary artery. The balloon was then inflated and 3 mL of cell suspension released carefully into the coronary artery over a few seconds (Figure 1). The entire procedure was performed under fluoroscopic guidance (OEC 9800 Plus; GE Medical Systems S.A., Buc Cedex, France) and with intermittent infusions of sodium and meglumine ioxitalamate (Telebrix Coronar; Guerbet, Rio de Janeiro, Brazil).
Figure 1.
Angiography image showing the guide wire and the angioplasty balloon catheter positioned within the circumflex branch of the left coronary artery.
Assessment of cardiac function
Serial electrocardiograms, echocardiograms, and blood pressure measurements were recorded in every animal prior to transplanting stem cells (M0-Baseline) and at monthly intervals up to 6 mo after the procedure (M1 to M6). A single experienced ultrasonographer was responsible for all echocardiographic examinations throughout the study.
A computerized electrocardiograph was used to record ECG traces, which were stored for offline measurements in accordance with the guidelines outlined by Tilley (16). Blood pressure was measured non-invasively with an oscillometry device, allowing the recording of systolic, diastolic, and mean arterial pressures. At least 3 consecutive measurements were made and averaged for each parameter.
A complete transthoracic echocardiographic examination was done for each dog using a Pandion S300 echocardiograph (PieMedical, Maastricht, The Netherlands) and a 5.0 MHz mechanical sector transducer. Echocardiographic images were recorded on videotape with a simultaneous lead II electrocardiogram for offline measurements. These measurements were taken by using a two-dimensional-guided M-mode on the standard right parasternal short-axis view at chordae tendineae level for measurement of left ventricular end-systolic (LVs) and end-diastolic (LVd) dimensions, interventricular septal thickness in systole (IVSs) and diastole (IVSd), left ventricular free wall thickness in systole (FWs) and diastole (FWd). These values were used for calculation of the ejection fraction (%EF) and fractional shortening (%FS). All M-mode measurements were performed in accordance with the recommendations of the American Society of Echocardiography (17).
Doppler studies from the left apical 4 and 5 chamber views (mitral and aorta) or the left cranial parasternal location (pulmonic and tricuspid) were taken. Gain and filter settings were adjusted individually to reduce background noise and result in clear flow profiles. Mitral and tricuspid peak velocities of early (PVME, PVTE) and late (PVMA, PVTA) diastole were acquired at the tips of the mitral and tricuspid valve leaflets, respectively. The measurement of peak velocity of aortic flow (PVAO) was recorded just distal to the aortic leaflets. The pre-ejection period (PEP) was measured from the electrocardiogram Q wave to the onset of the left-ventricular outflow. Left-ventricular ejection time (LVET) was determined as the duration of left ventricular outflow profile. Pulmonic measurement included the peak flow velocity (PVPUL). Isovolumic relaxation time (IVRT) was measured as the interval between aortic valve closure and the onset of mitral inflow. Doppler parameters were recorded using pulsed-wave Doppler, except for IVRT, which was recorded using continuous wave Doppler. All echocardiographic measurements used for analysis represent a mean of at least 3 sinus beats and not sinus beats following premature beats or prolonged pauses in the rhythm.
Several echocardiographic indices were calculated [end-systolic wall stress index (LVs/IVSs), end-diastolic wall stress index (LVd/IVSd), stroke index (aortic flow velocity integral × aortic area/body surface area), and cardiac index (stroke index × heart rate)/1000), as well as the PVME-to-PVMA-ratio, PVTE-to-PVTA-ratio, and PEP-to-LVET-ratio]. Also, the Tei index of myocardial performance (TEI) was calculated as shown elsewhere [(isovolumic contraction time + isovolumic relaxation time)/LVET] (18).
Statistical analyses
A repeated measures ANOVA was used to investigate differences over time. When the differences were determined by the analysis of variance to be significant, they were further analyzed by the post hoc Dunnett’s test to check for differences from the baseline values. For all tests, P < 0.05 was considered significant.
Results
The comparison of baseline echo parameters of Chagas-infected dogs with normal values in the literature for similarly sized dogs (Table 1) showed E/A reversal, and prolonged isovolumic relaxation time.
No complications occurred during the aspiration of bone marrow and its fractionation. Mean cell recovery was 24.1%, and mean cellular viability was 95.8%. However, moderate difficulty was experienced when trying to catheterize the right coronary artery in every dog, which was attributable to the reduced lumen of that artery. One of the dogs died during the procedure because of malignant irreversible arrhythmia. The autopsy of this animal disclosed air embolism and areas compatible with myocardial infarction. Another dog died 14 d after the procedure with no prior signs of complications, and its autopsy showed evidence of neither myocardial infarction nor coronary lesions. Although serial electrocardiograms had been recorded in that animal with no findings suggestive of arrhythmia, it is likely that paroxysmal arrhythmias were the cause of its death.
Although a significant difference was detected between the baseline duration of P wave and its 2-month post- transplantation value, it did not attain clinical significance because the values fell within the reference range for this parameter. Also, no significant changes were seen in the remainder of the electrocardiographic parameters, as well as in the blood pressure data (data not shown). The echocardiographic measurements are listed in Tables 2 and 3, and represent the mean values for the 3 remaining dogs. A significant increase was observed in PVAO from M3, whereas PEP, IVRT and TEI were reduced significantly from M4. Also, there were trends of reduction in LVs, WSIs, WSId, and PEP/LVET, and trends of increase in stroke index and cardiac index.
Table 2.
M-mode-derived parameters (mean ± standard deviation) in dogs with chronic Chagas disease (n = 3) undergoing an intracoronary autologous stem cell transplantation
| Post-transplantation
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline M0 | M1 1 month | M2 2 months | M3 3 months | M4 4 months | M5 5 months | M6 6 months | P ANOVA | |
| LVs | 2.03 ± 0.38 | 1.80 ± 0.34 | 1.80 ± 0.15 | 1.88 ± 0.25 | 1.88 ± 0.28 | 1.76 ± 0.25 | 1.76 ± 0.27 | 0.9009 |
| LVd | 2.99 ± 0.15 | 2.67 ± 0.24 | 2.99 ± 0.12 | 3.02 ± 0.22 | 2.68 ± 0.25 | 3.02 ± 0.18 | 2.95 ± 0.20 | 0.1561 |
| IVSs | 1.01 ± 0.10 | 1.04 ± 0.15 | 1.05 ± 0.08 | 1.04 ± 0.05 | 0.93 ± 0.09 | 0.95 ± 0.06 | 0.94 ± 0.06 | 0.4501 |
| IVSd | 0.69 ± 0.07 | 0.83 ± 0.05 | 0.75 ± 0.08 | 0.79 ± 0.14 | 0.83 ± 0.17 | 0.66 ± 0.04 | 0.77 ± 0.17 | 0.4781 |
| FWs | 1.07 ± 0.12 | 1.29 ± 0.32 | 1.16 ± 0.30 | 1.03 ± 0.10 | 1.15 ± 0.16 | 1.07 ± 0.26 | 1.12 ± 0.16 | 0.8261 |
| FWd | 0.85 ± 0.15 | 0.88 ± 0.19 | 0.73 ± 0.10 | 0.69 ± 0.13 | 0.69 ± 0.18 | 0.64 ± 0.15 | 0.64 ± 0.09 | 0.3089 |
| %EF | 61.00 ± 3.08 | 64.00 ± 5.72 | 65.33 ± 5.03 | 69.33 ± 6.03 | 69.33 ± 8.39 | 70.67 ± 2.08 | 68.00 ± 7.21 | 0.4066 |
| %FS | 32.33 ± 9.29 | 34.33 ± 2.74 | 35.33 ± 4.04 | 38.00 ± 4.58 | 39.00 ± 2.77 | 39.00 ± 1.00 | 36.67 ± 5.13 | 0.5833 |
| WSIs | 2.10 ± 0.70 | 1.70 ± 0.30 | 1.90 ± 0.20 | 1.80 ± 0.10 | 1.70 ± 0.10 | 1.60 ± 0.30 | 1.10 ± 0.30 | 0.0761 |
| WSId | 4.40 ± 0.20 | 3.90 ± 0.90 | 3.90 ± 1.50 | 2.70 ± 0.40 | 2.70 ± 0.70 | 2.90 ± 0.90 | 2.90 ± 1.10 | 0.1723 |
LVs — left-ventricular end-systolic dimension (cm); LVd — left-ventricular end-diastolic dimension (cm); IVSs — interventricular septal thickeness in systole (cm); IVSd — interventricular septal thickness in diastole (cm); FWs — left-ventricular free wall thickeness in systole (cm); FWd — left-ventricular free wall thickeness in diastole (cm); %EF — ejection fraction (%); %FS — fractional shortening (%); WSIs — end-systolic wall stress index; WSId — end-diastolic wall stress index.
Table 3.
Doppler-derived parameters (mean ± standard deviation) in dogs with chronic Chagas disease (n = 3) undergoing an intracoronary autologous stem cell transplantation
| Post-transplantation
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline M0 | M1 1 month | M2 2 months | M3 3 months | M4 4 months | M5 5 months | M6 6 months | P ANOVA | |
| PVPUL | 0.73 ± 0.15 | 0.77 ± 0.23 | 0.72 ± 0.15 | 0.77 ± 0.20 | 0.72 ± 0.07 | 0.76 ± 0.11 | 0.76 ± 0.18 | 0.9989 |
| PVAO | 0.80 ± 0.21 | 1.11 ± 0.02 | 1.11 ± 0.03 | 1.24 ± 0.18a | 1.15 ± 0.05a | 1.14 ± 0.08a | 1.16 ± 0.09a | 0.0111 |
| PVME | 0.52 ± 0.08 | 0.49 ± 0.10 | 0.45 ± 0.04 | 0.58 ± 0.17 | 0.54 ± 0.06 | 0.60 ± 0.12 | 0.57 ± 0.21 | 0.7660 |
| PVMA | 0.65 ± 0.18 | 0.72 ± 0.10 | 0.66 ± 0.07 | 0.72 ± 0.15 | 0.74 ± 0.15 | 0.78 ± 0.11 | 0.78 ± 0.24 | 0.8997 |
| PVTE | 0.34 ± 0.15 | 0.41 ± 0.09 | 0.42 ± 0.11 | 0.54 ± 0.20 | 0.65 ± 0.19 | 0.48 ± 0.05 | 0.47 ± 0.04 | 0.1892 |
| PVTA | 0.56 ± 0.16 | 0.70 ± 0.12 | 0.59 ± 0.13 | 0.59 ± 0.15 | 0.68 ± 0.23 | 0.74 ± 0.15 | 0.74 ± 0.15 | 0.6673 |
| PVME/PVMA | 0.82 ± 0.10 | 0.68 ± 0.06 | 0.67 ± 0.05 | 0.80 ± 0.07 | 0.75 ± 0.11 | 0.79 ± 0.24 | 0.75 ± 0.27 | 0.8442 |
| PVTE/PVTA | 0.60 ± 0.14 | 0.59 ± 0.02 | 0.72 ± 0.11 | 0.74 ± 0.07 | 0.79 ± 0.15 | 0.67 ± 0.14 | 0.65 ± 0.12 | 0.3495 |
| PEP | 69.67 ± 7.02 | 64.33 ± 7.51 | 63.33 ± 5.51 | 52.00 ± 6.73a | 50.67 ± 6.11a | 49.00 ± 5.57a | 51.00 ± 5.55a | 0.0051 |
| LVET | 217.7 ± 12.3 | 199.0 ± 35.8 | 219.0 ± 17.1 | 202.3 ± 15.8 | 217.7 ± 14.6 | 212.3 ± 15.7 | 215.0 ± 15.0 | 0.7939 |
| PEP/LVET | 0.32 ± 0.04 | 0.37 ± 0.12 | 0.34 ± 0.14 | 0.26 ± 0.05 | 0.21 ± 0.03 | 0.21 ± 0.04 | 0.21 ± 0.03 | 0.0855 |
| IVRT | 77.00 ± 13.00 | 63.00 ± 8.89 | 53.00 ± 12.07 | 58.33 ± 9.71 | 48.00 ± 6.56a | 48.33 ± 9.29a | 39.33 ± 6.04a | 0.0072 |
| SI | 49.04 ± 8.07 | 68.64 ± 10.80 | 77.65 ± 10.03 | 74.26 ± 19.81 | 73.38 ± 11.95 | 76.98 ± 8.22 | 78.90 ± 8.44 | 0.0864 |
| CI | 5.52 ± 0.66 | 7.66 ± 2.69 | 8.68 ± 2.83 | 8.05 ± 0.23 | 9.10 ± 2.00 | 8.00 ± 1.38 | 9.11 ± 1.26 | 0.2882 |
| TEI | 0.68 ± 0.02 | 0.64 ± 0.04 | 0.64 ± 0.05 | 0.51 ± 0.09 | 0.47 ± 0.03a | 0.46 ± 0.06a | 0.42 ± 0.10a | 0.0005 |
PVPUL — peak velocity of pulmonic flow (m/s); PVAO — peak velocity of aortic flow (m/s); PVME — peak velocity of mitral E wave (m/s); PVMA — peak velocity of mitral A wave (m/s); PVTE — peak velocity of tricuspid E wave (m/s); PVTA — peak velocity of tricuspid A wave (m/s); PVME/PVMA — PVME-to-PVMA-ratio; PVTE/PVTA — PVTE-to-PVTA-ratio; PEP — pre-ejection period (milliseconds); LVET — left-ventricular ejection time (milliseconds); PEP/LVET — PEP-to-LVET-ratio; IVRT — isovolumic relaxation time (milliseconds); SI — stroke index (mL/beat × m2); CI — cardiac index (L/min × m2); TEI — Tei index of myocardial performance.
Statistically different (P < 0.05) from M0 (baseline value).
Discussion
As in a previous study of Chagas disease in humans (13), there were no difficulties in collecting and fractionating the marrow. Also, our cell recovery rate was satisfactory and similar to that obtained in another study (12).
The procedure requires adequate training and specialized equipment. Also, one should consider that some of the delivered cells might not be retained in the damaged myocardium, but may spread randomly throughout the body after being transported to the systemic circulation (19,20). Although the transplanted cells were not marked, it is likely that a reasonable amount of cells were retained in the myocardium. Several studies have shown that intracoronary delivery of cells permits the retention of adequate amounts of cells in the myocardium. An adequate distribution of bone marrow cells was demonstrated in the myocardium of people with chronic refractory angina 2 wk after delivery into the coronary arteries (21). By using genetically marked stem cells delivered directly into the myocardium of rats with experimentally induced infarction, another study (22) demonstrated that most of the cells were retained in the scar and peri-scar area of the heart, and expressed the heavy chains of cardiomyocyte-specific myosin and troponin I-C.
Regarding the sudden death of 1 dog 14 d after the procedure, a similar case was documented 2 mo after transplanting stem cells to humans with Chagas disease (13). In that case, however, no causal or temporal relations were found to exist between the procedure and the death. Because late potentials have been demonstrated in dogs with Chagas disease (23), the death of that animal might have been attributable to the development of paroxysmal ventricular arrhythmias. One should be aware, however, that the supposed lack of electromechanical integration of the transplanted stem cells into the recipient myocardium might be responsible for the development of ventricular arrhythmias (24). Although the stem cell procedure itself may have been a potential cause of the death of that animal, no evidence of myocardial infarction or coronary injury was shown at necropsy to support this possibility, as opposed to the dog that died during the procedure. Nevertheless, that case was the very first animal to be included in this study; its death caused by malignant arrhythmias was later ascribed to errors in the technique of coronary catheterization, which resulted in air embolism and myocardial infarction. Changing the technique was enough to allow adequate catheterization of the coronaries in the subsequent animals.
The absence of clinically relevant alterations in the electrocardiographic and blood pressure measurements does not mean that there were no structural or functional changes in the electrical conduction system of the myocardium. Also, because ECG recordings were not continuous, the development of cardiac arrhythmias over time may be a possibility.
Although trends of amelioration have been identified in several echocardiographic parameters, significant changes were not demonstrated, and therefore the data may not support an improvement in cardiac function. Other investigators, however, have documented the augmentation of %FS and LVd in rats given autologous stem cells 2 wk after induction of myocardial infarction (22). Also, an increase in ejection fraction was demonstrated in rats with Chagas cardiomyopathy 4 wk after autologous mesenchymal stem cells were transplanted in conjunction with skeletal myoblasts (25).
Regarding diastolic function, a reverse mitral E:A ratio was documented in every animal at baseline examination. Impaired myocardial relaxation was in agreement with the prolonged isovolumic relaxation time seen in those dogs as well. It is well-documented that the damage to the muscular fibers and the interstitium caused by Chagas myocarditis may result in foci of fibrosis throughout the myocardium (11). Five months after the transplantation, mitral E:A ratio tended to normalize in 2 of the dogs. This finding, in conjunction with a significant decrease in IVRT, might be attributable to the restoration of left-ventricular diastolic compliance (26), although one should be aware that progression of diastolic disease and pseudo-normalization could also be implicated in this finding. In a canine model of chronic ischemia, a lower degree of cardiac fibrosis was demonstrated after mesenchymal stem cells were injected into the damaged myocardium (27). In another study, the marrow-derived endothelial progenitor cells were shown to be capable of inducing myocardial neovascularization, preventing myocyte apoptosis, as well as its progressive substitution by collagen, therefore, avoiding the process of myocardial remodeling (28).
Systolic and diastolic performances were assessed through the Tei index. This index is calculated from both isovolumic contraction and relaxation times, as well as the left-ventricular ejection time, to evaluate overall cardiac function. Because this index does not depend on ventricular geometry, heart rate, arterial pressure, preload and afterload, its decrease over time in this study is in agreement with other echocardiographic parameters that might reflect some improvement in cardiac function (18). The myocardial neovascularization induced by the transplantation of stem cells may eventually have induced the proliferation of cardiac myocytes, with beneficial effects on contractility, and restored the impaired ventricular compliance owing to myocardial fibrosis (25,27,28).
Several mechanisms, including angiogenesis leading to myocardial neovascularization, have been proposed to explain the beneficial effects of stem cell therapy. Because this was a clinical study, however, histological assessment of myocardial vessels was not possible. It is believed that endothelial progenitor cells increase tissue perfusion by differentiating into endothelial cells at the site of neovascularization (29), and by delivering angiogenic and arteriogenic cytokines to the myocardium (30). A study in pigs with chronic myocardial ischemia documented enhancement in the collateral coronary flow, capillary density, and regional myocardial contractility after non-fractionated bone-marrow-derived cells were injected through the endocardium (31). In a non-ischemic model of heart failure, an enhancement in microcirculation contributed to preserving myocardium and, therefore, to preventing deterioration of cardiac function (32).
The main limitations of this investigation are the small number of animals, and the absence of a non-transplanted control group. Because we did not label the delivered cells, it was not possible to determine the amount of cells that remained in the damaged myocardium of these dogs. The great variation among animals observed in some parameters may be attributable, therefore, to limited cell distribution throughout the myocardium. Also, the absence of a 24-hour Holter monitoring constitutes a marked limitation because of the arrhythmic potential of Chagas disease and coronary catheterization, which needed a more detailed analysis of heart rhythm. Histologic evaluation of the hearts would also provide important information regarding the beneficial or detrimental effects provoked by the procedure.
In conclusion, this study demonstrated that intracoronary transplantation of stem cells is a feasible technique in dogs with Chagas cardiomyopathy, with promising effects on cardiac function of these animals. However, because of the small number of animals, further studies are necessary to investigate cardiac function in a larger population of affected dogs, as well as the benefits of cell therapy in subjects with varying degrees of cardiac compromise. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Strauer B, Kornowski R. Stem cell therapy in perspective. Circulation. 2003;107:929–934. doi: 10.1161/01.cir.0000057525.13182.24. [DOI] [PubMed] [Google Scholar]
- 2.Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs S, Kornowski R, Weisz G, et al. Safety and feasibility of transendocardial autologous bone marrow transplantation in patients with advanced heart disease. Am J Cardiol. 2006;97:823–829. doi: 10.1016/j.amjcard.2005.09.132. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Kalka C, Isner JM. Stem cell therapy and gene transfer for regeneration. Gene Ther. 2000;7:421–457. doi: 10.1038/sj.gt.3301142. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Luttun A. The emerging role of the bone marrow-derived stem cells in (therapeutic) angiogenesis. Thromb Haemost. 2001;86:289–297. [PubMed] [Google Scholar]
- 6.Fuchs S, Baffour R, Zhour YF, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional functions in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726–1732. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 7.Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 8.Shake JG, Gruber PJ, Baumgartner WA, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: Engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. [DOI] [PubMed] [Google Scholar]
- 9.Tomita S, Mickie DA, Weisel RD, et al. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg. 2002;123:1132–1140. doi: 10.1067/mtc.2002.120716. [DOI] [PubMed] [Google Scholar]
- 10.Laranja FS, Andrade ZA. Forma crônica cardíaca da doença de Chagas no cão. Arq Bras Cardiol. 1980;35:377–380. [PubMed] [Google Scholar]
- 11.Oliveira-Alves R. PhD dissertation. Jaboticabal, São Paulo, Brazil: College of Agricultural and Veterinarian Sciences; 2003. Avaliações ecodopplercardiográfica, eletrocardiográfica computadorizada e dinâmica (sistema Holter) e clínico-patológica em cães com cardiomiopatia chagásica experimental. [Google Scholar]
- 12.Vilas-Boas F, Feitosa GS, Soares MBP, et al. Bone marrow cell transplantation to the myocardium of a patient with heart failure due to Chagas’ disease. Arq Brasil Cardiol. 2004;82:185–187. doi: 10.1590/s0066-782x2004000200010. [DOI] [PubMed] [Google Scholar]
- 13.Vilas-Boas F, Feitosa GS, Soares MBP, et al. Early results of bone marrow cell transplantation to the myocardium of patients with heart failure due to Chagas disease. Arq Brasil Cardiol. 2006;87:159–166. doi: 10.1590/s0066-782x2006001500014. [DOI] [PubMed] [Google Scholar]
- 14.Böyun A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation and of granulocytes by combining centrifugation and sedimentation at 1g. Scand J Clin Lab Invest suppl1. 1968;21:77–89. [PubMed] [Google Scholar]
- 15.Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta Radiol. 1953;39:368–376. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- 16.Tilley LP. Essentials of Canine and Feline Electrocardiography. 3rd ed. Philadelphia: Lea & Febiger; 1992. p. 470. [Google Scholar]
- 17.Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: Results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 18.Tei C, Ling LH, Hodge DO, et al. New index of combined systolic and diastolic myocardial performance: A simple and reproducible measure of cardiac function — A study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357–366. [PubMed] [Google Scholar]
- 19.Kornowski R, Fuchs S, Leon MB, Epstein SE. Delivery strategies to achieve therapeutic myocardial angiogenesis. Circulation. 2000;101:454–458. doi: 10.1161/01.cir.101.4.454. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama SI, Noboru F, Li Y, et al. A strategy of retrograde injection of bone marrow mononuclear cells into the myocardium for the treatment of ischemic heart disease. J Mol Cell Cardiol. 2006;40:24–34. doi: 10.1016/j.yjmcc.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Vicario J, Campo C, Piva J, et al. One-year follow-up of transcoronary sinus administration of autologous bone marrow in patients with chronic refractory angina. Cardiovasc Revasc Med. 2005;6:99–107. doi: 10.1016/j.carrev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Saito T, Kuang JQ, Lin CCH, Chiu RC. Transcoronary implantation of bone marrow stromal cells ameliorates cardiac function after myocardial infarction. J Thor Cardiovasc Surg. 2003;126:114–122. doi: 10.1016/s0022-5223(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira WL. PhD dissertation. Jaboticabal, São Paulo, Brazil: College of Agricultural and Veterinary Sciences; 2003. Estudo da eletrocardiografia de alta resolução em cães. [Google Scholar]
- 24.Menasché P. Stem cell therapy for heart failure. Are arrhythmias a real safety concern? Circulation. 2009;119:2735–2740. doi: 10.1161/CIRCULATIONAHA.108.812693. [DOI] [PubMed] [Google Scholar]
- 25.Guarita-Souza LC, Carvalho KA, Woitowicz V, et al. Simultaneous autologous transplantation of cocultured mesenchymal stem cells and skeletal myoblasts improves ventricular function in a murine model of Chagas disease. Circulation. 2006;113 (suppl 1):120–124. doi: 10.1161/CIRCULATIONAHA.105.000646. [DOI] [PubMed] [Google Scholar]
- 26.Berry MF, Engler AJ, Woo YJ, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circul Physiol. 2006;290:2196–2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 27.Silva GV, Litovsky S, Assad JA, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 28.Schuster MD, Kocher AA, Seki T, et al. Myocardial neovascularization by bone marrow angioblasts results in cardiomyocyte regeneration. Am J Physiol Heart Circul Physiol. 2004;287:525–532. doi: 10.1152/ajpheart.00058.2004. [DOI] [PubMed] [Google Scholar]
- 29.Kocher AA, Schuster MD, Szaboles MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblast prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nature Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 30.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells genes encoding a broad spectrum of arteriogenesis through paracrine mechanism. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 31.Kawamoto A, Tkebuchava T, Yamaguchi J, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neo-vascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 32.Ishida M, Tomita S, Nakatani T, et al. Bone marrow mononuclear cell transplantation had beneficial effects on doxorubicin-induced cardiomyopathy. J Heart Lung Transplant. 2004;23:436–445. doi: 10.1016/S1053-2498(03)00220-1. [DOI] [PubMed] [Google Scholar]
- 33.Sousa MG, Carareto R, De-Nardi AB, et al. Effects of isoflurane on echocardiographic parameters in healthy dogs. Vet Anaesth Analg. 2008;35:185–190. doi: 10.1111/j.1467-2995.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- 34.Kirberger RM, Bland-Van Den Berg P, Darazs B. Doppler echocardiography in the normal dog: Part II — factors influencing flow velocities and a comparison between left and right heart blood flow. Vet Radiol and Ultrasound. 1992;33:380–386. [Google Scholar]
- 35.Sousa MG, Carareto R, De-Nardi AB, et al. Effects of isoflurane on Tei-index of myocardial performance in healthy dogs. Can Vet J. 2007;48:277–282. [PMC free article] [PubMed] [Google Scholar]