Abstract
We have examined the effect of stearylamine (SA) in liposomes on the viability of Plasmodium falciparum in culture by studying the inhibition of incorporation of [3H]-hypoxanthine in the nucleic acid of parasites. Stearylamine in liposomes significantly inhibits the growth of the parasites depending on the phospholipids composition. The maximum inhibition was observed when SA was delivered through Soya phosphatidylcholine (SPC) liposomes. The chain length of alkyl group and density of SA in liposomes play a significant role in inhibiting the growth of the parasites. Incorporation of either cholesterol or Distearylphosphatidylethanolamine−Methoxy-Polyethylene glycol-2000 (DSPE-mPEG-2000) in Soya phosphatidylcholine-stearylamine (SPC-SA) liposomes improves the efficacy. Intraerythrocytic entry of intact SPC-SA liposomes into infected erythrocytes was visualized using fluorescent microscopy. No hemolysis was observed in uninfected erythrocytes, and slight hemolysis was noted in infected erythrocytes at high concentrations of SPC-SA liposomes. Overall, our data suggested SA in SPC-liposomes might have potential application in malaria chemotherapy.
1. Introduction
Malaria, a protozoan parasitic infection, is considered to be one of the most prevalent parasitic diseases afflicting the subtropical countries [1]. The quinoline group of antimalarial drugs such as chloroquine (CQ), mefloquine, and primaquine has been until recently, shown to be the most effective drugs for malaria chemotherapy because of their rapid onset of action and most important, their cost effectiveness, which have encouraged their wide use [2]. With the onset of CQ-resistant parasites, attempts have been made by several investigators to deliver chloroquine through liposomes for the treatment of chloroquine-resistant malaria [3–6]. The focus was also shifted either to develop novel chemotherapeutics or new modes of antimalarial drug delivery to overcome drug resistance mechanisms of the parasite [7]. Dramatic changes in the functional and structural characteristics of infected red blood cells (RBCs) are observed with Plasmodium infection and maturation. The alterations in lipid and protein composition-resulting from disturbance in membrane structure and function, ultimately lead to increased membrane fluidity [8]. The formation of “new permeable pathways” (NPPs) allows the entry of low molecular weight as well as nanosized molecules selectively to the parasitized RBCs [9, 10].
It has been previously reported by a number of investigators that liposomes consisting of stearylamine (SA) and phosphatidylcholine (PC) have antiprotozoan activity towards a number of pathogenic protozoan parasites like Trypanosoma cruzi [11], T. brucei gambiense [12], Toxoplasma gondii [13], and Leishmania donovani [14]. The aim of this study was to evaluate the antimalarial effect of SA-intercalated PC liposomes on infected erythrocytes in culture. The effect of various liposomal components/constituents like phospholipids, the density of cholesterol, density and chain length of alkyl group of SA, and DSPE-mPEG-2000 which are known to influence significantly the efficacy of liposomal drugs, was coherently studied. Fluorescent analogue of SA (octadecylrhodamine) was used both as a marker for SA intercalation as well as a tracking molecule to visualize the entry of SPC-SA (Soya phosphatidylcholine-Stearylamine) liposome into infected RBC.
2. Methods
2.1. Chemicals
All chemicals were obtained from Sigma Chemical Co., St. Louis, Mo, USA or GIBCO BRL, Gaithersburg, Md, USA unless otherwise indicated. Soya Phosphatidylcholine (SPC) was obtained as a gift from Lifecare Innovations Pvt. Ltd., Gurgoan, India. DSPE-mPEG-2000 (Distearoyl phosphatidyl ethanolamine-Methoxy-Polyethy-lene glycol-2000) was purchased from Avanti Polar Lipids, Inc., Alabaster, Ala, USA. Hypoxanthine Monohydrochloride, [3H(G)] was bought from American Radiolabeled Chemicals, Inc., St. Louis, Mo, USA. Human O+ serum was obtained from Innovative Research, Inc., Peary Court Novi, Mich, USA.
A fluorescent amine analogue, octadecylrhodamine was purchased from Invitrogen Corp., Grand Island, NY, USA. The stock solution was prepared by dissolving the compound at 1.25 mM in ethanol and was stored at −20°C protected from light.
2.2. In Vitro Culture of Plasmodium falciparum
The strain of Plasmodium falciparum used in the study was 3D7 obtained from National Institute of Malarial Research, New Delhi, India. This strain was maintained (a modified method of Trager and Jensen [15]) by serial passages in human erythrocytes cultured at 4% hematocrit in RPMI-1640 media supplemented with 10% human serum and incubated at 37°C under the atmosphere of mixed gas (containing 5% CO2, 5% O2, and 90% N2) in a plastic chamber. Heparinized whole O+ blood was collected from Rotary Blood Bank, New Delhi, and RBCs were separated under sterile conditions by centrifugation to remove serum and buffy coat. The levels of parasitemia were routinely monitored on blood smear with 5% Giemsa azure type B stain in phosphate buffer (20 mM, pH 7.2). For each experiment, samples of the stock culture were further diluted in culture medium up to 2% hematocrit and parasitemia of 1% in preparation for the addition to microtitration plates.
2.3. Liposomes Preparation
Liposomes were prepared by the hand-shak method as described earlier [16]. Briefly, SPC or EPC (Egg phosphatidylcholine) alone or with either SA or PA (Phosphatidic acid) in a molar ratio of 80 : 20 (40 μmole total lipids) and trace amount of octadecylrhodamine (R18) (41 nmoles/40 nmoles total lipid), as a marker for estimation of SA intercalation, was dissolved in chloroform (2-3 mL) in 100 mL round-bottom flask. The chloroform was evaporated to dryness at 30°C, under reduced pressure, by using rotary evaporator. The thin film, so formed, was desiccated for 1h, followed by hydration with 1ml phosphate buffer saline (PBS 20 mM, pH 7.2) for overnight at 4°C. On the following day, liposomes were sonicated at 25°C for 30 min in a bath-type sonicator (Branson 1510) at 40 kHz and extruded through 100 nm polycarbonate membrane. The liposomal SA was separated from free SA by ultracentrifugation (Beckman coulter ultracentrifuge, 300,000× g, 40 min, 4°C). The pellet was suspended in 1ml PBS (20 mM, pH 7.2) and finally filtered through 0.22 μm Millex GV Millipore membrane. Liposomes containing stearylalcohol, stearic acid and different chain length fatty acids were prepared as described above using molar ratio of SPC : other components 80 : 20. The recovery of liposomes was monitored by measuring phospholipid using Stewart's method [17] and SA was assessed fluorimetrically (excitation 560 nm/emission 590 nm). Recovery of liposomes was found to be 60–65% with 90–95% SA intercalated in all the liposomal formulations. Stearically stabilized liposomes were prepared as described above by adding various mol% (1–5 mol%) of DSPE-mPEG-2000 during the preparation of lipid film. The size of the liposomes was measured using Malvern Zetasizer, Nano ZS model, and it was found in the range of 90–110 nm (Figure 1). For the preparation of fluorescent labeled-liposomes for fluorescence microscopy, octadecylrhodamine (R18) (6 nmole/200 nmoles total lipid) was added in SA-SPC liposome suspension and incubated at room temperature for 15 min. Excess R18 was removed by ultracentrifugation (300,000× g, 40 min, 4°C), and the pellet obtained was resuspended in 20 mM PBS (final concentration 1 mg/mL of phospholipid).
Figure 1.
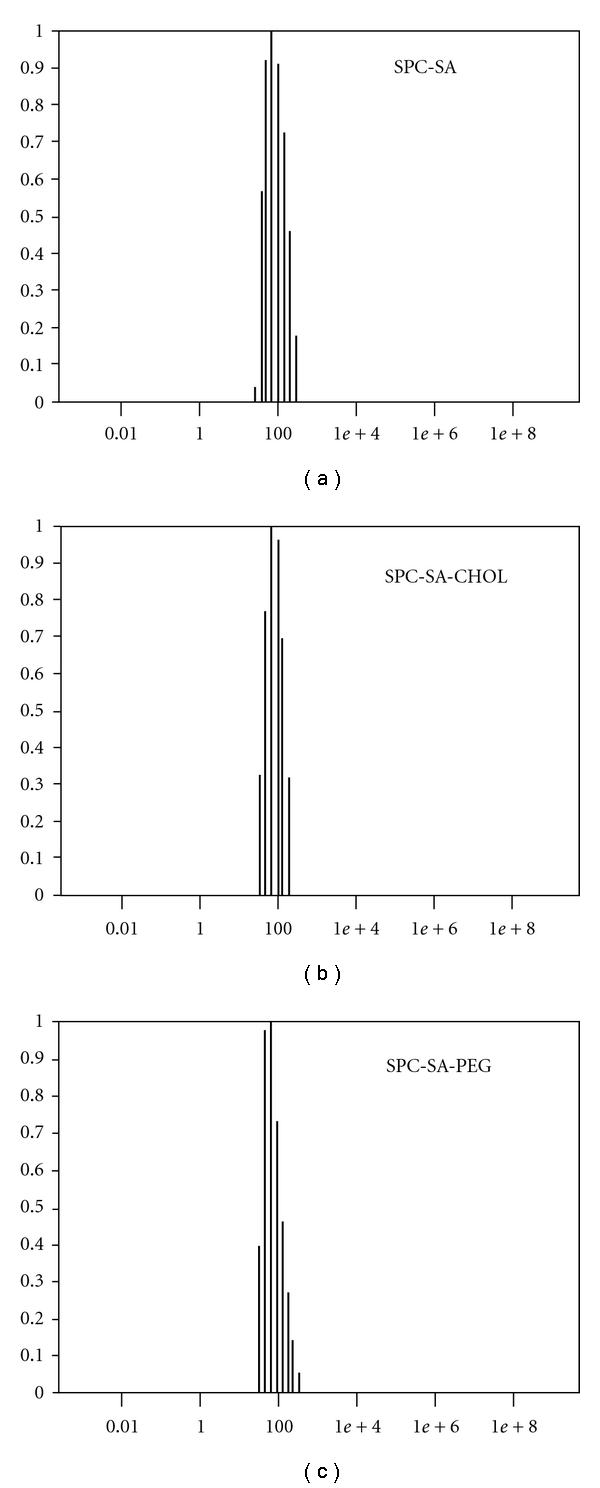
Particle size determination of SPC-SA liposomes using Malvern Zetasizer: Mean diameter of the liposomes was found to be (a) 100 nm for SPC-SA, (b) 120 nm for SPC-SA-CHOL (CHOL: Cholesterol, 20 mol%), and (c) 90 nm for SPC-SA-PEG (PEG, 5 mol%). x-axis denotes diameter of liposomes (in nm) and y-axis Intensity (%).
2.4. Evaluation of Liposomal Antimalarial Activity
Antimalarial effects of the different liposomal formulations was monitored by studying the inhibition of incorporation of [3H]-hypoxanthine in the nucleic acid of parasites [18]. In brief, liposomal preparations were serially diluted and added to Plasmodium falciparum infected erythrocyte suspension (2% final hematocrit and 1% parasitemia) in a 96-well tissue culture plate. After 24 h of incubation at 37°C, 0.2 μCi of [3H]-hypoxanthine was added to each well and cells were harvested 18 h later by using a Skatron Semiautomated cell harvester. [3H]-hypoxanthine incorporation in nucleic acid was measured in a liquid scintillation counter (Model: Tri-Carb 2900 TR, Perkin Elmer), and inhibition of growth was calculated by comparison with control (Control consists of complete medium in a substitute for the test molecule.) All data points were collected in triplicate for each experiment. The IC50 (concentration of SA required to inhibit 50% of growth of parasites as measured by incorporation of [3H]-hypoxanthine) of the data was generated using Origin Cal software (version 3).
2.5. Intracellular Accumulation of SA-Liposomes
The interaction of SA liposomes with infected erythrocytes was visualized by performing a time-dependent study using octadecylrhodamine (R18)-SPC-SA liposomes. The erythrocytes cultured at 2% hematocrit and high parasitemia (8–10%) were exposed to labeled liposomes at its IC50 for 15, 30, 45, and 120 min and kept at 37°C under the mixed gas atmosphere as mentioned previously. At the end of incubation, unbound liposomes were washed off by brief spin and cells were fixed with a mixture of 2% paraformaldehyde and 2.5% glutaraldehyde for 2h in 0.1 M PBS at 4°C. Staining of parasites was done by using DAPI stain (1 μg/mL) for 30 min at 37°C and after subsequent wash cells were visualized on a fluorescent microscope (Nikon Eclipse TE 2000) using rhodamine filter 96312/G2EC (excitation 540–525 nm/emission 620–660 nm) and DAPI filter (excitation 359 nm/emission 461 nm). Uninfected erythrocytes were taken as control.
2.6. Assessment of Hemolytic Activity
Hemolysis was measured in SPC-SA-liposome-treated sets of cultured infected and uninfected erythrocytes by measuring absorbance of Hb at 405 nm (soret peak of hemoglobin (Hb)) as described [19]. Briefly, increasing concentration of liposomal preparations was added in Plasmodium falciparum infected (2% hematocrit and 1% parasitemia) and uninfected erythrocyte (2% hematocrit) in a 96-well plate for 42 h at 37°C. After incubation, the plate was spun down briefly and absorbance of supernatant was measured at 405 nm. Mixing the erythrocytes with 1% of Triton-X 100 attained the complete hemolysis. Cells in PBS were taken as negative control.
3. Results
3.1. Effects of Various Liposomes on the Viability of Plasmodium falciparum In Vitro
The effect of various liposomes on the viability of Plasmodium falciparum strain 3D7 in culture was tested (Table 1). SPC liposome alone or having negatively charged lipid, phosphatidic acid (PA), had no effect on the viability of parasites. However, SPC liposomes having positively charged lipid, stearylamine (SA), significantly inhibited the growth of the parasites (IC50 = 6.87 μM (Figure 3) & 24.08 μM in terms of phospholipid in Table 1). To further dissect the role of alkyl and amino group of SA in plasmocidal activity, SPC liposomes containing either stearyl alcohol or stearic acid having no amine group were prepared and tested. Effect of spermidine—a polyamine having multiple amino groups and no alkyl group—was also examined. Stearyl moiety alone as in stearic alcohol and stearic acid did not show any effect as concluded by the lack of antimalarial activity. Even multiple amino groups as in spermidine had no effect on growth of parasites (Table 1). Different chain length fatty acids and alkyl alcohols ranging from tetradecyl to octadecyl (stearyl) did not show any inhibitory effect (Table 1). These results clearly suggest that both alkyl and amino groups of SA are essential in the inhibition of growth of Plasmodium falciparum in culture as stearylalcohol without amino group and spermidine without alkyl group but with three amino groups have no antimalarial activity.
Table 1.
Effects of Various Liposomes on the Viability of Plasmodium falciparum in vitro.
| Types of liposomes | IC50 in terms of phospholipid concentration (μM) |
|---|---|
| SPC | >208.33 |
| SPC: PA | >208.33 |
| SPC: SA | 24.08 ± 1.15 |
| SPC: Stearic acid (octadecanoic acid) | >208.33 |
| SPC: Stearyl alcohol (octadecyl alcohol) | >208.33 |
| SPC: myristic acid ( tetradecanoic acid) | >208.33 |
| SPC: Palmitic acid (hexadecanoic acid) | >208.33 |
| SPC: myristyl alcohol (tetradecyl alcohol) | >208.33 |
| SPC: Cetyl alcohol (hexadecyl alcohol) | >208.33 |
| Free spermidine | >1102 |
Cells with 1% parasitemia and 2% hematocrit in the presence of various concentrations of above mentioned liposomal formulations (6.5 μM–208 μM lipids) and free spermidine (34 μM–1102 μM) were treated for 42 h at 37°C (in between at 24 h, [3H] hypoxanthine was added). The cell-associated radioactivity was determined and inhibition of growth calculated by comparison with controls (without liposomes/spermidine).
Figure 3.
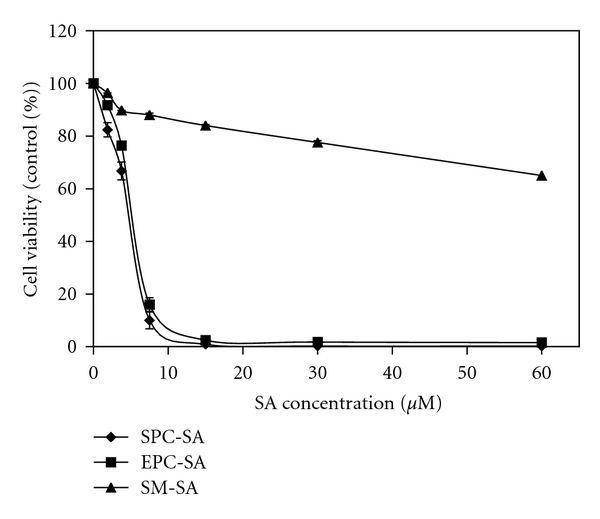
Effect of different composition of liposome on plasmocidal activity of SA: SPC, EPC, and sphingomyelin liposomes containing 20 mol% of SA were added in the cultured cells as described in materials and methods. A graph of cell viability (measured after 42 h) against SA concentration provides the IC50.
3.2. SA Density in Liposome Membrane Dictates Plasmocidal Activity
After incubating the cells with liposomes loaded with various mol% of SA, a linear increase in antimalarial activity was observed till 20 mol% (IC50 = 6.87 μM) after which there was no further enhancement. The liposomes consisting of 5 mol% SA showed very weak antimalarial activity (IC50 = 41.15 μM) (Figure 2). This indicates that the optimum mol% of SA in liposomes to confer maximum killing activity was 20 mol%. All subsequent experiments were carried out using 20 mole% SA in liposomes.
Figure 2.
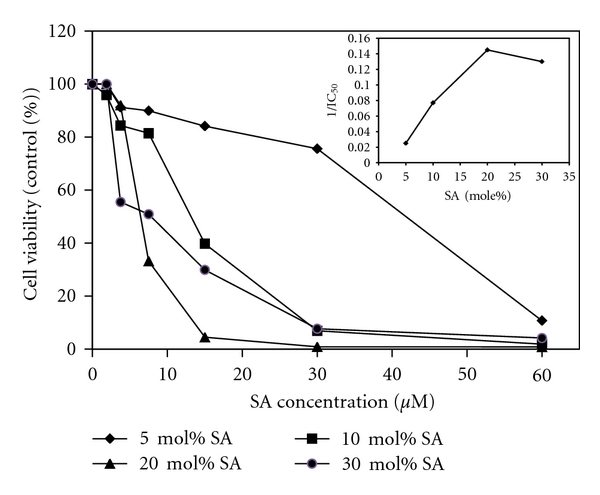
Effect of SA density in SPC liposome on the parasite viability: Infected human RBCs in the presence of liposome suspension (30 μM) containing 5, 10, 20, and 30 mol% SA was incubated at 37°C for 42 h as described in materials and methods. Inset: Relationship between surface density of SA in the liposome membrane and reciprocal of the IC50 required for killing the parasite (1/ IC50).
3.3. Role of Chain Length of Alkyl Amine on the Inhibition of Growth of Plasmodium falciparum in Culture
Plasmodium falciparum infected red blood cells suspension was incubated with liposomes composed of 80% soya PC and 20% alkyl amines of different chain length, and their viability was examined. The results depicted in Table II suggested that the chain length of the alkyl group of the aliphatic amines is directly proportional to antimalarial activity. The liposome containing decylamine has IC50 of 43.45 μM compared with that of octadecylamine (SA) having IC50 of 6.87 μM (Table 2) suggesting that the hydrophobic portion of alkyl amine plays a synergistic role along with the amino group in plasmocidal activity.
Table 2.
Effect of different chain length alkylamine in SPC liposome on parasite growth after 42 h in culture.
| Types of liposomes | IC50 (μM) |
|---|---|
| SPC: Decylamine CH3(CH2)9NH2 | 43.45 ± 4.45 |
| SPC: Dodecylamine CH3(CH2)11NH2 | 24.65 ± 2.69 |
| SPC: Tetradecylamine CH3(CH2)13NH2 | 13.59 ± 1.64 |
| SPC: Hexadecylamine CH3(CH2)15NH2 | 10.44 ± 1.20 |
| SPC: Octadecylamine CH3(CH2)17NH2 | 6.87 ± 0.44 |
Parasites were treated with indicated alkylamine SPC liposome for 42 h with concentration range of (1.88 μM–60 μM alkylamine). IC50 value was assessed by measuring [3H] hypoxanthine incorporation as described in Materials and Methods. The mean value ± standard deviation is indicated for each group, and values are representative of 3 separate experiments.
3.4. Antimalarial Activity of SA Liposome Is Phospholipid Dependent
The phospholipid is the main constituent of liposomes and phospholipid composition of liposomes significantly influence the biological efficacy of encapsulated drugs [20]. In order to ascertain whether incorporation of SA in different phospholipids has any effect on the inhibition of growth of Plasmodium falciparum in human erythrocytes in culture, we have incorporated SA in three different phospholipids like bovine brain sphingomyelin, egg phosphatidylcholine, and soya phosphatidylcholine and studied its effect on the anti-malarial efficacy of SA in culture. SA in SPC and EPC liposomes with IC50 of 6.87 μM and 7.30 μM, respectively, was equally effective as an anti-malarial agent (Figure 3). On the other hand, it had no effect when incorporated in sphingomyelin liposomes; clearly showing that anti-malarial efficacy of SA in liposomes is significantly dependent on the phospholipid composition of liposomes.
3.5. Incorporation of Stabilizers in SPC-SA Liposomes
It is well known that cholesterol increases the stability of liposomes in the physiological environment, and DSPE-mPEG-2000 increases the hydrophilicity on the surface of liposomes, consequently increasing the longevity of liposomes in the blood circulation and improving the therapeutic efficacy of a number of drugs [21, 22]. In our case, incorporating cholesterol and DSPE-mPEG-2000 in SA liposomes marginally increased the efficacy (Figures 4(a) and 4(b)) suggesting that these liposomes can be used as a delivery vehicle for SA for the treatment of malaria in vivo condition.
Figure 4.
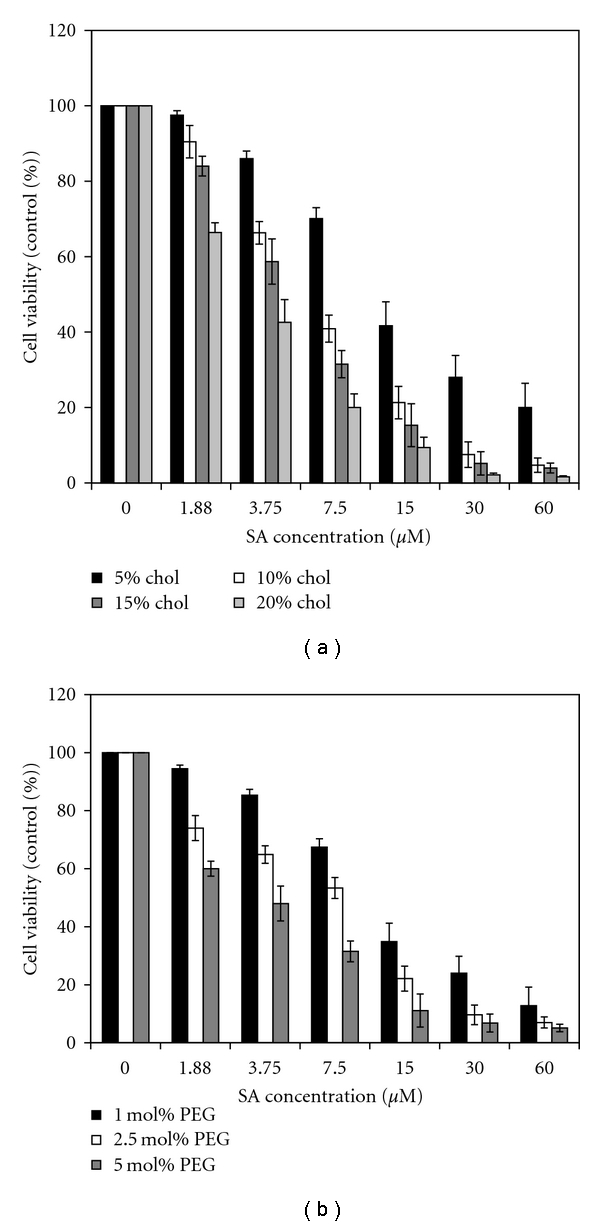
Effect of density of cholesterol or DSPE-mPEG-2000 in SPC-SA liposome on plasmocidal activity: cells (1% parasitemia and 2% hematocrit) in the presence of increasing concentrations (1.88 μM–60 μM SA) of SPC-SA liposome containing different density of (a) cholesterol (5, 10, 15, and 20 mol%) (b) DSPE-mPEG-2000 (1, 1.25, and 5 mol %) cultured for 42 h at 37°C. The results are expressed as a percentage of the incorporation of the radioactive precursors as compared to controls incubated in the absence of any liposome.
3.6. Direct Entry of SPC-SA Liposomes Specifically into Infected Erythrocytes
A time-dependent study was performed to probe the initiation of the interaction of SA liposome with infected RBCs. After 45 minutes exposure to the SPC-SA liposome, internalization of the liposome into infected RBCs began, which became completely evident after 2 h of incubation (Figure 5), indicating that SPC-SA liposome was able to find its way through the host cell membrane. However, uninfected erythrocytes showed the accumulation of liposomes at their surface even after 2 h of incubation clearly suggesting that the entry of SPC-SA liposome is moreover restricted to parasitized erythrocytes only.
Figure 5.
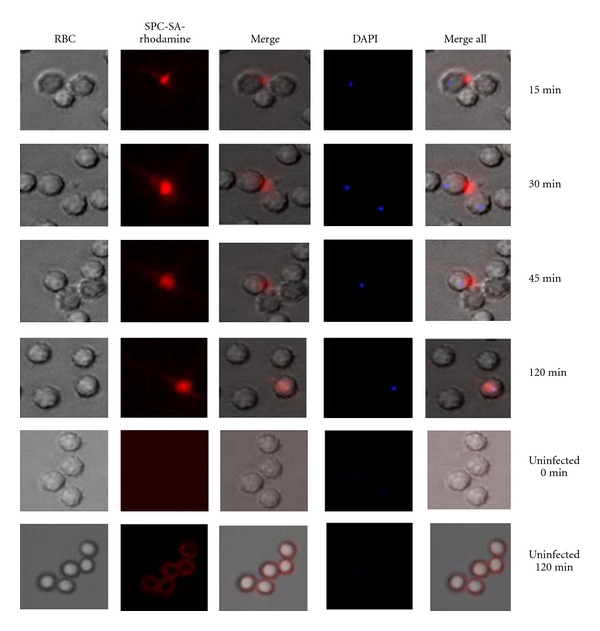
Intraerythrocytic uptake of SPC-SA liposome: parasite cultures were exposed for different time points with rhodaminated SPC-SA liposomes (5 μg/mL) (Panel: SPC-SA Rhodamine) at 37°C, fixed and further counterstained using DAPI (1 μg/mL), and kept in dark. Images were taken within 5 min after exposure to microscopic light to avoid photo bleaching. Uninfected erythrocytes were also treated under similar conditions. Panel RBC denotes phase contrast pictures of RBC, Merge denotes merger of phase contrast picture and rhodamine picture.
3.7. Plasmocidal Activity of SPC-SA Liposome without Hemolysis
To probe the mechanism of antimalarial activity of SA liposome, the ability to induce hemolysis of infected cells was investigated. No hemolysis was observed in uninfected erythrocytes. However, in infected erythrocytes at a higher dose (60–1.88 μM), hemolysis of about 5% was observed with the effect subsiding at the IC50 value of SPC-SA liposome (Table 3), suggesting that the antimalarial effect of these liposomes is independent of hemolysis.
Table 3.
Effect of various concentrations of SPC liposomes containing 20 mol% SA on the hemolysis of infected & uninfected erythrocytes.
| Concentration of liposomes (SA) μg/mL | % Hemolysis | |
|---|---|---|
| Infected RBC (1% parasitemia) |
Uninfected RBC | |
| 16.0 | 3.30 ± 0.34 | 0 |
| 8.0 | 1.91 ± 0.62 | 0 |
| 4.0 | 1.59 ± 0.85 | 0 |
| 2.0 | 0 | 0 |
| 1.0 | 0 | 0 |
| 0.5 | 0 | 0 |
SPC-SA liposomes at different concentrations were mixed with infected (1% parasitemia) and uninfected erythrocytes for 42 h as described in materials and methods. Percentage hemolysis was calculated using the expression % hemolysis = [A405 nm (sample) − A405 nm (negative control)]/A405 nm (positive control).
4. Discussion
Several studies have reported that SA in liposomes kills a number of pathogenic protozoan parasites in culture as well as in animal model [11, 13, 14, 23, 24]. The results of the current study showed for the first time the inhibition of growth and multiplication of Plasmodium falciparum by these liposomes. It has been reported that the interaction of liposomes with mammalian cells is significantly dependent on the composition, charge, size, rigidity, and hydrophilicity on the surface of the liposomes as well as the cell types [25]. Therefore, to examine the role of these parameters in the antimalarial activities of SA liposomes, modifiers were incorporated in SPC-SA liposomes. The maximum inhibition was observed when SA was delivered through soya-PC followed by egg-PC liposomes with no inhibition when SA was delivered through SM liposomes. Alone egg-PC or soya-PC liposomes or SPC-PA liposomes were unable to elicit any plasmocidal activity.
As stated earlier, phospholipid composition plays a significant role in determining the biological efficacy of encapsulated drugs in liposomes. It is known that sphingomyelin (SM) forms very rigid liposomes as compared to liposomes prepared by EPC or SPC. It has also been reported that fusion of liposomes with mammalian cells [26], transfer of individual lipid molecules from liposomes to the plasma membrane of the mammalian cells or lipoproteins [27, 28] and endocytosis of liposomes [29] are significantly dependent on the lipid composition and fluidity of the liposomes. The reduced efficacy of SA in SM liposomes in inhibiting the growth of Plasmodium falciparum as compared to EPC or SPC liposomes may be due to reduced fusion/interaction of rigid SM liposomes with infected erythrocytes as compared to fluid EPC or SPC liposomes.
Increasing the surface density of SA in the liposomal membrane resulted in a progressive increase in the inhibition of growth of parasites, and liposomes, containing <5 mol% were possibly inactive although SA has been 90–95% intercalated in all the liposomal formulations. The explanation for this observation has been attributed to polycationic nature of SA by earlier investigators [13]. However, there has been no report in the literature about the dependency of the hydrophobic domain of SA. In order to answer this question, liposomes composed of 80% soya PC and 20% alkylamines of different chain length were prepared, and their plasmocidal activity was examined. It was found that increasing the chain length of the aliphatic amines increases antimalarial activity (Table 2) suggesting that not only polycationic surface but also the hydrophobic portion of the alkyl amines is very important and involved in the antimalarial activity. This may explain why polycationic molecules, like spermidine, polylysine, polyhistidine, and polymyxin B that lacks hydrophobic domain, are unable to kill parasites.
The mechanism by which SPC-SA liposomes kill malaria parasite is not well understood at present. But reports suggest that these liposomes kill other protozoan parasites by direct interaction with the negatively charged surface molecules of the parasites [24]. Now, question is how malaria parasite infected mature red blood cell, which is a terminally differentiated cell and lacks endocytic activity, internalized positively charged SA incorporated into SPC liposomes? Infection of human erythrocytes by the malarial parasites, Plasmodium falciparum, results in complex membrane sorting and signaling events in the mature erythrocytes [30]. Host-signaling components in combination with parasite protein makes the intraerythrocytic environment conducive to induce formation of the membrane surrounding malaria vacuole [31]. Erythrocytes membrane contain detergent-resistant membrane (DRM) raft, which contains many proteins, and some of these proteins (minor DRM proteins) are enriched in the parasitophorous vacuolar membrane formed by the malaria parasite as it enter the erythrocytes [32]. These results suggest that entry of erythrocyte membrane protein into the cytosol occurs through membrane invagination in malaria-infected erythrocytes. It has also been reported that once inside the erythrocytes, malaria parasite remodels the erythrocyte membrane creating a number of transport pathways for importing nutrient molecules from the circulation rendering it more permissive for malaria parasite survival [33–35]. Reports suggested that channels called “New Permeability Pathways” or NPPs appear after 12–16 h of Plasmodium invasion, and small molecules (MW < 200 Da) gained access to the parasite through it [9, 10]. It has also been reported that parasite has direct access to extracellular nanosized (80 nm) latex bead particles[36]. To understand the mechanism of internalization of SPC-SA liposomes, we have tracked entry of octadecylrhodamine-SA liposomes (having size 90–110 nm) into infected RBCs by fluorescence microcopy. It was found that octadecyl rhodamine probe enters into the cytosol of infected erythrocytes (Figure 5). However, the exact mechanism of entry of the probe into cytosol and from cytosol of infected erythrocytes into parasitophorous vacuole is not well understood.
The spontaneous transfer of the probe octadecyl rhodamine to unlabelled membranes has been reported [37]. Further, Grellier et al. have demonstrated the existence of the unidirectional flux of lipid from HDL to the intracellular parasites via the erythrocyte plasma membrane without internalization of the lipoprotein particles [38]. They have also demonstrated that the cytoplasm of infected erythrocytes contains numerous vesicles and tubular structure. Tubular structures acts as connector between parasitophorous vacuolar membrane system and the erythrocytes membrane for transport of lipids. Our results suggest that both SPC and SA were transferred to the membrane of Plasmodium-infected erythrocytes following fusion between SPC-SA liposomes and infected erythrocytes. Then, SA was transported from the infected erythrocytes membrane to the parasites possibly via lateral diffusion followed by fusion with complex network of membranes occurring in the cytosol of Plasmodium-infected erythrocytes as reported by Grellier et al. [38].This may explain the labeling of infected erythrocytes with octadecyl rhodamine probe. Besides, various reports suggested that parasites take up hemoglobin from cytosol of RBC by plethora of pathways. A well-characterized vesicular pathway for internalization of host cytoplasm is via cytostome-derived invagination resembling endocytosis [39, 40]. Recently, Elliott et al. [41] showed that four different but overlapping pathways exist in Plasmodium falciparum for taking up hemoglobin from cytosol of RBC. They have shown that early ring-stage parasites undergoes profound morphological changes in which they fold like a cup onto themselves and during this process (termed as “Big Gulp”) engulfs a large amount of cytosol. In addition, different processes like endocytosis via cytostome, cytosomal tubes arising from cytostome and phagotrophy resembling phagocytosis occur at later stages [41]. A cytostome-mediated but vesicle-independent process model of hemoglobin uptake in Plasmodium falciparum has also been demonstrated [42]. Therefore, it is tempting to speculate that parasite use either one or all of the above pathways for taking membrane bound SA from cytosol. The interaction of SA with the malaria parasite in the parasitophorous vacuoles in erythrocytes may be facilitated by its high negative surface charge [43].
In the present study, it is clearly demonstrated for the first time that SA incorporated into SPC liposomes enter into malaria infected RBCs and are very effective in the inhibition of the growth and multiplication of the Plasmodium falciparum within erythrocytes. The low toxicity of these liposomes (Table 3) to the host erythrocytes may find SA-bearing liposomes an effective means of chemotherapy and control of Plasmodium falciparum infection in human.
Authors Contribution
G. H. Mustafa and N. Garg contributed equally to the paper.
Conflict of Interests
The authors report no conflict of interests.
Acknowledgments
These studies were supported in part by grant from the Department of Science and Technology (DST) and Special Assistance Programme (SAP) from University Grant Commission, Government of India. Gulam Mustafa Hasan, Neha Garg, Enna Dogra, and Ranu Surolia are supported by the “Council of Scientific and Industrial Research” research fellowship. The authors would like to thank Dr. C. R. Pillai (National Institute of Malaria Research, New Delhi, India) for providing Plasmodium falciparum (3D7) and the Rotary Blood Bank, New Delhi, India for continuous supplying of O+ blood. Dr. Debi P. Sarkar and Prashant Mani are acknowledged for kind help in the Fluorescent microscopy.
References
- 1.Kajfasz P. Malaria prevention. International Maritime Health. 2009;60(1-2):67–70. [PubMed] [Google Scholar]
- 2.Foley M, Tilley L. Quinoline antimalarials: mechanisms of action and resistance. International Journal for Parasitology. 1997;27(2):231–240. doi: 10.1016/s0020-7519(96)00152-x. [DOI] [PubMed] [Google Scholar]
- 3.Owais M, Varshney GC, Choudhury A, Chandra S, Gupta CM. Chloroquine encapsulated in malaria-infected erythrocyte-specific antibody-bearing liposomes effectively controls chloroquine-resistant Plasmodium berghei infections in mice. Antimicrobial Agents and Chemotherapy. 1995;39(1):180–184. doi: 10.1128/aac.39.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peeters PA, Huiskamp CWEM, Eling WMC, Crommelin DJA. Chloroquine containing liposomes in the chemotherapy of murine malaria. Parasitology. 1989;98(3):381–386. doi: 10.1017/s003118200006145x. [DOI] [PubMed] [Google Scholar]
- 5.Singh AM, Owais M, Varshney GC. Use of specific polyclonal antibodies for site specific drug targeting to malaria infected erythrocytes in vivo. Indian Journal of Biochemistry and Biophysics. 1993;30(6):411–413. [PubMed] [Google Scholar]
- 6.Peeters PA, Brunink BG, Eling WMC, Crommelin DJA. Therapeutic effect of chloroquine (CQ)-containing immunoliposomes in rats infected with Plasmodium berghei parasitized mouse red blood cells: comparison with combinations of antibodies and CQ or liposomal CQ. Biochimica et Biophysica Acta. 1989;981(2):269–276. doi: 10.1016/0005-2736(89)90037-0. [DOI] [PubMed] [Google Scholar]
- 7.Biot C, Chibale K. Novel approaches to antimalarial drug discovery. Infectious Disorders. 2006;6(2):173–204. doi: 10.2174/187152606784112155. [DOI] [PubMed] [Google Scholar]
- 8.Sherman IW, Greenan JR. Altered red cell membrane fluidity during schizogonic development of malarial parasites (Plasmodium falciparum and P. lophurae) Transactions of the Royal Society of Tropical Medicine and Hygiene. 1984;78(5):641–644. doi: 10.1016/0035-9203(84)90227-x. [DOI] [PubMed] [Google Scholar]
- 9.Biagini GA, Ward SA, Bray PG. Malaria parasite transporters as a drug-delivery strategy. Trends in Parasitology. 2005;21(7):299–301. doi: 10.1016/j.pt.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Pouvelle B, Spiegel R, Hsiao L, et al. Direct access to serum macromolecules by intraerythrocytic malaria parasites. Nature. 1991;353(6339):73–75. doi: 10.1038/353073a0. [DOI] [PubMed] [Google Scholar]
- 11.Yoshihara E, Tachibana H, Nakae T. Trypanocidal activity of the stearylamine-bearing liposome in vitro. Life Sciences. 1987;40(22):2153–2159. doi: 10.1016/0024-3205(87)90005-1. [DOI] [PubMed] [Google Scholar]
- 12.Tachibana H, Yoshihara E, Kaneda Y, Nakae T. In vitro lysis of the bloodstream forms of Trypanosoma brucei gambiense by stearylamine-bearing liposomes. Antimicrobial Agents and Chemotherapy. 1988;32(7):966–970. doi: 10.1128/aac.32.7.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tachibana H, Yoshihara E, Kaneda Y, Nakae T. Protection of toxoplasma gondii-infected mice by stearylamine-bearing liposomes. Journal of Parasitology. 1990;76(3):352–355. [PubMed] [Google Scholar]
- 14.Afrin F, Dey T, Anam K, Ali N. Leishmanicidal activity of stearylamine-bearing liposomes in vitro. Journal of Parasitology. 2001;87(1):188–193. doi: 10.1645/0022-3395(2001)087[0188:LAOSBL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 16.Bharadwaj S, Rathore SS, Ghosh PC. Enhancement of the cytotoxicity of liposomal ricin by the carboxylic ionophore monensin and the iysosomotropic amine NHCl in Chinese hamster ovary cells. International Journal of Toxicology. 2006;25(5):349–359. doi: 10.1080/10915810600846195. [DOI] [PubMed] [Google Scholar]
- 17.Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Analytical Biochemistry. 1980;104(1):10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 18.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrobial Agents and Chemotherapy. 1979;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krugliak M, Feder R, Zolotarev VY, et al. Antimalarial activities of dermaseptin S4 derivatives. Antimicrobial Agents and Chemotherapy. 2000;44(9):2442–2451. doi: 10.1128/aac.44.9.2442-2451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer LD, Tai LCL, Ko DSC, et al. Influence of vesicle size, lipid composition, and drug-to-lipid ratio on the biological activity of liposomal doxorubicin in mice. Cancer Research. 1989;49(21):5922–5930. [PubMed] [Google Scholar]
- 21.Torchilin VP, Omelyanenko VG, Papisov MI, et al. Poly( ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevity. Biochimica et Biophysica Acta. 1994;1195(1):11–20. doi: 10.1016/0005-2736(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 22.Kirby C, Clarke J, Gregoriadis G. Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochemical Journal. 1980;186(2):591–598. doi: 10.1042/bj1860591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dey T, Anam K, Afrin F, Ali N. Antileishmanial activities of stearylamine-bearing liposomes. Antimicrobial Agents and Chemotherapy. 2000;44(6):1739–1742. doi: 10.1128/aac.44.6.1739-1742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee A, Roychoudhury J, Ali N. Stearylamine-bearing cationic liposomes kill Leishmania parasites through surface exposed negatively charged phosphatidylserine. Journal of Antimicrobial Chemotherapy. 2008;61(1):103–110. doi: 10.1093/jac/dkm396. [DOI] [PubMed] [Google Scholar]
- 25.Rathore SS, Ghosh PC. Effect of surface charge and density of distearylphosphatidylethanolamine-mPEG-2000 (DSPE-mPEG-2000) on the cytotoxicity of liposome-entrapped ricin: effect of lysosomotropic agents. International Journal of Pharmaceutics. 2008;350(1-2):79–94. doi: 10.1016/j.ijpharm.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 26.Fraley R, Straubinger RM, Rule G, Springer EL, Papahadjopoulos D. Liposome-mediated delivery of deoxyribonucleic acid to cells: enhanced efficiency of delivery related to lipid composition and incubation conditions. Biochemistry. 1981;20(24):6978–6987. doi: 10.1021/bi00527a031. [DOI] [PubMed] [Google Scholar]
- 27.Gregoriadis G, Senior J. The phospholipid component of small unilamellar liposomes controls the rate of clearance of entrapped solutes from the circulation. FEBS Letters. 1980;119(1):43–46. doi: 10.1016/0014-5793(80)80994-x. [DOI] [PubMed] [Google Scholar]
- 28.Kirby C, Clarke J, Gregoriadis G. Cholesterol content of small unilamellar liposomes controls phospholipid loss to high density lipoproteins in the presence of serum. FEBS Letters. 1980;111(2):324–328. doi: 10.1016/0014-5793(80)80819-2. [DOI] [PubMed] [Google Scholar]
- 29.Lee KD, Hong K, Papahadjopoulos D. Recognition of liposomes by cells: in vitro binding and endocytosis mediated by specific lipid headgroups and surface charge density. Biochimica et Biophysica Acta. 1992;1103(2):185–197. doi: 10.1016/0005-2736(92)90086-2. [DOI] [PubMed] [Google Scholar]
- 30.Murphy SC, Hiller NL, Harrison T, Lomasney JW, Mohandas N, Haldar K. Lipid rafts and malaria parasite infection of erythrocytes. Molecular Membrane Biology. 2006;23(1):81–88. doi: 10.1080/09687860500473440. [DOI] [PubMed] [Google Scholar]
- 31.Taraschi TF, Trelka D, Martinez S, Schneider T, O’Donnell ME. Vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes. International Journal for Parasitology. 2001;31(12):1381–1391. doi: 10.1016/s0020-7519(01)00256-9. [DOI] [PubMed] [Google Scholar]
- 32.Murphy SC, Samuel BU, Harrison T, et al. Erythrocyte detergent-resistant membrane proteins: their characterization and selective uptake during malarial infection. Blood. 2004;103(5):1920–1928. doi: 10.1182/blood-2003-09-3165. [DOI] [PubMed] [Google Scholar]
- 33.Nagao E, Seydel KB, Dvorak JA. Detergent-resistant erythrocyte membrane rafts are modified by a Plasmodium falciparum infection. Experimental Parasitology. 2002;102(1):57–59. doi: 10.1016/s0014-4894(02)00143-1. [DOI] [PubMed] [Google Scholar]
- 34.Ginsburg H, Kutner S, Krugliak M, Cabantchik ZI. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Molecular and Biochemical Parasitology. 1985;14(3):313–322. doi: 10.1016/0166-6851(85)90059-3. [DOI] [PubMed] [Google Scholar]
- 35.Kutner S, Baruch D, Ginsburg H, Cabantchik ZI. Alterations in membrane permeability of malaria-infected human erythrocytes are related to the growth stage of the parasite. Biochimica et Biophysica Acta. 1982;687(1):113–117. doi: 10.1016/0005-2736(82)90178-x. [DOI] [PubMed] [Google Scholar]
- 36.Goodyer ID, Pouvelle B, Schneider TG, Trelka DP, Taraschi TF. Characterization of macromolecular transport pathways in malaria- infected erythrocytes. Molecular and Biochemical Parasitology. 1997;87(1):13–28. doi: 10.1016/s0166-6851(97)00039-x. [DOI] [PubMed] [Google Scholar]
- 37.Stegmann T, Schoen P, Bron R, et al. Evaluation of viral membrane fusion assays. Comparison of the octadecylrhodamine dequenching assay with the pyrene excimer assay. Biochemistry. 1993;32(42):11330–11337. doi: 10.1021/bi00093a009. [DOI] [PubMed] [Google Scholar]
- 38.Grellier P, Rigomier D, Clavey V, Fruchart JC, Schrevel J. Lipid traffic between high density lipoproteins and Plasmodium falciparum-infected red blood cells. Journal of Cell Biology. 1991;112(2):267–277. doi: 10.1083/jcb.112.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slomianny C. Three-dimensional reconstruction of the feeding process of the malaria parasite. Blood Cells. 1990;16(2-3):369–378. [PubMed] [Google Scholar]
- 40.Yayon A, Timberg R, Friedman S, Ginsburg H. Effects of chloroquine on the feeding mechanism of the intraerythrocytic human malarial parasite plasmodium falciparum. Journal of Protozoology. 1984;31(3):367–372. doi: 10.1111/j.1550-7408.1984.tb02981.x. [DOI] [PubMed] [Google Scholar]
- 41.Elliott DA, McIntosh MT, Hosgood HD, et al. Four distinct pathways of hemoglobin uptake in the malaria parasite Plasmodium falciparum. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(7):2463–2468. doi: 10.1073/pnas.0711067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazarus MD, Schneider TG, Taraschi TF. A new model for hemoglobin ingestion and transport by the human malaria parasite Plasmodium falciparum. Journal of Cell Science. 2008;121(11):1937–1949. doi: 10.1242/jcs.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seed TM, Aikawa M, Sterling C, Rabbege J. Surface properties of extracellular malaria parasites: morphological and cytochemical study. Infection and Immunity. 1974;9(4):750–761. doi: 10.1128/iai.9.4.750-761.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


