Abstract
Aromatase is a specific component of the cytochrome P450 enzyme system responsible for the transformation of androgen precursors into estrogens. This enzyme is encoded by the CYP19A1 gene located at chromosome 15q21.2, that is, expressed in ovary and testis, but also in many extraglandular sites such as the placenta, brain, adipose tissue, and bone. The activity of aromatase regulates the concentrations of estrogens with endocrine, paracrine, and autocrine effects on target issues including bone. Importantly, extraglandular aromatization of circulating androgen precursors is the major source of estrogen in men. Clinical and experimental evidences clearly indicate that aromatase activity and estrogen production are necessary for longitudinal bone growth, the attainment of peak bone mass, pubertal growth spurt, epiphyseal closure, and normal bone remodeling in young individuals. Moreover, with aging, individual differences in aromatase activity may significantly affect bone loss and fracture risk in men.
1. Background
Sex steroid hormones are important for the acquisition and maintenance of bone mass in both sexes [1, 2]. Alterations in their levels can be relevant in the pathogenesis of osteoporosis and fractures because their deficiency may lead to suboptimal acquisition of peak bone mass in young individuals or bone loss in adulthood. While estrogens effects in bone in females have been well established (as estrogen deficiency after menopause leads to an imbalance between bone resorption by osteoclasts and bone formation by osteoblasts), the role for estrogen in male skeletal health has only recently become appreciated [3–6]. In fact, even though alterations in circulating androgen have been associated with low bone mass and impaired bone strength [7], their primacy has been increasingly questioned as direct and indirect evidence has emerged suggesting that estrogens also play a major role in male skeletal health [3–7]. These new observations underscore the normal biosynthetic pathway by which estrogens are made in men, via the activity of aromatase (a cytochrome P450 product of the CYP19A1 gene) on circulating androgenic precursors (Figure 1).
Figure 1.
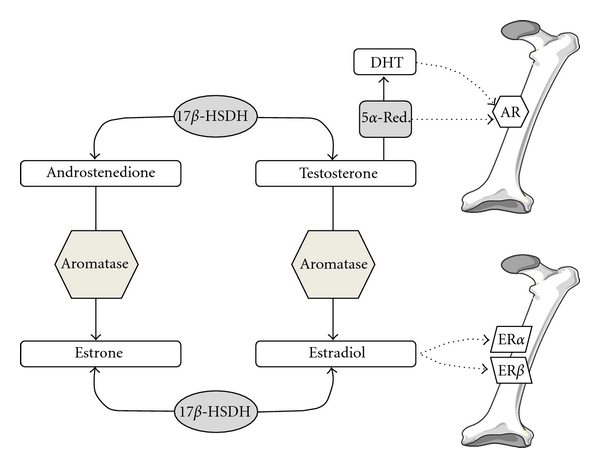
Proposed models of sex steroid hormones action on bone.
Of interest, several clinical and experimental studies on estrogen and/or aromatase deficiency also reinforced the hypothesis of a threshold estradiol level for skeletal sufficiency in the male [1–4]. In fact, either in cross-sectional or longitudinal analysis in aging men bone mineral density (BMD) and rates of bone loss at different skeletal sites were unrelated to serum estradiol concentrations when the latter were above the median value, while they were clearly associated with estradiol at concentrations below the median [8–10]. This hypothesis gained further support from a study in which raloxifene (a selective estrogen receptor modulator) was given to men of varying estradiol levels [11]. Subjects with serum total estradiol levels below 96 pmol/liter responded to raloxifene with a decrease in bone resorption markers while above this estrogen value, raloxifene caused an increase in bone resorption markers [11]. Overall, these observations clearly indicate that men need a sufficient concentration of estrogen, defined as a threshold value, for normal skeletal remodeling. In all these studies, the required concentration appeared to be remarkably similar, ranging from 90 to 110 pmol/liter in case of total estradiol or from 40 to 55 pmol/liter in case of bioavailable estradiol [5]. This apparent threshold value is higher than typical estradiol concentrations for postmenopausal women who are not receiving exogenous estrogens. On the other hand, premenopausal women and young men are typically above this apparent threshold level, while up to 50% of middle-aged men fall below this estradiol threshold and, thus, are at higher risk of bone loss as shown in several cross-sectional and longitudinal studies [1–4]. Importantly, estradiol levels above a given threshold are also important to prevent fractures, as recently demonstrated in a prospective study of men from the MrOS cohort [12].
This paper summarizes the evidence that aromatase activity plays an important role in the skeleton in men, either in young individuals or in the elderly, by its actions to convert androgens to estrogens in bone and other peripheral tissues.
2. Aromatase and Estrogen Production in Men
Aromatase is a specific component of the cytochrome P450 enzyme system that converts the delta 4-3-one A ring of C19 androgen precursors into the corresponding phenolic A ring characteristic of C18 estrogenic compounds
While in fertile women the ovary represents the major source of circulating estrogen, which functions as a circulating hormone to act on distal target tissues, in men the testes account at most for 15% of circulating estrogens, while the remaining 85% is due to peripheral aromatization of circulating androgen precursors in different tissues [13, 14]. These include the adipose tissue, the brain, the skin, the endothelium, and the bone. It has been demonstrated that testicular androgen precursors contribute more to the total amount of circulating estradiol than adrenal androgens [15]. In fact, dexamethasone-induced suppression of adrenal steroid synthesis moderately decreases estradiol concentrations [16], whereas orchidectomy (ORX) leads to a more dramatic suppression of plasma estradiol [17, 18]. Clearly, these extragonadal sites of estrogen biosynthesis lack the ability to synthesize C19 precursors from cholesterol, hence, their estrogen-producing activity totally depends on the availability of these circulating C19 androgenic steroids [19]. Moreover, the estrogen synthesized within these extragonadal compartments may be also locally active in a paracrine or intracrine fashion [13, 20].
In human bone, aromatase is expressed in osteoblast or osteoblast-like cells from fetal and normal tissue [21–23], in articular cartilage chondrocytes, in adipocytes adjacent to bone trabeculae, and in osteocytes, but not in osteoclasts [23]. Importantly, a recent study demonstrated that aromatase gene can be expressed in bone tissue in consistent amounts, at levels similar to those found in adipose tissue [24]. In particular, osteoblasts are the major source of aromatase within the bone microenvironment [24].
Aromatase is encoded by the CYP19A1 gene located at chromosome 15q21.2 [25] (Figure 2). Despite the presence of a common CYP19A1 gene, in a tissue-specific fashion, a number of untranslated initial exons are found in aromatase transcripts due to differential splicing by at least 10 different tissue specific promoters [26–28]. Only the 30-kb 3′ region of the gene (containing exons 2 to 10) encodes aromatase while a larger 93 Kb 5′ flanking region serves as the regulatory unit of the gene [27, 28]. Thus all the multiple exons 1 are not translated, so that the different splicing patterns lead to transcripts that are all translated as the same protein. Importantly, this complex structure of the promoter region of the gene defines the tissue-specific regulation of aromatase activity and estrogen biosynthesis. Thus, the ovary, testes, adipose tissue, brain, and bone each utilize their own promoters and associated enhancers and suppressors leading to different amounts of mRNA transcripts, mRNA stability and/or protein translation [26, 29, 30].
Figure 2.
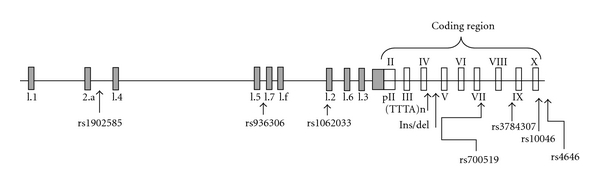
Aromatase gene (CYP19A1) with its promoters and untranslated first exons. Major polymorphic variants of the CYP19A1 gene are indicated.
In the skeleton, the majority of aromatase transcripts contain exon 1.4 and exon 1.6 [31–33]. Some minor transcripts by promoter I.3, PII and If have been also described [31]. Interestingly, experiments in osteoblast cell lines demonstrated that cortisol may induce aromatase gene expression transiently and that 1,25 dihydroxyvitamin D can maintain its expression, dependent on vitamin D receptor density [34–36]. Moreover, a more recent study demonstrated that Runx2 (a key regulator of osteoblast differentiation) directly increases aromatase gene expression in human osteoblast cells by increasing promoter I.4 and I.6 activity [37]. In keeping with this in vitro evidence, a marked decrease in skeletal aromatase expression has been described in Runx2-deficient mice [37].
3. Aromatase Deficiency and the Male Skeleton
During the past 2 decades, several clinical and experimental observations underscored the importance of local and peripheral aromatization of androgens into estrogen for skeletal homeostasis in males.
3.1. Aromatase Deficiency Syndrome
The discovery of human cases of aromatase deficiency preceded the construction of aromatase knockout animals and, in fact, provided the first insight into the role of estrogens and aromatase in male skeletal physiology.
Human aromatase deficiency is a very rare autosomal recessive syndrome characterized by congenital estrogen deprivation caused by loss-of-function mutations in the CYP19A1 gene [38]. In both genders, the overall severity of the phenotype may be variable according to residual enzyme activity [38, 39]. While affected females generally have ambiguous genitalia at birth and fail to develop secondary sexual characteristics, the affected male individuals have normal male sexual differentiation and pubertal maturation, and their clinical phenotype mostly develops after puberty [5, 40]. So far, there are at least 9 known cases of inactivating mutations in the CYP19A1 gene and aromatase deficiency in men [5, 41–49]. All these patients generally showed markedly low or undetectable estrogen levels while androgens were normal or even elevated. Interestingly, skeletal maturation and bone metabolism were severely impaired in all these subjects, with a similar phenotype to a previously described male case of loss of function mutation at the estrogen receptor alpha (ESR1) gene [50]. Common skeletal characteristics include tall stature and continued longitudinal growth due to unfused epiphyses, delayed bone age, lack of pubertal growth spurt, eunuchoid skeletal proportions, genu valgum, elevated bone resorption markers, and low bone mass. Moreover, other extraskeletal characteristics, such as lipid abnormalities, increased body weight, hyperinsulinemia, and various degrees of glucose impairment (including diabetes and acanthosis nigricans in 1 and 2 cases, resp.) have been also reported in these men.
As in part expected, in 2 of these cases, treatment with intramuscular testosterone did not give skeletal benefit to these patients, since aromatase deficiency does not lead to low testosterone levels [5, 47]. Conversely, estrogen treatment was associated with marked improvements in the skeletal phenotype. In fact, epiphyses closed quickly, longitudinal growth ceased, and BMD increased consistently at all assessed skeletal sites [41, 42, 47, 51, 52]. As counterpart, estrogen replacement in the male case of loss of function mutation at the ESR1 gene did not improve bone outcomes [5, 50]. Of interest, consistent with the threshold estradiol hypothesis for skeletal health, a clear dose-dependent effect of estrogen replacement therapy on bone mass was evidenced, since a very low dose of estradiol (below 25 mcg twice weekly) was not sufficient for maintaining a normal BMD in one of these aromatase-deficient men [52].
In a more recent study, the skeletal phenotype of a 16-year-old boy with aromatase deficiency was investigated by both DXA and peripheral quantitative computed tomography (pQCT) of the radius [46]. The use of the later technique allowed to assess additional characteristics of bone strength, such as cross-sectional area (CSA), cortical thickness, trabecular volumetric BMD, and cortical volumetric BMD. Consistent with the previous observations, estrogen replacement in this boy was associated with the normalization of sex hormone concentrations, reduced bone turnover rate, and increases in lumbar spine (+23%) and femoral neck (+14%) areal BMD. However, the gain in volumetric BMD (either estimated by the calculation of the bone mineral apparent density from DXA or assessed directly by pQCT) was limited at the lumbar spine and even absent at the femoral neck and the radius. Conversely longitudinal bone growth, cross-sectional area, and cortical thickness (as measured by pQCT) increased significantly by 8.5%, 46%, and 12%, respectively. Thus, it was clear that the observed increase in areal BMD was mainly driven by an increase in bone size, rather than bone density, particularly at peripheral sites. Interestingly, these changes are similar to those associated with normal pubertal growth and support the notion that in growing bones, except for the spine true density does not increase [53, 54]. On the contrary, periosteal diameter continues to expand and cortical thickness increases during normal male puberty because of reduced endocortical expansion and accelerated periosteal apposition [55]. These effects lead to increased bone size, and have classically been attributed to androgens, accounting for the greater areal density that is typical of the male skeleton. In fact, when females enter puberty, periosteal apposition is inhibited, an action classically believed to be an estrogen effect. However, according to the effects of estrogen administration on cross-sectional area and cortical thickness in this young boy with aromatase deficiency [46], some actions on bone size, previously attributed to androgens, must at least in part be an estrogen effect. Thus, it is likely that a biphasic, dose-dependent effect of estrogen at the periosteum could exist. At low levels (as observed in males and in early pubertal females) estrogen may stimulate periosteal apposition and increase bone size, whereas at higher concentrations (as observed in late pubertal and adult females) estrogen may inhibit cross-sectional bone growth.
Recently, the concomitant presence of mild hypogonadism in a man with aromatase deficiency has offered a useful model to study the effects of testosterone and estradiol replacement, separately or in combination [56]. As expected, in this man, estradiol treatment alone increased BMD with a greater gain than the one obtained with testosterone alone. However, the combination of testosterone (6 mg/day) and estrogen (25 mcg twice weekly) replacement led to a further increase in cortical thickness at the radius and the tibia as measured by pQCT, further supporting the concomitant importance of both sex steroids for periosteal apposition. In this case, an increase in volumetric BMD at the tibia and the radius as well as an increase of areal BMD of the lumbar spine and the femoral neck was also described after 2 years of combined therapy.
The skeletal consequences of aromatase deficiency have been illustrated further by studies of the aromatase knock-out mouse (ArKO) models [57–59]. Overall, these experimental observations generally provided the confirmation of the human gene disorders suggesting that estrogen may be more protective in the growing skeleton than androgens in man, but left open the issues of what, if any, are the roles of estrogen in regulating bone remodeling and bone loss in adult males.
3.2. Inhibition of Aromatase Activity: Clinical Studies in Adult Men
A first indirect indication about the importance of aromatase and the relative roles of estrogen versus testosterone in the adult male skeleton came from cross-sectional and longitudinal observations in middle-aged and elderly men. In fact, in most of these studies BMD and bone loss were more directly related to declining estrogen levels than declining androgen levels, particularly, when circulating bioavailable fractions of these steroids were considered [8–10, 60–66]. Moreover, in one of these studies, the ratio between estradiol and testosterone presumed to be an indirect index of aromatase activity, increased significantly with age, and was higher in normal than in osteoporotic subjects or in men with fragility fractures [10]. Consistent with these findings, other clinical evidences underlined the importance of estrogen on the adult male skeleton. Thus, in a preliminary observation, Taxel and Raisz described significant reductions in bone resorption markers in 9 elderly men treated short term with either 0.5 mg or 2.0 mg daily of micronized 17β-estradiol [67]. In a different study, Anderson et al. treated 21 eugonadal osteoporotic men with intramuscular testosterone and found a significant increase in lumbar spine BMD, which was correlated with changes in serum estradiol, but not circulating testosterone [68].
In order to definitively dissect out estrogen versus testosterone effects on the adult male skeleton, more dynamic short-term interventional observations have been performed, where aromatase activity was suppressed through the use of aromatase inhibitors. In a first study, Falahati-Nini et al. [69] examined the differential effects of estrogen versus testosterone replacement in a group of 59 elderly men (mean age 68 yr) following the induction of hypogonadism (by the use of a GnRH agonist, Lupron) and aromatase inhibition (with letrozole 2.5 mg daily). Of interest, the increase in bone resorption markers (deoxypyridinoline and N-telopeptide of type I collagen) associated with the use of the GnRH agonist was almost completely prevented by estradiol but not by testosterone therapy alone, indicating that the increase in bone resorption was due primarily to estrogen loss, not to testosterone loss. In the case of bone formation markers, there was evidence for stimulatory effects of both estrogen and testosterone on serum osteocalcin but not the amino-terminal propeptide of type I procollagen that only increased with estrogen replacement. Since osteocalcin is produced primarily by mature osteoblastic cells and osteocytes [70], these findings are consistent with an important role for both estrogen and testosterone in maintaining the functional integrity of these cells. Type I collagen, by contrast, is produced by cells of the entire osteoblastic lineage [71], and these data would suggest that it is primarily estrogen that regulates osteoblast differentiation. These results were in part replaced in a similar study performed in younger individuals, [72] following the induction of the hypogonadal state by the GnRH agonist, goserelin acetate. In this model, evidence was provided for independent effects of testosterone and estrogen on bone resorption. A subsequent study by Taxel et al. [73] with a longer observation period (9 weeks) also gave similar results, further indicating that treatment of elderly men with an aromatase inhibitor (in this specific-case anastrozole 2 mg/day) produces significant increases in bone resorption and decreases in bone formation. These effects on bone turnover may be less evident with lower doses (i.e., anastrozole 1 mg/day or less), or in case of borderline hypogonadism (testosterone levels less than 350 ng/dl), at least over a short-term period of treatment [74]. To this regard, a more recent study assessed the 12 months effects of a low dose of anastrozole (1.0 mg/day) on BMD and bone turnover markers in 69 men (aged 60 yr or older) with low or low-normal testosterone levels [75, 76]. Interestingly, with this longer observation period, despite an increase in testosterone levels at all time points, a statistically significant decrease in posterior-anterior spine BMD versus placebo was described, likely due to the parallel mild decrease in serum estradiol. Qualitatively similar changes, although nonsignificant, were observed at the other bone sites. Conversely, bone turnover markers were not significantly affected by aromatase inhibition, in this study.
4. Variation in Aromatase Activity and Bone Metabolism in Men
All the above clinical and experimental models (in which aromatase activity is absent or inhibited) have clearly shown that aromatase and estrogen production are important factors in male skeletal health. These models, however, were mainly based on conditions of complete estrogen deficiency, while they did not address the potential skeletal implications of interindividual variation in aromatase efficiency [5, 14]. Such differences in aromatase activity, and, hence, estrogen levels, might become particularly important in elderly males in whom age-related declines in testicular and adrenal androgen precursors are common.
4.1. Inherited Variation in Aromatase Activity
Several polymorphic regions have been detected in the human CYP19A1 gene that could be responsible for qualitative and/or quantitative differences in gene expression and aromatase activity (Figure 2). The most widely studied include a silent polymorphism (G/A at Val80) in exon 3, a tetranucleotide (TTTA)n tandem repeat polymorphism in intron 4, a Arg264Cys (C/T) substitution in exon 7, and a single nucleotide change (C/T) in exon 10. These polymorphisms were investigated in postmenopausal women or elderly men. In particular, several studies evidenced an association between the number of TTTA repeats and estrogen levels, breast cancer, or osteoporotic risk [77–83]. More recently other polymorphic variants within the promoter region of the CYP19A1 gene have been widely investigated and associated with BMD in both genders as well as with susceptibility to breast or uterine cancer in females [84–86]. Most of these evidences were confirmed in subsequent meta-analyses and large scale studies [87–91]. Thus, it is possible that the presence of particular CYP19A1 variants could be responsible of higher aromatase activity and increased estrogen production. If so, these polymorphisms should be protective for bone loss in elderly men or postmenopausal women while potentially also increasing the risk of estrogen-related cancer.
Of interest, the skeletal consequences of genetic variation in CYP19A1 appear to be modulated by fat mass, particularly in men. In fact, BMD differences associated with CYP19A1 genotypes were greater in male subjects with a normal BMI, while the association progressively decreased when overweight and obese men were analyzed [81] (Figure 3). This point suggests that fat mass may be a mitigating factor in the expression of CYP19A1 genotypes on bone. It is possible that with more adipose tissue, the associated increase in adipose aromatase activity dominates any effect of the polymorphisms on intrinsic aromatase activity.
Figure 3.
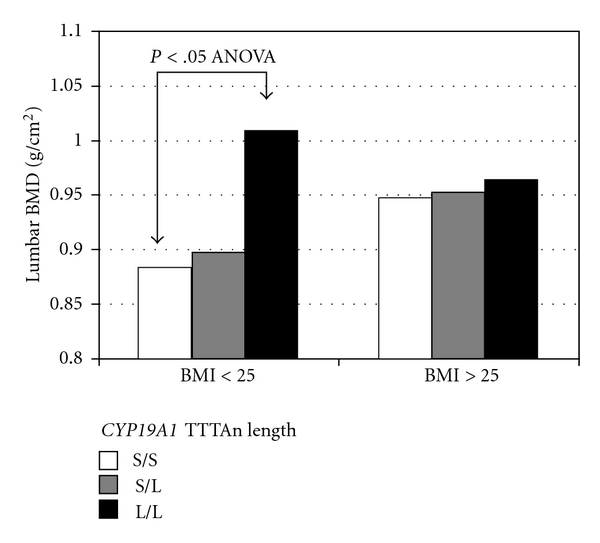
Lumbar BMD values according to CYP19A1 (TTTA)n repeat genotype in subjects divided by BMI in normal (BMI ≤ 25) and overweight or obese groups (BMI > 25), respectively. Subjects were grouped according to short (S, TTTA ≤ 9) and long (L, TTTA > 9) repeats number (adapted from [81]).
Given the importance of estrogen in bone growth, it is likely that genetic variation in CYP19A1 may be also relevant for young individuals, before the attainment of peak bone mass. Despite an initial study in 140 middle-aged Finnish men evidenced an association between the number of TTTA repeat sequences and height or BMI but not with BMD [92], a larger analysis in younger individuals confirmed that CYP19A1 polymorphisms significantly affect the attainment of peak bone mass [93]. Thus, in a well-characterized cohort of 1068 men at the age of peak bone mass (18.9 ± 0.6 years), both the TTTA repeat variation and a silent G/A polymorphism at Val80 of the CYP19A1 gene were predictors of areal BMD, lumbar spine, total body, and cortical bone size (cortical cross-sectional area and thickness) at 2 peripheral sites (radius and tibia).
To date, the molecular mechanisms through which the different CYP19A1 variants affect aromatase activity, and bone metabolism remain in great part unknown. In a preliminary analysis, higher in vitro aromatase efficiency and greater estrogen production were observed in fibroblasts from men with a high TTTA repeat genotype than in men bearing a low TTTA repeat genotype [81]. Even though, it is unlikely that this polymorphism might have a direct effect on aromatase expression and activity (due to its intronic location within the CYP19A1 gene), a different study described a strong degree of linkage disequilibrium between the TTTA repeat variation and the C-T substitution in exon 10, just 19 base-pairs downstream of the termination site of translation [78]. Interestingly, in that study, the T allele was associated with a higher number of TTTA repeats and showed a high-activity phenotype, with increased aromatase activity, increased aromatase mRNA levels, and with a switch in promoter usage from adipose tissue promoter to the more active ovary promoter. More recent studies also evidenced a functional role of other polymorphisms located within the complex promoter region of the CYP19A1 gene. In particular, a C/G polymorphisms in promoter I.2 (rs1062033) were associated with differences in gene transcription by interacting with the transcription factor CEBPβ [94], which affects aromatase expression in different tissues [95, 96]. In fact, the expression of the reporter luciferase gene in osteoblast cell lines was significantly higher in constructs bearing the G allele (which was also associated with higher BMD in population studies) than in those with the C allele, and this difference was particularly evident after cotransfection with CEBPβ. Consistent with these results, differential allelic expression was also evidenced in bone tissue samples, again indicating the G allele as the more overexpressed. Although these studies, in the aggregate, provide data to argue for the importance of CYP19A1 polymorphisms as determinants of estrogen production and bone strength, larger and more definitive studies are needed before any firm conclusion can be drawn.
Interestingly, epigenetic effects on aromatase transcription and activity have been also evidenced in recent studies. In fact, CpG methylation has been described as an important epigenetic mechanism for the regulation of CYP19A1 expression. To this regard, an in vitro study performed in skin fibroblast indicated that differences in methylation patterns may be responsible for the observed interindividual variation in promoter-driven expression of aromatase [97]. In particular, in this study, unmethylated constructs showed consistently higher promoter activity than methylated constructs.
4.2. Acquired Variation in Aromatase Activity
Besides the above genetic and epigenetic considerations, several additional mechanisms have been proposed in which aromatase activity could be modulated under certain circumstances in different tissues. It is known, for example, that aromatase is a specific marker of the undifferentiated adipose mesenchymal cell phenotype while it is less expressed in mature adipocytes. Thus, factors that stimulate adipocyte differentiation, such as PPARγ agonists could also lead to the downregulation of aromatase gene and a reduction in aromatase activity [98–100]. Of course, if there are more adipocytes, there could be more aromatase activity even with reduced production of estrogen per fat cell. Moreover, the skeletal effects of PPARγ agonists are more complex and mainly involve direct negative effects on bone cells [101]. Other agents acting on PPARγ and PPARα pathways, such as the phthalates (ubiquitous environmental toxins found in plasticizers) have been associated with a decrease in aromatase mRNA and aromatase activity in ovarian granulosa cells [102]. Moreover, the activation of PPARα pathway by fenofibrate in female mice significantly reduced aromatase mRNA and activity and was associated with a decrease in femoral BMD and uterine size [103]. Despite these different experimental evidences, the clinical relevance of these environmental agents on global aromatase activity and estrogen production in man remains unknown.
Several other contaminants may affect aromatase activity and estrogen production. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines that are widely used across the world. Their residues are frequent pollutants in the environment and are spread on most eaten transgenic plants, modified to tolerate high levels of these compounds in their cells. While up to 400 ppm of these residues are accepted in some feed, recent experimental studies demonstrated that aromatase transcription and activity were disrupted with subagricultural doses and with residues from 10 ppm [105]. In addition, a different study indicated that phytochemicals such as procyanidin B dimers contained in red wine and grape seeds inhibits aromatase activity in vitro and suppress aromatase-mediated breast tumor formation in vivo [106]. To this regard, it has been assumed that daily consumption of 125 mL of red wine would provide adequate amounts of procyanidin B dimers to suppress in situ aromatase in an average postmenopausal woman. By a different mechanism myosmine, a minor tobacco alkaloid widely occurring in food products of plant and animal origin inhibits the conversion of testosterone to estradiol by human aromatase with potential implications for sex hormone homoeostasis [107]. Another important and well-recognized modulator of aromatase efficiency in bone cells is vitamin D that has been shown to stimulate glucocorticoid-induced aromatase activity in cultured osteoblasts [35]. The magnitude of this effect varies largely among individuals, depending on the level of the vitamin D receptor [107]. Of interest, vitamin D receptor knock-out mice showed reduced aromatase activity with respect to wild-type animals [108].
Importantly aromatase activity may be also affected by pathological conditions. In this respect, it is known that increased androgen aromatization can occur in case of hepatocellular carcinoma [109], adrenocortical tumors [110], and testicular tumors [111, 112]. In all these neoplastic conditions, inappropriate amounts of aromatase enzyme are expressed and estrogen levels are increased. Elevated plasma estradiol concentrations also have been described in men with liver cirrhosis together with decreased plasma testosterone [112, 113]. In these patients, the metabolic clearance rate of estrogens seems to be unaltered, suggesting that the observed hyperestrogenism could be caused solely by an increase in aromatization of androgen precursors. Conversely, other pathological conditions may negatively affect aromatase activity and estrogen levels in males. In a preliminary study on elderly men Figura et al. described significant differences in estradiol levels in relation to Helicobacter pylori infection, independently from circulating testosterone levels [105]. Serum concentrations of estradiol were significantly lower in infected CagA-positive patients than CagA-negative patients (Figure 4), and this variation was associated with differences in bone turnover. The mechanism underlying this association is unknown and deserves further investigations. Indeed, aromatase activity and production of estradiol were recently demonstrated in gastric parietal cells [114]. Finally, more recent observations suggested that diabetes may negatively affect expression levels of aromatase at least in the ovary and the testis [115, 116]. The effects of this disorder on major extragonadal sites of aromatase activity including bone remains to be determined. Of interest, experimental studies also evidenced that oral antidiabetic agents such as metformin can decrease aromatase expression in both granulose-luteal cells and breast adipose cells while, on the opposite, insulin has been associated with enhanced aromatase expression in different cell lines [117, 118]. Importantly, since a recent study evidenced that metformin-induced inhibition of aromatase expression occurs via the downregulation of promoter II, I.3, and 1.4 [118], its potential negative effects on skeletal estrogen production and bone health should be investigated.
Figure 4.
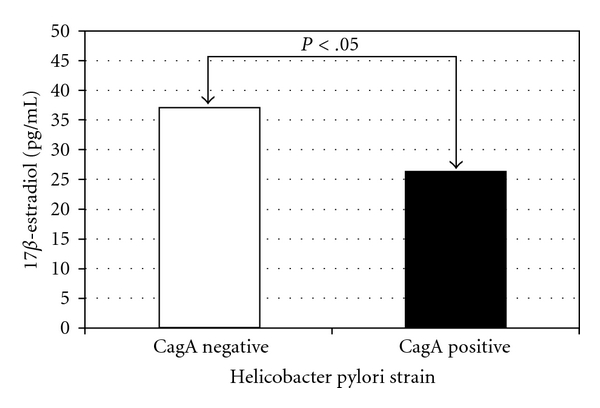
Variations in estrogens levels in elderly men affected by Helicobacter Pylori CagA positive or negative strains (adapted from [104]).
5. Conclusions
Extraglandular aromatization of circulating androgen precursors is the major source of estrogen in men. Several lines of clinical and experimental evidence now clearly indicate that aromatase activity and estrogen production are necessary in men (as well as in women) for longitudinal bone growth, the pubertal growth spurt, epiphyseal closure, normal bone remodeling, and the attainment of peak bone mass. Moreover, like in women, estrogen production from androgen precursors by peripheral aromatase activity (even within the bone) is also important for the maintenance of bone mass and the prevention of bone loss in aging men. Further studies are required to better understand how genetic, epigenetic, environmental, pathologic, and pharmacological influences might modulate aromatase activity, increasing or reducing estrogen production in ageing individuals, and thereby affecting skeletal health.
References
- 1.Riggs BL, Khosla S, Melton LJ., III Sex steroids and the construction and conservation of the adult skeleton. Endocrine Reviews. 2002;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 2.Khosla S. Update on estrogens and the skeleton. Journal of Clinical Endocrinology and Metabolism. 2010;95(8):3569–3577. doi: 10.1210/jc.2010-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khosla S, Melton LJ, III, Lawrence Riggs B. Clinical review 144: estrogen and the male skeleton. Journal of Clinical Endocrinology and Metabolism. 2002;87(4):1443–1450. doi: 10.1210/jcem.87.4.8417. [DOI] [PubMed] [Google Scholar]
- 4.Gennari L, Khosla S, Bilezikian JP. Estrogen and fracture risk in men. Journal of Bone and Mineral Research. 2008;23(10):1548–1551. doi: 10.1359/jbmr.0810c. [DOI] [PubMed] [Google Scholar]
- 5.Gennari L, Khosla S, Bilezikian JP. Estrogen effects on bone in the male skeleton. In: Bilezikian JP, Raisz LG, Martin J, editors. Principles of Bone Biology. 3rd edition. San Diego, Calif, USA: Elsevier Academic Press; 2008. pp. 1801–1818. [Google Scholar]
- 6.Vandenput L, Ohlsson C. Estrogens as regulators of bone health in men. Nature Reviews Endocrinology. 2009;5(8):437–443. doi: 10.1038/nrendo.2009.112. [DOI] [PubMed] [Google Scholar]
- 7.Clarke BL, Khosla S. Androgens and bone. Steroids. 2009;74(3):296–305. doi: 10.1016/j.steroids.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szulc P, Munoz F, Claustrat B, et al. Bioavailable estradiol may be an important determinant of osteoporosis in men: the MINOS study. Journal of Clinical Endocrinology and Metabolism. 2001;86(1):192–199. doi: 10.1210/jcem.86.1.7126. [DOI] [PubMed] [Google Scholar]
- 9.Khosla S, Melton LJ, III, Atkinson EJ, O’Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. Journal of Clinical Endocrinology and Metabolism. 2001;86(8):3555–3561. doi: 10.1210/jcem.86.8.7736. [DOI] [PubMed] [Google Scholar]
- 10.Gennari L, Merlotti D, Martini G, et al. Longitudinal association between sex hormone levels, bone loss, and bone turnover in elderly men. Journal of Clinical Endocrinology and Metabolism. 2003;88(11):5327–5333. doi: 10.1210/jc.2003-030736. [DOI] [PubMed] [Google Scholar]
- 11.Doran PM, Riggs BL, Atkinson EJ, Khosla S. Effects of raloxifene, a selective estrogen receptor modulator, on bone turnover markers and serum sex steroid and lipid levels in elderly men. Journal of Bone and Mineral Research. 2001;16(11):2118–2125. doi: 10.1359/jbmr.2001.16.11.2118. [DOI] [PubMed] [Google Scholar]
- 12.Mellström D, Vandenput L, Mallmin H, et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. Journal of Bone and Mineral Research. 2008;23(10):1552–1560. doi: 10.1359/jbmr.080518. [DOI] [PubMed] [Google Scholar]
- 13.Simpson ER. Role of aromatase in sex steroid action. Journal of Molecular Endocrinology. 2000;25(2):149–156. doi: 10.1677/jme.0.0250149. [DOI] [PubMed] [Google Scholar]
- 14.Gennari L, Nuti R, Bilezikian JP. Aromatase activity and bone homeostasis in men. Journal of Clinical Endocrinology and Metabolism. 2004;89(12):5898–5907. doi: 10.1210/jc.2004-1717. [DOI] [PubMed] [Google Scholar]
- 15.de Ronde W, Pols HAP, van Leeuwen JPTM, de Jong FH. The importance of oestrogens in males. Clinical Endocrinology. 2003;58(5):529–542. doi: 10.1046/j.1365-2265.2003.01669.x. [DOI] [PubMed] [Google Scholar]
- 16.Veldhuis JD, Lizarralde G, Iranmanesh A. Divergent effects of short term glucocorticoid excess on the gonadotropic and somatotropic axes in normal men. Journal of Clinical Endocrinology and Metabolism. 1992;74(1):96–102. doi: 10.1210/jcem.74.1.1727834. [DOI] [PubMed] [Google Scholar]
- 17.Bartsch W, Horst HJ, Becker H, Nehse G. Sex hormone binding globulin binding capacity, testosterone, 5α-dihydro-testosterone, oestradiol and prolactin in plasma of patients with prostatic carcinoma under various types of hormonal treatment. Acta Endocrinologica. 1977;85(3):650–664. doi: 10.1530/acta.0.0850650. [DOI] [PubMed] [Google Scholar]
- 18.Moorjani S, Dupont A, Labrie F, et al. Changes in plasma lipoproteins during various androgen suppression therapies in men with prostatic carcinoma: effects of orchiectomy, estrogen and combination treatment with luteinizing hormone-releasing hormone agonist and flutamide. Journal of Clinical Endocrinology and Metabolism. 1988;66(2):314–322. doi: 10.1210/jcem-66-2-314. [DOI] [PubMed] [Google Scholar]
- 19.Simpson E, Jones M, Davis S, Rubin G. Do intracrine mechanisms regulate aromatase expression? Journal of Steroid Biochemistry and Molecular Biology. 1999;69(1–6):447–452. doi: 10.1016/s0960-0760(99)00067-9. [DOI] [PubMed] [Google Scholar]
- 20.Labrie F, Bélanger A, Cusan L, Candas B. Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: intracrinology. Journal of Clinical Endocrinology and Metabolism. 1997;82(8):2403–2409. doi: 10.1210/jcem.82.8.4161. [DOI] [PubMed] [Google Scholar]
- 21.Bruch HR, Wolf L, Budde R, Romalo G, Schweikert HU. Androstenedione metabolism in cultured human osteoblast-like cells. Journal of Clinical Endocrinology and Metabolism. 1992;75(1):101–105. doi: 10.1210/jcem.75.1.1618995. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka S, Haji M, Nishi Y, Yanase T, Takayanagi R, Nawata H. Aromatase activity in human osteoblast-like osteosarcoma cell. Calcified Tissue International. 1993;52(2):107–109. doi: 10.1007/BF00308318. [DOI] [PubMed] [Google Scholar]
- 23.Sasano H, Uzuki M, Sawai T, et al. Aromatase in human bone tissue. Journal of Bone and Mineral Research. 1997;12(9):1416–1423. doi: 10.1359/jbmr.1997.12.9.1416. [DOI] [PubMed] [Google Scholar]
- 24.Hernández JL, Garcés CM, Sumillera M, et al. Aromatase expression in osteoarthritic and osteoporotic bone. Arthritis and Rheumatism. 2008;58(6):1696–1700. doi: 10.1002/art.23500. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Besman MJ, Sparkes RS, et al. Human aromatase: cDNA cloning, southern blot analysis, and assignment of the gene to chromosome 15. DNA. 1988;7(1):27–38. doi: 10.1089/dna.1988.7.27. [DOI] [PubMed] [Google Scholar]
- 26.Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis—some new perspectives. Endocrinology. 2001;142(11):4589–4594. doi: 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- 27.Sebastian S, Bulun SE. A highly complex organization of the regulatory region of the human CYP19 (aromatase) gene revealed by the human genome project. Journal of Clinical Endocrinology and Metabolism. 2001;86(10):4600–4602. doi: 10.1210/jcem.86.10.7947. [DOI] [PubMed] [Google Scholar]
- 28.Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. Journal of Steroid Biochemistry and Molecular Biology. 2003;86(3–5):219–224. doi: 10.1016/s0960-0760(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 29.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocrine Reviews. 2009;30(4):343–375. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Li R, Hu Y. The alternative noncoding exons 1 of aromatase (Cyp19) gene modulate gene expression in a posttranscriptional manner. Endocrinology. 2009;150(7):3301–3307. doi: 10.1210/en.2008-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shozu M, Simpson ER. Aromatase expression of human osteoblast-like cells. Molecular and Cellular Endocrinology. 1998;139(1–2):117–129. doi: 10.1016/s0303-7207(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 32.Shozu M, Zhao Y, Bulun SE, Simpson ER. Multiple splicing events involved in regulation of human aromatase expression by a novel promoter, I.6. Endocrinology. 1998;139(4):1610–1617. doi: 10.1210/endo.139.4.5878. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe M, Simpson ER, Pathirage N, Nakajin S, Clyne CD. Aromatase expression in the human fetal osteoblastic cell line SV-HFO. Journal of Molecular Endocrinology. 2004;32(2):533–545. doi: 10.1677/jme.0.0320533. [DOI] [PubMed] [Google Scholar]
- 34.Nawata H, Tanaka S, Tanaka S, et al. Aromatase in bone cell: association with osteoporosis in postmenopausal women. Journal of Steroid Biochemistry and Molecular Biology. 1995;53(1–6):165–174. doi: 10.1016/0960-0760(95)00031-t. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka S, Haji M, Takayanagi R, Tanaka S, Sugioka Y, Nawata H. 1,25-dihydroxyvitamin D3 enhances the enzymatic activity and expression of the messenger ribonucleic acid for aromatase cytochrome P450 synergistically with dexamethasone depending on the vitamin D receptor level in cultured human osteoblasts. Endocrinology. 1996;137(5):1860–1869. doi: 10.1210/endo.137.5.8612525. [DOI] [PubMed] [Google Scholar]
- 36.Yanase T, Suzuki S, Goto K, et al. Aromatase in bone: roles of vitamin D and androgens. Journal of Steroid Biochemistry and Molecular Biology. 2003;86(3–5):393–397. doi: 10.1016/s0960-0760(03)00349-2. [DOI] [PubMed] [Google Scholar]
- 37.Jeong JH, Jung YK, Kim HJ, et al. The gene for aromatase, a rate-limiting enzyme for local estrogen biosynthesis, is a downstream target gene of Runx2 in skeletal tissues. Molecular and Cellular Biology. 2010;30(10):2365–2375. doi: 10.1128/MCB.00672-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bulun SE. Aromatase deficiency and estrogen resistance: from molecular genetics to clinic. Seminars in Reproductive Medicine. 2000;18(1):31–39. doi: 10.1055/s-2000-13481. [DOI] [PubMed] [Google Scholar]
- 39.Belgorosky A, Guercio G, Pepe C, Saraco N, Rivarola MA. Genetic and clinical spectrum of aromatase deficiency in infancy, childhood and adolescence. Hormone Research. 2009;72(6):321–330. doi: 10.1159/000249159. [DOI] [PubMed] [Google Scholar]
- 40.Rochira V, Carani C. Aromatase deficiency in men: a clinical perspective. Nature Reviews Endocrinology. 2009;5(10):559–568. doi: 10.1038/nrendo.2009.176. [DOI] [PubMed] [Google Scholar]
- 41.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. Journal of Clinical Endocrinology and Metabolism. 1995;80(12):3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 42.Carani C, Qin K, Simoni M, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. The New England Journal of Medicine. 1997;337(2):91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 43.Deladoëy J, Flück C, Bex M, Yoshimura N, Harada N, Mullis PE. Aromatase deficiency caused by a novel P450 arom gene mutation: impact of absent estrogen production on serum gonadotropin concentration in a boy. Journal of Clinical Endocrinology and Metabolism. 1999;84(11):4050–4054. doi: 10.1210/jcem.84.11.6135. [DOI] [PubMed] [Google Scholar]
- 44.Herrmann BL, Saller B, Janssen OE, et al. Impact of estrogen replacement therapy in a male with congenital aromatase deficiency caused by a novel mutation in the CYP19 gene. Journal of Clinical Endocrinology and Metabolism. 2002;87(12):5476–5484. doi: 10.1210/jc.2002-020498. [DOI] [PubMed] [Google Scholar]
- 45.Mittre Hervé MH, Kottler ML, Pura M. Human gene mutations. Gene symbol: CYP19: disease: aromatase deficiency. Human Genetics. 2004;114(2):p. 224. [PubMed] [Google Scholar]
- 46.Bouillon R, Bex M, Vanderschueren D, Boonen S. Estrogens are essential for male pubertal periosteal bone expansion. Journal of Clinical Endocrinology and Metabolism. 2004;89(12):6025–6029. doi: 10.1210/jc.2004-0602. [DOI] [PubMed] [Google Scholar]
- 47.Maffei L, Murata Y, Rochira V, et al. Dysmetabolic syndrome in a man with a Novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. Journal of Clinical Endocrinology and Metabolism. 2004;89(1):61–70. doi: 10.1210/jc.2003-030313. [DOI] [PubMed] [Google Scholar]
- 48.Maffei L, Rochira V, Zirilli L, et al. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clinical Endocrinology. 2007;67(2):218–224. doi: 10.1111/j.1365-2265.2007.02864.x. [DOI] [PubMed] [Google Scholar]
- 49.Lanfranco F, Zirilli L, Baldi M, et al. A novel mutation in the human aromatase gene: insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone. 2008;43(3):628–635. doi: 10.1016/j.bone.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. The New England Journal of Medicine. 1994;331(16):1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 51.Bilezikian JP, Morishima A, Bell J, Grumbach MM. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. The New England Journal of Medicine. 1998;339(9):599–603. doi: 10.1056/NEJM199808273390905. [DOI] [PubMed] [Google Scholar]
- 52.Rochira V, Faustini-Fustini M, Balestrieri A, Carani C. Estrogen replacement therapy in a man with congenital aromatase deficiency: effects of different doses of transdermal estradiol on bone mineral density and hormonal parameters. Journal of Clinical Endocrinology and Metabolism. 2000;85(5):1841–1845. doi: 10.1210/jcem.85.5.6583. [DOI] [PubMed] [Google Scholar]
- 53.Seeman E. Clinical review 137: sexual dimorphism in skeletal size, density, and strength. Journal of Clinical Endocrinology and Metabolism. 2001;86(10):4576–4584. doi: 10.1210/jcem.86.10.7960. [DOI] [PubMed] [Google Scholar]
- 54.Sundberg M, Gärdsell P, Johnell O, Ornstein E, Karlsson MK, Sernbo I. Pubertal bone growth in the femoral neck is predominantly characterized by increased bone size and not by increased bone density—a 4-year longitudinal study. Osteoporosis International. 2003;14(7):548–558. doi: 10.1007/s00198-003-1406-3. [DOI] [PubMed] [Google Scholar]
- 55.Seeman E. Pathogenesis of bone fragility in women and men. The Lancet. 2002;359(9320):1841–1850. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 56.Rochira V, Zirilli L, Madeo B, et al. Skeletal effects of long-term estrogen and testosterone replacement treatment in a man with congenital aromatase deficiency: evidences of a priming effect of estrogen for sex steroids action on bone. Bone. 2007;40(6):1662–1668. doi: 10.1016/j.bone.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 57.Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(12):6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Honda SI, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochemical and Biophysical Research Communications. 1998;252(2):445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- 59.Toda K, Saibara T, Okada T, Onishi S, Shizuta Y. A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19) Journal of Endocrinology. 2001;168(2):217–220. doi: 10.1677/joe.0.1680217. [DOI] [PubMed] [Google Scholar]
- 60.Slemenda CW, Longcope C, Zhou L, Hui SL, Peacock M, Johnston CC. Sex steroids and bone mass in older men. Positive associations with serum estrogens and negative associations with androgens. Journal of Clinical Investigation. 1997;100(7):1755–1759. doi: 10.1172/JCI119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greendale GA, Edelstein S, Barrett-Connor E. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo study. Journal of Bone and Mineral Research. 1997;12(11):1833–1843. doi: 10.1359/jbmr.1997.12.11.1833. [DOI] [PubMed] [Google Scholar]
- 62.Khosla S, Melton LJ, III, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. Journal of Clinical Endocrinology and Metabolism. 1998;83(7):2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 63.Ongphiphadhanakul B, Rajatanavin R, Chanprasertyothin S, Piaseu N, Chailurkit L. Serum oestradiol and oestrogen-receptor gene polymorphism are associated with bone mineral density independently of serum testosterone in normal males. Clinical Endocrinology. 1998;49(6):803–809. doi: 10.1046/j.1365-2265.1998.00631.x. [DOI] [PubMed] [Google Scholar]
- 64.Center JR, Nguyen TV, Sambrook PN, Eisman JA. Hormonal and biochemical parameters in the determination of osteoporosis in elderly men. Journal of Clinical Endocrinology and Metabolism. 1999;84(10):3626–3635. doi: 10.1210/jcem.84.10.6051. [DOI] [PubMed] [Google Scholar]
- 65.Amin S, Zhang Y, Sawin CT, et al. Association of hypogonadism and estradiol levels with bone mineral density in elderly men from the Framingham study. Annals of Internal Medicine. 2000;133(12):951–963. doi: 10.7326/0003-4819-133-12-200012190-00010. [DOI] [PubMed] [Google Scholar]
- 66.Barrett-Connor E, Mueller JE, von Mühlen DG, Laughlin GA, Schneider DL, Sartoris DJ. Low levels of estradiol are associated with vertebral fractures in older men, but not women: the Rancho Bernardo study. Journal of Clinical Endocrinology and Metabolism. 2000;85(1):219–223. doi: 10.1210/jcem.85.1.6327. [DOI] [PubMed] [Google Scholar]
- 67.Taxel P, Raisz LG. The effect of estrogen therapy on older men with low bone mass. Journal of Bone and Mineral Research. 1997;12:p. S353. [Google Scholar]
- 68.Anderson FH, Francis RM, Peaston RT, Wastell HJ. Androgen supplementation in eugonadal men with osteoporosis: effects of six months’ treatment on markers of bone formation and resorption. Journal of Bone and Mineral Research. 1997;12(3):472–478. doi: 10.1359/jbmr.1997.12.3.472. [DOI] [PubMed] [Google Scholar]
- 69.Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. Journal of Clinical Investigation. 2000;106(12):1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lian JB, Stein GS, Canalis E, Gehron Robey P, Boskey AL. Bone formation: osteoblast lineage cells, growth factors, matrix proteins, and the mineralization process. In: Favus MF, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 4th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 1999. pp. 14–38. [Google Scholar]
- 71.Orwoll E. Gender differences in the skeleton: osteoporosis. Journal of Women’s Health. 1995;4(4):429–431. [Google Scholar]
- 72.Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS. Differential effects of androgens and estrogens on bone turnover in normal men. Journal of Clinical Endocrinology and Metabolism. 2003;88(1):204–210. doi: 10.1210/jc.2002-021036. [DOI] [PubMed] [Google Scholar]
- 73.Taxel P, Kennedy DG, Fall PM, Willard AK, Clive JM, Raisz LG. The effect of aromatase inhibition on sex steroids, gonadotropins, and markers of bone turnover in older men. Journal of Clinical Endocrinology and Metabolism. 2001;86(6):2869–2874. doi: 10.1210/jcem.86.6.7541. [DOI] [PubMed] [Google Scholar]
- 74.Leder BZ, Finkelstein JS. Effect of aromatase inhibition on bone metabolism in elderly hypogonadal men. Osteoporosis International. 2005;16(12):1487–1494. doi: 10.1007/s00198-005-1890-8. [DOI] [PubMed] [Google Scholar]
- 75.Burnett-Bowie SAM, Roupenian KC, Dere ME, Lee H, Leder BZ. Effects of aromatase inhibition in hypogonadal older men: a randomized, double-blind, placebo-controlled trial. Clinical Endocrinology. 2009;70(1):116–123. doi: 10.1111/j.1365-2265.2008.03327.x. [DOI] [PubMed] [Google Scholar]
- 76.Burnett-Bowie SAM, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. Journal of Clinical Endocrinology and Metabolism. 2009;94(12):4785–4792. doi: 10.1210/jc.2009-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siegelmann-Danieli N, Buetow KH. Constitutional genetic variation at the human aromatase gene (Cyp19) and breast cancer risk. British Journal of Cancer. 1999;79(3–4):456–463. doi: 10.1038/sj.bjc.6690071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kristensen VN, Harada N, Yoshimura N, et al. Genetic variants of CYP19 (aromatase) and breast cancer risk. Oncogene. 2000;19(10):1329–1333. doi: 10.1038/sj.onc.1203425. [DOI] [PubMed] [Google Scholar]
- 79.Masi L, Becherini L, Gennari L, et al. Polymorphism of the aromatase gene in postmenopausal italian women: distribution and correlation with bone mass and fracture risk. Journal of Clinical Endocrinology and Metabolism. 2001;86(5):2263–2269. doi: 10.1210/jcem.86.5.7450. [DOI] [PubMed] [Google Scholar]
- 80.Van Pottelbergh I, Goemaere S, Kaufman JM. Bioavailable estradiol and an aromatase gene polymorphism are determinants of bone mineral density changes in men over 70 years of age. Journal of Clinical Endocrinology and Metabolism. 2003;88(7):3075–3081. doi: 10.1210/jc.2002-021691. [DOI] [PubMed] [Google Scholar]
- 81.Gennari L, Masi L, Merlotti D, et al. A polymorphic CYP19 TTTA repeat influences aromatase activity and estrogen levels in elderly men: effects on bone metabolism. Journal of Clinical Endocrinology and Metabolism. 2004;89(6):2803–2810. doi: 10.1210/jc.2003-031342. [DOI] [PubMed] [Google Scholar]
- 82.Somner J, McLellan S, Cheung J, et al. Polymorphisms in the P450 c17 (17-hydroxylase/17,20-lyase) and P450 c19 (aromatase) genes: association with serum sex steroid concentrations and bone mineral density in postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 2004;89(1):344–351. doi: 10.1210/jc.2003-030164. [DOI] [PubMed] [Google Scholar]
- 83.Ahsan H, Whittemore AS, Chen Y, et al. Variants in estrogen-biosynthesis genes CYP17 and CYP19 and breast cancer risk: a family-based genetic association study. Breast Cancer Research. 2005;7(1):R71–R81. doi: 10.1186/bcr951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riancho JA. Polymorphisms in the CYP19 gene that influence bone mineral density. Pharmacogenomics. 2007;8(4):339–352. doi: 10.2217/14622416.8.4.339. [DOI] [PubMed] [Google Scholar]
- 85.Talbott KE, Gammon MD, Kibriya MG, et al. A CYP19 (aromatase) polymorphism is associated with increased premenopausal breast cancer risk. Breast Cancer Research and Treatment. 2008;111(3):481–487. doi: 10.1007/s10549-007-9794-2. [DOI] [PubMed] [Google Scholar]
- 86.Yang HP, Bosquet JG, Li Q, et al. Common genetic variation in the sex hormone metabolic pathway and endometrial cancer risk: pathway-based evaluation of candidate genes. Carcinogenesis. 2010;31(5):827–833. doi: 10.1093/carcin/bgp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eriksson AL, Lorentzon M, Vandenput L, et al. Genetic variations in sex steroid-related genes as predictors of serum estrogen levels in men. Journal of Clinical Endocrinology and Metabolism. 2009;94(3):1033–1041. doi: 10.1210/jc.2008-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Setiawan VW, Doherty JA, Shu XO, et al. Two estrogen-related variants in CYP19A1 and endometrial cancer risk: a pooled analysis in the epidemiology of endometrial cancer consortium. Cancer Epidemiology Biomarkers and Prevention. 2009;18(1):242–247. doi: 10.1158/1055-9965.EPI-08-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Limer KL, Pye SR, Thomson W, et al. Genetic variation in sex hormone genes influences heel ultrasound parameters in middle-aged and elderly men: results from the European male aging study (EMAS) Journal of Bone and Mineral Research. 2009;24(2):314–323. doi: 10.1359/jbmr.080912. [DOI] [PubMed] [Google Scholar]
- 90.Travis RC, Schumacher F, Hirschhorn JN, et al. CYP19A1 genetic variation in relation to prostate cancer risk and circulating sex hormone concentrations in men from the breast and prostate cancer cohort consortium. Cancer Epidemiology Biomarkers and Prevention. 2009;18(10):2734–2744. doi: 10.1158/1055-9965.EPI-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma X, Qi X, Chen C, et al. Association between CYP19 polymorphisms and breast cancer risk: results from 10,592 cases and 11,720 controls. Breast Cancer Research and Treatment. 2010;122(2):495–501. doi: 10.1007/s10549-009-0693-6. [DOI] [PubMed] [Google Scholar]
- 92.Remes T, Väisänen SB, Mahonen A, et al. Aerobic exercise and bone mineral density in middle-aged Finnish men: a controlled randomized trial with reference to androgen receptor, aromatase, and estrogen receptor α gene polymorphisms. Bone. 2003;32(4):412–420. doi: 10.1016/s8756-3282(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 93.Lorentzon M, Swanson C, Eriksson AL, Mellström D, Ohlsson C. Polymorphisms in the aromatase gene predict areal BMD as a result of affected cortical bone size: the GOOD study. Journal of Bone and Mineral Research. 2006;21(2):332–339. doi: 10.1359/JBMR.051026. [DOI] [PubMed] [Google Scholar]
- 94.Riancho JA, Sañudo C, Valero C, et al. Association of the aromatase gene alleles with BMD: epidemiological and functional evidence. Journal of Bone and Mineral Research. 2009;24(10):1709–1718. doi: 10.1359/jbmr.090404. [DOI] [PubMed] [Google Scholar]
- 95.Yang S, Fang Z, Suzuki T, et al. Regulation of aromatase P450 expression in endometriotic and endometrial stromal cells by CCAAT/enhancer binding proteins (C/EBPs): decreased C/EBPβ in endometriosis is associated with overexpression of aromatase. Journal of Clinical Endocrinology and Metabolism. 2002;87(5):2336–2345. doi: 10.1210/jcem.87.5.8486. [DOI] [PubMed] [Google Scholar]
- 96.Ishikawa H, Fencki V, Marsh EE, et al. CCAAT/enhancer binding protein β regulates aromatase expression via multiple and novel cis-regulatory sequences in uterine leiomyoma. Journal of Clinical Endocrinology and Metabolism. 2008;93(3):981–991. doi: 10.1210/jc.2007-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Demura M, Bulun SE. CpG dinucleotide methylation of the CYP19 I.3/II promoter modulates cAMP-stimulated aromatase activity. Molecular and Cellular Endocrinology. 2008;283(1–2):127–132. doi: 10.1016/j.mce.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 98.Yanase T, Mu YM, Nishi Y, et al. Regulation of aromatase by nuclear receptors. Journal of Steroid Biochemistry and Molecular Biology. 2001;79(1–5):187–192. doi: 10.1016/s0960-0760(01)00161-3. [DOI] [PubMed] [Google Scholar]
- 99.Mu YM, Yanase T, Nishi Y, Takayanagi R, Goto K, Nawata H. Combined treatment with specific ligands for PPARγ:RXR nuclear receptor system markedly inhibits the expression of cytochrome P450arom in human granulosa cancer cells. Molecular and Cellular Endocrinology. 2001;181(1–2):239–248. doi: 10.1016/s0303-7207(00)00457-3. [DOI] [PubMed] [Google Scholar]
- 100.Mu YM, Yanase T, Nishi Y, et al. Insulin sensitizer, troglitazone, directly inhibits aromatase activity in human ovarian granulosa cells. Biochemical and Biophysical Research Communications. 2000;271(3):710–713. doi: 10.1006/bbrc.2000.2701. [DOI] [PubMed] [Google Scholar]
- 101.Lovekamp-Swan T, Jetten AM, Davis BJ. Dual activation of PPARα and PPARγ by mono-(2-ethylhexyl) phthalate in rat ovarian granulosa cells. Molecular and Cellular Endocrinology. 2003;201(1–2):133–141. doi: 10.1016/s0303-7207(02)00423-9. [DOI] [PubMed] [Google Scholar]
- 102.Toda K, Okada T, Miyaura C, Saibara T. Fenofibrate, a ligand for PPARγ, inhibits aromatase cytochrome P450 expression in the ovary of mouse. Journal of Lipid Research. 2003;44(2):265–270. doi: 10.1194/jlr.M200327-JLR200. [DOI] [PubMed] [Google Scholar]
- 103.Gasnier C, Dumont C, Benachour N, Clair E, Chagnon MC, Séralini GE. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology. 2009;262(3):184–191. doi: 10.1016/j.tox.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 104.Figura N, Gennari L, Merlotti D, et al. Prevalence of helicobacter pylori infection in male patients with osteoporosis and controls. Digestive Diseases and Sciences. 2005;50(5):847–852. doi: 10.1007/s10620-005-2651-4. [DOI] [PubMed] [Google Scholar]
- 105.Longcope C, Pratt JH, Fineberg E. Estrogen and androgen dynamics in liver disease. Journal of Endocrinological Investigation. 1984;7(6):629–634. doi: 10.1007/BF03349497. [DOI] [PubMed] [Google Scholar]
- 106.Doering IL, Richter E. Inhibition of human aromatase by myosmine. Drug Metabolism Letters. 2009;3(2):83–86. doi: 10.2174/187231209788654045. [DOI] [PubMed] [Google Scholar]
- 107.Takayanagi R, Goto K, Suzuki S, Tanaka S, Shimoda S, Nawata H. Dehydroepiandrosterone (DHEA) as a possible source for estrogen formation in bone cells: correlation between bone mineral density and serum DHEA-sulfate concentration in postmenopausal women, and the presence of aromatase to be enhanced by 1,25-dihydroxyvitamin D3 in human osteoblasts. Mechanisms of Ageing and Development. 2002;123(8):1107–1114. doi: 10.1016/s0047-6374(01)00394-3. [DOI] [PubMed] [Google Scholar]
- 108.Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000;141(4):1317–1324. doi: 10.1210/endo.141.4.7403. [DOI] [PubMed] [Google Scholar]
- 109.Bulun SE, Noble LS, Takayama K, et al. Endocrine disorders associated with inappropriately high aromatase expression. Journal of Steroid Biochemistry and Molecular Biology. 1997;61(3–6):133–139. [PubMed] [Google Scholar]
- 110.Young J, Bulun SE, Agarwal V, et al. Aromatase expression in a feminizing adrenocortical tumor. Journal of Clinical Endocrinology and Metabolism. 1996;81(9):3173–3176. doi: 10.1210/jcem.81.9.8784064. [DOI] [PubMed] [Google Scholar]
- 111.Wan Y. PPARγ in bone homeostasis. Trends in Endocrinology and Metabolism. 2010;21(12):722–728. doi: 10.1016/j.tem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 112.Aiginger P, Kolbe H, Kuhbock J, Spona J, Geyer G. The endocrinology of testicular germinal cell tumours. Acta Endocrinologica. 1981;97(3):419–426. doi: 10.1530/acta.0.0970419. [DOI] [PubMed] [Google Scholar]
- 113.Gordon GG, Olivo J, Rafil F, Southren AL. Conversion of androgens to estrogens in cirrhosis of the liver. Journal of Clinical Endocrinology and Metabolism. 1975;40(6):1018–1026. doi: 10.1210/jcem-40-6-1018. [DOI] [PubMed] [Google Scholar]
- 114.Ueyama T, Shirasawa N, Numazawa M, et al. Gastric parietal cells: potent endocrine role in secreting estrogen as a possible regulator of gastro-hepatic axis. Endocrinology. 2002;143(8):3162–3170. doi: 10.1210/endo.143.8.8974. [DOI] [PubMed] [Google Scholar]
- 115.Burul-Bozkurt N, Pekiner C, Kelicen P. Diabetes alters aromatase enzyme levels in gonadal tissues of rats. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2010;382(1):33–41. doi: 10.1007/s00210-010-0518-5. [DOI] [PubMed] [Google Scholar]
- 116.Prabhu A, Xu Q, Manigrasso MB, et al. Expression of aromatase, androgen and estrogen receptors in peripheral target tissues in diabetes. Steroids. 2010;75(11):779–787. doi: 10.1016/j.steroids.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brown KA, Hunger NI, Docanto M, Simpson ER. Metformin inhibits aromatase expression in human breast adipose stromal cells via stimulation of AMP-activated protein kinase. Breast Cancer Research and Treatment. 2010;123(2):591–596. doi: 10.1007/s10549-010-0834-y. [DOI] [PubMed] [Google Scholar]
- 118.Rice S, Pellatt L, Ramanathan K, Whitehead SA, Mason HD. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology. 2009;150(10):4794–4801. doi: 10.1210/en.2009-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]


