Abstract
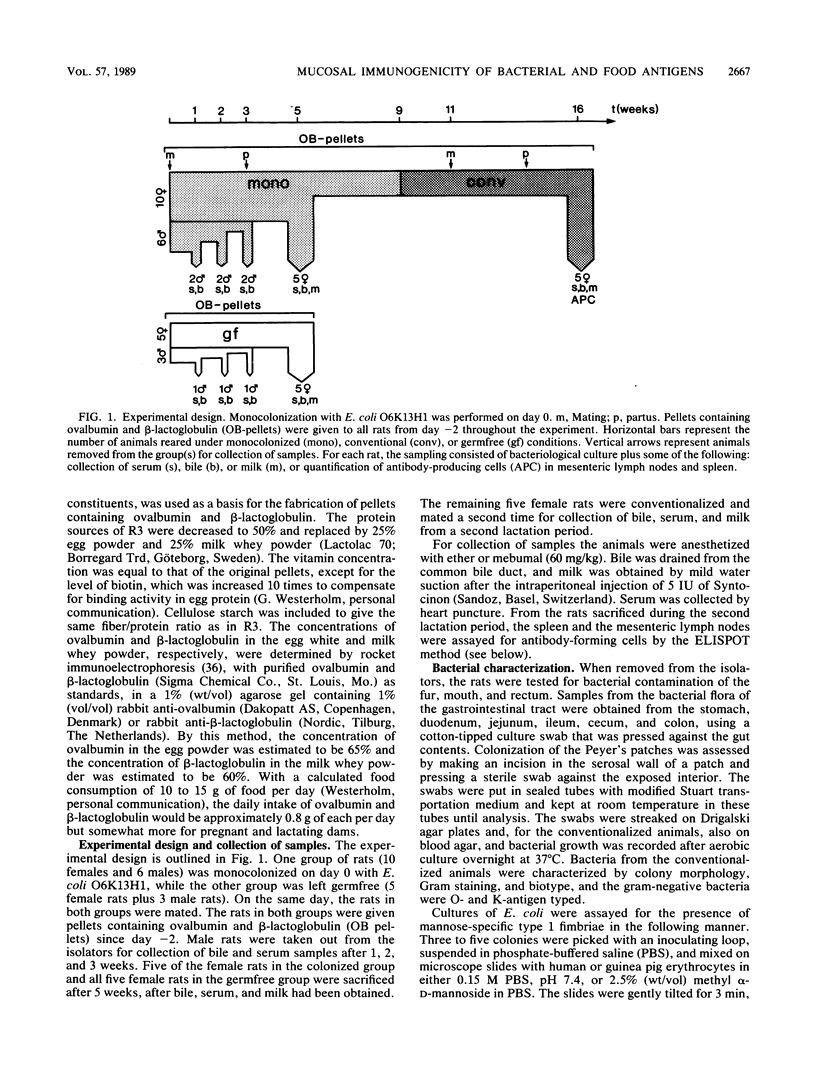
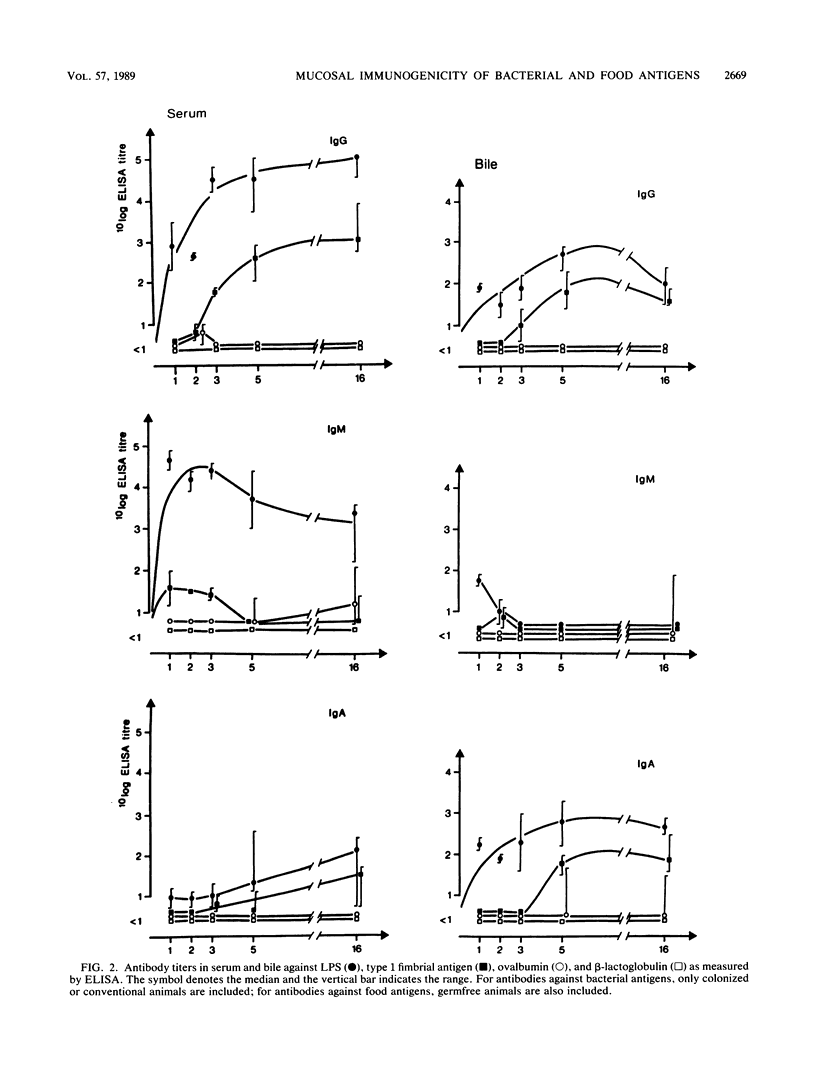
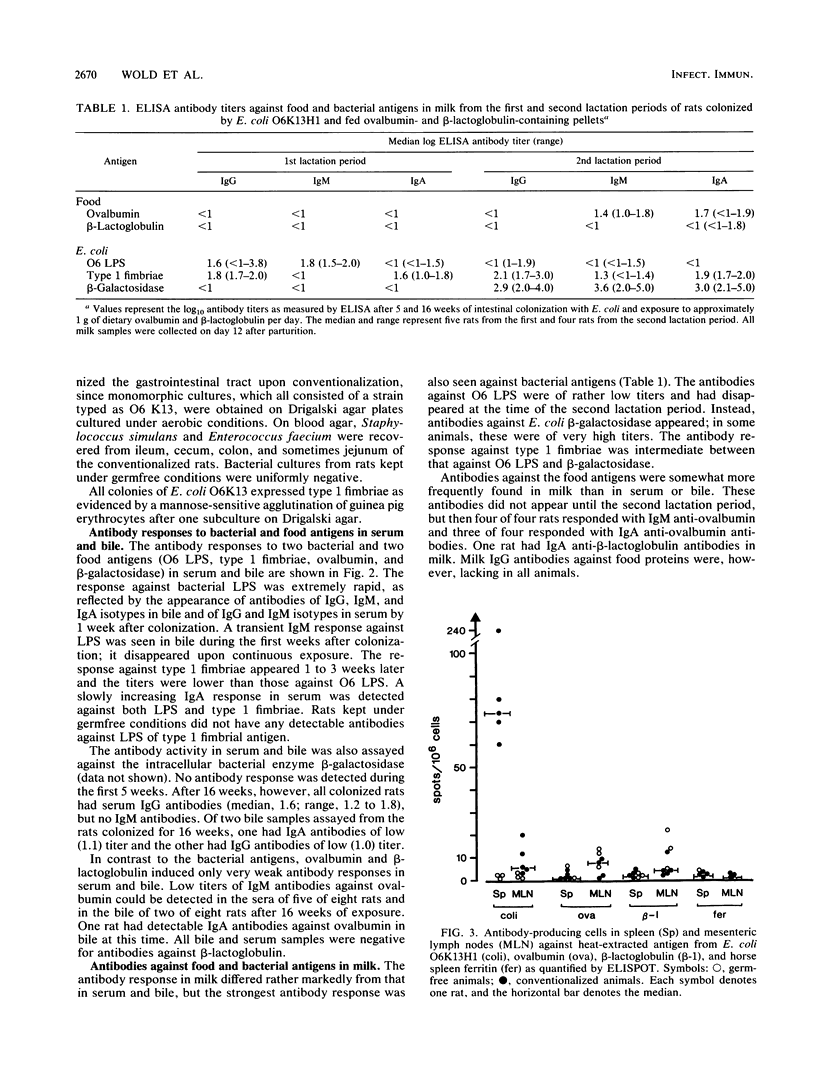
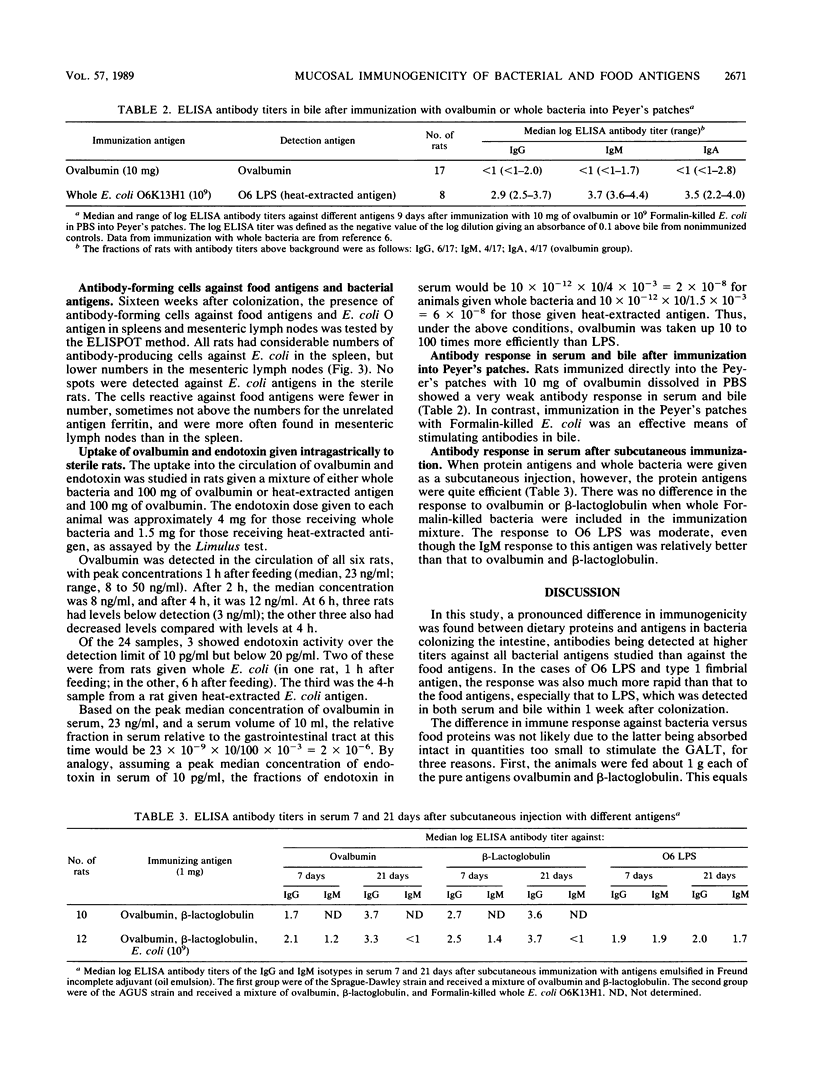
The mucosa-associated lymphoid tissue may deviate from its systemic counterpart in being able to discriminate between microbial and nonmicrobial antigens. To study this, the systemic and mucosal antibody responses to bacterial and food antigens were followed in parallel in female rats during two pregnancies and lactation periods. Germfree rats were monocolonized with an Escherichia coli O6K13H1 strain, and their diet was switched to pellets containing large amounts of ovalbumin and beta-lactoglobulin. Antibodies against O6 lipopolysaccharide already appeared in serum and bile 1 week after colonization, and those against type 1 fimbriae appeared a few weeks later. Serum immunoglobulin G antibodies against the E. coli enzyme beta-galactosidase were found in moderate titers in all rats after 16 weeks of exposure. In contrast, few rats had detectable antibody levels against the dietary proteins ovalbumin and beta-lactoglobulin in serum or bile even after 16 weeks of exposure. In the milk, antibodies against E. coli beta-galactosidase and type 1 fimbriae reached the highest titers, while moderate titers were found against the food antigens and against O6 lipopolysaccharide. The difference in immune reactivity against bacterial versus dietary antigens was not likely due to insufficient amounts of the latter reaching lymphoid tissue, since (i) uptake studies indicated that ovalbumin was more efficiently taken up than endotoxin and (ii) the same difference in antigenicity between ovalbumin and E. coli was seen after immunization directly into Peyer's patches. We therefore suggest that a prerequisite for a strong mucosal antibody response is that the antigen be encountered by the gut-associated lymphoid tissue within microorganisms capable of stimulating antigen presentation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandtzaeg P., Valnes K., Scott H., Rognum T. O., Bjerke K., Baklien K. The human gastrointestinal secretory immune system in health and disease. Scand J Gastroenterol Suppl. 1985;114:17–38. doi: 10.3109/00365528509093765. [DOI] [PubMed] [Google Scholar]
- Coulie P. G., Cayphas S., Vink A., Uyttenhove C., Van Snick J. Interleukin-HP1-related hybridoma and plasmacytoma growth factors induced by lipopolysaccharide in vivo. Eur J Immunol. 1987 Aug;17(8):1217–1220. doi: 10.1002/eji.1830170821. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C. C., Nilsson L. A., Nygren H., Ouchterlony O., Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Dec 16;65(1-2):109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C. C., Nilsson L. A., Tarkowski A., Ouchterlony O., Jeansson S., Gretzer C. An immunoenzyme procedure for enumerating fibronectin-secreting cells. J Immunoassay. 1984;5(3-4):291–302. doi: 10.1080/01971528408063013. [DOI] [PubMed] [Google Scholar]
- Dahlgren U. I., Ahlstedt S., Hanson L. A. The localization of the antibody response in milk or bile depends on the nature of the antigen. J Immunol. 1987 Mar 1;138(5):1397–1402. [PubMed] [Google Scholar]
- Dahlgren U., Ahlstedt S., Andersson T., Hedman L., Hanson L. A. IgA antibodies in rat bile are not solely derived from thoracic duct lymph. Scand J Immunol. 1983 Jun;17(6):569–574. doi: 10.1111/j.1365-3083.1983.tb00825.x. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Source and protective function of coproantibodies in intestinal disease. Am J Clin Nutr. 1972 Dec;25(12):1357–1363. doi: 10.1093/ajcn/25.12.1357. [DOI] [PubMed] [Google Scholar]
- GUSTAFSSON B. E. Lightweight stainless steel systems for rearing germfree animals. Ann N Y Acad Sci. 1959 May 8;78:17–28. doi: 10.1111/j.1749-6632.1959.tb53092.x. [DOI] [PubMed] [Google Scholar]
- Hohmann A., Schmidt G., Rowley D. Intestinal and serum antibody responses in mice after oral immunization with Salmonella, Escherichia coli, and Salmonella-Escherichia coli hybrid strains. Infect Immun. 1979 Jul;25(1):27–33. doi: 10.1128/iai.25.1.27-33.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Eggertsen G., Hanson L. A., Lincoln K. Immunodiffusion studies on Escherichia coli. 1. Identification of O, K and H antigens in an O6 strain. Acta Pathol Microbiol Scand. 1969;76(2):304–318. [PubMed] [Google Scholar]
- Husby S., Jensenius J. C., Svehag S. E. ELISA quantitation of IgG subclass antibodies to dietary antigens. J Immunol Methods. 1985 Oct 10;82(2):321–331. doi: 10.1016/0022-1759(85)90364-3. [DOI] [PubMed] [Google Scholar]
- Husby S., Jensenius J. C., Svehag S. E. Passage of undegraded dietary antigen into the blood of healthy adults. Quantification, estimation of size distribution, and relation of uptake to levels of specific antibodies. Scand J Immunol. 1985 Jul;22(1):83–92. doi: 10.1111/j.1365-3083.1985.tb01862.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Fujiyama Y., Hagiwara K., Kondoh H. Resistance of normal serum IgA and secretory IgA to bacterial IgA proteases: evidence for the presence of enzyme-neutralizing antibodies in both serum and secretory IgA, and also in serum IgG. Microbiol Immunol. 1987;31(11):1097–1106. doi: 10.1111/j.1348-0421.1987.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Levich J. D., Signorella A. P., Wittenberg G., Weigle W. O. Macrophage handling of a tolerogen and the role of IL 1 in tolerance induction in a helper T cell clone in vitro. J Immunol. 1987 Jun 1;138(11):3675–3679. [PubMed] [Google Scholar]
- Lim P. L., Rowley D. Intestinal absorption of bacterial antigens in normal adult mice. II. A comparative study of techniques. Int Arch Allergy Appl Immunol. 1985;76(1):30–36. doi: 10.1159/000233657. [DOI] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K. C., Jewell D. P. Characterization of antigen-presenting activity of intestinal mononuclear cells isolated from normal and inflammatory bowel disease colon and ileum. Immunology. 1988 Dec;65(4):543–549. [PMC free article] [PubMed] [Google Scholar]
- Markmann J., Lo D., Naji A., Palmiter R. D., Brinster R. L., Heber-Katz E. Antigen presenting function of class II MHC expressing pancreatic beta cells. Nature. 1988 Dec 1;336(6198):476–479. doi: 10.1038/336476a0. [DOI] [PubMed] [Google Scholar]
- Mayrhofer G., Pugh C. W., Barclay A. N. The distribution, ontogeny and origin in the rat of Ia-positive cells with dendritic morphology and of Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol. 1983 Feb;13(2):112–122. doi: 10.1002/eji.1830130206. [DOI] [PubMed] [Google Scholar]
- McKenzie D. Alloantigen presentation by B cells. Requirement for IL-1 and IL-6. J Immunol. 1988 Nov 1;141(9):2907–2911. [PubMed] [Google Scholar]
- Peri B. A., Theodore C. M., Losonsky G. A., Fishaut J. M., Rothberg R. M., Ogra P. L. Antibody content of rabbit milk and serum following inhalation or ingestion of respiratory syncytial virus and bovine serum albumin. Clin Exp Immunol. 1982 Apr;48(1):91–101. [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Kaper J. B., Mekalanos J. J. Determinants of immunogenicity and mechanisms of protection by virulent and mutant Vibrio cholerae O1 in rabbits. Infect Immun. 1988 Jan;56(1):142–148. doi: 10.1128/iai.56.1.142-148.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Kaper J. B., Mekalanos J. J., Cray W. C., Jr, Richardson K. Determinants of the immunogenicity of live virulent and mutant Vibrio cholerae O1 in rabbit intestine. Infect Immun. 1987 Feb;55(2):477–481. doi: 10.1128/iai.55.2.477-481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Witmer M. D. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. C., Parrott M. V. The induction of tolerance to a soluble protein antigen by oral administration. Immunology. 1974 Oct;27(4):631–639. [PMC free article] [PubMed] [Google Scholar]
- Walker W. A., Cornell R., Davenport L. M., Isselbacher K. J. Macromolecular absorption. Mechanism of horseradish peroxidase uptake and transport in adult and neonatal rat intestine. J Cell Biol. 1972 Aug;54(2):195–205. doi: 10.1083/jcb.54.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. A., Isselbacher K. J. Uptake and transport of macromolecules by the intestine. Possible role in clinical disorders. Gastroenterology. 1974 Sep;67(3):531–550. [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Wells C. L., Maddaus M. A., Erlandsen S. L., Simmons R. L. Evidence for the phagocytic transport of intestinal particles in dogs and rats. Infect Immun. 1988 Jan;56(1):278–282. doi: 10.1128/iai.56.1.278-282.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aizpurua H. J., Russell-Jones G. J. Oral vaccination. Identification of classes of proteins that provoke an immune response upon oral feeding. J Exp Med. 1988 Feb 1;167(2):440–451. doi: 10.1084/jem.167.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]


