Abstract
Auditory brainstem responses (ABRs) were recorded in adult budgerigars, canaries, and zebra finches in quiet and in three levels of white noise for tone stimuli between 1 and 4 kHz. Similar to behavioral results, masked ABR thresholds increased linearly with increasing noise levels. When the three species are considered together, ABR-derived CRs were higher than behavioral CRs by 18–23 dB between 2 and 4 kHz and by about 30 dB at 1 kHz. This study clarifies the utility of using ABRs for estimating masked auditory thresholds in natural environmental noises in species that cannot be tested behaviorally.
INTRODUCTION
Birds do not live in a quiet world. The study of auditory sensitivity in a background of noise provides a closer estimate to what birds experience in the natural world. While behavioral measures of hearing are the most relevant to what occurs in nature, psychoacoustic methods can be time consuming. The auditory brainstem response (ABR) (a far-field potential produced in response to a short acoustic stimulus) has been used to quickly estimate absolute auditory sensitivity for pure tones in a wide range of animals, birds included (Brittan-Powell et al., 2002, 2010). The purpose of the current study was to determine whether the ABR could also be used to estimate hearing thresholds masked by noise. Here, we compare ABR thresholds in three species of birds for which behavioral measures of both absolute and masked thresholds are known: the budgerigar (Melopsittacus undulatus), the canary (Serinus canarius), and the zebra finch (Taeniopygia guttata).
For the masking of pure tones by broadband white noise, the ratio between the sound pressure level of the tone at hearing threshold and the spectrum level (dB/Hz) of the masking noise in the frequency region of the tone remains constant over a wide range of noise levels (Hawkins and Stevens, 1950) and is called the critical ratio (CR) (Zwicker et al., 1957). For most vertebrate species tested, including the canary and the zebra finch, behavioral CR measures increase by about 3 dB per octave over most of the species’ hearing range (Okanoya and Dooling, 1987, 1990; Lohr et al., 2004). Henry and Lucas (2010) used notched-noise masking and ABRs to determine auditory filter shapes in songbirds and found that auditory filters increased in bandwidth with increasing frequency in four species. This suggests that tone thresholds in the presence of broadband noise, as measured by the ABR, would also show increasing CRs with frequency. The budgerigar, however, is a known exception to the rule and shows the smallest behavioral CRs between 2 and 4 kHz (Dooling and Saunders, 1975). Here, we test whether masked thresholds measured in birds using the ABR method show the same comparative patterns of results across species and frequency as behavioral experiments.
METHODS
Adult budgerigars, canaries, and zebra finches (five birds of each species) served as subjects in this experiment. The birds were housed in an aviary at the University of Maryland. Each bird had free access to food and water, except on the day of the experiment, prior to sedation. All experimental procedures were in agreement with the Animal Care and Use Committee at the University of Maryland.
Experiments were executed in a walk-in sound isolation chamber with an overall noise floor level of 27 dB sound pressure level (SPL). The general procedures were modeled after those of Brittan-Powell et al. (2002), except where noted. After sedation [intramuscular injection of ketamine 50 mg/kg and diazepam 2 mg/kg], the bird was positioned so that the center of the speaker (JBL 2105H) was 20 cm from the bird’s right ear. ABRs were recorded with subdermal needle electrodes. The stimulus presentation, ABR acquisition, and analysis were managed through Tucker Davis Technologies (TDT) hardware and software packages (System 3). Stimuli consisted either of pure tones or of pure tones simultaneously presented with continuous masking noise. Each ABR corresponded to the averaged response of 300 acquisitions; each stimulus was presented twice to ensure the replicability of the ABR. Tone trains were generated using TDT SigGen software and a RP2.1 real time processor. The signal processor was connected to a PA5 programmable attenuator and then to input A of a SM5 signal mixer. Masking noise was generated with an audio generator (Minirator MR1) and flattened by two equalizers connected in series (Symetrics 551E) before going to input B of the signal mixer. The output of the mixer transmitted either the tone stimuli alone, sampled at 50 kHz (hearing sensitivity in quiet and no-noise control) or the complex stimuli (tone and noise; masking experiment) to the speaker in the sound isolation chamber.
An unmasked ABR audiogram (frequencies between 0.5 and 8 kHz) of each bird was obtained before starting the masking experiment. In the presence of masking noise, only midrange frequencies were tested (1, 2, 2.86, and 4 kHz). Each test session consisted of four different conditions: three noise levels (44, 54, and 64 dB SPL, corresponding to spectrum levels of 6, 16, and 26 dB/Hz), and one no-noise control presented in random order. No-noise controls performed during the masking experiment were compared to absolute thresholds measured before the masking experiment to ensure that no threshold shift had occurred from the exposure to noise.
Hearing thresholds were estimated in two ways. First, the ABR threshold was defined as the stimulus intensity halfway between the trace with a discernable response and the trace without a response (Brittan-Powell et al., 2010). Secondly, amplitude-intensity functions were computed for wave 1 at 1, 2, 2.86, and 4 kHz for each species, and the threshold at each frequency was defined as the x-intercept at 0 μV using linear regression (Brittan-Powell et al., 2002). When compared by Wilcoxon signed rank tests, both methods yielded similar audiogram shapes, but thresholds determined visually were higher than those determined by linear regression (p < 0.001), both in quiet and in noise, by about 10 dB. Difference measurements were calculated by subtracting the behavioral CR obtained from published data from the one obtained with the ABR by visual inspection in the present study. Visual inspection thresholds and CRs were analyzed using two-way analyses of variance, Holm–Sidak pairwise multiple comparison tests and linear regressions. Results were considered significant if p < 0.05.
RESULTS
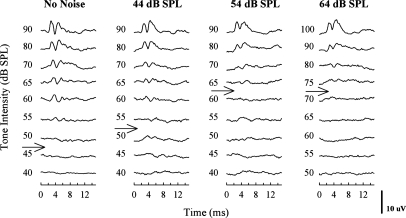
The waveforms showed a series of replicable peaks, occurring within the first 10 ms after sound reached the bird’s ear for both unmasked and masked conditions (Fig. 1). As the intensity of stimulation decreased, the peak latency of the waves increased and the amplitudes decreased. Unmasked audiograms were comparable in shape and showed the same frequency range of best hearing sensitivity, but for each species, ABR thresholds were higher than the corresponding behavioral thresholds.
Figure 1.
Representative ABR waveforms in response to a 2 kHz pure tone recorded from a single zebra finch. Tone stimuli were presented over a range of intensities without noise (left), and simultaneously with broadband white noise at 44 dB SPL (second column), 54 dB SPL (third column), and 64 dB SPL (right column). Each waveform corresponds to the average of 300 acquisitions. The arrows indicate thresholds estimated by visual detection.
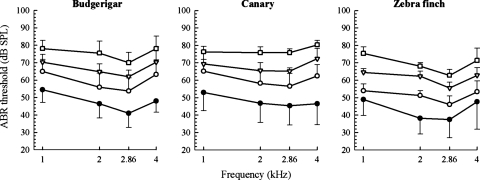
In the masking condition, ABR peak amplitudes decreased as background noise increased, resulting in elevated thresholds, for the three species (Fig. 2). For all birds at all frequencies, there was a nearly linear relation between ABR masked threshold and noise level. Averaged across frequencies, the slopes and correlation coefficients for these regressions were the following: budgerigar (slope: 0.79, R: 0.67), canary (slope: 0.82; R: 0.77), and zebra finch (slope: 0.91; R: 0.81).
Figure 2.
ABR thresholds in budgerigars, canaries, and zebra finches, in three increasing noise levels (open symbols; circles: 6 dB/Hz; triangles: 16 dB/Hz; squares: 26 dB/Hz), compared to the no-noise control (closed circles). Error bars are standard deviations.
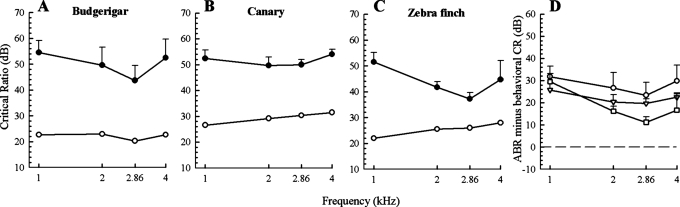
Critical ratios taken from ABR measurements were calculated by subtracting each noise spectrum level from the corresponding sound pressure level at masked threshold. CRs were independent of noise levels in each species. Figures 3A–3C compare ABR-derived CRs at 26 dB/Hz with behavioral CRs from published data for all three species. ABR-derived CRs showed significant effects of species (F(2, 35) = 7.19; p < 0.05) and frequency (F(3, 35) = 19.62; p < 0.001) as well as a significant interaction between the two factors (F(6, 35) = 2.86; p < 0.05). CRs for canaries were not significantly different across frequencies and were larger than the two other species at 2.86 kHz. Zebra finches had smaller ABR-derived CRs than the two other species at 2, 2.86, and 4 kHz. CRs were smaller at 2.86 kHz than at 4 kHz in budgerigars and in zebra finches. Interestingly, CRs at 1 kHz were similar in all three species yet higher than CRs at 2 and 2.86 kHz in budgerigars and zebra finches and also higher than CRs at 4 kHz in zebra finches. Figure 3D shows the difference between CRs as determined by ABR for each species in the present study and CRs as previously measured behaviorally from these three species. In all cases, ABR-derived CRs are higher than behaviorally determined CRs, with budgerigars showing the greatest difference between the two methods, while zebra finches show the least. Taken together, ABR-derived CRs are higher than behavioral CRs by 18 dB at 2.86 kHz—the frequency of best hearing for all three species. The difference between ABR and behavioral measures of the CR increases at lower and higher frequencies. At 1 kHz, the CR as measured by ABR overestimates the behavioral CR by almost 30 dB.
Figure 3.
ABR-derived critical ratio function at 26 dB/Hz (closed circles) compared to the behavioral critical ratio function (open circles; Dooling and Saunders, 1975; Okanoya and Dooling, 1987, 1990; Hashino and Sokabe, 1989; Farabaugh et al., 1998; Lohr et al., 2004) in (A) budgerigars, (B) canaries, and (C) zebra finches. (D): Difference between ABR and behavioral measures of CR, in budgerigars (circles), canaries (triangles), and zebra finches (squares). Error bars are standard deviations.
DISCUSSION
The morphology of the ABR waveforms and the unmasked thresholds were quite similar across the species studied and gave a good estimate of the shape of the behavioral audiograms as shown previously in these species (Brittan-Powell et al., 2002, 2010; Zevin et al., 2004). The three species showed lower ABR thresholds at midrange frequencies than at both ends of the hearing range, with the region of best sensitivity within the frequency region of each species’ vocalizations (Dooling and Saunders, 1975; Hashino and Okanoya, 1989; Wright et al., 2004), allowing for good intraspecific communication in a quiet environment.
In everyday life, however, noise surrounds us. This study aimed at determining whether masked ABR thresholds could be useful for estimating the effects of noise on the audibility thresholds, and thus allow a better judgment on the impact of environmental noise on communication. Masked ABR amplitudes in response to tonal stimuli were reduced compared to ABR amplitudes recorded in quiet, suggesting a decrease in both the effective stimulus level and the number and synchrony of neurons contributing to the response (Nousak and Stapells, 2005). Similar to findings in humans, ABR thresholds increased in all birds in the presence of noise, at all levels presented. The three species showed a nearly linear increase in masked ABR thresholds with increasing noise levels at all frequencies, a finding that is typical of behavioral thresholds in response to increased noise in most vertebrates, including these specific bird species (Okanoya and Dooling, 1987). The slopes of best-fitting linear functions in this study were a little less than unity, meaning that threshold shifts were slightly smaller than expected, suggesting a reduction in the intensity discrimination threshold. Comparatively, these results on increase in masking with increase in noise level from ABR measurements were similar to those obtained behaviorally by Okanoya and Dooling (1987) which also showed slopes very near 1 for all three species. Thus, the increase in masked ABR thresholds is perfectly in line with the increase seen in masked behavioral thresholds in these species.
The ABR-derived CR function paralleled the shape of the behavioral CR function for the budgerigar, especially between 2 and 4 kHz, and, like behavioral studies, also suggests that the budgerigar has greater spectral resolution for sounds in this region. This corresponds to the frequency region of two of its important vocal signals, the contact call and the warble song, suggesting that hearing in this species may be optimized for the reception of species-specific vocalizations in noise (Dooling and Saunders, 1975). In contrast, most vertebrates have a pattern of increasing CR with increasing frequency. Behavioral studies in canaries (Okanoya and Dooling, 1987; Lohr et al., 2004) and finches (Okanoya and Dooling, 1990) show this pattern as well. In the current study, canaries showed an increasing ABR-derived CR between 2 and 4 kHz (similar to behavior) while zebra finches showed an increasing ABR-derived CR only from 2.86 to 4 kHz.
The ABR-derived CR function in zebra finches differed considerably from that measured behaviorally in an earlier study (Okanoya and Dooling, 1990), and the reason for this is unclear. Though the use of noise is often intended to improve frequency and place specificity of the ABR by limiting the portion of the cochlea from which a response is generated, it is conceivable that other portions of the cochlea might still be responsive to the stimulus (Hall, 2007).
The present experiment measured pure tone masked thresholds in three species of birds using the ABR and compared these results with earlier behavioral measures of masked thresholds in the same species. As was seen in behavioral studies, ABR measurements show that the amount of masking caused by noise increases roughly linearly over at least a 30 dB range. On average, in the region of best hearing for all species, the ABR overestimates the actual CR by about 18 dB and slightly more at lower and higher frequencies. There is also a reasonably good correspondence between ABR masked thresholds and behavioral masked thresholds for budgerigars in that both measures show decreasing CRs from 1 to 2.86 kHz and show the smallest CRs occurring at 2.86 kHz. While this study shows that the ABR can be useful for obtaining a broad estimate of the effects of noise on hearing in birds in species that cannot be easily tested behaviorally, it also reveals species differences in the relation between behavioral and ABR measures of masked thresholds. This means that while the ABR masked threshold may provide a reasonable approximation of masked thresholds measured behaviorally, it may not provide a precise estimate of a particular species’ behavioral masked thresholds across the range of hearing. Thus, in circumstances where masked thresholds at specific frequencies are critical for determining the effects of noise on hearing and communication in natural environments, behavioral methods are clearly preferred. The amount of masking depends on (1) the level of the noise but also its spectral composition, (2) the level and spectrum of the sender’s vocalization at the receiver, and (3) the receiver’s CR function. Noise can mask communication signals thereby degrading or eliminating effective communication between individuals. Knowledge of an animal’s CR is essential for estimating communication at a distance in a noisy environment. The current results may prove useful in studies examining the effect of anthropogenic noise on the characteristics of bird vocalizations (e.g., Slabbekoorn and den Boer-Visser, 2006).
ACKNOWLEDGMENTS
This work was supported by NIH R01DC000198, R01DC001372, and P30DC004664 to R.J.D. Article publié avec le concours de la Fondation Universitaire de Belgique (pour I.C.N.).
References
- Brittan-Powell, E. F., Dooling, R. J., and Gleich, O. (2002). “Auditory brainstem responses in adult budgerigars (Melopsittacus undulatus),” J. Acoust. Soc. Am. 112, 999–1008. 10.1121/1.1494807 [DOI] [PubMed] [Google Scholar]
- Brittan-Powell, E. F., Dooling, R. J., Ryals, B., and Gleich, O. (2010). “Electrophysiological and morphological development of the inner ear in Belgian Waterslager Canaries,” Hear. Res. 269, 56–69. 10.1016/j.heares.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling, R. J., and Saunders, J. C. (1975). “Hearing in the parakeet (Melopsittacus undulatus): absolute thresholds, critical ratios, frequency difference limens, and vocalizations,” J. Comp. Physiol. Psychol. 88, 1–20. 10.1037/h0076226 [DOI] [PubMed] [Google Scholar]
- Farabaugh, S. M., Dent, M. L., and Dooling, R. J. (1998). “Hearing and vocalizations of wild-caught Australian budgerigars (Melopsittacus undulatus),” J. Comp. Psychol. 112, 74–81. 10.1037/0735-7036.112.1.74 [DOI] [PubMed] [Google Scholar]
- Hashino, E., and Okanoya, K. (1989). “Auditory sensitivity in the zebra finch (Poephila guttata castanotis),” J. Acoust. Soc. Jpn. 10, 51–52. [Google Scholar]
- Hashino, E., and Sokabe, M. (1989). “Kanamycin induced low-frequency hearing loss in the budgerigar (Melopsittacus undulatus),” J. Acoust. Soc. Am. 85, 289–294. 10.1121/1.397736 [DOI] [PubMed] [Google Scholar]
- Hawkins, J. E., and Stevens S. S. (1950). “The masking of pure tones and of speech by white noise,” J. Acoust. Soc. Am. 22, 6–13. 10.1121/1.1906581 [DOI] [Google Scholar]
- Hall, J. W. (2007). “Frequency specific auditory brainstem response (ABR) and auditory steady-state response (ASSR),” in New Handbook of Auditory Evoked Responses (Allyn and Bacon, Boston, MA: ), pp. 258–312. [Google Scholar]
- Henry, K. S., and Lucas J. R. (2010). “Habitat-related differences in the frequency selectivity of auditory filters in songbirds,” Funct. Ecol. 24, 614–624. 10.1111/j.1365-2435.2009.01674.x [DOI] [Google Scholar]
- Lohr, B., Lauer, A., Newman, M. R., and Dooling, R. J. (2004). “Hearing in the red-billed firefinch Lagonosticta senegala and the Spanish timbrado canary Serinus canaria: the influence of natural and artificial selection on auditory abilities and vocal structure,” Bioacoustics 14, 83–98. [Google Scholar]
- Nousak, J. K., and Stapells, D. R. (2005). “Auditory brainstem and middle latency responses to 1 kHz tones in noise-masked normally-hearing and sensorineurally hearing-impaired adults,” Int. J. Audiol. 44, 331–344. 10.1080/14992020500060891 [DOI] [PubMed] [Google Scholar]
- Okanoya, K., and Dooling, R. J. (1987). “Hearing in passerines and psittacine birds: a comparative study of absolute and masked thresholds,” J. Comp. Psychol. 101, 7–15. 10.1037/0735-7036.101.1.7 [DOI] [PubMed] [Google Scholar]
- Okanoya, K., and Dooling, R. J. (1990). “Detection of auditory sinusoids of fixed and uncertain frequency by budgerigars (Melopsittacus undulatus) and zebra finches (Poephila guttata),” Hear. Res. 50, 175–184. 10.1016/0378-5955(90)90043-O [DOI] [PubMed] [Google Scholar]
- Slabbekoorn, H., and den Boer-Visser, A. (2006). “Cities change the songs of birds,” Curr. Biol. 16, 2326–2331. 10.1016/j.cub.2006.10.008 [DOI] [PubMed] [Google Scholar]
- Wright, T. F., Brittan-Powell, E. F., Dooling, R. J., and Mundinger, P. C. (2004). “Sex-linked inheritance of hearing and song in the Belgian Waterslager canary,” Proc. R. Soc. London, Ser. B 271, S409–S412. 10.1098/rspb.2003.2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevin, J. D., Seidenberg, M. S., and Bottjer, S. W. (2004). “Limits on reacquisition of song in adult zebra finches exposed to white noise,” J. Neurosci. 24, 5849–5862. 10.1523/JNEUROSCI.1891-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker, E. Flottorp, G., and Stevens, S. S. (1957). “Critical band width in loudness summation,” J. Acoust. Soc. Am. 29, 548–557. 10.1121/1.1908963 [DOI] [Google Scholar]