Abstract
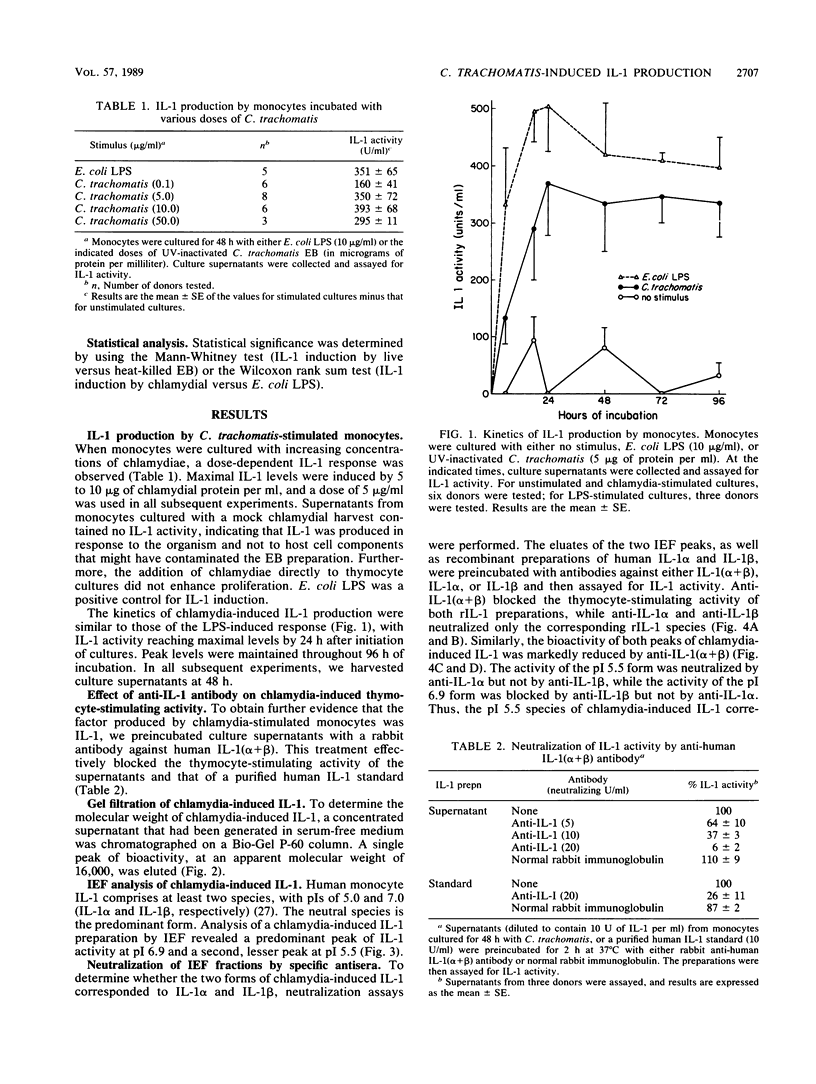
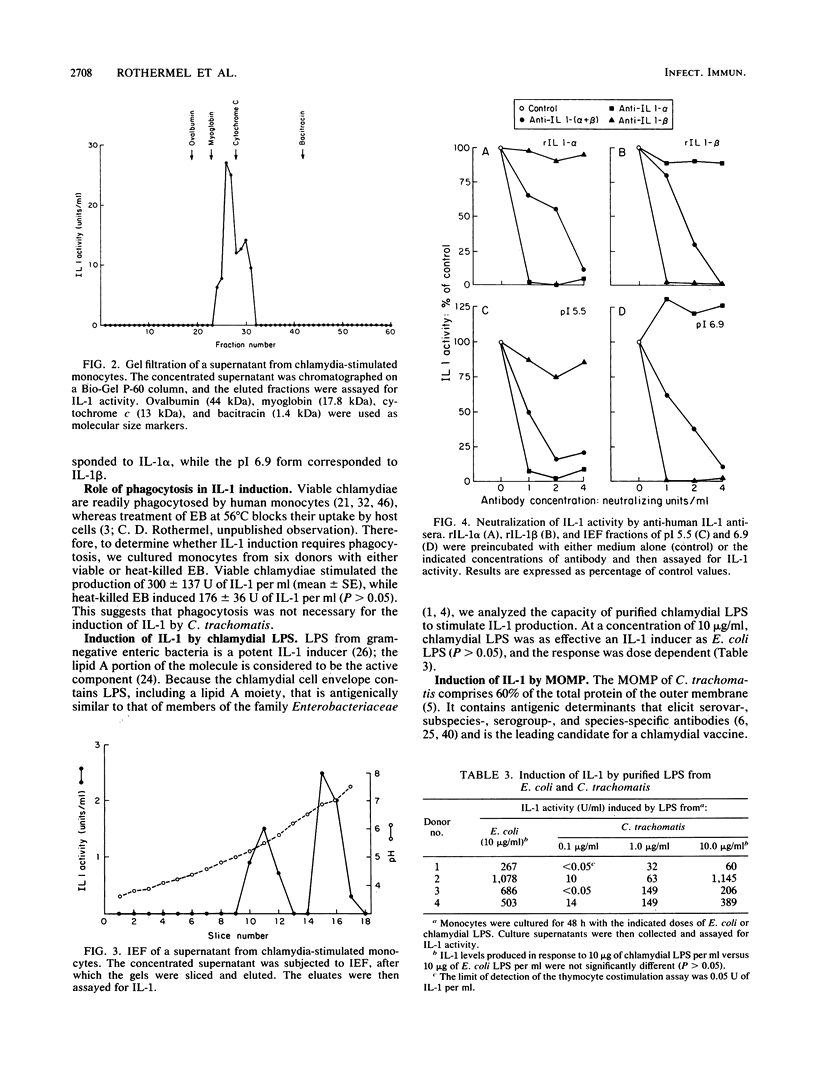
Human diseases caused by the intracellular bacterium Chlamydia trachomatis include genital tract infections and blinding trachoma. Chlamydial infections are characterized by chronic inflammation and scarring, and development of such complications is thought to be immunologically mediated. In this study, we show that coculture of C. trachomatis (serovar L2) with human blood monocytes induced the production of interleukin-1 (IL-1), an important mediator of inflammation, tissue remodeling, and scarring. IL-1 was produced in response to UV-inactivated elementary bodies containing from 0.1 to 50 micrograms of protein per ml, with a maximal response at 5 to 10 micrograms/ml. IL-1 activity was detected by 6 h of incubation and was maximal by 24 h. Peak levels were maintained throughout 96 h of incubation. Rabbit antibody to human IL-1(alpha + beta) effectively neutralized the thymocyte-stimulating activity of the supernatants. The apparent molecular weight of chlamydia-induced IL-1 was 16,000, as determined by gel filtration on a Bio-Gel P-60 column. Isoelectric focusing yielded two peaks of activity, with pIs of 5.5 and 6.9. Neutralization studies with antisera against human IL-1 alpha and IL-1 beta showed that the acidic and neutral peaks corresponded to IL-1 alpha and IL-1 beta, respectively, with IL-1 beta predominating. Heat-killed chlamydiae, which are not internalized by monocytes, were effective IL-1 inducers, indicating that phagocytosis was not required for IL-1 induction. Purified C. trachomatis lipopolysaccharide was also an effective IL-1 inducer, suggesting that the response to intact organisms may be largely a response to chlamydial lipopolysaccharide. Finally, purified chlamydial major outer membrane protein induced low but detectable IL-1 activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brade L., Nurminen M., Mäkelä P. H., Brade H. Antigenic properties of Chlamydia trachomatis lipopolysaccharide. Infect Immun. 1985 May;48(2):569–572. doi: 10.1128/iai.48.2.569-572.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T., Spagnuolo P. J., Goldsmith G. H., Aikawa M. Interaction of Borrelia spirochetes with human mononuclear leukocytes causes production of leukocytic pyrogen and thromboplastin. J Lab Clin Med. 1982 May;99(5):709–721. [PubMed] [Google Scholar]
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Hitchcock P. J. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect Immun. 1984 May;44(2):306–314. doi: 10.1128/iai.44.2.306-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982 Mar;35(3):1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Dukovich M., Severin J. M., White S. J., Yamazaki S., Mizel S. B. Stimulation of fibroblast proliferation and prostaglandin production by purified recombinant murine interleukin 1. Clin Immunol Immunopathol. 1986 Mar;38(3):381–389. doi: 10.1016/0090-1229(86)90248-5. [DOI] [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Francus T., Manzo G., Canki M., Thompson L. C., Szabo P. Two peaks of interleukin 1 expression in human leukocytes cultured with tobacco glycoprotein. J Exp Med. 1989 Jul 1;170(1):327–332. doi: 10.1084/jem.170.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Grayston J. T., Wang S. P., Yeh L. J., Kuo C. C. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985 Nov-Dec;7(6):717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- Johnson A. P., Osborn M. F., Thomas B. J., Hetherington C. M., Taylor-Robinson D. Immunity to reinfection of the genital tract of marmosets with Chlamydia trachomatis. Br J Exp Pathol. 1981 Dec;62(6):606–613. [PMC free article] [PubMed] [Google Scholar]
- Johnson A. P. Pathogenesis and immunology of chlamydial infections of the genital tract. Rev Infect Dis. 1985 Nov-Dec;7(6):741–745. doi: 10.1093/clinids/7.6.741. [DOI] [PubMed] [Google Scholar]
- Lachman L. B., Shih L. C., Brown D. C. Interleukin 1 from human leukemic monocytes. Methods Enzymol. 1985;116:467–479. doi: 10.1016/s0076-6879(85)16038-6. [DOI] [PubMed] [Google Scholar]
- Mabey D. C., Robertson J. N., Ward M. E. Detection of Chlamydia trachomatis by enzyme immunoassay in patients with trachoma. Lancet. 1987 Dec 26;2(8574):1491–1492. doi: 10.1016/s0140-6736(87)92623-7. [DOI] [PubMed] [Google Scholar]
- Manor E., Sarov I. Fate of Chlamydia trachomatis in human monocytes and monocyte-derived macrophages. Infect Immun. 1986 Oct;54(1):90–95. doi: 10.1128/iai.54.1.90-95.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Monnickendam M. A., Darougar S., Treharne J. D., Tilbury A. M. Development of chronic conjunctivitis with scarring and pannus, resembling trachoma, in guinea-pigs. Br J Ophthalmol. 1980 Apr;64(4):284–290. doi: 10.1136/bjo.64.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Newhall W. J., 5th, Terho P., Wilde C. E., 3rd, Batteiger B. E., Jones R. B. Serovar determination of Chlamydia trachomatis isolates by using type-specific monoclonal antibodies. J Clin Microbiol. 1986 Feb;23(2):333–338. doi: 10.1128/jcm.23.2.333-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton R. C. Human monocyte production of interleukin-1: parameters of the induction of interleukin-1 secretion by lipopolysaccharides. J Leukoc Biol. 1986 Mar;39(3):299–311. doi: 10.1002/jlb.39.3.299. [DOI] [PubMed] [Google Scholar]
- Patton D. L. Immunopathology and histopathology of experimental chlamydial salpingitis. Rev Infect Dis. 1985 Nov-Dec;7(6):746–753. doi: 10.1093/clinids/7.6.746. [DOI] [PubMed] [Google Scholar]
- Patton D. L., Kuo C. C., Wang S. P., Halbert S. A. Distal tubal obstruction induced by repeated Chlamydia trachomatis salpingeal infections in pig-tailed macaques. J Infect Dis. 1987 Jun;155(6):1292–1299. doi: 10.1093/infdis/155.6.1292. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Robb R. J. Human interleukin 2. Methods Enzymol. 1985;116:493–525. doi: 10.1016/s0076-6879(85)16040-4. [DOI] [PubMed] [Google Scholar]
- Rothermel C. D., Byrne G. I., Havell E. A. Effect of interferon on the growth of Chlamydia trachomatis in mouse fibroblasts (L cells). Infect Immun. 1983 Jan;39(1):362–370. doi: 10.1128/iai.39.1.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (first of three parts). N Engl J Med. 1978 Feb 23;298(8):428–435. doi: 10.1056/NEJM197802232980805. [DOI] [PubMed] [Google Scholar]
- Schachter J., Moncada J., Dawson C. R., Sheppard J., Courtright P., Said M. E., Zaki S., Hafez S. F., Lorincz A. Nonculture methods for diagnosing chlamydial infection in patients with trachoma: a clue to the pathogenesis of the disease? J Infect Dis. 1988 Dec;158(6):1347–1352. doi: 10.1093/infdis/158.6.1347. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Schmidt J. A. Purification and partial biochemical characterization of normal human interleukin 1. J Exp Med. 1984 Sep 1;160(3):772–787. doi: 10.1084/jem.160.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielvogel R. L., Kersey J. H., Goltz R. W. Mononuclear cell stimulation of fibroblast collagen synthesis. Clin Exp Dermatol. 1978 Mar;3(1):25–30. doi: 10.1111/j.1365-2230.1978.tb01454.x. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Taylor H. R., Johnson S. L., Schachter J., Caldwell H. D., Prendergast R. A. Pathogenesis of trachoma: the stimulus for inflammation. J Immunol. 1987 May 1;138(9):3023–3027. [PubMed] [Google Scholar]
- Taylor H. R. Ocular models of chlamydial infection. Rev Infect Dis. 1985 Nov-Dec;7(6):737–740. doi: 10.1093/clinids/7.6.737. [DOI] [PubMed] [Google Scholar]
- Wahl S. M. The role of lymphokines and monokines in fibrosis. Ann N Y Acad Sci. 1985;460:224–231. doi: 10.1111/j.1749-6632.1985.tb51170.x. [DOI] [PubMed] [Google Scholar]
- Watkins N. G., Hadlow W. J., Moos A. B., Caldwell H. D. Ocular delayed hypersensitivity: a pathogenetic mechanism of chlamydial-conjunctivitis in guinea pigs. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7480–7484. doi: 10.1073/pnas.83.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong E. C., Chi E. Y., Kuo C. C. Differential antimicrobial activity of human mononuclear phagocytes against the human biovars of Chlamydia trachomatis. J Immunol. 1987 Aug 15;139(4):1297–1302. [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S. J., Caldwell H. D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989 Feb;57(2):636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


