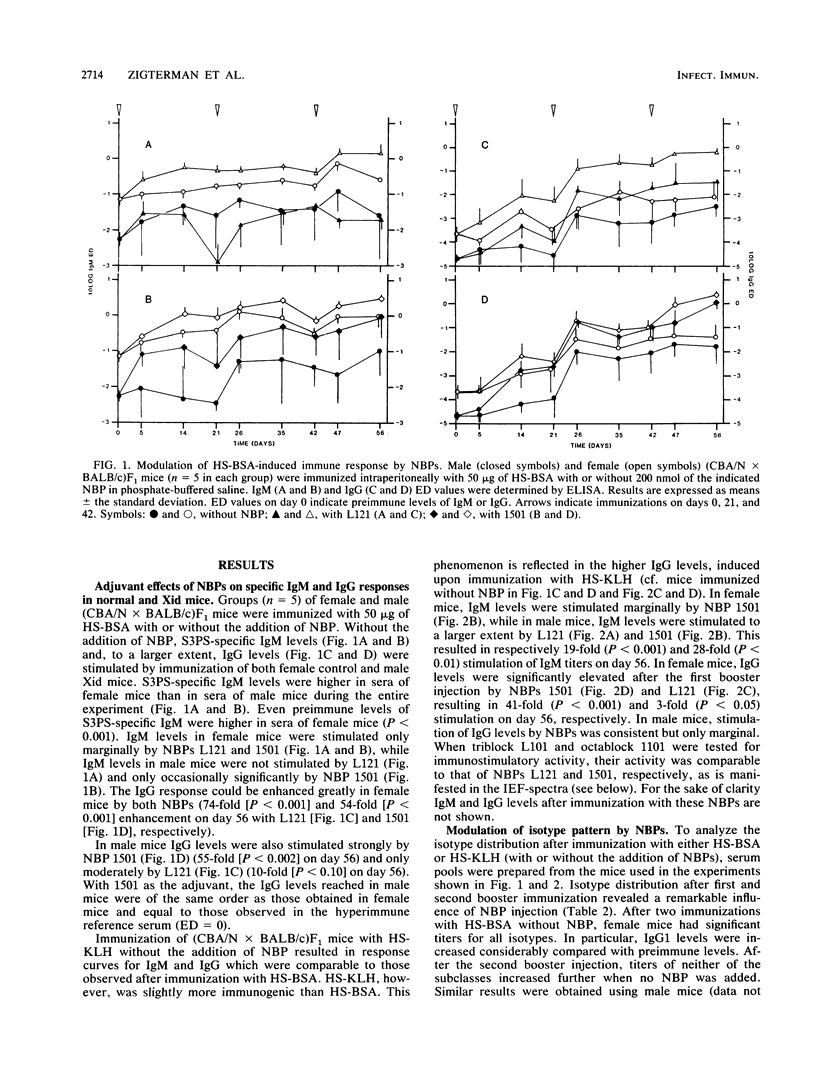
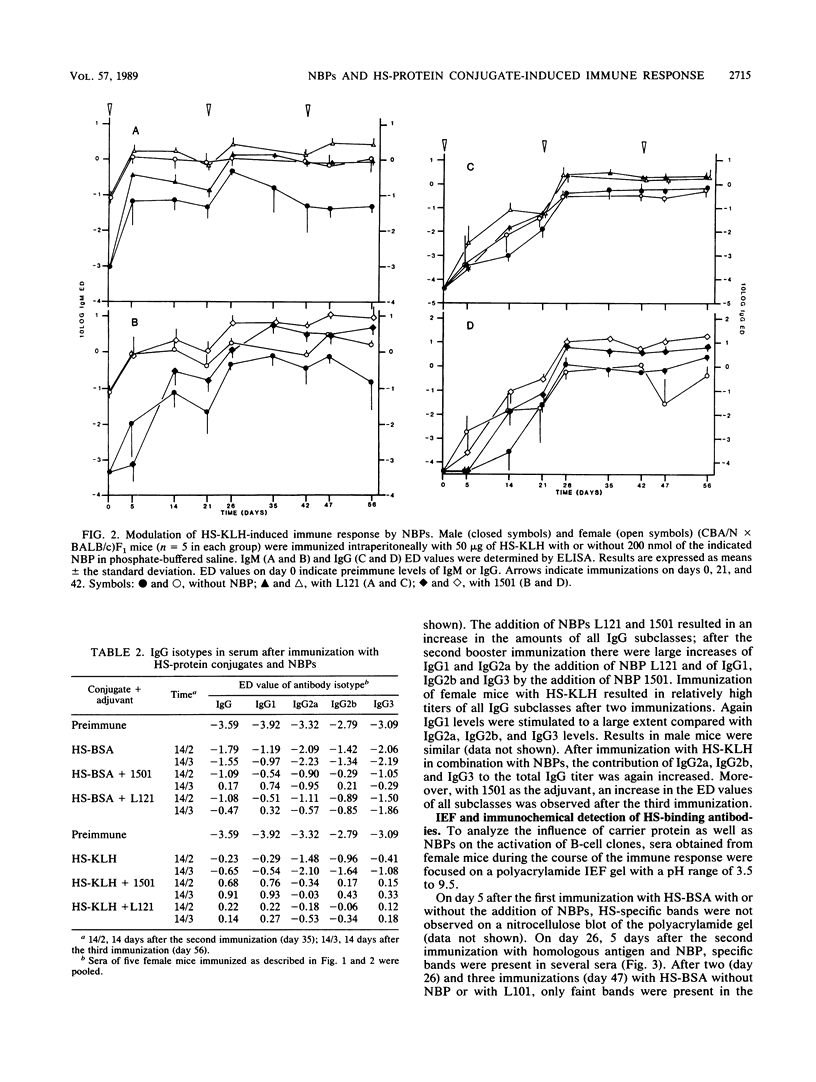
Abstract
Nonionic block polymer surfactants (NBPs) were tested for the capacity to stimulate the antibody response against hexasaccharide (HS), derived from Streptococcus pneumoniae type 3 capsular polysaccharide (S3PS), which was conjugated to proteins. The immune response was evaluated in the (CBA/N x BALB/c)F1 progeny, in which female mice are phenotypically normal whereas male mice carry an X-chromosome-linked immunodeficiency. NBPs L101, L121, 1101, and 1501 were able to increase anti-HS immunoglobulin M (IgM) and IgG levels in both normal and X-chromosome-linked immunodeficient mice (with up to 74-fold stimulation of antibody titers). Distribution of S3PS-specific antibodies over the various IgG isotypes was restricted after immunization with either HS-bovine serum albumin or HS-keyhole limpet hemocyanin (HS-KLH). Addition of NBPs (in particular 1501) resulted in a more diverse immune response with either antigen as judged by isotype distribution. Isoelectric focusing of individual sera and subsequent detection of S3PS-binding antibodies in these sera by immunochemical staining revealed a restricted number of different spectrotypes in the course of the immune response. Upon immunization of mice with HS-KLH, spectra of secreted antibodies were slightly more complex and more densely stained than after immunization with HS-bovine serum albumin. Furthermore, NBPs 1101 and 1501 appeared to be able to stimulate the secretion of antibodies, which were secreted only in small amounts without the use of NBPs. Different explanations for increased spectrotype diversity after immunization with KLH as the carrier and after administration of NBPs as the adjuvant are discussed.
Full text
PDF



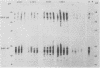
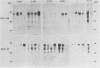
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Byars N. E. An adjuvant formulation that selectively elicits the formation of antibodies of protective isotypes and of cell-mediated immunity. J Immunol Methods. 1986 Dec 24;95(2):157–168. doi: 10.1016/0022-1759(86)90402-3. [DOI] [PubMed] [Google Scholar]
- Anderson P., Insel R. A. A polysaccharide-protein complex from Haemophilus influenzae type b. I. Activity in weanling rabbits and human T lymphocytes. J Infect Dis. 1981 Dec;144(6):509–520. doi: 10.1093/infdis/144.6.509. [DOI] [PubMed] [Google Scholar]
- Austrian R. Polysaccharide vaccines. Ann Inst Pasteur Microbiol. 1985 Nov-Dec;136B(3):295–307. doi: 10.1016/s0769-2609(85)80075-2. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Rudbach J. A., Prescott B., Caldes G., Evans C., Stashak P. W. Influence of multiple genes on the magnitude of the antibody response to bacterial polysaccharide antigens. Infect Immun. 1984 Jul;45(1):56–61. doi: 10.1128/iai.45.1.56-61.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz C. D., Ward J. I., Meier K., Hendley J. O., Brunell P. A., Barkin R. A., Zahradnik J. M., Samuelson J., Gordon L. Safety and immunogenicity of Haemophilus influenzae type b polysaccharide and polysaccharide diphtheria toxoid conjugate vaccines in children 15 to 24 months of age. J Pediatr. 1987 Apr;110(4):509–514. doi: 10.1016/s0022-3476(87)80540-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Miles H. B. Vaccination against Streptococcus pneumoniae in childhood: lack of demonstrable benefit in young Australian children. J Infect Dis. 1984 Jun;149(6):861–869. doi: 10.1093/infdis/149.6.861. [DOI] [PubMed] [Google Scholar]
- Goebel W. F. STUDIES ON ANTIBACTERIAL IMMUNITY INDUCED BY ARTIFICIAL ANTIGENS : I. IMMUNITY TO EXPERIMENTAL PNEUMOCOCCAL INFECTION WITH AN ANTIGEN CONTAINING CELLOBIURONIC ACID. J Exp Med. 1939 Feb 28;69(3):353–364. doi: 10.1084/jem.69.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M., Apicella M. A., Greenwood B., Mäkelä P. H. Vaccines against encapsulated bacteria: a global agenda. Rev Infect Dis. 1987 Jan-Feb;9(1):176–188. doi: 10.1093/clinids/9.1.176. [DOI] [PubMed] [Google Scholar]
- Hunter R. L., Bennett B. The adjuvant activity of nonionic block polymer surfactants. II. Antibody formation and inflammation related to the structure of triblock and octablock copolymers. J Immunol. 1984 Dec;133(6):3167–3175. [PubMed] [Google Scholar]
- Insel R. A., Anderson P. W. Oligosaccharide-protein conjugate vaccines induce and prime for oligoclonal IgG antibody responses to the Haemophilus influenzae b capsular polysaccharide in human infants. J Exp Med. 1986 Feb 1;163(2):262–269. doi: 10.1084/jem.163.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel R. A., Kittelberger A., Anderson P. Isoelectric focusing of human antibody to the Haemophilus influenzae b capsular polysaccharide: restricted and identical spectrotypes in adults. J Immunol. 1985 Oct;135(4):2810–2816. [PubMed] [Google Scholar]
- Jennings H. J. Capsular polysaccharides as human vaccines. Adv Carbohydr Chem Biochem. 1983;41:155–208. doi: 10.1016/s0065-2318(08)60058-x. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Roy R., Gamian A. Induction of meningococcal group B polysaccharide-specific IgG antibodies in mice by using an N-propionylated B polysaccharide-tetanus toxoid conjugate vaccine. J Immunol. 1986 Sep 1;137(5):1708–1713. [PubMed] [Google Scholar]
- Kamerling J. P., Gerwig G. J., Vliegenthart J. F., Clamp J. R. Characterization by gas-liquid chromatography-mass spectrometry and proton-magnetic-resonance spectroscopy of pertrimethylsilyl methyl glycosides obtained in the methanolysis of glycoproteins and glycopeptides. Biochem J. 1975 Dec;151(3):491–495. doi: 10.1042/bj1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. O. The epidemiology of pneumococcal disease in infants and children. Rev Infect Dis. 1981 Mar-Apr;3(2):246–253. doi: 10.1093/clinids/3.2.246. [DOI] [PubMed] [Google Scholar]
- Koch C., Skjødt K., Laursen I. A simple immunoblotting method after separation of proteins in agarose gel. J Immunol Methods. 1985 Nov 28;84(1-2):271–278. doi: 10.1016/0022-1759(85)90434-x. [DOI] [PubMed] [Google Scholar]
- Lepow M. L., Samuelson J. S., Gordon L. K. Safety and immunogenicity of Haemophilus influenzae type b-polysaccharide diphtheria toxoid conjugate vaccine in infants 9 to 15 months of age. J Pediatr. 1985 Feb;106(2):185–189. doi: 10.1016/s0022-3476(85)80284-5. [DOI] [PubMed] [Google Scholar]
- Metcalf E. S., Scher I., Klinman N. R. Susceptibility to in vitro tolerance induction of adult B cells from mice with an X-linked B-cell defect. J Exp Med. 1980 Feb 1;151(2):486–491. doi: 10.1084/jem.151.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Prescott B., Cross S. S., Stashak P. W., Baker P. J. Regulation of the antibody response to type III pneumococcal polysaccharide. V. Ontogeny of factors influencing the magnitude of the plaque-forming cell response. J Immunol. 1976 Feb;116(2):279–287. [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Nakashima S., Kamikawa H. Accelerated expansion of antibody heterogeneity by complete Freund's adjuvant during the response to bacterial alpha-amylase. Immunology. 1984 Dec;53(4):837–845. [PMC free article] [PubMed] [Google Scholar]
- Rijkers G. T., Dollekamp E. G., Zegers B. J. The in vitro B-cell response to pneumococcal polysaccharides in adults and neonates. Scand J Immunol. 1987 May;25(5):447–452. doi: 10.1111/j.1365-3083.1987.tb02215.x. [DOI] [PubMed] [Google Scholar]
- Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- Schuler W., Lehle G., Weiler E., Kölsch E. Immune response against the T-independent antigen alpha (1 leads to 3) dextran. I. Demonstration of an unexpected IgG response of athymic and germ-free-raised euthymic BALB/c mice. Eur J Immunol. 1982 Feb;12(2):120–125. doi: 10.1002/eji.1830120205. [DOI] [PubMed] [Google Scholar]
- Snippe H., De Reuver M. J., Strickland F., Willers J. M., Hunter R. L. Adjuvant effect of nonionic block polymer surfactants in humoral and cellular immunity. Int Arch Allergy Appl Immunol. 1981;65(4):390–398. doi: 10.1159/000232780. [DOI] [PubMed] [Google Scholar]
- Snippe H., van Houte A. J., van Dam J. E., De Reuver M. J., Jansze M., Willers J. M. Immunogenic properties in mice of hexasaccharide from the capsular polysaccharide of Streptococcus pneumoniae type 3. Infect Immun. 1983 Jun;40(3):856–861. doi: 10.1128/iai.40.3.856-861.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein K. E., Bona C., Lieberman R., Chien C. C., Paul W. E. Regulation of the anti-inulin antibody response by a nonallotype-linked gene. J Exp Med. 1980 May 1;151(5):1088–1102. doi: 10.1084/jem.151.5.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein K. E., Zopf D. A., Johnson B. M., Miller C. B., Paul W. E. The immune response to an isomaltohexosyl-protein conjugate, a thymus-dependent analogue of alpha(1 replaced by 6) dextran. J Immunol. 1982 Mar;128(3):1350–1354. [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Coupling of acid labile Salmonella specific oligosaccharides to macromolecular carriers. J Immunol Methods. 1979;25(4):323–335. doi: 10.1016/0022-1759(79)90025-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Vogel F. R., Chedid L. A. Current status of immunological adjuvants. Annu Rev Immunol. 1986;4:369–388. doi: 10.1146/annurev.iy.04.040186.002101. [DOI] [PubMed] [Google Scholar]
- Wicker L. S., Scher I. X-linked immune deficiency (xid) of CBA/N mice. Curr Top Microbiol Immunol. 1986;124:87–101. doi: 10.1007/978-3-642-70986-9_6. [DOI] [PubMed] [Google Scholar]
- Zigterman G. J., Snippe H., Jansze M., Ernste E. B., De Reuver M. J., Willers J. M. Nonionic block polymer surfactants enhance immunogenicity of pneumococcal hexasaccharide-protein vaccines. Infect Immun. 1988 May;56(5):1391–1393. doi: 10.1128/iai.56.5.1391-1393.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigterman G. J., Snippe H., Jansze M., Willers J. M. Adjuvant effects of nonionic block polymer surfactants on liposome-induced humoral immune response. J Immunol. 1987 Jan 1;138(1):220–225. [PubMed] [Google Scholar]
- Zigterman G. J., Verheul A. F., Ernste E. B., Rombouts R. F., De Reuver M. J., Jansze M., Snippe H., Willers J. M. Measurement of the humoral immune response against Streptococcus pneumoniae type 3 capsular polysaccharide and oligosaccharide containing antigens by ELISA and ELISPOT techniques. J Immunol Methods. 1988 Jan 21;106(1):101–107. doi: 10.1016/0022-1759(88)90277-3. [DOI] [PubMed] [Google Scholar]
- Zigterman J. W., van Dam J. E., Snippe H., Rotteveel F. T., Jansze M., Willers J. M., Kamerling J. P., Vliegenthart J. F. Immunogenic properties of octasaccharide-protein conjugates derived from Klebsiella serotype 11 capsular polysaccharide. Infect Immun. 1985 Feb;47(2):421–428. doi: 10.1128/iai.47.2.421-428.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]