Abstract
Fibrosis is a deregulated and ultimately defective form of tissue repair that underlies a large number of chronic human diseases, as well as obesity and aging. The pathogenesis of fibrosis involves multiple cell types and extracellular signals, of which transforming growth factor- β (TGF-β) is pre-eminent. The prevalence of fibrosis is rising worldwide, and to date no agents has shown clinical efficacy in the attenuating or reversing the process. Recent studies implicate the immediate-early response transcription factor Egr-1 in the pathogenesis of fibrosis. Egr-1 couples acute changes in the cellular environment to sustained alterations in gene expression, and mediates a broad spectrum of biological responses to injury and stress. In contrast to other ligand-activated transcription factors such as NF-κB, c-jun and Smad2/3 that undergo post-translational modification such as phosphorylation and nuclear translocation, Egr-1 activity is regulated via its biosynthesis. Aberrant Egr-1 expression or activity is implicated in cancer, inflammation, atherosclerosis, and ischemic injury and recent studies now indicate an important role for Egr-1 in TGF-β-dependent profibrotic responses. Fibrosis in various animal models and human diseases such as scleroderma (SSc) and idiopathic pulmonary fibrosis (IPF) is accompanied by aberrant Egr-1 expression. Moreover Egr-1 appears to be required for physiologic and pathological connective tissue remodeling, and Egr-1-null mice are protected from fibrosis. As a novel profibrotic mediator, Egr-1 thus appears to be a promising potential target for the development of anti-fibrotic therapies.
Keywords: Egr-1, TGF-β, fibrosis, Scleroderma (systemic sclerosis), fibroblast
Introduction
Fibrosis is characterized by relentless accumulation of collagen-rich extracellular matrix (ECM) that eventually results in disrupted tissue architecture and organ failure. Because fibrosis accounts for as much as 40% of all deaths worldwide and appears to be increasing in prevalence (Wynn, 2010), understanding its pathogenesis and developing therapeutic strategies to block its progression are urgent scientific challenges. Both the initiation and persistence of pathological fibrosis involve activation and differentiation of mesenchymal cells (Kalluri, 2009). Transforming growth factor-β (TGF-β) is a pivotal signal for triggering fibrogenic responses, generally acting in concert with hypoxia, reactive oxygen species (ROS), Wnt and Notch signaling, along with chemokines, cytokines and mechanical stress signals (Wei et al, 2010). TGF-β responses are mediated through both canonical (Smad-dependent) and Smad-independent intracellular signal transduction mechanisms (Rahimi et al, 2007). Early growth response-1 (Egr-1) is a novel Smad-independent mediator of TGF-β signaling. This zinc finger DNA-binding protein belongs to a family of ligand-inducible early response genes (Egrs) that are implicated in diverse physiological processes, and in the pathogenesis of multiple inherited and acquired human diseases. Emerging studies reveal a novel function for Egr-1 as an important mediator of TGF-β-induced responses. Moreover, abnormal Egr-1 expression and function has been linked to animal models of fibrosis, as well as human fibrotic disorders including idiopathic pulmonary fibrosis and scleroderma. This review focuses on recent insights into the regulation and complex functional role of Egr-1 and related proteins in fibroblast biology, connective tissue homeostasis, physiologic tissue repair and pathological fibrosis.
Egr-1 and the early growth response gene family
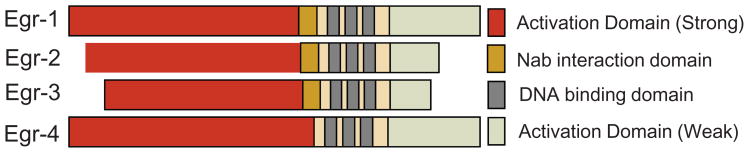
The Egr-1 gene, located on human chromosome 5q31, encodes an 80 kDa DNA-binding transcription factor (Khachigian and Collins, 1998). In resting cells, Egr-1 expression is low or undetectable. However, Egr-1 expression is elicited by a large number of extracellular stimuli, typically in a rapid and transient manner. The Egr-1 protein (shown in Fig. 1) consists of transactivation and repression domains, along with three DNA-binding zinc fingers that recognize GC-rich sequences present in target gene promoters (Gashler A and Sukhatme, 1995). An inhibitory domain, located between the activation domain and the DNA-binding domain serves as a recognition site for Egr-1/NGFI-A binding proteins 1 (Nab1) and Nab2 (Svaren et al., 1996; Russo et al, 1995). These cofactors repress Egr-1-dependent transcriptional activity via recruitment of the inhibitory nucleosomal remodeling and deacetylation (NuRD) complex to the promoter-bound Egr-1 (Srinivasan et al, 2006). The levels of Nab1/2 and the ratio of Egr-1/Nab1/2 in a particular cell are thus of extreme importance for setting the level of Egr-1 activity. The expression of Nab2 is itself controlled by Egr-1, enabling Egr-1 to regulate its own biological activity via a negative feedback loop (Ehrengruber et al, 2000). Other members of the Egr family are listed in Table 1.
Fig. 1.
Egr-1 and related proteins of the Egr gene family. Egr-1 consists of activation domains, an inhibitory (Nab Interaction) domain and a DNA-binding domain.
Table 1.
Biological activities regulated via Egrs.
| Gene | Prominent Functions | Mouse Knockout phenotype | Disease associations |
|---|---|---|---|
| Egr-1 | Growth, development, differentiation, apoptosis, inflammation, fibrosis | Homozygous Egr-1 null overtly healthy; reduced body size; females infertile | Prostrate cancer, lung cancer, breast cancer, schizophrenia, SSc |
| Egr-2 | Formation and maintenance of peripheral nerve myelin, hindbrain development, T cell regulation, apoptosis | Homozygous Egr-2 null had congenital hypomyelinating neuropathy; uniformly die within days after birth | Charcot-Marie-Tooth disease, Dejerine-Sottas syndrome, congenital hypomyelinating neuropathy, SLE, schizophrenia, SSc |
| Egr-3 | Sympathetic nervous system development, regulation of T-cell activation, survival and proliferation | Egr-3 null mice had profound ataxia; lack of muscle spindle | 607A-G SNP associated with Schizophrenia |
| Egr-4 | Central nervous system function (not well known) | Homozygous Egr-4 null overtly healthy; males infertile | Unknown |
| Nab1* | Peripheral nerve differentiation, cardiac hypertrophy | Nab1-null mice produced at the expected Mendelian ratios, overtly healthy. | Unknown |
| Nab2* | Vascular homeostasis, differentiation of endothelial cells, fibroblasts, inflammation, peripheral nerve myelination | Nab2 null overtly healthy; thick dermis; increased collagen accumulation | Unknown |
Nab1/Nab2 double null mice have severe hypomyelination and suffer early lethality SSc, Scleroderma; SLE Systemic Lupus Erythematosus; SNP, single nucleotide polymorphisms
Egr-1 activity is controlled at the transcriptional level
Egr-1 activity is determined largely by regulation of its biosynthesis, which is under tight control. Egr-1 expression is induced by environmental stimuli characteristically associated with injury and stress. The list includes growth factors, cytokines, T cell receptor ligation, hormones, thrombin, shear stress and mechanical forces, neurotransmitters, ultraviolet light, reactive oxygen species (ROS), ischemia-reperfusion and hypoxia (Hjoberg et al, 2004; Yan SF, 2000; Gaggioli et al, 2005; Guha et al, 2001; Li et al, 2007; Kaufmann et al, 2002; Rossler et al, 2009; Lohoff et al, 2010).
Much is now known about how these extracellular stimuli induce the expression of Egr-1. The human Egr-1 gene promoter harbors five consensus serum response elements (SREs) that are recognized by serum response factor (SRF) and ternary complex factors (TCF) such as Elk-1 and related Ets family oncogenes (Buchwalter et al, 2004). The induction of Egr-1 characteristically involves the ERK kinase cascade converging on Elk-1 (Hasan et al, 2008; Kachigian, 2006; Midgley et al, 2004; Hjoberg et al, 2004). Phosphorylation enhances Elk-1 DNA binding activity, resulting in Egr-1 transcription. The Egr-1 gene promoter also contains an inhibitory Egr-1 binding site, which enables Egr-1 to negatively regulate its own expression (Cao et al, 1993). This feedback mechanism normally allows prompt extinction of Egr-1-dependent signaling.
Profibrotic signals induce Egr-1
We recently discovered that another potent stimulus for Egr-1 expression is TGF-β, which causes rapid and transient up-regulation of Egr-1 transcription in normal human fibroblasts via a Smad-independent MEK1-ERK1/2- Elk-1 pathway (Bhattacharyya et al, 2008). Moreover TGF-β enhanced the interaction of endogenous Egr-1 with the consensus Egr-1-binding element of the human COL1A2 promoter in vitro and in vivo (Chen et al, 2006). Insulin-like growth factor binding protein-5 (IGFBP-5), a member of IGFBP family that is upregulated in lung fibrosis, also induces Egr-1 via the MAPK signaling pathway (Beattie et al, 2006). Like TGF-β, IGFBP5 stimulates collagen production via Egr-1 (Yasuoka et al, 2009).
The range of Egr-1’s biological activities induces fibrogenesis
Studies during the past two decades have implicated aberrant Egr-1 expression or function in carcinogenesis, inflammation, atherosclerosis, ischemic injury as well as fibrosis (Krones-Herzig et al, 2005; Tureyen et al, 2008; Du et al, 2000; Khachigian, 2006; Yan SF, 2000; Bhattacharyya et al, 2008; Wu et al, 2009). It was therefore a surprising observation that mice lacking Egr-1 appeared to show no overt phenotype, with the exception of infertility in females (Lee SL, 1996). Upon its induction by environmental signals, Egr-1 regulates genes involved in broad range of responses, including many that are important for fibrogenesis (Table 3). These include the profibrotic cytokine TGF-β, platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF) and vascular endothelial growth factor (VEGF), along with plasminogen activator inhibitor-1 (PAI-1), hemin, fibronectin, tissue inhibitor of metalloproteinase (TIMP1) and Osteopontin (Baron et al, 2006; Midgley et al, 2004; Theil and Cibelli, 2002; Hjoberg et al, 2004; Hasan et al, 2008; Guha et al, 2001; Yan et al, 2000; Freidrich et al, 2008; Yu et al, 2010). Moreover, in HepG2 cells, Egr-1 induces epithelial-mesenchymal transition (EMT), an important cellular response involved in fibrosis, by upregulating Snail (Grotegut et al, 2006). In normal skin and lung fibroblasts, forced expression of Egr-1 was sufficient to cause up-regulation of COL1A2 promoter activity, and further enhanced the stimulation induced by TGF-β (Chen et al, 2006). Fibroblasts lacking Egr-1 showed attenuated TGF-β responses despite intact Smad signaling, and forced expression of ectopic Egr-1 in these cells could restore TGF-β sensitivity (Chen et al, 2006). Subcutaneous administration of bleomycin in mice results in scleroderma-like dermal fibrosis and enhanced local TGF-β signaling (Wu and Varga, 2008). Bleomycin induced scleroderma was accompanied by increased Egr-1 accumulation in lesional fibroblasts (Bhattacharyya et al, 2008). The up-regulation of TGF-β signaling upon bleomycin treatment was attenuated in Egr-1-null mice. Treatment of wildtype mice with rosiglitazone, a PPAR-γ agonist, prevented dermal fibrosis (Wu et al, 2008). Remarkably, Egr-1 up-regulation in lesional skin was also prevented, implicating Egr-1 as a potential target for the anti-fibrotic effects of rosiglitazone.
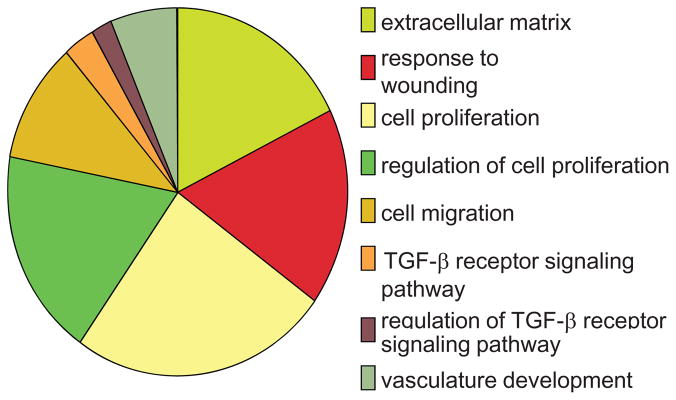
In order to get an unbiased view of genes regulated by Egr-1 in fibroblasts, we performed microarray analysis. Genome-wide expression profiling identified 647 genes whose expression was significantly altered by Egr-1 (Bhattacharyya S, Du P et al, unpublished). Gene Ontology (GO) analysis (Fig. 3) showed that genes associated with cell proliferation, TGF-β signaling, wound healing, extracellular matrix synthesis (ECM), and vascular development were strongly represented in the “Egr-1 response gene signature”. One of these genes is the NADPH oxidase 4 (NOX4), which showed > eight-fold increase in Egr-1-expressing fibroblasts. NOX4 catalyzes the generation of superoxide and other ROS. NOX4 expression is strongly induced by TGF-β in a variety of cell types, and the consequent generation of ROS is implicated in mediating TGF-β responses including myofibroblast transformation (Hecker et al, 2009). Chronic hypoxia was shown to induce elevated NOX4 in pulmonary artery smooth muscle cells via Egr-1 (Djordjevic et al., 2005; Sturrock et al., 2006; Diebold I, 2010; Gupte and Wolin, 2008). In SSc patients, TGF-β and hypoxia together might be responsible for sustained Egr-1 expression, resulting in increased NOX4 activity and oxidative stress, which further exacerbates fibrogenesis (Gabrielli et al, 2009).
Fig. 3.
Normal human dermal fibroblasts were infected with Ad-EGFP or Ad-Egr-1m (100 MOI). At the end of 24 or 48 h incubation, total RNA was isolated and subjected to genomewide transcriptional analysis using Illumina Microarray chips. Genome-wide expression profiling of Egr-1 regulated genes. GO analysis showing biological processes significantly enriched with Egr-1 regulated genes(p < 0.001). A subset of the biological processes is shown.
Deciphering profibrotic Egr-1 signaling: the roles of c-Abl and p300
Recent studies have yielded new insights into how Egr-1 mediates fibrotic responses. The c-Abl oncoprotein is a non-receptor tyrosine kinase that is constitutively activated in patients with chronic myelogenous leukemia (CML) (Rowley, 1973; Lugo et al, 1990). Mutated Bcr-Abl drives uncontrolled myeloid cell proliferation due to constitutive MEK1 phosphorylation (Kharbanda et al, 2000). It was recently shown that TGF-β directly induced the activity of c-Abl (Bhattacharyya, 2008; Wilkes and Leof, 2006). The response was specific for mesenchymal cells, and was not seen in epithelial cells. Ectopic c-Abl stimulated collagen gene expression, whereas its inhibition abrogated the stimulation by TGF-β. Moreover, pharmacological blockade of c-Abl in vivo using imatinib mesylate prevented fibrosis in animal models (Daniels et al, 2004; Wang et al, 2005; Wilkes and Leof, 2006; Distler et al, 2007). We found that in normal skin fibroblasts ectopic c-Abl directly induced Egr-1 expression, and c-Abl was both necessary and sufficient for Egr-1stimulation (Bhattacharyya et al, 2009). Moreover, genetic and pharmacological approaches demonstrated that Egr-1 was also indispensable for c-Abl-induced collagen stimulation (Bhattacharyya et al, 2008). Together, these findings indicate that fibrotic TGF-β responses involve c-Abl stimulation of Egr-1, and the pathway is therefore amenable to disruption by small molecule kinase inhibitors such as imatinib. Indeed, this mechanistic model for TGF-β-induced fibrogenesis involving c-Abl accounts for the potent antifibrotic efficacy of imatinib in animal models, and provides the rationale for current clinical trials of c-Abl inhibitors in SSc and other fibrotic diseases (Daniels et al., 2004; Wang et al, 2005; Wilkes and Leof, 2006; Distler et al, 2007).
The phosphoprotein p300/CBP is a ubiquitously expressed transcriptional coactivator with diverse biological functions, including a critical role in Smad-dependent TGF-β responses (Ghosh and Varga, 2007). Overexpression of p300 is sufficient by itself to stimulate collagen gene expression and p300 further enhances Smad-dependent TGF-β responses, whereas loss of p300 results in attenuation (Ghosh et al, 2000; Bhattacharyya et al. 2005). We found that TGF-β stimulate p300 expression via Egr-1 (Ghosh et al, unpublished). This response is similar to serum induction of p300, which was also shown to be mediated via Egr-1 (Yu et al, 2004). These studies place c-Abl upstream of Egr-1 in the TGF-β fibrotic response, and show Egr-1 itself to be upstream of p300, the indispensible coactivator for Smad-dependent fibrotic gene expression (Fig. 2A).
Fig. 2.
Regulation of TGF-β responses by Egr-1 and its inhibitor. A. TGF-β binds to the TβR1–TβR2 complex causing activation of Smad2/3. The pSmad2/3-Smad4 complex then translocates into the nucleus, where it binds to Smad binding elements (SBE) of target genes. The coactivator p300 is recruited to the Smad complex to activate COL1A2 transcription. TGF-β also activates a non-Smad pathway via c-Abl which in turn stimulates Egr-1 via MAP kinase. Egr-1 then binds to the Egr-1 binding element (EBS) to further stimulate COL1A2 transcription. B: Nab2 modulates Egr-1 activity and TGF-β signaling. In unstimulated fibroblasts (basal state), small amounts of Egr-1 and Nab2 are constitutively associated with the COL1A2 promoter (left upper panel). During early TGF-β stimulation (30min–4h) (right upper panel), Egr-1 is induced and Egr-1 is recruited to the COL1A2 promoter, where it enhances histone H4 hyperacetylation and stimulates transcription. Sustained TGF-β stimulation (2–48h) (left lower panel) leads to increased Nab2 expression and its accumulation in the Egr-1-COL1A2 transcriptional complex, where recruitment of HDAC1 resulting in H4 histone deacetylation and transcriptional silencing. In pathological fibrosis associated with constitutive Egr-1 expression (exemplified by scleroderma fibroblasts), defective Nab2 induction or function might results in unopposed Egr-1 signaling and target gene transcription (right lower panel).
Complex and dynamic cross-regulation of Egr family members in profibrotic TGF-β signaling
In addition to Egr-1, the early response gene family also includes Egr-2, Egr-3, and Egr-4, along with their inhibitors Nab1 and Nab2 which have complex and dynamic interactions with each other (Table 1). Members of the family play important roles in cell growth, differentiation, and apoptosis induced by environmental stimuli. While highly homologous, the distinct Egr’s show only partially overlaps patterns of tissue expression and biological activity.
Egr-2 is also induced by TGF-β and mediates profibrotic responses
Egr-2 plays important roles in immune regulation as an inhibitor of T cell activation, and is critical for maintaining immune tolerance (Collins et al, 2008). Mice with spontaneous lupus-like disease show diminished Egr-2 expression (Sela et al, 2008), and genetic targeting of Egr-2 in T cells results in expansion of autoreactive T cells, and loss of self tolerance (Zhu et al, 2008) #250}. Egr-2 is also essential for hindbrain development and peripheral nerve myelination, and in striking contrast to Egr-1-null mice, Egr-2-null mice suffer early lethality (Collins et al, 2008; Le et al, 2005). We recently began to investigate the distinct role of Egr-2 in fibrosis. We found that Egr-2 functions as an intracellular mediator of collagen gene stimulation and related fibrotic responses elicited by TGF-β (Feng et al, 2011). However, in contrast to Egr-1, which showed rapid up-regulation in response to TGF- β, stimulation of Egr-2 was delayed but sustained. Interestingly, Egr-1 itself induced Egr-2 expression. Ectopic expression of Egr-2 in normal fibroblasts directly induced the expression of a large number of profibrotic genes. Furthermore, Egr-2 level were found to be elevated in fibrotic skin and lung biopsies from patients with SSc. Thus, Egr-2 plays a distinct and functionally non-redundant role in ECM regulation and fibrogenesis.
The corepressor Nab2 blocks Egr-1-dependent TGF-β responses
A member of the Egr gene family Nab2 is a 55 kD nuclear protein, that was originally identified based on its ability to interact with Egr-1 in a yeast two-hybrid assay (Svaren et al, 1996). As a corepressor, Nab2 lacks DNA-binding activity, but can positively or negatively modulate the transcription of Egr-1 target genes via direct interaction with Egr-1 (Svaren et al, 1998). Many of the same environmental signals inducing Egr-1, also stimulates Nab2 expression (Bhattacharyya et al, 2009). Overexpression of Nab2 expression in normal fibroblasts blocked Egr-1-dependent transcription, and prevented the stimulation of collagen synthesis and myofibroblasts differentiation induced by TGF-β (Bhattacharyya et al, 2009). Inhibition involved HDAC1 recruitment to the COL1A2 promoter, and was accompanied by reduced histone H4 acetylation at the COL1A2 locus (Fig. 2B). A physiologic role for Nab2 in suppressing TGF-β signaling is indicated by loss-of- function studies: mice with targeted deletion of Nab2 displayed increased collagen deposition in the dermis, and Nab2−/− MEFs showed constitutively elevated collagen synthesis (Bhattacharyya et al, 2009). Therefore, Nab2 appears to be a novel endogenous inhibitor of TGF-β activity via negative regulation of Egr-1-dependent signaling. A recent study highlighted the complex relationship between the Egr’s and Nab2 by demonstrating that Egr-1, Egr-2, and Egr-3 can each activate Nab2 transcription which is in turn repressed by Nab2, thus establishing a cellular context-dependent negative feedback loop (Kumbrink et al, 2010)
Egr-1 implicated in fibrosis: insights from animal models
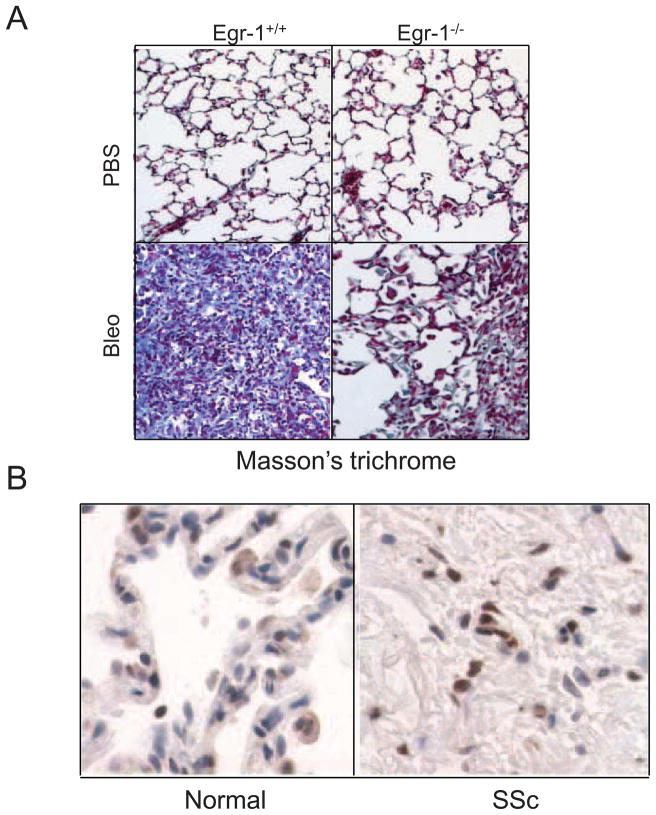
The key role of Egr-1 in fibrosis is highlighted by recent studies showing aberrant expression in several animal model of fibrosis. For instance, elevated Egr-1 was noted in the lungs of transgenic mice expressing TGF-β (Lee et al, 2004) or IL-13 (Cho et al, 2006), and in the gut in a mouse model of Crohn’s disease (Fichtner-Feigl et al, 2008). We showed that the expression of Egr-1 was elevated in lesional skin from mice with bleomycin-induced scleroderma, with expression localized principally to fibroblastic cells in the dermis (Bhattacharyya et al, 2008). On the other hand, Egr-1 deficiency blunted the development of cardiac hypertrophy induced by catecholamine (Saadane et al, 2000). We recently showed that Egr-1 null mice were protected from bleomycin-induced skin and lung fibrosis (Fig. 4) (Wu et al, 2009). Curiously, recent studies indicated that TGF-α-induced lung fibrosis and CCl4-induced hepatic fibrosis appeared to be exacerbated, rather than attenuated, in mice lacking Egr-1 raising the possibility that Egr-1 in fact has a more context-dependent role in fibrogenesis, possibly determined by the initiating nature of injury and cell type where it is active (Kramer et al, 2009; Pritchard et al, 2010). To further investigate the role of Egr-1 in fibrosis, we generated transgenic mice with fibroblast-specific Egr-1 overexpression by using a 6-kb enhancer from the pro 2(I) chain of the mouse type I collagen (Col1a2) gene. These transgenic mice did not develop spontaneous fibrosis, but did show a robust and exaggerated healing of incisional wounds (Wu et al, 2009).
Fig. 4.
Egr-1 modulates lung fibrosis in mice and human. A. Reduced lung fibrosis in mice lacking Egr-1. Egr-null mice and wild-type littermates received daily s.c. injections of bleomycin or PBS for 14 days. Fourteen days after the last injections, the lungs were harvested and examined with Masson’s trichrome stain (Original magnification x400). B. Elevated Egr-1 expression in SSc lung. Lesional lung tissue from patients with SSc- associated end-stage fibrosis (n=3) and healthy controls (n=3) were examined by immunohistochemistry with antibodies to Egr-1. Reprentative images are shown (original magnification x1000).
Aberrant Egr-1 regulation and expression in human fibrosing disorders
Emerging evidence implicates aberrant Egr-1 expression in human fibrotic disorder. Elevated Egr-1 expression has been demonstrated in atherosclerotic plaques where Egr-1 levels correlated with collagen accumulation (Bot et al, 2009). Elevated Egr-1 expression has also been reported in lesional skin and lung biopsies from patients with diffuse SSc (Fig. 5) and lungs of patients with idiopathic pulmonary fibrosis (IPF) (Bhattacharyya et al, 2008; Yasuka et al, 2009). Genome-wide expression profiling comparing lungs from IPF patients with stable versus rapidly progressive disease showed elevated Egr-1 expression to be associated with rapid progression (Boon et al, 2009). Intriguingly, elevated Egr-1 gene expression in peripheral blood cells from SSc patients was associated with pulmonary arterial hypertension in patients with SSc (Grigoryev et al, 2008).
Further evidence implicating Egr-1 signaling in SSc comes from recent DNA microarray studies. The “Egr-1 responsive gene signature” defined by 647 genes whose expression was significantly regulated by Egr-1 in normal fibroblasts was strongly associated with skin biopsies from the ‘diffuse-proliferation’ subset of SSc patients (Milano et al, 2008; Sargent et al, 2009; Bhattacharyya S, Sargent J et al, Ms. in preparation).
Summary and perspectives
Emerging insights from in vitro experiments, transgenic animal models and human diseases point to an important novel role for Egr-1 in physiological and pathological tissue repair and fibrosis. Egr-1 expression is induced by fibrogenic stimuli, and Egr-1 in turn can regulate the expression of extracellular matrix components, matrix remodeling enzymes and fibrogenic cytokines such as TGF-β, and drive myofibroblast differentiation and EMT. Egr-1 shows persistent elevation in fibrotic lesions in a variety of human fibrosing diseases, and in animal models of fibrosis. Microarray studies reveal the “Egr-1-responsive gene signature” in skin biopsies from SSc patients in the ‘diffuse-proliferation’ subset (Bhattacharyya et al, unpublished). Moreover, elevated Egr-1 expression in the lungs predicts rapid progression of fibrosis in IPF patients (Boon et al, 2009).
The identification of novel signaling pathways and mediators that are altered in fibrosis and contribute to tissue damage opens the door to the development of strategies for their selective targeting. Blocking Egr-1 expression or biological activity therefore appears to be a potential novel approach to control pathological fibrogenesis. It is noteworthy that several drugs in current clinical use display potent inhibitory activity for Egr-1 induction or activity. These include mycophenolate mofetil, cyclosporine, simvastatin, imatinib mesylate, and insulin-sensitizing peroxisome proliferator activated receptor-γ ligands such as rosiglitazone (Farivar et al, 2005; Bea et al 2003; Bhattacharyya et al 2008; Okada et al, 2002; Wu et al 2009). Some of these agents have already shown efficacy in animal models of fibrosis, and may be candidates in the treatment of SSc, IPF and other fibrotic conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baron V, Adamson ED, Calogero A, Ragona G, Mercola D. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther. 2006;13:115–124. doi: 10.1038/sj.cgt.7700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bea F, Blessing E, Shelley MI, Shultz JM, Rosenfeld ME. Simvastatin inhibits expression of tissue factor in advanced atherosclerotic lesions of apolipoprotein E deficient mice independently of lipid lowering: potential role of simvastatin-mediated inhibition of Egr-1 expression and activation. Atherosclerosis. 2003;167:187–194. doi: 10.1016/s0021-9150(02)00387-8. [DOI] [PubMed] [Google Scholar]
- Beattie J, Allan GJ, Lochrie JD, Flint DJ. Insulin-like growth factor-binding protein-5 (IGFBP-5): a critical member of the IGF axis. Biochem J. 2006;395:1–19. doi: 10.1042/BJ20060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Chen SJ, Wu M, Blankenship MW, Ning H, Lakos G, Mori Y, Chang E, Nihijima C, Takehara K, Feghali-Bostwick C, Varga J. Smad-Independent Transforming Growth Factor-β Regulation of Early Growth Response-1 and Sustained Expression in Fibrosis. Implications for Scleroderma. Am J Path. 2008;173:1085–1099. doi: 10.2353/ajpath.2008.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Ishida W, Wu M, Wilkes M, Mori Y, Hinchcliff M, Leof E, Varga J. A non-Smad mechanism of fibroblast activation by transforming growth factor-b via c-Abl and Egr-1: selective modulation by imatinib mesylate. Oncogene. 2008:1–13. doi: 10.1038/onc.2008.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Ghosh AK, Pannu J, Mori Y, Takagawa S, Chen G, Trojanowska M, Gilliam AC, Varga J. Fibroblasts expression of the coactivator p300 governs the intensity of profibrotic response to TGF-β. Arthritis Rheum. 2005;52:1248–1258. doi: 10.1002/art.20996. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Wei J, Melichian DS, Milbrandt J, Takehara K, Varga J. The transcriptional cofactor Nab2 is induced by TGF-β and suppresses fibroblast activation: physiological roles and impaired expression in scleroderma. PLoS One. 2009;26:4, e7620. doi: 10.1371/journal.pone.0007620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot PT, Hoefer IE, Sluijter JP, van Vliet P, Smits AM, Lebrin F, Moll F, de Vries JP, Doevendans P, Piek JJ, Pasterkamp G, Goumans MJ. Increased expression of the transforming growth factor-beta signaling pathway, endoglin, and early growth response-1 in stable plaques. Stroke. 2009;40:439–447. doi: 10.1161/STROKEAHA.108.522284. [DOI] [PubMed] [Google Scholar]
- Boon K, Bailey NW, Yang J, Steel MP, Groshong S, Kervitsky D, Brown KK, Schwarz MI, Schwartz DA. Molecular phenotypes distinguish patients with relatively stable from progressive idiopathic pulmonary fibrosis (IPF) PLoS One. 2009;4(4):e5134. doi: 10.1371/journal.pone.0005134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwalter G, Gross C, Wasylyk B. Ets ternary complex transcription factors. Gene. 2004;324:1–14. doi: 10.1016/j.gene.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Cao X, Mahendran R, Guy GR, Tan YH. Detection and characterization of cellular EGR-1 binding to its recognition site. J Biol Chem. 1993;268:16949–16957. [PubMed] [Google Scholar]
- Chen SJ, Ning H, Ishida W, Sodin-Semrl S, Takagawa S, Mori Y, Varga J. The early-immediate gene EGR-1 is induced by transforming growth factor-beta and mediates stimulation of collagen gene expression. J Biol Chem. 2006;281:21183–21197. doi: 10.1074/jbc.M603270200. [DOI] [PubMed] [Google Scholar]
- Cho SJ, Kang MJ, Homer RJ, Kang HR, Zhang X, Lee PJ, Elias JA, Lee CG. Role of early growth response-1 (Egr-1) in interleukin-13-induced inflammation and remodeling. J Biol Chem 2006. 2006;281:8161–8168. doi: 10.1074/jbc.M506770200. [DOI] [PubMed] [Google Scholar]
- Collins S, Lutz MA, Zarek PE, Anders RA, Kersh GJ, Powell JD. Opposing regulation of T cell function by Egr-1/NAB2 and Egr-2/Egr-3. Eur J Immunol. 2008;38:528–536. doi: 10.1002/eji.200737157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold I, Petry A, Hess J, Görlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell. 2010;21:2087–2096. doi: 10.1091/mbc.E09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler JH, Jüngel A, Huber LC, Schulze-Horsel U, Zwerina J, Gay RE, et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007;56:311–322. doi: 10.1002/art.22314. [DOI] [PubMed] [Google Scholar]
- Djordjevic T, BelAiba RS, Bonello S, Pfeilschifter J, Hess J, Görlach A. Human urotensin II is a novel activator of NADPH oxidase in human pulmonary artery smooth muscle cells. Arterioscle Thromb Vasc Biol. 2005;25:519–525. doi: 10.1161/01.ATV.0000154279.98244.eb. [DOI] [PubMed] [Google Scholar]
- Du B, Fu C, Kent KC, Bush H, Jr, Schulick AH, Kreiger K, Collins T, McCaffrey TA. Elevated Egr-1 in human atherosclerotic cells transcriptionally represses the transforming growth factor-beta type II receptor. J Biol Chem. 2000;275:39039–39047. doi: 10.1074/jbc.M005159200. [DOI] [PubMed] [Google Scholar]
- Ehrengruber MU, Muhlebach SG, Sohrman S, Leutenegger CM, Lester HA, et al. Modulation of early growth response (EGR) transcription factor-dependent gene expression by using recombinant adenovirus. Gene. 2000;258:63–69. doi: 10.1016/s0378-1119(00)00445-5. [DOI] [PubMed] [Google Scholar]
- Farivar AS, MacKinnon-Patterson B, Barnes AD, Mulligan MS. The effect of anti-inflammatory properties of mycophenolate mofetil on the development of lung reperfusion injury. J Heart Lung Transplant. 2005;24:2235–2242. doi: 10.1016/j.healun.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Fang F, Ooka K, Bhattacharyya S, Wei J, Wu M, Du P, Lin S, Del Galdo F, Feghali-Bostwick CA, Varga J. The early growth response gene Egr-2 (Krox20) is a novel transcriptional target of transforming growth factor-! that is up-regulated in systemic sclerosis and mediates profibrotic responses. Am J Path. 2011 doi: 10.1016/j.ajpath.2011.01.035. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farivar AS, Mackinnin-Patterson BC, Barnes AD, McCourtie AS, Mulligan MS. Cyclosporine modulates the response to hypoxia-reoxygenation in pulmonary artery endothelial cells. Ann Thorac Surg. 2005;79:1010–1016. doi: 10.1016/j.athoracsur.2004.08.078. [DOI] [PubMed] [Google Scholar]
- Fichtner-Feigl S, Young CA, Kitani A, Geissler EK, Schlitt HJ, Strober W. IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology. 2008;135:2003–2013. doi: 10.1053/j.gastro.2008.08.055. [DOI] [PubMed] [Google Scholar]
- Friedrich B, Janessa A, Artunc F, Aicher WK, Müller GA, Lang F, Risler T, Alexander D. DOCA and TGF-beta induce early growth response gene-1 (Egr-1) expression. Cell Physiol Biochem. 2008;22:465–474. doi: 10.1159/000185495. [DOI] [PubMed] [Google Scholar]
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- Gaggioli C, Deckert M, Robert G, Abbe P, Batoz M, Ehrengruber MU, Ortonne JP, Ballotti R, Tartare-Deckert S. HGF induces fibronectin matrix synthesis in melanoma cells through MAP kinase-dependent signaling pathway and induction of Egr-1. Oncogene. 2005;24:1423–1433. doi: 10.1038/sj.onc.1208318. [DOI] [PubMed] [Google Scholar]
- Gashler A, Sukhatme VP. Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol. 1995;50:191–224. doi: 10.1016/s0079-6603(08)60815-6. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Varga J. The transcriptional coactivator and acetyltransferase p300 in fibroblast biology and fibrosis. J Cell Physiol. 2007;213:663–71. doi: 10.1002/jcp.21162. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Yuan W, Mori Y, Varga J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF- ßeta involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene. 2000;19:3546–3555. doi: 10.1038/sj.onc.1203693. [DOI] [PubMed] [Google Scholar]
- Grigoryev DN, Mathai SC, Fisher MR, Girgis RE, Zaiman AL, Housten-Harris T, Cheadle C, Gao L, Hummers LK, Champion HC, Garcia JG, Wigley FM, Tuder RM, Barnes KC, Hassoun PM. Identification of candidate genes in scleroderma-related pulmonary arterial hypertension. Transl Res. 2008;151:197–207. doi: 10.1016/j.trsl.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotegut S, von Schweinitz D, Christofori G, Lehembre F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J. 2006;25:3534–3545. doi: 10.1038/sj.emboj.7601213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, O’Connell MA, Pawlinski R, Hollis A, McGovern P, Yan SF, Stern D, Mackman N. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood. 2001;98:1429–1439. doi: 10.1182/blood.v98.5.1429. [DOI] [PubMed] [Google Scholar]
- Gupte SA, Wolin MS. Oxidant and redox signaling in vascular oxygen sensing: implications for systemic and pulmonary hypertension. Antioxid Redox Signal. 2008;10:1137–1152. doi: 10.1089/ars.2007.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan RN, Schafer AI. Hemin Upregulates Egr-1 Expression in Vascular Smooth Muscle Cells via ROS ERK-1/2 Elk-1 and NF-{kappa}B. Circ Res. 2008;102:42–50. doi: 10.1161/CIRCRESAHA.107.155143. [DOI] [PubMed] [Google Scholar]
- Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjoberg J, Le L, Imrich A, Subramaniam V, Mathew SI, Vallone J, Haley KJ, Green FH, Shore SA, Silverman ES. Induction of early growth-response factor 1 by platelet-derived growth factor in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L817–825. doi: 10.1152/ajplung.00190.2003. [DOI] [PubMed] [Google Scholar]
- Khachigian LM, Collins T. Early growth response factor 1: a pleiotropic mediator of inducible gene expression. J Mol Med. 1998;76:613–616. doi: 10.1007/s001090050258. [DOI] [PubMed] [Google Scholar]
- Khachigian LM. Early growth response-1 in cardiovascular pathobiology. Circ Res. 2006;98:186–191. doi: 10.1161/01.RES.0000200177.53882.c3. [DOI] [PubMed] [Google Scholar]
- Kalluri R. EMT: When epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K, Thiel G. Epidermal growth factor and thrombininduced proliferation of immortalized human keratinocytes is coupled to the synthesis of Egr-1, a zinc finger transcriptional regulator. J Cell Biochem. 2002;85:381–391. doi: 10.1002/jcb.10145. [DOI] [PubMed] [Google Scholar]
- Kharbanda SR, Ren P, Pandey TD, Shafman SM, Feller RR, Weichselbaum, et al. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature. 1995;376:785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- Kramer EL, Mushaben EM, Pastura PA, Acciani TH, Deutsch GH, Khurana Hershey GK, Korfhagen TR, Hardie WD, Whitsett JA, Le Cras TD. Egr-1 Suppresses EGFR-mediated airway hyperresponsiveness and lung remodeling in mice. Am J Respir Cell Mol Biol. 2009;41:415–425. doi: 10.1165/rcmb.2008-0470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krones-Herzig A, Mittal S, Yule K, Liang H, English C, Urcis R, Soni T, Adamson ED, Mercola D. Early growth response 1 acts as a tumor suppressor in vivo and in vitro via regulation of p53. Cancer Res. 2005;65:5133–5143. doi: 10.1158/0008-5472.CAN-04-3742. [DOI] [PubMed] [Google Scholar]
- Kumbrink J, Kirsch KH, Johnson JP. EGR1, EGR2, and EGR3 activate the expression of their coregulator NAB2 establishing a negative feedback loop in cells of neuroectodermal and epithelial origin. J Cell Biochem. 2010;111:207–17. doi: 10.1002/jcb.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci U S A. 2005;102:2596–2601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, Homer RJ, Elias JA. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, Milbrandt J. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1) Science. 1996;273:1219–1221. doi: 10.1126/science.273.5279.1219. [DOI] [PubMed] [Google Scholar]
- Li CJ, Ning W, Matthay MA, Feghali-Bostwick CA, Choi AM. MAPK pathway mediates EGR-1-HSP70-dependent cigarette smoke-induced chemokine production. Am J Physiol Lung Cell Mol Physio. 2007;292:L1297–L1303. doi: 10.1152/ajplung.00194.2006. [DOI] [PubMed] [Google Scholar]
- Lohoff M, Giaisi M, Köhler R, Casper B, Krammer PH, Li-Weber M. Early growth response protein-1 (Egr-1) is preferentially expressed in T helper type 2 (Th2) cells and is involved in acute transcription of the Th2 cytokine interleukin-4. J Biol Chem. 2010;285:1643–1652. doi: 10.1074/jbc.M109.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- Midgley VC, Levon M. Fibroblast growth factor-2 induction of platelet-derived growth factor-C chain transcription in vascular smooth muscle cells is ERK-dependent but not JNK-dependent and mediated by Egr-1. J Biol Chem. 2004;279:40289–40295. doi: 10.1074/jbc.M406063200. [DOI] [PubMed] [Google Scholar]
- Milano A, Pendergrass SA, Sargent JL, George LK, McCalmont TH, Connolly MK, Whitfield ML. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS One. 2008;16(3 7):e2696. doi: 10.1371/journal.pone.0002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Wen F, Yan SF. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) activation suppresses ischemic induction of Egr-1 and its inflammatory gene targets. FASEB J. 2002;16:1861–1868. doi: 10.1096/fj.02-0503com. [DOI] [PubMed] [Google Scholar]
- Pritchard MT, Cohen JI, Roychowdhury S, Pratt BT, Nagy LE. Early growth response-1 attenuates liver injury and promotes hepatoprotection after carbon tetrachloride exposure in mice. J Hepatol. 2010;53:655–662. doi: 10.1016/j.jhep.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi RA, Leof EB. TGF-beta signaling: a tale of two responses. J Cell Biochem. 2007;102:593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- Russo MW, Sevetson BR, Milbrandt J. Identification of NAB1, a repressor of NGFI-A- and Krox20-mediated transcription. Proc Natl Acad Sci U S A. 1995;92:6873–6877. doi: 10.1073/pnas.92.15.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössler OG, Thiel G. Thrombin induces Egr-1 expression in fibroblasts involving elevation of the intracellular Ca2+ concentration, phosphorylation of ERK and activation of ternary complex factor. BMC Mol Biol. 2009;10:40. doi: 10.1186/1471-2199-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrinefluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Saadane N, Alpert L, Chalifour LE. Altered molecular response to adrenoreceptor-induced cardiac hypertrophy in Egr-1-deficient mice. Am J Physiol Heart Circ Physiol. 2000;278:H796–805. doi: 10.1152/ajpheart.2000.278.3.H796. [DOI] [PubMed] [Google Scholar]
- Sargent JL, Milano A, Bhattacharyya S, Varga J, Connolly MK, Chang HW, Whitfield ML. A TGF!-responsive Gene Signature is Associated with a Subset of Diffuse Scleroderma with Increased Disease Severity. J Inves Derm 2009. 2009;130:694–705. doi: 10.1038/jid.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela U, Dayan M, Hershkoviz R, Lider O, Mozes E. A peptide that ameliorates lupus up-regulates the diminished expression of early growth response factors 2 and 3. J Immunol. 2008;180:1584–1591. doi: 10.4049/jimmunol.180.3.1584. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Mager GM, Ward RM, Mayer J, Svaren J. NAB2 represses transcription by interacting with the CHD4 subunit of the nucleosome remodeling and deacetylase (NuRD) complex. J Biol Chem. 2006;281:15129–15137. doi: 10.1074/jbc.M600775200. [DOI] [PubMed] [Google Scholar]
- Svaren J, Sevetson BR, Golda T, Stanton JJ, Swirnoff AH, Milbrandt J. Novel mutants of NAB corepressors enhance activation by Egr transactivators. EMBO J. 1998;17:6010–6019. doi: 10.1093/emboj/17.20.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaren J, Sevetson BR, Apel ED, Zimonjic DB, Popescu NC, Milbrandt J. NAB2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and differentiative stimuli. Mol Cell Biol. 1996;16:3545–53. doi: 10.1128/mcb.16.7.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Brooks N, Bowen K, Svaren J, Vemuganti R. Transcription factor early growth response-1 induction mediates inflammatory gene expression and brain damage following transient focal ischemia. J Neurochem. 2008;105:1313–1324. doi: 10.1111/j.1471-4159.2008.05233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wilkes MC, Leof EB, Hirschberg R. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J. 2005;19:1–11. doi: 10.1096/fj.04-2370com. [DOI] [PubMed] [Google Scholar]
- Wei J, Bhattacharyya S, Tourtellotte WG, Varga J. Fibrosis in systemic sclerosis: Emerging concepts and implications for targeted therapy. Autoimmun Rev. 2010 doi: 10.1016/j.autrev.2010.09.015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Melichian DS, Chang E, Warner-Blankenship M, Ghosh AK, Varga J. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. Am J Pathol. 2009;174:519–533. doi: 10.2353/ajpath.2009.080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Melichian DS, de la Garza M, Gruner K, Bhattacharyya S, Barr L, Nair A, Shahrara S, Sporn PH, Mustoe TA, Tourtellotte WG, Varga J. Essential Roles for Early Growth Response Transcription Factor Egr-1 in Tissue Fibrosis and Wound Healing. Am J Pathol. 2009;175:1041–1065. doi: 10.2353/ajpath.2009.090241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Varga J. In perspective: murine models of scleroderma. Curr Rheumatol Rep. 2008;10:173–182. doi: 10.1007/s11926-008-0030-9. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Fibrosis under arrest. Nat Med. 2010;16:523–525. doi: 10.1038/nm0510-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes MC, Leof EB. Transforming growth factor beta activation of c-Abl is independent of receptor internalization and regulated by phosphatidylinositol 3-kinase and PAK2 in mesenchymal cultures. J Biol Chem. 2006;281:27846–27854. doi: 10.1074/jbc.M603721200. [DOI] [PubMed] [Google Scholar]
- Yan SF, Fujita T, Lu J, Okada K, Shan Zou Y, Mackman N, Pinsky DJ, Stern DM. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med. 2000;6:1355–1361. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- Yasuoka H, Hsu E, Ruiz XD, Steinman RA, Choi AM, Feghali-Bostwick CA. The fibrotic phenotype induced by IGFBP-5 is regulated by MAPK activation and egr-1-dependent and -independent mechanisms. Am J Pathol. 2009;175:605–615. doi: 10.2353/ajpath.2009.080991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HW, Liu QF, Liu GN. Positive regulation of the Egr-1/osteopontin positive feedback loop in rat vascular smooth muscle cells by TGF-beta, ERK, JNK, and p38 MAPK signaling. Biochem Biophys Res Commun. 2010;396:451–456. doi: 10.1016/j.bbrc.2010.04.115. [DOI] [PubMed] [Google Scholar]
- Yu J, de Belle I, Liang H, Adamson ED. Coactivating factors p300 and CBP are transcriptionally crossregulated by Egr1 in prostate cells, leading to divergent responses. Mol Cell. 2004;15:83–94. doi: 10.1016/j.molcel.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Zhu B, Symonds AL, Martin JE, Kioussis D, Wraith DC, Li S, Wang P. Early growth response gene 2 (Egr-2) controls the self-tolerance of T cells and prevents the development of lupuslike autoimmune disease. J Exp Med. 2008;205:2295–2307. doi: 10.1084/jem.20080187. [DOI] [PMC free article] [PubMed] [Google Scholar]