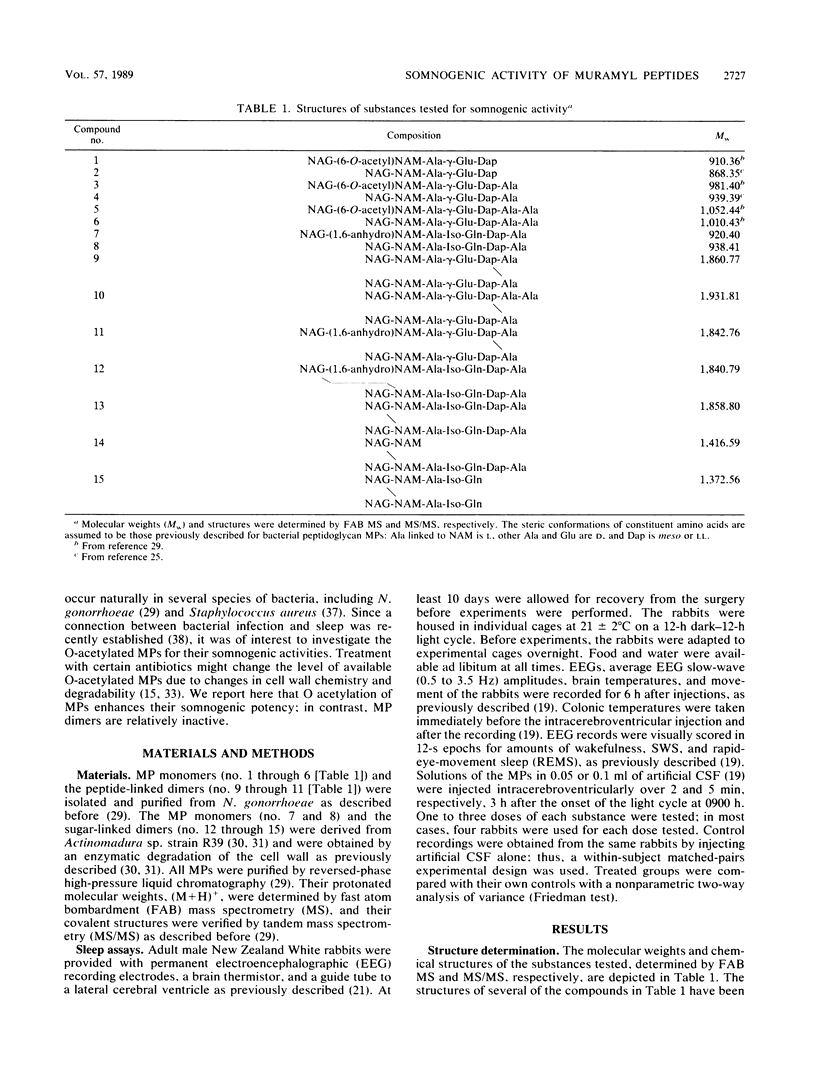
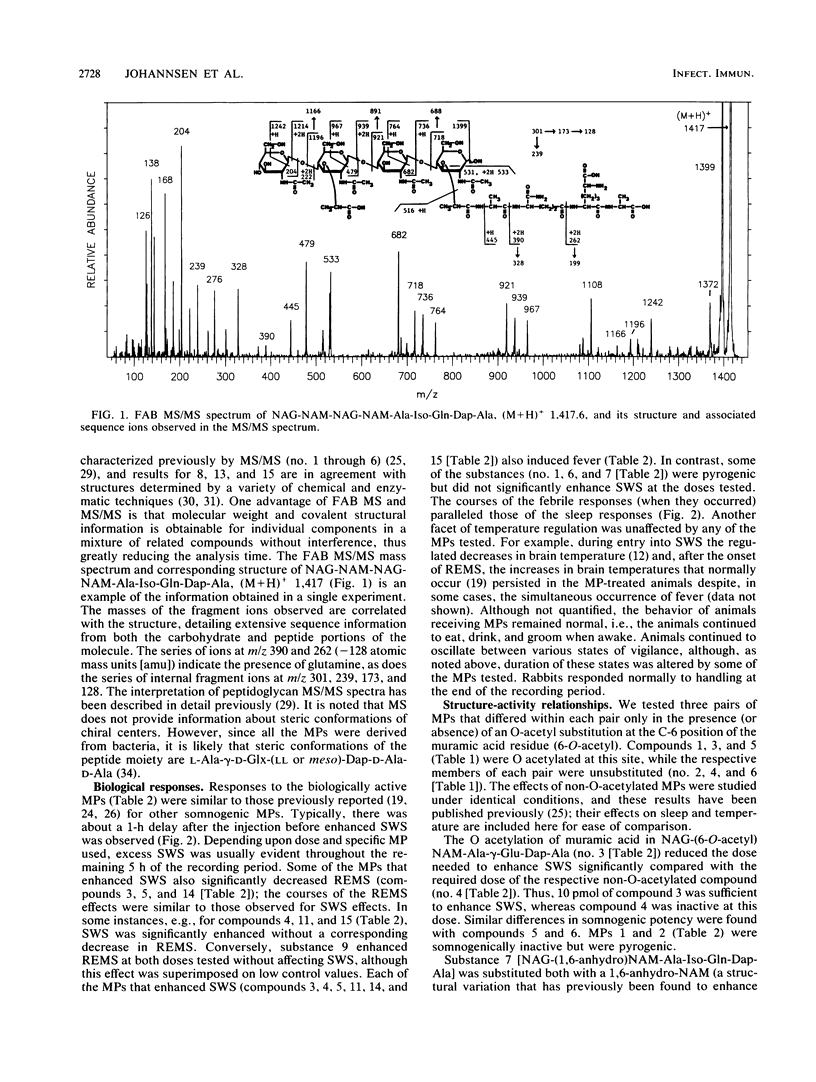
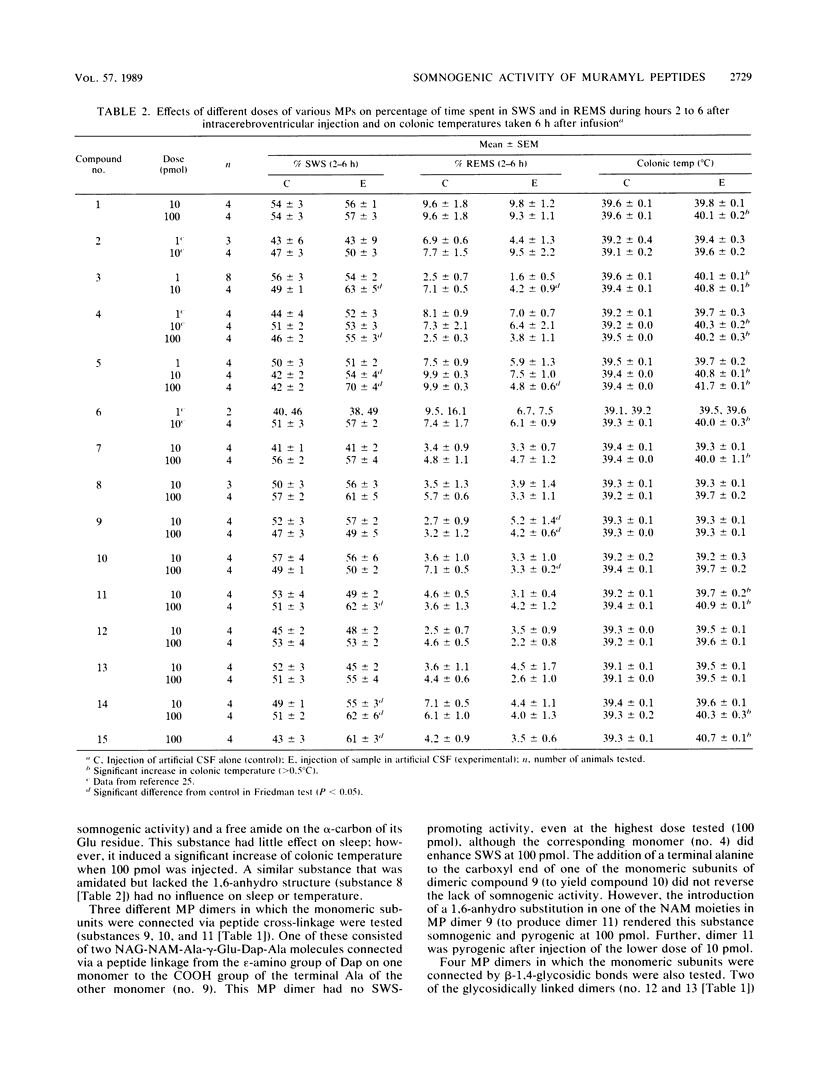
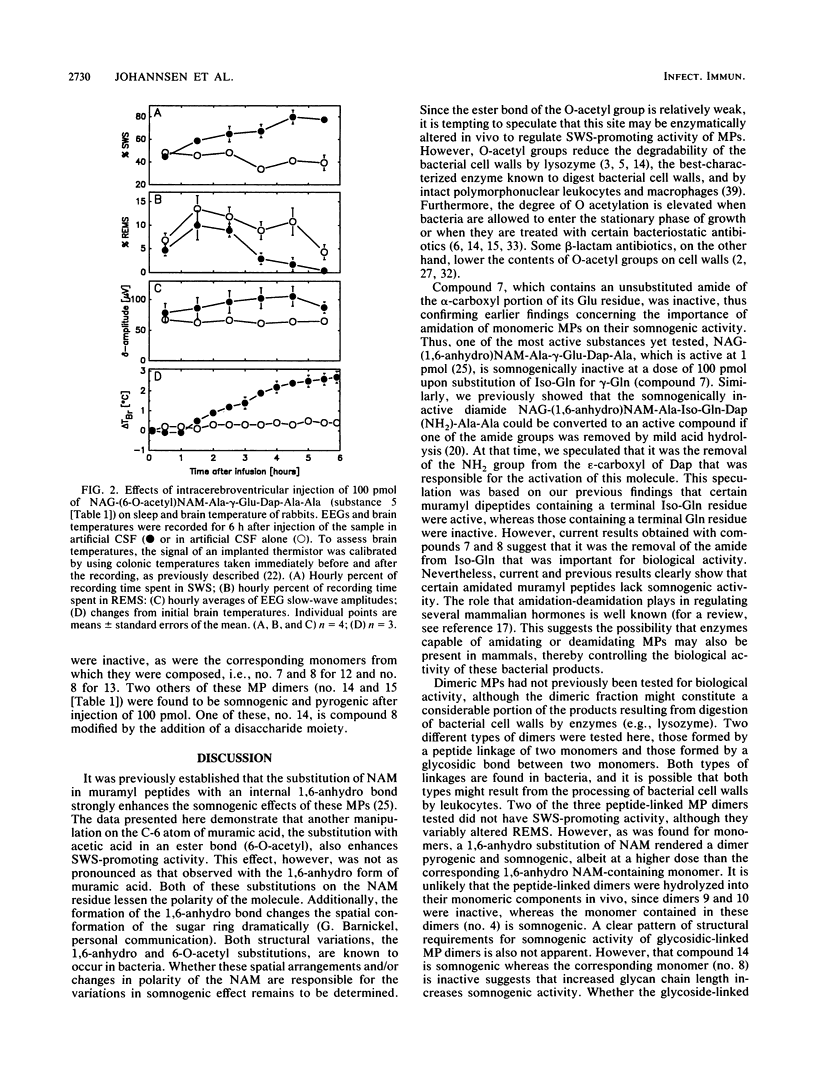
Abstract
Slow-wave sleep-promoting factors in brain and urine were identified as muramyl peptides (MPs), the building blocks of bacterial cell wall peptidoglycan. In this study, structural variations of MPs that occur naturally in bacterial peptidoglycan were investigated for somnogenic activity. Monomeric and dimeric MPs were isolated and purified from Neisseria gonorrhoeae and Actinomadura sp. strain R39. The structures of these MPs were verified by fast atom bombardment mass spectroscopy and tandem mass spectroscopy. After intracerebroventricular administration of MPs, electroencephalograms and brain temperatures of rabbits were recorded for 6 h and were analyzed to determine durations of slow-wave sleep, rapid-eye-movement sleep, and wakefulness. The 6-O acetylation of muramic acid enhanced the somnogenic effects of certain monomeric MPs relative to their non-O-acetylated (but otherwise identical) counterparts. Two monomeric MPs containing an unsubstituted amide (i.e., Iso-Gln) were inactive, thus confirming previous results showing that amidation of a variety of MPs can block somnogenic activity. Two peptide-cross-linked MP dimers tested had no effect on slow-wave sleep, although a third peptide-cross-linked MP containing a 1,6-anhydro muramyl end on one of its monomeric subunits, a structure that enhances somnogenic potency of un-cross-linked monomers, was somnogenic. Two dimers connected by glycosidic bonds and containing an Iso-Gln moiety were inactive. Two other glycosidically linked dimers that also contained an Iso-Gln moiety, but were of lower molecular weight, were somnogenic. In summary, 6-O acetylation of muramic acid in somnogenic MPs enhances activity, and as a class, peptide-linked dimeric MPs tend to be less active than their constituent monomers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Lederer E. Muramyl peptides: immunomodulators, sleep factors, and vitamins. Med Res Rev. 1984 Apr-Jun;4(2):111–152. doi: 10.1002/med.2610040202. [DOI] [PubMed] [Google Scholar]
- BRUMFITT W., WARDLAW A. C., PARK J. T. Development of lysozyme-resistance in Micrococcus lysodiekticus and its association with an increased O-acetyl content of the cell wall. Nature. 1958 Jun 28;181(4626):1783–1784. doi: 10.1038/1811783a0. [DOI] [PubMed] [Google Scholar]
- Blundell J. K., Perkins H. R. Effects of beta-lactam antibiotics on peptidoglycan synthesis in growing Neisseria gonorrhoeae, including changes in the degree of O-acetylation. J Bacteriol. 1981 Aug;147(2):633–641. doi: 10.1128/jb.147.2.633-641.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breder C. D., Dinarello C. A., Saper C. B. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988 Apr 15;240(4850):321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Krueger J. M. Induction of interleukin 1 by synthetic and naturally occurring muramyl peptides. Fed Proc. 1986 Oct;45(11):2545–2548. [PubMed] [Google Scholar]
- Farrar W. L., Hill J. M., Harel-Bellan A., Vinocour M. The immune logical brain. Immunol Rev. 1987 Dec;100:361–378. doi: 10.1111/j.1600-065x.1987.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Kilian P. L., Ruff M. R., Hill J. M., Pert C. B. Visualization and characterization of interleukin 1 receptors in brain. J Immunol. 1987 Jul 15;139(2):459–463. [PubMed] [Google Scholar]
- Fencl V., Koski G., Pappenheimer J. R. Factors in cerebrospinal fluid from goats that affect sleep and activity in rats. J Physiol. 1971 Aug;216(3):565–589. doi: 10.1113/jphysiol.1971.sp009541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Heller H. C., Glotzbach S. F. Thermoregulation during sleep and hibernation. Int Rev Physiol. 1977;15:147–188. [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollès P. A possible physiological function of lysozyme. Biomedicine. 1976 Sep 30;25(8):275–276. [PubMed] [Google Scholar]
- Krieger D. T. Brain peptides: what, where, and why? Science. 1983 Dec 2;222(4627):975–985. doi: 10.1126/science.6139875. [DOI] [PubMed] [Google Scholar]
- Krueger J. M., Bacsik J., García-Arrarás J. Sleep-promoting material from human urine and its relation to factor S from brain. Am J Physiol. 1980 Feb;238(2):E116–E123. doi: 10.1152/ajpendo.1980.238.2.E116. [DOI] [PubMed] [Google Scholar]
- Krueger J. M., Davenne D., Walter J., Shoham S., Kubillus S. L., Rosenthal R. S., Martin S. A., Biemann K. Bacterial peptidoglycans as modulators of sleep. II. Effects of muramyl peptides on the structure of rabbit sleep. Brain Res. 1987 Feb 17;403(2):258–266. doi: 10.1016/0006-8993(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Krueger J. M., Karnovsky M. L., Martin S. A., Pappenheimer J. R., Walter J., Biemann K. Peptidoglycans as promoters of slow-wave sleep. II. Somnogenic and pyrogenic activities of some naturally occurring muramyl peptides; correlations with mass spectrometric structure determination. J Biol Chem. 1984 Oct 25;259(20):12659–12662. [PubMed] [Google Scholar]
- Krueger J. M., Kubillus S., Shoham S., Davenne D. Enhancement of slow-wave sleep by endotoxin and lipid A. Am J Physiol. 1986 Sep;251(3 Pt 2):R591–R597. doi: 10.1152/ajpregu.1986.251.3.R591. [DOI] [PubMed] [Google Scholar]
- Krueger J. M., Majde J. A., Blatteis C. M., Endsley J., Ahokas R. A., Cady A. B. Polyriboinosinic:polyribocytidylic acid enhances rabbit slow-wave sleep. Am J Physiol. 1988 Nov;255(5 Pt 2):R748–R755. doi: 10.1152/ajpregu.1988.255.5.R748. [DOI] [PubMed] [Google Scholar]
- Krueger J. M., Pappenheimer J. R., Karnovsky M. L. Sleep-promoting effects of muramyl peptides. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6102–6106. doi: 10.1073/pnas.79.19.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. M., Pappenheimer J. R., Karnovsky M. L. The composition of sleep-promoting factor isolated from human urine. J Biol Chem. 1982 Feb 25;257(4):1664–1669. [PubMed] [Google Scholar]
- Krueger J. M., Rosenthal R. S., Martin S. A., Walter J., Davenne D., Shoham S., Kubillus S. L., Biemann K. Bacterial peptidoglycans as modulators of sleep. I. Anhydro forms of muramyl peptides enhance somnogenic potency. Brain Res. 1987 Feb 17;403(2):249–257. doi: 10.1016/0006-8993(87)90062-x. [DOI] [PubMed] [Google Scholar]
- Krueger J. M., Walter J., Karnovsky M. L., Chedid L., Choay J. P., Lefrancier P., Lederer E. Muramyl peptides. Variation of somnogenic activity with structure. J Exp Med. 1984 Jan 1;159(1):68–76. doi: 10.1084/jem.159.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. H., Gmeiner J. Modification of peptidoglycan structure by penicillin action in cell walls of Proteus mirabilis. Eur J Biochem. 1979 Apr;95(3):487–495. doi: 10.1111/j.1432-1033.1979.tb12988.x. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Karnovsky M. L., Krueger J. M., Pappenheimer J. R., Biemann K. Peptidoglycans as promoters of slow-wave sleep. I. Structure of the sleep-promoting factor isolated from human urine. J Biol Chem. 1984 Oct 25;259(20):12652–12658. [PubMed] [Google Scholar]
- Martin S. A., Rosenthal R. S., Biemann K. Fast atom bombardment mass spectrometry and tandem mass spectrometry of biologically active peptidoglycan monomers from Neisseria gonorrhoeae. J Biol Chem. 1987 Jun 5;262(16):7514–7522. [PubMed] [Google Scholar]
- Qoronfleh M. W., Wilkinson B. J. Effects of growth of methicillin-resistant and -susceptible Staphylococcus aureus in the presence of beta-lactams on peptidoglycan structure and susceptibility to lytic enzymes. Antimicrob Agents Chemother. 1986 Feb;29(2):250–257. doi: 10.1128/aac.29.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Gfell M. A., Folkening W. J. Influence of protein synthesis inhibitors on regulation of extent of O-acetylation of gonococcal peptidoglycan. Infect Immun. 1985 Jul;49(1):7–13. doi: 10.1128/iai.49.1.7-13.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Release of soluble peptidoglycan from growing conococci: demonstration of anhydro-muramyl-containing fragments. Infect Immun. 1980 Sep;29(3):914–925. doi: 10.1128/iai.29.3.914-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Isolation of 4-O-beta-N-acetylmuramyl-N-acetylglucosamine and 4-O-beta-N, 6-O-diacetylmuramyl-N-acetylglucosamine and the structure of the cell wall polysaccharide of Staphylococcus aureus. Biochem Biophys Res Commun. 1966 Jan 4;22(1):48–56. doi: 10.1016/0006-291x(66)90601-2. [DOI] [PubMed] [Google Scholar]
- Toth L. A., Krueger J. M. Alteration of sleep in rabbits by Staphylococcus aureus infection. Infect Immun. 1988 Jul;56(7):1785–1791. doi: 10.1128/iai.56.7.1785-1791.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


