Abstract
Coordinated transcription factor networks have emerged as the master regulatory mechanisms of stem cell pluripotency and differentiation. Many stem cell-specific transcription factors, including the pluripotency transcription factors, OCT4, NANOG, and SOX2 function in combinatorial complexes to regulate the expression of loci, which are involved in embryonic stem (ES) cell pluripotency and cellular differentiation. This review will address how these pathways form a reciprocal regulatory circuit whereby the equilibrium between stem cell self-renewal, proliferation, and differentiation is in perpetual balance. We will discuss how distinct epigenetic repressive pathways involving polycomb complexes, DNA methylation, and microRNAs cooperate to reduce transcriptional noise and to prevent stochastic and aberrant induction of differentiation. We will provide a brief overview of how these networks cooperate to modulate differentiation along hematopoietic and neuronal lineages. Finally, we will describe how aberrant functioning of components of the stem cell regulatory network may contribute to malignant transformation of adult stem cells and the establishment of a “cancer stem cell” phenotype and thereby underlie multiple types of human malignancies.
Introduction
This review discusses the emerging evidence that complex reciprocal regulatory circuits involving the NANOG, OCT4, and SOX2 pluripotency transcription factors, polycomb repressive complexes (PRC), and microRNAs regulate stem cell pluripotency and differentiation. These factors cooperate in the transcriptional and epigenetic regulation of key stem cell genes. We will examine the roles of each component of this circuit in pluripotent embryonic stem (ES) cells derived from the embryonic inner cell mass and in experimentally induced pluripotent stem (iPS) cells derived from adult fibroblasts. We will evaluate the potential roles of these factors in guiding stem cell differentiation and discuss how deregulation of these networks may contribute to carcinogenesis and the adoption of a “cancer stem cell” phenotype.
Pluripotent stem cells possess the unique ability to self-renew and differentiate into all of the cell lineages present in the embryo and adult. ES cells are pluripotent cells derived from the inner cell mass of the early stage blastocyst (please refer to reference [1] for a thorough review of the history of stem cell research and the molecular characteristics of undifferentiated stem cells) (Fig. 1A). The vitamin A metabolite, all-trans-retinoic acid can induce the differentiation of both mouse and human ES cells [2,3]. Mouse [4,5] and human [6] ES cells share this extraordinary differentiation capacity and possess similar, though not identical, molecular characteristics [7]. In the absence of serum, mouse ES cells require both the leukemic inhibitory factor (LIF) and bone morphogenetic proteins (BMPs) for the maintenance of the undifferentiated state [8]. These features of mouse ES cell culture requirements were central to elucidating the role of STAT3 [9], BMP-SMAD [8,10], and the inhibitor of differentiation (ID) [11] pathways in the maintenance of mouse ES cells. In the presence of serum, activation of STAT3 by LIF-cytokine is sufficient to maintain mouse ES cells in an undifferentiated state [9,12]. However, this is not the case for human ES cells [13]. These differences in tissue culture requirements likely contributed to the challenges in derivation of pluripotent human ES cells, which were overcome in 1998 by Thomson and colleagues, who reported the successful isolation and characterization of human ES cells [6]. In contrast to mouse ES cells, BMPs induce differentiation [14] and STAT3 activation by LIF is not sufficient to maintain self-renewal of human ES cells [13]. Suppression of BMP signaling by basic fibroblast growth factor (bFGF) contributes, but is not sufficient, to maintain human ES cell self-renewal [14,15]. Interestingly, mouse pluripotent stem cells derived from the E5.5-E6.5 postimplantation embryos (epiblast stem cells, Epi-SCs) fail to self-renew in the presence of LIF or BMP4; however, they share the requirement of FGF4 and activin/nodal signaling for self-renewal [16,17], as is the case for human ES cells. This suggests that self-renewal and pluripotency in human ES cells may be a more complicated process that requires more cooperative factors compared with that in mouse ES cells. Stem cell lines derived from the mouse blastocyst, in the presence of FGF, activin, and the Wnt pathway activator, BIO [7], termed FAB-SCs, share characteristics of both Epi-SCs and human ES cells and express the pluripotency factors Nanog, Oct4, and Sox2, but are not pluripotent [18]. Yet, treatment of FAB-SCs with LIF/BMP4 induces latent pluripotent capacity in these cells, which is attributable to the induction of E-cadherin expression [18]. These results indicate that parallel molecular pathways exist in mouse and human ES cells through which cells can achieve similar transcriptional and epigenetic states consistent with pluripotency. Indeed, it now appears that in the absence of any extrinsic signal, self-renewal is the default program of ES cells [19].
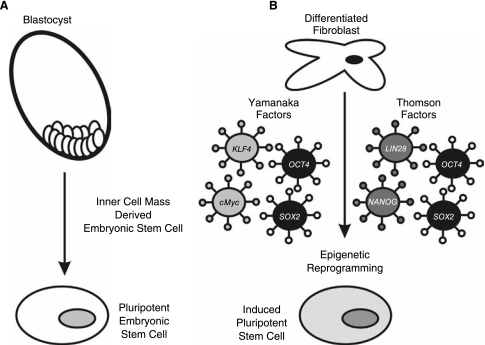
FIG. 1.
Pluripotent stem cells can be derived from cells isolated from the inner cell mass of early stage blastocysts (A) or experimentally derived by epigenetic reprogramming of differentiated adult cell types (B). Greatest reprogramming efficiency is achieved when combinations of 4 factors, OCT4, SOX2, c-MYC, and KLF4, or OCT4, SOX2, NANOG, and LIN28 genes are introduced into the differentiated cell. However, OCT4 and SOX2 appear critically required to induce pluripotency.
Further insight into the critical signaling determinants of pluripotency were revealed by the experimental induction of pluripotency by somatic cell nuclear transfer [20,21], nuclear reprogramming/cell fusion experiments [22–24], and most recently by retroviral introduction of 4 critical genes, now sometimes referred to as the Yamanaka factors: Oct4, Sox2, Klf4, and c-Myc (Fig. 1B), or a combination of OCT4, SOX2, NANOG, and LIN28 [25]. This technique has permitted the reprogramming of multiple distinct mouse and human differentiated cell types to yield iPS cells [26–34]. The highest efficiencies of induced pluripotency are achieved when all 4 factors were utilized; however, c-Myc [35] and Klf4 [36] have been shown to be dispensable for somatic cell reprogramming to pluripotency under specific culture conditions. Specifically, the histone deacetylase inhibitor valproic acid (VPA) both enhances the efficiency of iPS derivation by the combined 4 factors and permits the derivation of iPS cells using just Oct4 and Sox2 [37]. These studies indicate that Oct4 and Sox2 are critical factors required for maintaining self-renewal and pluripotency of mouse and human stem cells. Indeed Oct4 was sufficient to induce pluripotency in adult neural stem cells, which express endogenous Sox2, c-Myc, and Klf4 [38], whereas Klf4 could induce pluripotency from Epi-SC [39]. In the context of this review, these experiments have been pivotal in revealing the importance of OCT4 in pluripotency and that each pluripotency transcription factor possesses a unique epigenetic function to influence pluripotency and differentiation of stem cells. Recent advances in nonviral methodologies to introduce reprogramming factors into differentiated cells represent major advances toward the ultimate clinical application of these iPS cells [40–42]. A related but distinct combination of 4 critical genes, OCT4, SOX2, NANOG, and LIN28, can also induce pluripotency of human somatic cell types [25]. OCT4 and SOX2 are central to the iPS methodologies described by the Yamanaka-laboratory [26,27,29–31,35]. Later in this review we will address the pivotal functions of the NANOG homeodomain protein in pluripotency [43,44] and the recently described roles of LIN28 in regulation of microRNA processing in stem cells [45]. Further recent studies have determined that the timing of expression of pluripotency-associated factors appears to directly influence the generation of iPS cells. A combination of OCT4, NANOG, SOX2, LIN28, c-MYC, and KLF4 enhances iPS derivation by a factor of ~10, and reduces the time of reprogramming from 26 to 17 days [46]. Recently, Zhao and colleagues reported that a timely knockdown of p53 (p53-siRNA) combined with forced expression of UTF1 was able to increase the efficacy of iPS formation by ~100-fold, in a background consisting of fibroblasts pretransduced with the classic Yamanaka factors [47]. These remarkable studies reinforce the concept of pluripotency as a reprogrammable state established as the outcome of a transcriptional circuit involving key stem cell transcription factors and microRNAs.
Epigenetic events involving reversible histone modifications and DNA methylation govern stem cell self-renewal and differentiation
The crucial role of epigenetics in modulating the transcriptional outcome and thereby regulating cell fate decisions has emerged over the last decade. Epigenetics can be defined as “heritable” or transmitted changes in the chromatin structure independent of the underlying DNA sequence. These changes include a functionally diverse array of distinct covalent histone modifications [48] and methylation of the DNA CpG islands [49] (Fig. 2). Although DNA methylation primarily mediates transcriptional repression [49], different histone modifications such as acetylation, methylation, phosphorylation, and ubiquitination play a more complex role in regulating gene transcription [50]. The transcriptional status of a locus/gene, whether it is expressed or repressed, is modulated by local histone covalent modifications. Indeed the specific histone residues modified and the nature of the covalent modification constitute a histone code, which segregates the genome into regions which are active, repressed, or are poised for activation [48]. Several studies have provided compelling evidence that distinct chromatin organization and epigenetic signatures exist within ES cells and govern their intrinsic ability to self-renew and differentiate into multiple lineages [51–61]. An elegant study reported by Meshorer and colleagues [62] revealed hyper-dynamic association of architectural chromatin proteins (including heterochromatin protein-1 and histones) within ES cell chromatin, as compared to lineage committed neural progenitor cells (NPCs) [62]. Additionally, overall chromatin architecture, as assessed by heterochromatin organization, differed in ES cells as compared to the NPCs. While the heterochromatin pattern was more dispersed in ES cells, it formed compact and discrete foci in NPCs [62]. There have also been reports of global increases in acetylation of histone 3 (H3) and histone 4 (H4) in ES cells indicative of an open chromatin structure [63]. However, histone acetylation is dynamic during stem cell differentiation and this reflects the important roles of histone deacetylases (HDACs) in guiding stem cell differentiation and embryonic development [64,65]. Histone deacetylase I (HDAC1), the mammalian homolog of yeast RPD3, functions to deacetylate core histones and thereby contributes to chromatin condensation and transcriptional repression. HDAC1 is also implicated as a key regulator of genome activation in 1-cell embryos [65]. HDAC1 also participates in the nucleosome remodeling–histone deacetylase (NuRD) complex that plays essential roles in stem cell biology [66]. HDAC1, and the closely related HDAC2, are also the enzymatic components of the distinct Nanog–Oct4–deacetylase (NODE) complex, which functions to control developmentally regulated and pro-differentiation genes [64]. Consistent with this critical function, an HDAC1−/− null mutation in mice results in embryonic lethality because of severely retarded proliferation and development [67,68]. HDAC1 is also found in complexes with the YinYang1 (YY1) polycomb transcription factor and other polycomb complex members, including the EED/EZH2 components of the polycomb repressive complex-2 [69]. HDAC1 can also interact with the de novo DNA methyltransferase 3a (Dnmt3a) [70,71] and with Ddx5/p68 [67], establishing a link between epigenetic transcriptional regulation and the RNA helicases, which play diverse roles in miRNA biogenesis and transcriptional regulation [72] (see section on microRNAs). Thus, HDAC1 contributes to distinct and dynamic transcriptional repressive complexes with essential roles in stem cell differentiation and development.
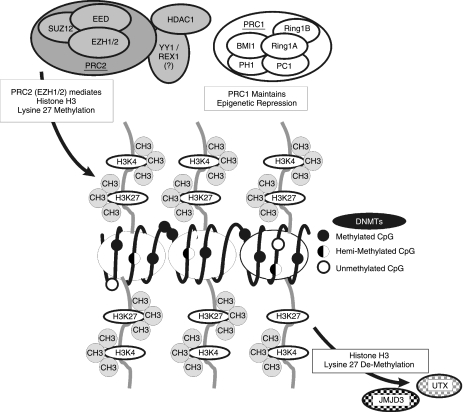
FIG. 2.
Bivalent chromatin domains, composed of activating (histone H3K4 trimethylation) and repressive (H3K27 trimethylation) histone tail modifications (indicated in gray) are a hallmark of developmentally regulated genes. Transcriptional repression of pro-differentiation genes is maintained in pluripotent stem cells by polycomb repressive complexes (PRC) 1 and 2. The core PCR2 includes the EZH2 H3K27 methyltransferase, SUZ12, and EED. However, HDAC1 and YY1 have been shown to interact with PRC2. PRC1 includes Bmi1, the Ring1A/B ubiquitin ligases, and mammalian homologs of Drosophila polyhomeotic (Ph) and polycomb (Pc). Transcriptional repression can be relieved by the combined actions of the JMJD3 and UTX H3K27 demethylases. DNA CpG methylation also contributes to the developmental regulation of gene expression. Individual CpG islands may be unmethylated (○), partially/hemi-methylated ( ), or fully methylated (•).
), or fully methylated (•).
Recent advances in the use of combined genome microarray and chromatin immunoprecipitation (ChIP-chip) technology have enabled researchers to study patterns of genome-wide histone modifications. This technology has proven essential for the identification of the characteristic “epigenetic signature” of pluripotent stem cells. Bernstein and colleagues used a combination of ChIP and oligonucleotide tiling arrays spanning ~2.5% of the mouse genome comprising of a subset of highly conserved noncoding elements to identify the histone modification patterns in mouse ES cells [59]. This study reported a novel chromatin pattern, “bivalent domain” that harbors both the “repressive” trimethylated histone H3 lysine 27 (H3K27me3) and “activating” trimethylated histone H3 lysine4 (H3K4me3) modifications [59] (Fig. 2). The majority of these bivalent domains were enriched at the promoters of genes encoding transcription factors that regulate developmentally important genes in pluripotent stem cells. A subsequent study utilized ChIP and high throughput DNA sequencing techniques (ChIP-Seq) to compare the chromatin states (including methylation of H3K4, H3K27, H3K36) of mouse ES cells, neural progenitors, and embryonic fibroblasts [73]. Comparison of the genomic distribution of these histone modifications and their correlation with the CpG content of 17,762 mouse promoters in undifferentiated and differentiated cell types studied permitted the identification and segregation of promoters as active, repressed, or poised for distinct developmental fates. Bivalent chromatin domains are also present in human ES cells [54,55]. The large scale of these studies enabled the identification of distinct categories of genes that exclusively possess the H3K4me3 or H3K27me3 marks, genes containing a combination of H3K4me3 and H3K27me3, and genes that lack both modifications (see Table 1 for examples). The activating H3K4me3 modification is associated with genes implicated in essential biological functions such as cell proliferation and metabolism. Consistent with the reports on the mouse epigenome, combinations of H3K27me3 and H3K4me3 were found to be associated with genes involved in important developmental functions such as neurogenesis, ectoderm formation, and transcriptional regulation [54,55]. Notably, the Hox cluster family was present in the class of genes with colocalized H3K4me3 and H3K27me3 epigenetic marks in both mouse and human studies. The Hox family of transcription factors has an essential role in axial patterning during embryogenesis, and Hox genes are well-established targets of polycomb group (PcG) proteins [58,74,75]. Similarly, promoter regions of developmentally important genes occupied by LIF-activated STAT3 in mouse ES cells exhibit these bivalent chromatin states [76]. This suggests that integration of additional signaling pathways is necessary to convert extrinsic LIF signaling into meaningful epigenetic and developmental decisions in mouse ES cells. Therefore, there is convincing evidence that bivalent modification in ES cells is associated with repression of genes involved in development, possibly due to the PcG-mediated repressive H3K27me3 mark. In addition, it is likely that these genes are “poised” for activation, most likely due to the presence of the H3K4me3 mark. However, how these opposing modifications are incorporated at these bivalent sites in ES cells remains to be determined. Another key question pertains to the precise function of these bivalent domains. It is still unclear if the existence of such domains is solely important for proper differentiation or if they play a role in establishment and/or maintenance of pluripotency. Although initial studies suggested that bivalent domains were possibly a feature unique to stem cells, several differentiated cell lineages have been shown to possess bivalent sites. These include mouse neuronal precursor cells, mouse embryonic fibroblasts, human T cells, and human lung fibroblasts [77–79]. Together, these studies have identified the existence of a dynamic chromatin architecture in undifferentiated ES cells, which undergoes global reorganization during differentiation.
Table 1.
Epigenetic Features and Transcription Factor Occupancy of Stem Cell Loci
| Gene ID | Function | Kim: 2008 (mES) Boyer: 2005 (hES) | Loh: 2006 (mES) | Marson: 2008 (mES) | Marson: 2008 SUZ12 | Ku: EZH2 (PRC2) | Ku: Suz12 (PRC2) | Ku: Ring1B (PRC1) | Ku: ESC Promoter State | Ku: NPC Promoter State | Ku: CpG (mES) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pou5f1 | TF activity | NOS | NO | NOST | – | – | – | – | K4 | None | ICP |
| Nanog | TF activity | NOS | NO | N | – | – | – | – | K4 | None | ICP |
| Sox2 | TF activity | NOS | NO | NOST | – | – | – | – | K4 | K4 | HCP |
| Osr2 | TF activity | NOS | – | NOST | Suz12 (CpG) | EZH2 | Suz12 | Ring1B | K4 + K27 | K4 | HCP |
| Olfml2a | ECM Binding | NOS | – | NO | – | – | – | – | K4 | None | HCP |
| Hist1h3i | DNA binding | NOS | – | NOST | – | – | – | – | K4 | K4 | HCP |
| Rest | Repressor | NOS | NO | NOS | – | – | – | – | K4 | None | HCP |
| Sfrp1 | Binding (Wnt) | NOS | – | NOST | Suz12 (CpG) | EZH2 | – | Ring1B | K4 + K27 | K27 | HCP |
| Zfp36l1 | TF activity | NOS | NO | NOST | – | – | – | – | K4 | K4 | HCP |
| Thbs2 | Structural | NOS | N | NOST | – | – | – | – | None | None | ICP |
| Ror1 | RTK activity | NOS | N | NOST | Suz12 (CpG) | EZH2 | – | – | K4 + K27 | K4 | HCP |
| Jarid2 | Repressor | NOS | NO | NOST | – | – | – | – | K4 | K4 | HCP |
| Zic5 | DNA binding | NOS | N | NOST | – | EZH2 | – | Ring1B | K4 | K4 | HCP |
| Rfx4 | DNA binding | NOS | NO | NOST | – | EZH2 | Suz12 | Ring1B | K4 + K27 | K4 | HCP |
| Lrrc2 | Protein Binding | NOS | NO | NOST | – | – | – | – | K4 | None | ICP |
| Spred1 | SCF-receptor | NOS | N | NOST | – | – | – | – | K4 | K4 | HCP |
| Fgfr2 | FGFR activity | NOS | N | NOST | – | EZH2 | Suz12 | Ring1B | K4 + K27 | K4 | HCP |
| Gad2 | E-decarboxylase | NOS | – | NOST | Suz12 (CpG) | EZH2 | Suz12 | Ring1B | K4 + K27 | None | HCP |
| Hoxb1 | TF activity | NOS | – | NOST | Suz12 (CpG) | EZH2 | Suz12 | Ring1B | K27 | K27 | HCP |
| Pipox | Oxidase | NOS | O | NOST | – | – | – | – | K4 | K4 | LC P |
| Grap2 | SH3/SH2 adaptor | NOS | O | NOST | – | – | – | – | None | None | LC P |
| Spic | TF activity | NOS | – | NOST | – | – | – | – | None | None | ICP |
| Hist1h1b | DNA binding | NOS | – | NOST | – | – | – | – | K4 | K4 | HCP |
| Rif1 | Binding | NOS | O | NOST | – | – | – | – | K4 | K4 | HCP |
| Lefty2 | TGFbetaR | NOS | – | NOST | – | – | – | – | K4 + K27 | None | HCP |
| Lrrtm3 | Protein Binding | NOS | – | NOST | Suz12 | – | Suz12 | – | K27 | K4 | ICP |
| Cripto1 | Growth Factor | NOS | NO | NOST | – | – | – | – | K4 | None | ICP |
| Neurog1 | TF activity | NOS | – | NOST | Suz12 (CpG) | EZH2 | Suz12 | Ring1B | K4 + K27 | K27 | HCP |
| Nkx2-2 | TF activity | NOS | – | NOST | Suz12 (CpG) | EZH2 | Suz12 | Ring1B | K27 | K4 | HCP |
| Pax6 | TF activity | NOS | – | NOST | Suz12 (CpG) | EZH2 | Suz12 | Ring1B | K4 + K27 | K4 | HCP |
| Zic2 | DNA binding | NOS | – | NOST | Suz12 (CpG) | EZH2 | Suz12 | Ring1B | K4 + K27 | K4 | HCP |
| Dkk1 | Transducer (Wnt) | NOS | O | NOST | Suz12 (CpG) | EZH2 | Suz12 | Uncl. | K4 + K27 | None | ICP |
| *ZFP42 | TF activity | NO | – | NOS | – | EZH2 | – | – | K4 | None | ICP |
To illustrate the convergence of pluripotency transcription factor networks with epigenetic processes, we selected a subset of ES cell genes identified as Oct4, Nanog, and Sox2 target genes in both human (Boyer et al., 2005) and mouse ES cells (Kim et al., 2008)
This dataset was then examined in independent datasets for promoter occupancy by Oct4, Nanog [99] and for Nanog, Oct4, SOX2, TCF4 and polycomb complexes [144], bivalent histone methylation marks (K4 and/or K27), and DNA promoter methylation classification [87]. *Zfp42/Rex1 is included for comparison (see Fig. 3).
Abbreviations: TF, transcription factor; N, Nanog; O, OCT4/Pou5f1; S, SOX2; T, TCF; –, not detected; Suz12/EZH2, PRC2; Ring1b, PRC1; K4, histone H3 lysine 4 trimethylation; K27, histone H3 lysine 27 trimethylation; HCP, high CpG promoter containing a 500 bp region with GC content ≥0.55, observed to expected CpG ratio ≥0.6; LCP, low CpG promoter containing no 500 bp region and with observed to expected CpG ratio ≥0.4 and ICP, intermediate CpG promoter, CpG distribution between HCP and LCP; ESC, embryonic stem cell; NPC, neural precursor cell.
DNA methylation and stem cell differentiation
DNA methylation (Fig. 2) is an epigenetic modification typically associated with gene silencing (reviewed in detail in [49]). Methylation of DNA CpG islands plays a critical role in physiological processes of transcriptional repression, X-inactivation, and genomic imprinting [80]. Three different DNA methyltransferase (Dnmt1, Dnmt3a, Dnmt3b) isoforms have been characterized. Dnmt1 exhibits a substrate preference for hemimethylated CpG and functions primarily to maintain DNA CpG methylation, whereas Dnmt3a and Dnmt3b are critical for de novo DNA methylation [80,81] (Fig. 2). Dnmts play essential functions in embryonic differentiation and development [52,82,83]. However, the exact roles of DNA CpG methylation in stem cell differentiation and how this may relate to other epigenetic regulatory mechanisms remain poorly understood. The genome-wide DNA methylation profiles analyses have revealed that DNA CpG methylation is a dynamic epigenetic trait resulting in distinct patterns of methylated CpG regions in undifferentiated stem cells and cells undergoing differentiation [52]. Interestingly, DNA methylation is associated with the majority (87%) of ES cell-repressed genes, which lack the bivalent histone domain [84]. This finding suggests that DNA methylation is a potential repressive mechanism for this class of genes, which is in a silenced state in ES cells (see Table 1). DNA methylation can also contribute to the silencing of the key pluripotency transcription factors as ES cells move toward a differentiated state. Dnmt3a and Dnmt3b were shown to methylate Oct3/4 and Nanog promoters in embryonal carcinoma and ES cells upon differentiation [81]. Consistent with this, the expression of several genes associated with pluripotency, including Nanog 1 and Zfp42/Rex1, is characterized by demethylated promoters in ES cells and the genes are silenced and methylated in differentiated mouse fibroblasts [84]. This reflects the distinct global DNA methylation patterns that contribute to stem cell differentiation. Nonetheless, the functional interplay between DNA methylation and histone modifications remains poorly understood. Indeed, the correlation between DNA methylation and PcG-mediated repression has been debated. Comparison of the genes targeted by PcG complex and those enriched by methyl-DNA immunoprecipitation revealed that polycomb-mediated repressive marks and DNA methylation are not associated with a common pool of target genes in ES cells [84]. Although there have been reports of functional interdependence of these epigenetic mechanisms [84,85], these and other results [86,87] suggest that DNA methylation and PcG-mediated transcriptional repression represent 2 distinct but convergent epigenetic silencing mechanisms.
The NANOG/OCT4/SOX2 pluripotency transcription factor network control embryonic stem cell differentiation by epigenetically modulating the expression of pro-differentiation loci
The transcription factors Oct4, Nanog, and Sox2 play essential roles in the regulation of pluripotency in both human and mouse ES cells [43,88–90]. Down-regulation of the Oct4 protein leads to a loss of maintenance of ES cell pluripotency as the cells differentiate [43,89]. Surprisingly, however, the overexpression of Oct4 also induces differentiation in ES cells [88]. This suggests that precise levels of Oct4 must be sustained for the maintenance of pluripotency, further implying that Oct4 levels are tightly regulated by the cell. Nanog is a homeodomain protein that is predominantly expressed in pluripotent cells and is essential for early embryo development [43]. Evidence from the derivation of iPS cells (described earlier) and other studies [43,89] indicates that while Nanog is not required for the establishment of pluripotency in ES cells, it does function to maintain the self-renewal capacity of these cells and its expression has been shown to suppress differentiation [31,44,91]. Consistent with this role, Nanog overexpression enables the propagation of mouse ES cells in a leukemia inhibitory factor (LIF)-free environment [43] and human ES cells in the absence of feeder cells [92]. Conversely, the loss or deficiency of Nanog results in ES cell differentiation [93,94]. Nanog [64], like Oct4 [95], forms distinct transcriptional repressive complexes, but more recent work has shown that Nanog possess 2 potent transcriptional activating domains [96,97]. This suggests that like Oct4 [98], Nanog can function as a transcriptional activator. The Sox2 transcription factor is a member of the SRY-related HMG box (Sox) transcription factor family [90,97]. Sox2 is expressed in pluripotent cells and multipotent embryonic and extraembryonic cells. Although Sox2 remains less well characterized than either Oct4 or Nanog [43,88,89], Sox2 is known to play a major role in the regulation of cell fate and with Oct4 appears essential for the derivation of iPS cells [36].
Oct4 and Nanog have long been recognized to be master transcriptional organizers of ES cell self-renewal [53,74, 96,99–101]. It is clear that Oct4 and Nanog form a mutual, interdependent transcriptional network with Sox2. Oct4 and Sox2 have been known to act synergistically to regulate their own transcription [102] and as well as the expression of other key stem cell genes including FGF4 [103], Nanog [104], and Zfp42/Rex1 (Fig. 3) [98,105,106]. The pluripotency transcription factors can form combinatorial complexes that include Nanog homodimers [107] and heteromeric complexes including OCT4–SOX2 [104], Oct4–Nanog [108], and Nanog–Sall4 [109] proteins. As described earlier, the levels of Oct4 must be precisely maintained [88]. The exact mechanisms regulating OCT4 levels in ES cells remain to be determined, but appear to involve direct regulation by Sox2 [90]. Furthermore, Oct4 has been shown to suppress its own expression [100]. This Oct4 self-suppression creates a negative feedback loop to counter the actions of Sox2 and Nanog, thereby fine-tuning the levels of Oct4 present in the undifferentiated stem cells [100]. One functional consequence of fluctuating Oct4 levels is seen on the expression of Nanog. Oct4 actively regulates Nanog in a biphasic manner such that lower Oct4 levels up-regulate Nanog, whereas higher Oct4 levels result in down-regulation of Nanog expression [99].
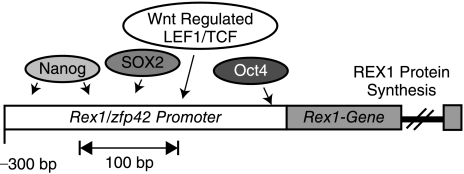
FIG. 3.
Convergent stem cell regulatory networks control expression of key stem cell genes. One classical representative of this transcriptional network is the Zfp42/Rex1 gene. Expression of the Zfp42/Rex1 gene has long been used as a marker of undifferentiated stem cells and is regulated by Nanog, Sox2, and Oct4, and by the Wnt pathway. Expression of Zfp42/Rex1 is also subject to epigenetic regulation by polycomb complexes and DNA methylation (see Table 1).
The pluripotency transcription factors function collaboratively to regulate the state of differentiation of ES cells. Two major studies have demonstrated that Oct4, Nanog, and Sox2 share a substantial fraction of target genes and, in fact, co-occupy genes in both mouse and human ES cells [74,99]. As reported by Boyer and colleagues, these genes occupy collectively about 10% of the promoters in the human genome. About half of the promoter regions bound by OCT4 were also bound by SOX2 and 90% of these doubly bound genes were in turn bound by NANOG [74]. Moreover, the OCT4, SOX2, and NANOG-binding sites were in close proximity, further confirming that the proteins work in concert. Data from mouse ES cells also indicate that Nanog and Oct4 colocalize in many gene regions [99] (see Table 1). Also, stem cell-associated genes were activated by Oct4 and Nanog, while inactive genes typically were implicated in cell lineage determination and development. Both studies also found that Oct4 and Nanog bound to gene regions associated with loci encoding microRNAs. However, in no case was a common microRNA identified as a potential Nanog or Oct4 target [74,99]. This may reflect intrinsic differences in the gene expression profiles of mouse and human ES cells or may be attributable in part to the different experimental techniques, ChIP-chip and ChIP-PET (paired-end ditag sequences), employed in the 2 studies.
The use of biotinylated-tag approaches has permitted biochemical characterization of the Nanog complex [101] and analysis of stem cell promoter occupancy of 9 stem cell transcription factors (including Oct4, Nanog, Sox2, c-Myc, Klf4, Zfp42/Rex1) [53]. These studies demonstrated that Nanog, its target genes, and protein partners interacted with a large number of proteins involved in early development in vivo. This method demonstrated that Oct4, Nanog, Sox2, and other associated proteins simultaneously occupy the promoters of a substantial number of genes. This supports the hypothesis that ES cell-associated transcription factors function as protein complexes. Genes whose promoters were bound by several transcription factors tended to be activated in ES cells, including the stem cell markers, Nanog, and Rex1. In contrast, genes involved in differentiation and therefore repressed in ES cells have promoters that tended to be bound by just one factor. Moreover, the authors indicated that this pluripotency protein network is also associated with other complexes such as PRC1, which functions in the repression of transcription [109–111]. In conclusion, Oct4, Nanog, Sox2, and a number of associated transcription factor proteins such as Sall4 [109,110] activate and maintain the expression of genes involved in self-renewal, while simultaneously repressing genes that mediate differentiation. Thus Oct4, Nanog, and Sox2 form a self-reinforcing and intricately connected network that preserves ES cell character. Hence, elucidating the various interactions between these proteins and their targets and protein partners remains a critical step in enhancing our understanding of the molecular basis of pluripotency.
Polycomb group proteins
Polycomb group (PcG) proteins play essential roles in epigenetic regulation of ES cell differentiation and development [56,57,75,112]. PcG proteins can cooperate with OCT4 and NANOG in the regulation of differentiation [58,75]. Important members of the polycomb family of proteins include the enhancer of zeste-1 and 2 (EZH1 and 2) histone H3K27 methyltransferases [113], embryonic ectoderm development (EED), suppressor of Zeste (SUZ12), B lymphoma Mo-MLV insertion region 1 (BMI1), and Ring1A/B. The zinc finger protein YinYang1 (YY1) can recruit polycomb complexes to chromatin [69] and on this basis is considered a polycomb protein. PcG proteins form multimeric PRC [75,114] (Fig. 2). The exact subunit and isoform composition of each PRC can vary dependent upon cellular conditions and differentiation [115–118]. In general, the EZH2 containing PRC2, which also requires SUZ12 and EED, is the complex that initiates polycomb repression. Distinct PRC2-related complexes with variant EED isoforms and/or SirT1 deacetylases have also been reported and exhibit distinct histone substrate specificities [115]. Polycomb epigenetic repression is maintained by PRC1, a large multi-subunit complex containing chromodomain proteins (CBC2/4/8), BMI1, and Ring1A/B ubiquitin-ligase activity (reviewed in [119]). YY1 can interact with the EED [120] and EZH2 [69] components of PRC2. YY1 can also interact with PRC1 under certain conditions [121].
The PRC2 proteins, EZH2, EED, and SUZ12, occupy and repress the promoter/regulatory domains of genes that participate in differentiation and cell fate decisions [57]. As described earlier, PcG proteins are also implicated in the establishment of “bivalent chromatin domains” that possess both repressive (histone H3 lysine 27 trimethylation; H3K27me3) and active (histone H3 lysine 4 trimethylation; H3K4me3) histone marks. Genes located within these bivalent chromatin domains, though epigenetically repressed, still possess the capacity for transcriptional activation in response to a differentiation stimulus [54,59,73]. During retinoic acid-induced differentiation of mouse teratocarcinoma cells, retinoic acid acting via its receptors causes PRC2 complexes to exit gene promoters transcriptionally activated by RA [122,123]. Thus PcG proteins have the epigenetic authority to maintain repression of developmentally regulated genes and thereby contribute to cell fate decisions [124].
The gene clusters repressed by the PcG complex change during differentiation. As repression of pro-differentiation loci is relieved, PcG-mediated epigenetic constraints are imposed on pluripotency genes such as OCT4 [54–56,112,125]. The mechanisms controlling this reciprocal regulatory network and the sequence of events that balance pluripotency versus differentiation remain unknown. Expression of the Suz12 [56,112], Yy1 [126], and Ezh2 [127,128] polycomb genes is necessary for embryonic development. However, a recent study shows that Eed, and by extension its PRC2 partners, Ezh2 and Suz12, are not required to maintain ES cell pluripotency, but are necessary to repress expression of lineage-specific genes [129]. Thus PRCs can be considered to regulate patterns of differentiation (see Table 1).
Histone lysine methylation can be reversed by an enzymatically diverse group of transcriptional coregulators. JMJD3 and UTX are H3K27me3-demethylases that reverse PcG-mediated repression of Hox genes in mammalian ES cells upon differentiation [130,131] (Fig. 2). Given the ability of JMJD3 to reverse the repressive epigenetic mark (H3K27me3), which is associated with repression of several differentiation genes in ES cells, it would be reasonable to speculate about a role for Jmjd3 in activation of pro-differentiation genes. Indeed Jmjd3 is essential for commitment of ES cells to the neural fate [132]. Jmjd3 mediates the transition to the neural fate by activating key neurogenesis genes namely, Nestin, Pax6, and Sox1. The increased expression of neural programming genes correlates with the loss of the H3K27me3 repressive mark via the catalytic activity of Jmjd3 [132]. JMJD3 has also been shown to regulate the activation of epidermal differentiation genes in primary human keratinocytes, thus expanding their role in regulating differentiation in epidermis, yet another self-renewing tissue [133].
The Jmjd1a and Jmjd2c histone lysine H3K9me3-demethylases also play important roles in mediating the epigenetic regulation of pluripotency in mouse ES cells and are directly regulated by Oct4 [134]. Depletion of Jmjd1a and Jmjd2c by siRNA in mouse ES cells led to morphological changes, induced cellular differentiation, and increased expression of different lineage-specific markers [134]. Subsequent microarray analysis in combination with functional assays revealed downstream targets of these demethylases. Jmjd1a was found to be associated with the promoter of the Tcl1 gene, which encodes the Tcl1 cofactor of the Akt1 kinase. Tcl1 regulates ES cell self-renewal [135]. Furthermore, Jmjd2c associates with the Nanog promoter and thereby regulates the expression of Nanog [134].
MicroRNAs and stem cells
Recent studies have revealed that microRNAs (miRNAs), a family of nonprotein encoding transcripts of ~20–25 nucleotides, play essential roles in regulating gene expression (see [136,137] for recent expansive reviews of miRNA biogenesis and function). A subset of miRNAs is preferentially expressed in undifferentiated stem cells [138] and plays essential roles in proliferation, pluripotency, and differentiation [139,140]. The pattern of miRNA expression changes during ES cell differentiation [141]. Mouse ES cells deficient in the Dicer [142] and Microprocessor (Drosha-DGCR8-Ddx5) [72,143] components of the miRNA processing apparatus exhibit defects in ES cell differentiation and development. The promoter regions of miRNAs that function in stem cells are typically occupied by key members of the stem cell transcription factor network, including Nanog, Oct4, and Sox2 [144,145]. Interestingly, Nanog, Oct4, and Sox2 are themselves subject to miRNA [146] and PcG [112] modulation during ES cell differentiation. A recent study showed that RA-induced differentiation of mES was associated with an up-regulation of miRNAs (miR-134, miR-296, miR-470), which target the coding sequences of the Nanog, Oct4, and Sox2 pluripotency transcription factors [147]. The miR-124 contributes to neuronal differentiation [148]. Collectively, these reports highlight the emerging view that miRNAs regulate, and are themselves regulated by, distinct transcriptional repressive mechanisms [45,144]. The best characterized miRNA network involves the ES-specific miR-290 cluster, which regulates Oct4-methylation in differentiating ES cells [149]. This illustrates that miRNAs play an indirect role in the control of de novo DNA CpG methylation in differentiating ES cells [149].
Convergence of distinct epigenetic repressive pathways and stem cell differentiation
There is evidence of functional convergence between the epigenetic repressive mechanisms, mediated by the PRC, DNA methyltransferase-3 (Dnmt3)-mediated promoter methylation, miRNAs and Oct4/Nanog, which participate in regulating ES cell differentiation [52,150,151]. ES cell chromatin is poised to initiate broad ranging and extensive transcriptional responses to pro-differentiation cues [59,125]. Therefore, one common effect of each of these parallel transcriptional repressive mechanisms is to reduce transcriptional “noise” [152]. This dampening down of “background” transcriptional events that could adversely bias patterns and timing of differentiation is achieved by a complex inter-regulation of the pluripotency network [153,154]. For example, expression of the EZH2 component of the polycomb repressive complex-2 is directly regulated by the miRNA-101 [155]. Conversely, the SUZ12 component of the polycomb complex and associated histone H3K27 methylation epigenetic mark were found to be associated with a subset of miRNAs inactive in undifferentiated ES cells [144]. The expression of these miRNA loci increases as the polycomb-mediated repression is relieved enabling these miRNAs to guide ES cell differentiation [144]. The YY1-PcG transcription factor also directly regulates transcription of the miR-29 miRNA by recruiting HDAC1 and EZH2 to regulatory regions in the miR-29 promoter region [156]. Similarly, miRNAs appear to regulate the stable silencing of pluripotency-associated genes, such as Oct4, by de novo DNA methylation [149]. Oct4 in turn regulates expression of the Eed component of the PRC2 [157] and recruits PRC1 to target loci silenced in undifferentiated stem cells [151,158]. Thus the NANOG, OCT4, SOX2 stem cell transcription factors, miRNAs, polycomb, and DNA methyltransferase complexes cooperate in a complex mutual regulatory circuit during ES cell differentiation (Figs. 2 and 4). The recently identified convergence of Oct4–Sox2 and miRNA pathways in the regulation of the ES cell cycle [159] suggests that the complexity of these networks is only now being deciphered.
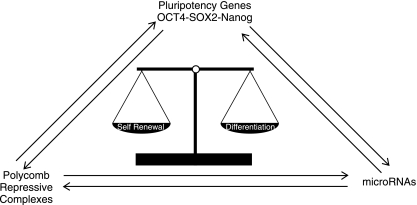
FIG. 4.
The reciprocal regulatory circuit composed of Oct4–Sox2–Nanog; polycomb repressive complexes and microRNAs regulate the transcriptional responses necessary to balance self-renewal and differentiation.
As noted earlier, the Zfp42/Rex1 gene is a convergence point of critical stem cell differentiation pathways. Zfp42/Rex1 is regulated by Nanog [105], Sox2 [105], and Oct4 [98,99,106,160] (Fig. 3). Expression of Rex1 is also influenced by the Wnt pathway [7]. REX1 is conserved in human and mouse stem cells [161]. We [161] and others [162] have shown that the ZFP42/REX1 stem cell protein is a member of the YinYang1 (YY1) subfamily of zinc finger transcription factors, which includes the Drosophila polycomb protein, pleiohomeotic (PHO). The exact mechanism of how PRCs are targeted to the correct DNA regions remains poorly understood. Polycomb response elements (PREs) have been identified in the Drosophila genome [163]. However, no such features have been definitely identified in the mouse or human genomes. Pho is involved in the recruitment of polycomb complexes to response elements in Drosophila [163–165]. YY1 has been shown to compensate partially for loss of Pho function in Drosophila [166]. This indicates that YY1, and by extension REX1, could play an analogous role targeting PcG complexes to specific loci in higher organisms. Studies have revealed that expression of Rex1 is limited to human [101,167–171] and mouse [171] ES cells, a variety of adult stem cells [172,173] and iPS cells derived from distinct mouse and human somatic cell types [26,28,31,35]. Following stem cell differentiation, the Rex1/Zfp42 locus is subject to extensive histone H3 lysine 9 dimethylation, a G9a-mediated epigenetic mark consistent with tissue-specific transcriptional repression [51] and DNA methylation [84]. Biotin-affinity labeling of the REX1 protein has revealed that the REX1 interactome involves potential interactions with other key stem cell proteins, including OCT4 [101]. The functional significance of these potential REX1 interactions remains unexplored. More recently, the genome-wide distribution of 9 stem cell transcription factors, including Rex1, was analyzed by bioChIP-on-Chip in mouse ES cells [53]. This study revealed that Rex1 promoter occupancy most frequently clustered with epigenetic marks consistent with transcriptional activation (histone H3 lysine 4 trimethylation; H3K4me3). These studies have confirmed the importance of Rex1 in epigenetic regulation in undifferentiated stem cells. The effects of ectopic expression of REX1 in ES cells have also been studied [174,175]. Zhang and colleagues found that Rex1 reduced the self-renewal capacity of mouse ES cells, suggesting that Rex1 plays a role in regulating the self-renewal potential of cells in which it is expressed, whereas Masui and colleagues saw little effect [174,175]. These reports may reflect differences in the levels of REX1 expressed in the distinct experimental systems used in the studies [174,175]. These results may indicate that the ability of stem cells to self-renew is sensitive to subtle differences in Rex1 protein levels, as is also the case for Oct4 [88]. We have found that in the presence of retinoic acid, Rex1 null ES cells display an increased tendency to differentiate along all 3 germ lines, suggesting a role for Rex1 in retinoic acid-induced differentiation [176].
Transcriptional networks and lineage determination during stem cell differentiation
The functional interplay of each component of the stem cell regulatory circuit (Fig. 4) is perhaps best understood in the establishment of neuronal and hematopoietic lineages. Mouse embryonic stem cells can be effectively guided into becoming hematopoietic progenitors in serum-free media when exposed to BMP4, activin A, bFGF, and VEGF consecutively [177]. When co-cultured with murine stromal cells human ES cells can undergo definitive hematopoiesis [178], generating an easily accessible population for the dissection of transcription factors involved in lineage commitment. The accessibility of human blood has also enabled the identification of miR-155 as key regulators in the erythroid and myeloid lineage differentiation responses [179]. Similarly, in vitro neuronal differentiation protocols have been reported (refer to [77] for an example of human neural progenitor derivation from ES cells in a defined system). Mohn and colleagues have characterized the epigenetic changes that occur upon differentiation of pluripotent ES cells toward neuronal lineages [77]. This study revealed that the genome-wide distributions of H3K27me3 (a surrogate mark of polycomb repression) and DNA CpG methylation exhibited stage-specific distributions during differentiation from ES cells to neural progenitors to terminally differentiated pyramidal neurons [77]. Another key epigenetic regulator of neuronal differentiation of stem cells is RE1-silencing transcription factor (REST) [180,181]. Although a potential central role for REST in the maintenance of stem cell self-renewal and pluripotency remains controversial [182–184], experimental depletion of REST by siRNA in ES cells impairs neuronal differentiation and development [185]. REST coordinates the epigenetic changes necessary for neural differentiation by interacting with and targeting HDAC1/2 and histone lysine demethylase 1 (LSD1/AOF2) to appropriate loci [186], during lineage commitment and neuronal differentiation [180,181]. Expression of REST is itself subject to miRNA regulation by miR-9/miR-9*, which are regulated by REST [187]. Thus, REST regulates neuronal differentiation by forming a reciprocal regulatory circuit composed of miR-NAs and diverse epigenetic mechanisms.
Stem cell transcriptional networks and human cancer
Small numbers of stem cells are believed to exist in most if not all adult tissues (reviewed in detail in [188]). Adult stem cells can evade the stringent genetic controls of their normal pathways of cellular differentiation and proliferation and can give rise to cancer. Cancer stem/initiating cells have been defined as a subset of cancer cells that have the exclusive ability of self-renewal and cause the heterogeneous lineages of cancer cells that comprise the tumor [189,190]. These “cancer stem cells” are implicated in cancer initiation, malignant potential, metastatic progression, and in the posttreatment recurrence of many human cancers types [191]. Stem cell-specific proteins, including OCT4, NANOG, and ZFP42/REX1 are implicated in some cancers [161,192,193]. Histologically poorly differentiated tumors showed preferential overexpression of genes normally enriched in ES cells. Activation targets of NANOG, OCT4, SOX2, and c-MYC are more frequently overexpressed in poorly differentiated tumors than in well-differentiated tumors [194]. It appears that the genes active in both ES cells and cancer stem cells are controlled by a few master regulatory genes, one of which is c-MYC. As noted earlier, c-MYC can contribute to epigenetic reprogramming and induction of pluripotency from differentiated fibroblasts (see section on iPS cells). However, c-MYC is also sufficient to reactivate the ES cell-like program in cancer cells [195]. These results suggest that aberrant activation of an ES cell-like transcriptional program in adult differentiated cells may induce pathological self-renewal characteristic of cancer stem cells.
There is also conclusive evidence implicating aberrant polycomb function in malignancy [196]. As described earlier, polycomb complexes contribute to the epigenetic regulation of key gene networks involved in stem cell self-renewal [197], differentiation, and proliferation and achieve this via dynamic regulation of chromatin/histone modifications (eg, via lysine methylation) associated with the promoter and regulatory regions of polycomb target genes [56,57,112,114,198,199]. This polycomb epigenetic stem cell gene signature has also been observed in cancer cells [200] and PRCs play important roles in cancer stem cells [201]. There is a wealth of evidence that overexpression of the EZH2 polycomb gene occurs in multiple human malignancies (eg, see [196,202]). Indeed genomic loss of miR-101 leads to increased EZH2 levels [155,203]. Although the exact mechanisms by which EZH2 contributes to carcinogenesis remain poorly defined, recent evidence indicates EZH2 overexpression can contribute to the inappropriate silencing of tumor suppressor genes [114]. For example, the pro-differentiation tumor suppressor gene, retinoic acid receptor β2 (RARβ2) was shown to be a PcG target [57], providing a plausible link between polycomb and epigenetic regulation of this important tumor suppressor gene whose expression has been found to be reduced or lost in many in human malignancies. For these reasons understanding the mechanisms by which the complex interplay of the pluripotency transcription factors, PRC, and miRNAs balance self-renewal and cellular proliferation (Fig. 4) is essential for our understanding of both the earliest events of embryonic differentiation and human carcinogenesis.
Acknowledgments
This research was supported by DOD-PCRP WXWH-081-0317 (V.K.), HHMI Gilliam Fellowship (N.C.R.); NIH-NRSA F31CA123703-02 (K.B.S.), NIH-NRSA T32MH018882 (S.M.S.), NIH R01CA097543, and NIH R01CA043786 (L.J.G.). This investigation was supported by grant UL1RR024996 of the Clinical and Translation Science Center at Weill Cornell Medical College (Pilot Award, N.P.M.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Yu J. Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22:1987–1997. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen AC. Gudas LJ. An analysis of retinoic acid-induced gene expression and metabolism in AB1 embryonic stem cells. J Biol Chem. 1996;271:14971–14980. doi: 10.1074/jbc.271.25.14971. [DOI] [PubMed] [Google Scholar]
- 3.Mongan NP. Gudas LJ. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation. 2007;75:853–870. doi: 10.1111/j.1432-0436.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- 4.Evans MJ. Kaufman MH. Establishment in culture of pluripotent cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 5.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson JA. Itskovitz-Eldor J. Shapiro SS. Waknitz MA. Swiergiel JJ. Marshall VS. Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 2008;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 7.Sato N. Meijer L. Skaltsounis L. Greengard P. Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3 specific inhibitor. Nat Med. 2008;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 8.Ying QL. Nichols J. Chambers I. Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2008;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda T. Nakamura T. Nakao K. Arai T. Katsuki M. Heike T. Yokota T. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 2008;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki A. Raya A. Kawakami Y. Morita M. Matsui T. Nakashima K. Gage FH. Rodriguez-Esteban C. Izpisúa Belmonte JC. Nanog binds to Smad1 and blocks bone morphogenetic protein-induced differentiation of embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:10294–10299. doi: 10.1073/pnas.0506945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benezra R. Davis RL. Lockshon D. Turner DL. Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 2008;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 12.Williams RL. Hilton DJ. Pease S. Willson TA. Stewart CL. Gearing DP. Wagner EF. Metcalf D. Nicola NA. Gough NM. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 2008;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 13.Daheron L. Opitz SL. Zaehres H. Lensch MW. Andrews PW. Itskovitz-Eldor J. Daley GQ. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2008;22:770–778. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- 14.Xu RH. Chen X. Li DS. Li R. Addicks GC. Glennon C. Zwaka TP. Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2008;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 15.Xu RH. Peck RM. Li DS. Feng X. Ludwig T. Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2008;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 16.Brons IG. Smithers LE. Trotter MW. Rugg-Gunn P. Sun B. Chuva de Sousa Lopes SM. Howlett SK. Clarkson A. Ahrlund-Richter L. Pedersen RA. Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2008;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 17.Tesar PJ. Chenoweth JG. Brook FA. Davies TJ. Evans EP. Mack DL. Gardner RL. McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2008;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 18.Chou YF. Chen HH. Eijpe M. Yabuuchi A. Chenoweth JG. Tesar P. Lu J. McKay RD. Geijsen N. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell. 2008;135:449–461. doi: 10.1016/j.cell.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ying QL. Wray J. Nichols J. Batlle-Morera L. Doble B. Woodgett J. Cohen P. Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne JA. Pedersen DA. Clepper LL. Nelson M. Sanger WG. Gokhale S. Wolf DP. Mitalipov SM. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2008;450:497–502. doi: 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- 21.Wilmut I. Schnieke AE. McWhir J. Kind AJ. Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 2008;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 22.Lluis F. Pedone E. Pepe S. Cosma MP. Periodic activation of Wnt/beta-catenin signaling enhances somatic cell reprogramming mediated by cell fusion. Cell Stem Cell. 2008;3:493–507. doi: 10.1016/j.stem.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Silva J. Chambers I. Pollard S. Smith A. Nanog promotes transfer of pluripotency after cell fusion. Nature. 2008;441:997–1001. doi: 10.1038/nature04914. [DOI] [PubMed] [Google Scholar]
- 24.Tada M. Takahama Y. Abe K. Nakatsuji N. Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol. 2008;11:1553–1558. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- 25.Yu J. Vodyanik MA. Smuga-Otto K. Antosiewicz-Bourget J. Frane JL. Tian S. Nie J. Jonsdottir GA. Ruotti V. Stewart R. Slukvin II. Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 26.Aoi T. Yae K. Nakagawa M. Ichisaka T. Okita K. Takahashi K. Chiba T. Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2008;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Hanna J. Markoulaki S. Schorderet P. Carey BW. Beard C. Wernig M. Creyghton MP. Steine EJ. Cassady JP. Foreman R. Lengner CJ. Dausman JA. Jaenisch R. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamanaka S. Induction of pluripotent stem cells from mouse fibroblasts by four transcription factors. Cell Prolif. 2008;41(Suppl 1):51–56. doi: 10.1111/j.1365-2184.2008.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi K. Okita K. Nakagawa M. Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2008;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Park IH. Zhao R. West JA. Yabuuchi A. Huo H. Ince TA. Lerou PH. Lensch MW. Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 33.Lowry WE. Richter L. Yachechko R. Pyle AD. Tchieu J. Sridharan R. Clark AT. Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci USA. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stadtfeld M. Brennand K. Hochedlinger K. Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Curr Biol. 2008;18:890–894. doi: 10.1016/j.cub.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa M. Koyanagi M. Tanabe K. Takahashi K. Ichisaka T. Aoi T. Okita K. Mochiduki Y. Takizawa N. Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 36.Huangfu D. Osafune K. Maehr R. Guo W. Eijkelenboom A. Chen S. Muhlestein W. Melton DA. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 37.Huangfu D. Maehr R. Guo W. Eijkelenboom A. Snitow M. Chen AE. Melton DA. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JB. Sebastiano V. Wu G. Arauzo-Bravo MJ. Sasse P. Gentile L. Ko K. Ruau D. Ehrich M. van den Boom D. Meyer J. Hubner K. Bernemann C. Ortmeier C. Zenke M. Fleischmann BK. Zaehres H. Scholer HR. Oct4-induced pluripotency in adult neural stem cells. Cell. 2008;136:411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 39.Guo G. Yang J. Nichols J. Hall JS. Eyres I. Mansfield W. Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2008;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okita K. Nakagawa M. Hyenjong H. Ichisaka T. Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 41.Woltjen K. Michael IP. Mohseni P. Desai R. Mileikovsky M. Hämäläinen R. Cowling R. Wang W. Liu P. Gertsenstein M. Kaji K. Sung H. Nagy A. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2008;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaji K. Norrby K. Paca A. Mileikovsky M. Mohseni P. Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2008;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chambers I. Colby D. Robertson M. Nichols J. Lee S. Tweedie S. Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2008;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 44.Chambers I. Silva J. Colby D. Nichols J. Nijmeijer B. Robertson M. Vrana J. Jones K. Grotewold L. Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2008;450:1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- 45.Viswanathan SR. Daley GQ. Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao J. Wu Z. Wang Y. Cheng L. Cui C. Gao Y. Chen T. Rao L. Chen S. Jia N. Dai H. Xin S. Kang J. Pei G. Xiao L. Enhanced efficiency of generating induced pluripotent stem (iPS) cells from human somatic cells by a combination of six transcription factors. Cell Res. 2008;18:600–603. doi: 10.1038/cr.2008.51. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y. Yin X. Qin H. Zhu F. Liu H. Yang W. Zhang Q. Xiang C. Hou P. Song Z. Liu Y. Yong J. Zhang P. Cai J. Liu M. Li H. Li Y. Qu X. Cui K. Zhang W. Xiang T. Wu Y. Zhao Y. Liu C. Yu C. Yuan K. Lou J. Ding M. Deng H. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 2008;3:475–479. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Jenuwein T. Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki MM. Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- 50.Mellor J. Dudek P. Clynes D. A glimpse into the epigenetic landscape of gene regulation. Curr Opin Genet Dev. 2008;18:116–122. doi: 10.1016/j.gde.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Wen B. Wu H. Shinkai Y. Irizarry RA. Feinberg AP. Large histone H3 lysine 9 dimethylated chromatin blocks distinguish differentiated from embryonic stem cells. Nat Genet. 2008;41:246–250. doi: 10.1038/ng.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fouse SD. Shen Y. Pellegrini M. Cole S. Meissner A. Van Neste L. Jaenisch R. Fan G. Promoter CpG methylation contributes to ES cell gene regulation in parallel with Oct4/Nanog, PcG complex, and histone H3 K4/K27 trimethylation. Cell Stem Cell. 2008;2:160–169. doi: 10.1016/j.stem.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J. Chu J. Shen X. Wang J. Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao XD. Han X. Chew JL. Liu J. Chiu KP. Choo A. Orlov YL. Sung WK. Shahab A. Kuznetsov VA. Bourque G. Oh S. Ruan Y. Ng HH. Wei CL. Whole-genome mapping of histone H3 Lys4 and 27 trimethylations reveals distinct genomic compartments in human embryonic stem cells. Cell Stem Cell. 2008;1:286–298. doi: 10.1016/j.stem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Pan G. Tian S. Nie J. Yang C. Ruotti V. Wei H. Jonsdottir GA. Stewart R. Thomson JA. Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell. 2008;1:299–312. doi: 10.1016/j.stem.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Pasini D. Bracken AP. Hansen JB. Capillo M. Helin K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol Cell Biol. 2008;27:3769–3779. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bracken AP. Dietrich N. Pasini D. Hansen KH. Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2008;20:1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee TI. Jenner RG. Boyer LA. Guenther MG. Levine SS. Kumar RM. Chevalier B. Johnstone SE. Cole MF. Isono K. Koseki H. Fuchikami T. Abe K. Murray HL. Zucker JP. Yuan B. Bell GW. Herbolsheimer E. Hannett NM. Sun K. Odom DT. Otte AP. Volkert TL. Bartel DP. Melton DA. Gifford DK. Jaenisch R. Young RA. Control of developmental regulators by polycomb in human embryonic stem cells. Cell. 2008;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bernstein BE. Mikkelsen TS. Xie X. Kamal M. Huebert DJ. Cuff J. Fry B. Meissner A. Wernig M. Plath K. Jaenisch R. Wagschal A. Feil R. Schreiber SL. Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2008;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 60.Wiblin AE. Cui W. Clark AJ. Bickmore WA. Distinctive nuclear organisation of centromeres and regions involved in pluripotency in human embryonic stem cells. J Cell Sci. 2008;118:3861–3868. doi: 10.1242/jcs.02500. [DOI] [PubMed] [Google Scholar]
- 61.Azuara V. Perry P. Sauer S. Spivakov M. Jorgensen HF. John RM. Gouti M. Casanova M. Warnes G. Merkenschlager M. Fisher AG. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2008;8:532–538. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- 62.Meshorer E. Yellajoshula D. George E. Scambler PJ. Brown DT. Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell. 2008;10:105–116. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kimura H. Tada M. Nakatsuji N. Tada T. Histone code modifications on pluripotential nuclei of reprogrammed somatic cells. Mol Cell Biol. 2008;24:5710–5720. doi: 10.1128/MCB.24.13.5710-5720.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang J. Wan M. Zhang Y. Gu P. Xin H. Jung SY. Qin J. Wong J. Cooney AJ. Liu D. Songyang Z. Nanog and Oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nat Cell Biol. 2008;10:731–739. doi: 10.1038/ncb1736. [DOI] [PubMed] [Google Scholar]
- 65.Ma P. Schultz RM. Histone deacetylase 1 (HDAC1) regulates histone acetylation, development, and gene expression in preimplantation mouse embryos. Dev Biol. 2008;319:110–120. doi: 10.1016/j.ydbio.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y. Ng HH. Erdjument-Bromage H. Tempst P. Bird A. Reinberg D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 2008;13:1924–1935. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson BJ. Bates GJ. Nicol SM. Gregory DJ. Perkins ND. Fuller-Pace FV. The p68 and p72 DEAD box RNA helicases interact with HDAC1 and repress transcription in a promoter-specific manner. BMC Mol Biol. 2004;5:11. doi: 10.1186/1471-2199-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacobs AM. Nicol SM. Hislop RG. Jaffray EG. Hay RT. Fuller-Pace FV. SUMO modification of the DEAD box protein p68 modulates its transcriptional activity and promotes its interaction with HDAC1. Oncogene. 2007;26:5866–5876. doi: 10.1038/sj.onc.1210387. [DOI] [PubMed] [Google Scholar]
- 69.Caretti G. Di Padova M. Micales B. Lyons GE. Sartorelli V. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 2008;18:2627–2638. doi: 10.1101/gad.1241904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fuller-Pace FV. DExD/H box RNA helicases: multifunctional proteins with important roles in transcriptional regulation. Nucleic Acids Res. 2006;34:4206–4215. doi: 10.1093/nar/gkl460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clark EL. Coulson A. Dalgliesh C. Rajan P. Nicol SM. Fleming S. Heer R. Gaughan L. Leung HY. Elliott DJ. Fuller-Pace FV. Robson CN. The RNA helicase p68 is a novel androgen receptor coactivator involved in splicing and is overexpressed in prostate cancer. Cancer Res. 2008;68:7938–7946. doi: 10.1158/0008-5472.CAN-08-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fukuda T. Yamagata K. Fujiyama S. Matsumoto T. Koshida I. Yoshimura K. Mihara M. Naitou M. Endoh H. Nakamura T. Akimoto C. Yamamoto Y. Katagiri T. Foulds C. Takezawa S. Kitagawa H. Takeyama K. O'Malley BW. Kato S. DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nat Cell Biol. 2008;9:604–611. doi: 10.1038/ncb1577. [DOI] [PubMed] [Google Scholar]
- 73.Mikkelsen TS. Ku M. Jaffe DB. Issac B. Lieberman E. Giannoukos G. Alvarez P. Brockman W. Kim TK. Koche RP. Lee W. Mendenhall E. O'Donovan A. Presser A. Russ C. Xie X. Meissner A. Wernig M. Jaenisch R. Nusbaum C. Lander ES. Bernstein BE. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2008;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boyer LA. Lee TI. Cole MF. Johnstone SE. Levine SS. Zucker JP. Guenther MG. Kumar RM. Murray HL. Jenner RG. Gifford DK. Melton DA. Jaenisch R. Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2008;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boyer LA. Plath K. Zeitlinger J. Brambrink T. Medeiros LA. Lee TI. Levine SS. Wernig M. Tajonar A. Ray MK. Bell GW. Otte AP. Vidal M. Gifford DK. Young RA. Jaenisch R. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2008;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 76.Kidder BL. Yang J. Palmer S. Stat3 and c-Myc genome-wide promoter occupancy in embryonic stem cells. PLoS ONE. 2008;3:e3932. doi: 10.1371/journal.pone.0003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohn F. Weber M. Rebhan M. Roloff TC. Richter J. Stadler MB. Bibel M. Schubeler D. Lineage-specific polycomb targets and de novo DNA methylation define restriction and potential of neuronal progenitors. Mol Cell. 2008;30:755–766. doi: 10.1016/j.molcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 78.Barski A. Cuddapah S. Cui K. Roh TY. Schones DE. Wang Z. Wei G. Chepelev I. Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2008;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 79.Roh TY. Cuddapah S. Cui K. Zhao K. The genomic landscape of histone modifications in human T cells. Proc Natl Acad Sci USA. 2008;103:15782–15787. doi: 10.1073/pnas.0607617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Okano M. Bell DW. Haber DA. Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 2008;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 81.Li JY. Pu MT. Hirasawa R. Li BZ. Huang YN. Zeng R. Jing NH. Chen T. Li E. Sasaki H. Xu GL. Synergistic function of DNA methyltransferases Dnmt3a and Dnmt3b in the methylation of Oct4 and Nanog. Mol Cell Biol. 2008;27:8748–8759. doi: 10.1128/MCB.01380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meissner A. Mikkelsen TS. Gu H. Wernig M. Hanna J. Sivachenko A. Zhang X. Bernstein BE. Nusbaum C. Jaffe DB. Gnirke A. Jaenisch R. Lander ES. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–770. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jackson M. Krassowska A. Gilbert N. Chevassut T. Forrester L. Ansell J. Ramsahoye B. Severe global DNA hypomethylation blocks differentiation and induces histone hyperacetylation in embryonic stem cells. Mol Cell Biol. 2008;24:8862–8871. doi: 10.1128/MCB.24.20.8862-8871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farthing CR. Ficz G. Ng RK. Chan CF. Andrews S. Dean W. Hemberger M. Reik W. Global mapping of DNA methylation in mouse promoters reveals epigenetic reprogramming of pluripotency genes. PLoS Genet. 2008;4:e1000116. doi: 10.1371/journal.pgen.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vire E. Brenner C. Deplus R. Blanchon L. Fraga M. Didelot C. Morey L. Van Eynde A. Bernard D. Vanderwinden JM. Bollen M. Esteller M. Di Croce L. de Launoit Y. Fuks F. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2008;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 86.McGarvey KM. Greene E. Fahrner JA. Jenuwein T. Baylin SB. DNA methylation and complete transcriptional silencing of cancer genes persist after depletion of EZH2. Cancer Res. 2008;67:5097–5102. doi: 10.1158/0008-5472.CAN-06-2029. [DOI] [PubMed] [Google Scholar]
- 87.Ku M. Koche RP. Rheinbay E. Mendenhall EM. Endoh M. Mikkelsen TS. Presser A. Nusbaum C. Xie X. Chi AS. Adli M. Kasif S. Ptaszek LM. Cowan CA. Lander ES. Koseki H. Bernstein BE. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet. 2008;4:e1000242. doi: 10.1371/journal.pgen.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niwa H. Miyazaki J. Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2008;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 89.Mitsui K. Tokuzawa Y. Itoh H. Segawa K. Murakami M. Takahashi K. Maruyama M. Maeda M. Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2008;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 90.Masui S. Nakatake Y. Toyooka Y. Shimosato D. Yagi R. Takahashi K. Okochi H. Okuda A. Matoba R. Sharov AA. Ko MS. Niwa H. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2008;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 91.Torres J. Watt FM. Nanog maintains pluripotency of mouse embryonic stem cells by inhibiting NFkappaB and cooperating with Stat3. Nat Cell Biol. 2008;10:194–201. doi: 10.1038/ncb1680. [DOI] [PubMed] [Google Scholar]
- 92.Darr H. Mayshar Y. Benvenisty N. Overexpression of NANOG in human ES cells enables feeder-free growth while inducing primitive ectoderm features. Development. 2008;133:1193–1201. doi: 10.1242/dev.02286. [DOI] [PubMed] [Google Scholar]
- 93.Zaehres H. Lensch MW. Daheron L. Stewart SA. Itskovitz-Eldor J. Daley GQ. High-efficiency RNA interference in human embryonic stem cells. Stem Cells. 2008;23:299–305. doi: 10.1634/stemcells.2004-0252. [DOI] [PubMed] [Google Scholar]
- 94.Hough SR. Clements I. Welch PJ. Wiederholt KA. Differentiation of mouse embryonic stem cells after RNA interference-mediated silencing of OCT4 and Nanog. Stem Cells. 2008;24:1467–1475. doi: 10.1634/stemcells.2005-0475. [DOI] [PubMed] [Google Scholar]
- 95.Liu L. Roberts RM. Silencing of the gene for the beta subunit of human chorionic gonadotropin by the embryonic transcription factor Oct-3/4. J Biol Chem. 1996;271:16683–16689. doi: 10.1074/jbc.271.28.16683. [DOI] [PubMed] [Google Scholar]
- 96.Pan G. Pei D. The stem cell pluripotency factor NANOG activates transcription with two unusually potent subdomains at its C terminus. J Biol Chem. 2005;280:1401–1407. doi: 10.1074/jbc.M407847200. [DOI] [PubMed] [Google Scholar]
- 97.Avilion AA. Nicolis SK. Pevny LH. Perez L. Vivian N. Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ben-Shushan E. Thompson JR. Gudas LJ. Bergman Y. Rex-1, a gene encoding a transcription factor expressed in the early embryo, is regulated via Oct-3/4 and Oct-6 binding to an octamer site and a novel protein, Rox-1, binding to an adjacent site. Mol Cell Biol. 2008;18:1866–1878. doi: 10.1128/mcb.18.4.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loh YH. Wu Q. Chew JL. Vega VB. Zhang W. Chen X. Bourque G. George J. Leong B. Liu J. Wong KY. Sung KW. Lee CW. Zhao XD. Chiu KP. Lipovich L. Kuznetsov VA. Robson P. Stanton LW. Wei CL. Ruan Y. Lim B. Ng HH. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2008;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 100.Pan G. Li J. Zhou Y. Zheng H. Pei D. A negative feedback loop of transcription factors that controls stem cell pluripotency and self-renewal. FASEB J. 2008;20:1730–1732. doi: 10.1096/fj.05-5543fje. [DOI] [PubMed] [Google Scholar]
- 101.Wang J. Rao S. Chu J. Shen X. Levasseur DN. Theunissen TW. Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2008;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 102.Chew JL. Loh YH. Zhang W. Chen X. Tam WL. Yeap LS. Li P. Ang YS. Lim B. Robson P. Ng HH. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2008;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ambrosetti DC. Basilico C. Dailey L. Synergistic activation of the fibroblast growth factor 4 enhancer by Sox2 and Oct-3 depends on protein–protein interactions facilitated by a specific spatial arrangement of factor binding sites. Mol Cell Biol. 2008;17:6321–6329. doi: 10.1128/mcb.17.11.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodda DJ. Chew JL. Lim LH. Loh YH. Wang B. Ng HH. Robson P. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2008;280:24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 105.Shi W. Wang H. Pan G. Geng Y. Guo Y. Pei D. Regulation of the pluripotency marker Rex-1 by Nanog and Sox2. J Biol Chem. 2008;281:23319–23325. doi: 10.1074/jbc.M601811200. [DOI] [PubMed] [Google Scholar]
- 106.Hosler BA. Rogers MB. Kozak CA. Gudas LJ. An octamer motif contributes to the expression of the retinoic acid-regulated zinc-finger gene Rex-1 (Zfp-42) in F9 teratocarcinoma cells. Mol Cell Biol. 2008;13:2919–2928. doi: 10.1128/mcb.13.5.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang J. Levasseur DN. Orkin SH. Requirement of Nanog dimerization for stem cell self-renewal and pluripotency. Proc Natl Acad Sci USA. 2008;105:6326–6331. doi: 10.1073/pnas.0802288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang L. Rayner S. Katoku-Kikyo N. Romanova L. Kikyo N. Successful co-immunoprecipitation of Oct4 and Nanog using cross-linking. Biochem Biophys Res Commun. 2008;361:611–614. doi: 10.1016/j.bbrc.2007.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu Q. Chen X. Zhang J. Loh YH. Low TY. Zhang W. Zhang W. Sze SK. Lim B. Ng HH. Sall4 interacts with Nanog and co-occupies Nanog genomic sites in embryonic stem cells. J Biol Chem. 2008;281:24090–24094. doi: 10.1074/jbc.C600122200. [DOI] [PubMed] [Google Scholar]
- 110.Yang J. Chai L. Fowles TC. Alipio Z. Xu D. Fink LM. Ward DC. Ma Y. Genome-wide analysis reveals Sall4 to be a major regulator of pluripotency in murine-embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:19756–19761. doi: 10.1073/pnas.0809321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lim CY. Tam WL. Zhang J. Ang HS. Jia H. Lipovich L. Ng HH. Wei CL. Sung WK. Robson P. Yang H. Lim B. Sall4 regulates distinct transcription circuitries in different blastocyst-derived stem cell lineages. Cell Stem Cell. 2008;3:543–554. doi: 10.1016/j.stem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 112.Pasini D. Bracken AP. Jensen MR. Lazzerini Denchi E. Helin K. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J. 2008;23:4061–4071. doi: 10.1038/sj.emboj.7600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shen X. Liu Y. Hsu YJ. Fujiwara Y. Kim J. Mao X. Yuan GC. Orkin SH. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32:491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bracken AP. Kleine-Kohlbrecher D. Dietrich N. Pasini D. Gargiulo G. Beekman C. Theilgaard-Monch K. Minucci S. Porse BT. Marine JC. Hansen KH. Helin K. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2008;21:525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuzmichev A. Margueron R. Vaquero A. Preissner TS. Scher M. Kirmizis A. Ouyang X. Brockdorff N. Abate-Shen C. Farnham P. Reinberg D. Composition and histone substrates of polycomb repressive group complexes change during cellular differentiation. Proc Natl Acad Sci USA. 2008;102:1859–1864. doi: 10.1073/pnas.0409875102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Otte AP. Kwaks TH. Gene repression by Polycomb group protein complexes: a distinct complex for every occasion? Curr Opin Genet Dev. 2003;13:448–454. doi: 10.1016/s0959-437x(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 117.Kuzmichev A. Jenuwein T. Tempst P. Reinberg D. Different EZH2-containing complexes target methylation of histone H1 or nucleosomal histone H3. Mol Cell. 2008;14:183–193. doi: 10.1016/s1097-2765(04)00185-6. [DOI] [PubMed] [Google Scholar]
- 118.Kim SY. Levenson JM. Korsmeyer S. Sweatt JD. Schumacher A. Developmental regulation of Eed complex composition governs a switch in global histone modification in brain. J Biol Chem. 2008;282:9962–9972. doi: 10.1074/jbc.M608722200. [DOI] [PubMed] [Google Scholar]
- 119.Spivakov M. Fisher AG. Epigenetic signatures of stem-cell identity. Nat Rev Genet. 2007;8:263–271. doi: 10.1038/nrg2046. [DOI] [PubMed] [Google Scholar]
- 120.Satijn DP. Hamer KM. den Blaauwen J. Otte AP. The polycomb group protein EED interacts with YY1, and both proteins induce neural tissue in Xenopus embryos. Mol Cell Biol. 2008;21:1360–1369. doi: 10.1128/MCB.21.4.1360-1369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Levine SS. Weiss A. Erdjument-Bromage H. Shao Z. Tempst P. Kingston RE. The core of the polycomb repressive complex is compositionally and functionally conserved in flies and humans. Mol Cell Biol. 2008;22:6070–6078. doi: 10.1128/MCB.22.17.6070-6078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gillespie R. Gudas L. RAR isotype specificity in F9 teratocarcinoma stem cells results from the differential recruitment of coregulators to retinoic acid response elements. J Biol Chem. 2007;282:33421–33434. doi: 10.1074/jbc.M704845200. [DOI] [PubMed] [Google Scholar]
- 123.Gillespie RF. Gudas LJ. Retinoid regulated association of transcriptional co-regulators and the polycomb group protein SUZ12 with the retinoic acid response elements of Hoxa1, RARbeta(2), and Cyp26A1 in F9 embryonal carcinoma cells. J Mol Biol. 2007;372:298–316. doi: 10.1016/j.jmb.2007.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pietersen AM. van Lohuizen M. Stem cell regulation by polycomb repressors: postponing commitment. Curr Opin Cell Biol. 2008;20:201–207. doi: 10.1016/j.ceb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 125.Golob JL. Paige SL. Muskheli V. Pabon L. Murry CE. Chromatin remodeling during mouse and human embryonic stem cell differentiation. Dev Dyn. 2008;237:1389–1398. doi: 10.1002/dvdy.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Donohoe ME. Zhang X. McGinnis L. Biggers J. Li E. Shi Y. Targeted disruption of mouse Yin Yang 1 transcription factor results in peri-implantation lethality. Mol Cell Biol. 2008;19:7237–7244. doi: 10.1128/mcb.19.10.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.O'Carroll D. Erhardt S. Pagani M. Barton SC. Surani MA. Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2008;21:4330–4336. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Erhardt S. Su IH. Schneider R. Barton S. Bannister AJ. Perez-Burgos L. Jenuwein T. Kouzarides T. Tarakhovsky A. Surani MA. Consequences of the depletion of zygotic and embryonic enhancer of zeste 2 during preimplantation mouse development. Development. 2008;130:4235–4248. doi: 10.1242/dev.00625. [DOI] [PubMed] [Google Scholar]
- 129.Chamberlain SJ. Yee D. Magnuson T. Polycomb repressive complex 2 is dispensable for maintenance of embryonic stem cell pluripotency. Stem Cells. 2008;26:1496–1505. doi: 10.1634/stemcells.2008-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee MG. Villa R. Trojer P. Norman J. Yan KP. Reinberg D. Di Croce L. Shiekhattar R. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science. 2008;318:447–450. doi: 10.1126/science.1149042. [DOI] [PubMed] [Google Scholar]
- 131.Agger K. Cloos PA. Christensen J. Pasini D. Rose S. Rappsilber J. Issaeva I. Canaani E. Salcini AE. Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2008;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- 132.Burgold T. Spreafico F. De Santa F. Totaro MG. Prosperini E. Natoli G. Testa G. The histone H3 lysine 27-specific demethylase Jmjd3 is required for neural commitment. PLoS ONE. 2008;3:e3034. doi: 10.1371/journal.pone.0003034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sen GL. Webster DE. Barragan DI. Chang HY. Khavari PA. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev. 2008;22:1865–1870. doi: 10.1101/gad.1673508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Loh YH. Zhang W. Chen X. George J. Ng HH. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 2008;21:2545–2557. doi: 10.1101/gad.1588207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ivanova N. Dobrin R. Lu R. Kotenko I. Levorse J. DeCoste C. Schafer X. Lun Y. Lemischka IR. Dissecting self-renewal in stem cells with RNA interference. Nature. 2008;442:533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- 136.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gangaraju VK. Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol. 2009;10:116–125. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Houbaviy HB. Murray MF. Sharp PA. Embryonic stem cell-specific MicroRNAs. Dev Cell. 2008;5:351–358. doi: 10.1016/s1534-5807(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 139.Barroso-delJesus A. Romero-Lopez C. Lucena-Aguilar G. Melen GJ. Sanchez L. Ligero G. Berzal-Herranz A. Menendez P. Embryonic stem cell-specific miR302–367 cluster: human gene structure and functional characterization of its core promoter. Mol Cell Biol. 2008;28:6609–6619. doi: 10.1128/MCB.00398-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Y. Baskerville S. Shenoy A. Babiarz JE. Baehner L. Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet. 2008;40:1478–1483. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lakshmipathy U. Love B. Goff LA. Jornsten R. Graichen R. Hart RP. Chesnut JD. MicroRNA expression pattern of undifferentiated and differentiated human embryonic stem cells. Stem Cells Dev. 2008;16:1003–1016. doi: 10.1089/scd.2007.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kanellopoulou C. Muljo SA. Kung AL. Ganesan S. Drapkin R. Jenuwein T. Livingston DM. Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2008;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Y. Medvid R. Melton C. Jaenisch R. Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2008;39:380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Marson A. Levine SS. Cole MF. Frampton GM. Brambrink T. Johnstone S. Guenther MG. Johnston WK. Wernig M. Newman J. Calabrese JM. Dennis LM. Volkert TL. Gupta S. Love J. Hannett N. Sharp PA. Bartel DP. Jaenisch R. Young RA. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Barroso-Del Jesus A. Lucena-Aguilar G. Menendez P. The miR-302–367 cluster as a potential stemness regulator in ESCs. Cell Cycle. 2008;8:1496–1505. doi: 10.4161/cc.8.3.7554. [DOI] [PubMed] [Google Scholar]
- 146.Tay Y. Zhang J. Thomson AM. Lim B. Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–1128. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 147.Tay YM. Tam WL. Ang YS. Gaughwin PM. Yang H. Wang W. Liu R. George J. Ng HH. Perera RJ. Lufkin T. Rigoutsos I. Thomson AM. Lim B. MicroRNA-134 modulates the differentiation of mouse embryonic stem cells, where it causes post-transcriptional attenuation of Nanog and LRH1. Stem Cells. 2008;26:17–29. doi: 10.1634/stemcells.2007-0295. [DOI] [PubMed] [Google Scholar]
- 148.Visvanathan J. Lee S. Lee B. Lee JW. Lee SK. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2008;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sinkkonen L. Hugenschmidt T. Berninger P. Gaidatzis D. Mohn F. Artus-Revel CG. Zavolan M. Svoboda P. Filipowicz W. MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nat Struct Mol Biol. 2008;15:259–267. doi: 10.1038/nsmb.1391. [DOI] [PubMed] [Google Scholar]
- 150.Chi AS. Bernstein BE. Developmental biology. Pluripotent chromatin state. Science. 2009;323:220–221. doi: 10.1126/science.1166261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van der Stoop P. Boutsma EA. Hulsman D. Noback S. Heimerikx M. Kerkhoven RM. Voncken JW. Wessels LF. van Lohuizen M. Ubiquitin E3 ligase Ring1b/Rnf2 of polycomb repressive complex 1 contributes to stable maintenance of mouse embryonic stem cells. PLoS ONE. 2008;3:e2235. doi: 10.1371/journal.pone.0002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chang HH. Hemberg M. Barahona M. Ingber DE. Huang S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453:544–547. doi: 10.1038/nature06965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen X. Xu H. Yuan P. Fang F. Huss M. Vega VB. Wong E. Orlov YL. Zhang W. Jiang J. Loh YH. Yeo HC. Yeo ZX. Narang V. Govindarajan KR. Leong B. Shahab A. Ruan Y. Bourque G. Sung WK. Clarke ND. Wei CL. Ng HH. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 154.MacArthur BD. Please CP. Oreffo RO. Stochasticity and the molecular mechanisms of induced pluripotency. PLoS ONE. 2008;3:e3086. doi: 10.1371/journal.pone.0003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Varambally S. Cao Q. Mani RS. Shankar S. Wang X. Ateeq B. Laxman B. Cao X. Jing X. Ramnarayanan K. Brenner JC. Yu J. Kim JH. Han B. Tan P. Kumar-Sinha C. Lonigro RJ. Palanisamy N. Maher CA. Chinnaiyan AM. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang H. Garzon R. Sun H. Ladner KJ. Singh R. Dahlman J. Cheng A. Hall BM. Qualman SJ. Chandler DS. Croce CM. Guttridge DC. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell. 2008;14:369–381. doi: 10.1016/j.ccr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ura H. Usuda M. Kinoshita K. Sun C. Mori K. Akagi T. Matsuda T. Koide H. Yokota T. STAT3 and Oct-3/4 control histone modification through induction of Eed in embryonic stem cells. J Biol Chem. 2008;283:9713–9723. doi: 10.1074/jbc.M707275200. [DOI] [PubMed] [Google Scholar]
- 158.Endoh M. Endo TA. Endoh T. Fujimura Y. Ohara O. Toyoda T. Otte AP. Okano M. Brockdorff N. Vidal M. Koseki H. Polycomb group proteins Ring1A/B are functionally linked to the core transcriptional regulatory circuitry to maintain ES cell identity. Development. 2008;135:1513–1524. doi: 10.1242/dev.014340. [DOI] [PubMed] [Google Scholar]
- 159.Card DA. Hebbar PB. Li L. Trotter KW. Komatsu Y. Mishina Y. Archer TK. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol Cell Biol. 2008;28:6426–6438. doi: 10.1128/MCB.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rosfjord E. Rizzino A. The octamer motif present in the Rex-1 promoter binds Oct-1 and Oct-3 expressed by EC cells and ES cells. Biochem Biophys Res Commun. 1994;203:1795–1802. doi: 10.1006/bbrc.1994.2395. [DOI] [PubMed] [Google Scholar]
- 161.Mongan NP. Martin KM. Gudas LJ. The putative human stem cell marker, rex-1 (Zfp42): structural classification and expression in normal human epithelial and carcinoma cell cultures. Mol Carcinog. 2008;45:887–900. doi: 10.1002/mc.20186. [DOI] [PubMed] [Google Scholar]
- 162.Kim JD. Faulk C. Kim J. Retroposition and evolution of the DNA-binding motifs of YY1, YY2 and REX1. Nucleic Acids Res. 2008;35:3442–3452. doi: 10.1093/nar/gkm235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mohd-Sarip A. Cleard F. Mishra RK. Karch F. Verrijzer CP. Synergistic recognition of an epigenetic DNA element by Pleiohomeotic and a Polycomb core complex. Genes Dev. 2008;19:1755–1760. doi: 10.1101/gad.347005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Klymenko T. Papp B. Fischle W. Kocher T. Schelder M. Fritsch C. Wild B. Wilm M. Muller J. A Polycomb group protein complex with sequence-specific DNA-binding and selective methyl-lysine-binding activities. Genes Dev. 2008;20:1110–1122. doi: 10.1101/gad.377406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang L. Brown JL. Cao R. Zhang Y. Kassis JA. Jones RS. Hierarchical recruitment of polycomb group silencing complexes. Mol Cell. 2008;14:637–646. doi: 10.1016/j.molcel.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 166.Atchison L. Ghias A. Wilkinson F. Bonini N. Atchison ML. Transcription factor YY1 functions as a PcG protein in vivo. EMBO J. 2008;22:1347–1358. doi: 10.1093/emboj/cdg124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Henderson JK. Draper JS. Baillie HS. Fishel S. Thomson JA. Moore H. Andrews PW. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells. 2008;20:329–337. doi: 10.1634/stemcells.20-4-329. [DOI] [PubMed] [Google Scholar]
- 168.Brivanlou AH. Gage FH. Jaenisch R. Jessell T. Melton D. Rossant J. Setting standards for human embryonic stem cells. Science. 2008;300:913–916. doi: 10.1126/science.1082940. [DOI] [PubMed] [Google Scholar]
- 169.Richards M. Tan SP. Tan JH. Chan WK. Bongso A. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells. 2008;22:51–64. doi: 10.1634/stemcells.22-1-51. [DOI] [PubMed] [Google Scholar]
- 170.Abeyta MJ. Clark AT. Rodriguez RT. Bodnar MS. Reijo-Pera RA. Firpo MT. Unique gene expression signatures of independently derived human embryonic stem cell lines. Hum Mol Gen. 2008;13:601–608. doi: 10.1093/hmg/ddh068. [DOI] [PubMed] [Google Scholar]
- 171.Rogers MB. Hosler BA. Gudas LJ. Specific expression of a retinoic acid-regulated zinc finger gene, Rex-1, in preimplantation embryos, trophoblast and spermatocytes. Development. 2008;113:815–824. doi: 10.1242/dev.113.3.815. [DOI] [PubMed] [Google Scholar]
- 172.D'Ippolito G. Diabira S. Howard GA. Menei P. Roos BA. Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2008;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 173.Baal N. Reisinger K. Jahr H. Bohle RM. Liang O. Munstedt K. Rao CV. Preissner KT. Zygmunt MT. Expression of transcription factor Oct-4 and other embryonic genes in CD133 positive cells from human umbilical cord blood. Thromb Haemost. 2008;92:767–775. doi: 10.1160/TH04-02-0079. [DOI] [PubMed] [Google Scholar]
- 174.Zhang JZ. Gao W. Yang HB. Zhang B. Zhu ZY. Xue YF. Screening for genes essential for mouse embryonic stem cell self-renewal using a subtractive RNA interference library. Stem Cells. 2008;24:2661–2668. doi: 10.1634/stemcells.2006-0017. [DOI] [PubMed] [Google Scholar]
- 175.Masui S. Ohtsuka S. Yagi R. Takahashi K. Ko MS. Niwa H. Rex1/Zfp42 is dispensable for pluripotency in mouse ES cells. BMC Dev Biol. 2008;8:45. doi: 10.1186/1471-213X-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Scotland K. Chen S. Sylvester R. Gudas LJ. Analysis of Rex1 (Zfp42) function in embryonic stem cell differentiation. Dev Dyn. 2009 Jul 17;238:1863–1877. doi: 10.1002/dvdy.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pearson S. Sroczynska P. Lacaud G. Kouskoff V. The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to Bmp4, activin A, bFGF and VEGF. Development. 2008;135:1525–1535. doi: 10.1242/dev.011767. [DOI] [PubMed] [Google Scholar]
- 178.Ma F. Ebihara Y. Umeda K. Sakai H. Hanada S. Zhang H. Zaike Y. Tsuchida E. Nakahata T. Nakauchi H. Tsuji K. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci USA. 2008;105:13087–13092. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Georgantas RW., III Hildreth R. Morisot S. Alder J. Liu CG. Heimfeld S. Calin GA. Croce CM. Civin CI. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc Natl Acad Sci USA. 2008;104:2750–2755. doi: 10.1073/pnas.0610983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jorgensen HF. Terry A. Beretta C. Pereira CF. Leleu M. Chen ZF. Kelly C. Merkenschlager M. Fisher AG. REST selectively represses a subset of RE1-containing neuronal genes in mouse embryonic stem cells. Development. 2008;136:715–721. doi: 10.1242/dev.028548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Johnson R. Teh CH. Kunarso G. Wong KY. Srinivasan G. Cooper ML. Volta M. Chan SS. Lipovich L. Pollard SM. Karuturi RK. Wei CL. Buckley NJ. Stanton LW. REST regulates distinct transcriptional networks in embryonic and neural stem cells. PLoS Biol. 2008;6:e256. doi: 10.1371/journal.pbio.0060256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Buckley NJ. Johnson R. Sun YM. Stanton LW. Is REST a regulator of pluripotency? Nature. 2008;457:E5–E6. doi: 10.1038/nature07784. discussion E7. [DOI] [PubMed] [Google Scholar]
- 183.Jorgensen HF. Chen ZF. Merkenschlager M. Fisher AG. Is REST required for ESC pluripotency? Nature. 2008;457:E4–E5. doi: 10.1038/nature07783. discussion E7. [DOI] [PubMed] [Google Scholar]
- 184.Singh SK. Kagalwala MN. Parker-Thornburg J. Adams H. Majumder S. REST maintains self-renewal and pluripotency of embryonic stem cells. Nature. 2008;453:223–227. doi: 10.1038/nature06863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sun YM. Cooper M. Finch S. Lin HH. Chen ZF. Williams BP. Buckley NJ. Rest-mediated regulation of extracellular matrix is crucial for neural development. PLoS ONE. 2008;3:e3656. doi: 10.1371/journal.pone.0003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zheng D. Zhao K. Mehler MF. Profiling RE1/REST-mediated histone modifications in the human genome. Genome Biol. 2008;10:R9. doi: 10.1186/gb-2009-10-1-r9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Packer AN. Xing Y. Harper SQ. Jones L. Davidson BL. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington's disease. J Neurosci. 2008;28:14341–14346. doi: 10.1523/JNEUROSCI.2390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Blanpain C. Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Clarke MF. Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 190.Hill RP. Perris R. “Destemming” cancer stem cells. J Natl Cancer Inst. 2007;99:1435–1440. doi: 10.1093/jnci/djm136. [DOI] [PubMed] [Google Scholar]
- 191.Dalerba P. Cho RW. Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2008;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 192.Chiou SH. Yu CC. Huang CY. Lin SC. Liu CJ. Tsai TH. Chou SH. Chien CS. Ku HH. Lo JF. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14:4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 193.Raman JD. Mongan NP. Liu L. Tickoo SK. Nanus DM. Scherr DS. Gudas LJ. Decreased expression of the human stem cell marker, Rex-1 (zfp-42), in renal cell carcinoma. Carcinogenesis. 2008;27:499–507. doi: 10.1093/carcin/bgi299. [DOI] [PubMed] [Google Scholar]
- 194.Ben-Porath I. Thomson MW. Carey VJ. Ge R. Bell GW. Regev A. Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wong DJ. Liu H. Ridky TW. Cassarino D. Segal E. Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Varambally S. Dhanasekaran SM. Zhou M. Barrette TR. Kumar-Sinha C. Sanda MG. Ghosh D. Pienta KJ. Sewalt RG. Otte AP. Rubin MA. Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2008;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 197.Kamminga LM. Bystrykh LV. de Boer A. Houwer S. Douma J. Weersing E. Dontje B. de Haan G. The Polycomb group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood. 2006;107:2170–2179. doi: 10.1182/blood-2005-09-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dietrich N. Bracken AP. Trinh E. Schjerling CK. Koseki H. Rappsilber J. Helin K. Hansen KH. Bypass of senescence by the polycomb group protein CBX8 through direct binding to the INK4A-ARF locus. EMBO J. 2008;26:1637–1648. doi: 10.1038/sj.emboj.7601632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bracken AP. Pasini D. Capra M. Prosperini E. Colli E. Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2008;22:5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Widschwendter M. Fiegl H. Egle D. Mueller-Holzner E. Spizzo G. Marth C. Weisenberger DJ. Campan M. Young J. Jacobs I. Laird PW. Epigenetic stem cell signature in cancer. Nat Genet. 2008;39:157–158. doi: 10.1038/ng1941. [DOI] [PubMed] [Google Scholar]
- 201.Liu S. Dontu G. Mantle ID. Patel S. Ahn NS. Jackson KW. Suri P. Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2008;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Raman JD. Mongan NP. Tickoo SK. Boorjian SA. Scherr DS. Gudas LJ. Increased expression of the polycomb group gene, EZH2, in transitional cell carcinoma of the bladder. Clin Cancer Res. 2008;11:8570–8576. doi: 10.1158/1078-0432.CCR-05-1047. [DOI] [PubMed] [Google Scholar]
- 203.Friedman JM. Liang G. Liu CC. Wolff EM. Tsai YC. Ye W. Zhou X. Jones PA. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2008;69:2623–2629. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]