Abstract
There have been reports of marrow cells converting into pulmonary epithelial cells after marrow transplantation in irradiated mice. We evaluated the impact of whole bone marrow (WBM) infusion in mice, with or without total body irradiation (TBI), treated with saline or monocrotaline (MCT), which induces pulmonary hypertension (PH). C57BL/6 mice were injected with MCT or saline weekly for 4 weeks. Cohorts were then infused with saline vehicle (vehicle) or WBM from C57BL/-Tg(UBC-GFP)30Scha/J mice, with or without previous TBI (WBM or WBM/TBI). Four weeks later, right ventricular peak pressures (RVPP), right ventricular free wall-to-body weight ratios (RV/BW), and pulmonary vessel wall thickness-to-blood vessel diameter ratios (PVWT/D) were determined. WBM infusion and WBM following TBI induced increases in RVPP and RV/BW in saline-treated mice, while only TBI-exposed mice showed additional increases in PVWT/D. MCT increased RVPP, RV/BW, and PVWT/D in mice given vehicle or WBM alone, but not in mice given WBM/TBI. RVPP and RV/BW were not significantly lower in MCT mice given WBM/TBI than in MCT mice treated with vehicle, but MCT-treated mice given WBM or TBI/WBM had significantly lower PVWT/D compared to MCT-treated mice given saline vehicle. No donor WBM-derived pulmonary vascular cells were detected, suggesting that the observed effects of WBM infusion may be due to paracrine effects separate from cell conversions. The observation of PH after marrow infusion suggests an additional mechanism for lung toxicity seen in marrow transplantation. In conclusion, WBM alone appears to increase RVPP and RV/BW in normal mice but the combination of WBM and TBI attenuates MCT-induced PH.
Introduction
Pulmonary arterial hypertension is a disease characterized by uncontrolled proliferation of pulmonary vascular endothelial cells and proliferation and hypertrophy of pulmonary vascular smooth muscle cells. Whether these abnormal growth responses are stimulated by overexpression of a variety of growth factors or occur from loss of normal apoptotic mechanisms is unclear, but their effect is to “remodel” the pulmonary circulation by decreasing luminal diameter and increasing pulmonary vascular resistance. The chronic elevation of right ventricular (RV) afterload from pulmonary vascular remodeling causes RV hypertrophy and eventually, RV failure. Although recently developed medical therapies have been shown to reduce pulmonary arterial pressure, increase cardiac output, and improve patient functional status and survival, no cure has been found for this devastating disease.
The possibility of using adult bone marrow-derived stem cells as a form of cell-based therapy is a novel and intriguing concept and has recently become an active area of investigation. Interest in this field is based on a growing number of animal studies that have described the ability of adult bone marrow-derived stem cells to differentiate into an expanding repertoire of nonhematopoietic cells, including lung cells [1–19]. However, in spite of all that has been published, the mechanism that governs this phenomenon remains unclear. Injury to the lung appears to be a critical variable. Previous studies have used radiation [1–5]-, bleomycin [6–8]-, and elastase [9]-induced lung injury models to increase homing of transplanted marrow cells to the lung. The reported rates of marrow conversion to lung cells in these studies have varied considerably and may reflect differences in the type of transplanted marrow cells used, the mode of lung injury, the strain of mouse, and the time from transplantation to analysis.
While many studies have focused on identifying and quantifying marrow cell conversion to lung cells, others have looked at the effect of marrow cell infusion on remodeling of the injured lung. Ortiz et al. [6] transplanted mesenchymal stem cells (MSC) into bleomycin-injured mice and found a significant reduction in lung inflammation and collagen content compared with nontransplanted controls. Ishizawa et al. [9] showed that elastase-injured, G-CSF-mobilized mice transplanted with fetal liver cells had fewer emphysematous changes compared with nontransplanted controls. In both cases, attenuation of lung injury could not be explained by conversion alone as a disproportionately low number of marrow cell-derived lung cells were detected. Less is known about the ability of transplanted marrow cells to improve organ function after injury. Zhao et al. [20] demonstrated that infusion of marrow-derived endothelial-like progenitor cells (ELPCs) prevented the development of pulmonary hypertension (PH) and RV hypertrophy in monocrotaline (MCT)-injured rats. Animals given ELPCs transduced with human endothelial NO-synthase (eNOS) showed preservation of pulmonary vasculature, partial reversal of PH and improved survival compared to controls. Interestingly, the low degree of engraftment of marrow cells in the distal arteriolar bed could not account for the degree of vascular repair that was observed. Together, these data suggest that marrow cell attenuation of lung injury depends more on altering the microenvironment of the injured lung rather than mass production of marrow-derived lung cells.
In the present study, we hypothesized that marrow cell transplantation could attenuate MCT-induced pulmonary vascular remodeling by replacing abnormally proliferating vascular endothelial and smooth muscle cells with marrow-converted lung cells. We examined the effect of marrow cell transplantation, with or without prior exposure to a myeloablative dose of radiation, on hemodynamic and histological changes in the pulmonary circulation of normal mice and mice with MCT-induced PH. Myeloablative irradiation was done in an attempt to increase bone marrow chimerism and increase the number of transplanted cells that remain viable. We found that marrow cell transplantation with or without myeloablative irradiation increased pulmonary vascular resistance in control mice. However, marrow cell transplantation following myeloablative irradiation blunted the progression of PH, RV hypertrophy, and pulmonary vascular remodeling in mice given MCT. These changes occurred without significant conversion of marrow cells to structural lung cells, suggesting that marrow cell transplantation has modulatory effects on vascular remodeling in the pulmonary circulation that are independent of cell conversion.
Materials and Methods
Experimental animals
Six- to eight-week-old male C57BL/-Tg(UBC-GFP)30Scha/J mice (uGFP; Jackson Laboratory, Bar Harbor, ME) were used for whole bone marrow (WBM) harvest and were bred in our animal facility by mating homozygous uGFP+ mice. Cohorts of 6- to 8-week-old female C57BL/6 mice (Jackson Laboratory) were used for MCT injury or as control (saline) mice. Animals were given ad libitum access to food and water. All studies were approved by the Institutional Animal Care and Use Committee of Roger Williams Medical Center.
Marrow cell harvest
Male uGFP+ mice were anesthetized with inhaled halothane and sacrificed by cervical dislocation. WBM was obtained by flushing the femurs, tibias, and pelvic bones with sterile 1X phosphate buffered solution (PBS) supplemented with 5% heat-inactivated fetal calf serum (HICFS). Cells were centrifuged at 350g for 10 min, 4°C and resuspended in fresh PBS/5% HIFCS.
Experimental design
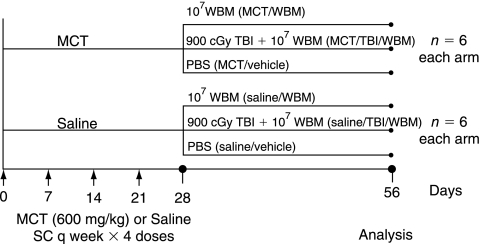
Female C57BL/6 mice were used for all groups. Six experimental groups of six mice each were studied as follows: Subcutaneous injections of saline once weekly for 4 weeks followed by (1) PBS vehicle infusion (saline/vehicle), (2) 1 × 107 uGFP+ WBM cells (saline/WBM), (3) 1 × 107 uGFP+ WBM cells 1 h after exposure to 900 centigray (cGy) total body irradiation (TBI) using a Gammacell 40 Exactor Irradiator (MDS Nordion) at 110 cGy/min given as two equal doses, 3 h apart (saline/TBI/WBM). The other three groups were given 600 mg/kg of MCT (Sigma-Aldrich, St. Louis, MO) in saline vehicle once weekly for 4 weeks followed by (4) PBS vehicle infusion (MCT/vehicle), (5) 1 × 107 uGFP+ WBM cells (MCT/WBM), or (6) 1 × 107 uGFP+ WBM cells 1 h after exposure to 900 cGy TBI (MCT/TBI/WBM). Hemodynamic measurements and lung harvest occurred 28 days after PBS, WBM, or TBI + WBM treatment (Day 56, Fig. 1).
FIG. 1.
Experimental design. Saline- and MCT-treated mice receiving either no further treatment (saline/vehicle, MCT/vehicle mice), marrow cell infusion (saline/WBM, MCT/WBM mice), or total body irradiation followed by marrow cell transplantation (saline/TBI/WBM, MCT/TBI/WBM mice).
Hemodynamic measurements
Mice were anesthetized with ketamine and xylazine (100 and 10 mg/kg, respectively) and weighed. Tracheas were cannulated with a blunted 20-gauge needle and lungs were ventilated with room air using an inspiratory pressure of 9 cm H 2O and end-expiratory pressure of 2 cm H 2O. The right carotid artery was cannulated with a 25-gauge angiocatheter connected to a Statham P23-G pressure transducer with a short segment of polyurethane-50 (P-50) tubing. Carotid arterial pressure was recorded continuously on a polygraph. Carotid artery peak pressure (CAPP) was measured as the average peak pressure (PP) recorded over a 1- to 3-min period during which pressure recordings remained stable. Right ventricular PP (RVPP) was measured as previously described [21,22]. Briefly, the anterior chest wall was shaved and a 26-gauge needle attached to a Statham P23-G pressure transducer by a short segment of P-50 tubing was inserted directly into the right ventricle using a transthoracic approach. RVPP was recorded as described earlier for CAPP measurements.
After completion of hemodynamic measurements, mice were sacrificed by exsanguination and hearts and lungs were removed en bloc. Cardiac atria were removed and hearts were dissected into right and left ventricular free walls and interventricular septa. Each section of the heart was blotted dry on sterile gauze to remove excess blood and weighed. Wet weight measurements were normalized to body weight (mg/g) and expressed as the right ventricular free wall weight-to-body weight (RV/BW) ratio.
Determination of peripheral blood chimerism of transplanted mice
Peripheral blood was obtained from the tail veins of mice 1 day prior to sacrifice and uGFP+ cells were quantified by Fluorescence Activated Cell Sorter (FACS) analysis. Peripheral blood chimerism was determined by comparing the percent of uGFP+ cells in samples to the percent of uGFP+ cells in a positive control (peripheral blood from an uGFP+ mouse) after background subtraction.
Lung histology
The pulmonary vasculature of recipient mice was flushed with PBS and lungs were fixed by intratracheal infusion of ice-cold PLP (balanced phosphate solution with 2% paraformaldehyde, sodium m-periodate, and l-lysine) at a pressure of 25 cm H 2O. Lungs were transferred to a 7% sucrose solution overnight and then placed into ethanol solutions of increasing concentrations, mineral spirits, and paraffin for embedding. Right and left lungs from each mouse were embedded in separate blocks of paraffin. Hematoxylin and eosin staining was performed on 5 μm sections from the left and right lungs, which were then used for histological analysis. The first 10 blood vessels (on end) from sections of the right and left lung of each mouse, with diameters of 25–100 μm, were used for analysis. The pulmonary vessel wall thickness (including adventitia, media, and intima)-to-blood vessel wall diameter (PVWT/D) ratio was determined.
Immunohistochemical labeling
Five micrometer lung sections were deparaffinized and labeled with biotinylated anti-CD45 antibody (Vector) alone or with other antibodies, including anti-von Willebrand factor for endothelium (Dako, Carpinteria, CA), anti-vimentin for fibroblasts (BD Pharmingen Inc., San Diego, CA), and anti-actin α-smooth muscle-Cy3 for smooth muscle (Sigma-Aldrich, St. Louis, MO). Anti-GFP antibody was not used as the GFP signal was well preserved with fixation. We have previously reported that the GFP signal from transplanted GFP+ marrow cells seen in lung sections of irradiated mice represents true GFP positivity and not artifact from auto-fluorescence. The use of anti-GFP Alexafluor-488 does not enhance this signal [1,23]. Isotype antibody labeling was performed a control to confirm the appropriate labeling pattern for all antibodies.
CD45/von Willebrand factor
Samples were permeabilized with 0.1% Triton-X and blocked with the following solutions: 20% horse serum, 0.3% hydrogen peroxide in 0.3% horse serum, and avidin/biotin (Avidin/Biotin Blocking Kit). Anti-CD45 (1:100) and anti-von Willebrand antibodies (1:60) were applied to each sample and incubated for 2 h at 37°C. The CD45 signal was amplified by using the TSA Biotin System (Perkin Elmer Life Sciences, Walton, MA) per product protocol. The secondary antibodies AMCA Avidin (CD45, 1:100) and AlexaFluor 594 F(ab′)2 fragment of goat anti-rabbit IgG (von Willebrand, 1:400) were applied to each sample and incubated at room temperature for 30 min. Vectashield (Vector) was added to all samples.
CD45/actin α-smooth muscle-Cy3
Samples were permeabilized and blocked as described earlier. An additional mouse-on-mouse block step (M.O.M. Kit, Vector) was performed. Anti-CD45 (1:100) and anti-actin α-smooth muscle-Cy3 (1:50) were applied to each sample and incubated for 1.5 h at 37°C. The CD45 signal was amplified by using the TSA Biotin System as described earlier. The secondary antibody AMCA Avidin (1:100) was applied to each sample and incubated at room temperature for 30 min. Vectashield (Vector) was added to all samples.
CD45/vimentin
Samples were incubated with Proteinase K (Sigma-Aldrich, St. Louis, MO, 5 μg/mL) for 5 min at 37°C and blocked as described earlier (including the mouse-on-mouse blocking step). Anti-CD45 (1:100) and anti-vimentin antibodies (1:5,000) were applied to each sample and incubated for 1 h at room temperature. The CD45 signal was amplified by using the TSA Biotin System as described earlier. The secondary antibodies AMCA Avidin (1:100) and AlexaFluor 594 F(ab′)2 fragment of goat anti-mouse IgG (1:1,000) were applied to each sample and incubated at room temperature for 30 min. Vectashield (Vector) was added to all samples.
CD45 only
Samples were permeabilized and blocked as described earlier. Anti-CD45 (1:100) was applied to each sample and incubated for 1 h at room temperature. The CD45 signal was amplified by using the TSA Biotin System as described earlier. The secondary antibody AMCA Texas Red (1:100) was applied to each sample and incubated at room temperature for 30 min. Vectashield (Vector) with 0.4 μmol of the nuclear counterstain DAPI (4,6-diamidino-2-phenyindole; Sigma-Aldrich, St. Louis, MO) was added to all samples.
CD11b/Gr-1 and CD3/B220
Samples were digested with Proteinase K for 3 min at 37°C then washed with PBS. Samples were fixed with 10% buffered formalin for 2 min then blocked with 20% horse serum. Anti-CD11b APC and anti-Gr-1APC antibodies (BD Pharmingen) were added (1:25 dilution for each). Alternatively, anti-CD3 and anti-B220 antibodies (BD Pharmingen) were added (1:25 dilution for each). Incubation with primary antibodies was for 1 h at room temperature. Vectashield and DAPI were added to all samples. Cells that were both CD11b+ and Gr-1+ were defined as myeloid cells, whereas cells that were both CD3+ and B220+ were defined as lymphoid cells.
Fluorescence microscopy
Samples were visualized using conventional and deconvolution fluorescence microscopy (Zeiss Axioplan 2 microscope, Carl Zeiss, Thornwood, NY) at room temperature. Eight to ten random 63× high power fields from least two sections over 100 μm apart of each sample were counted. Using deconvolution microscopy, selected sections were photographed at 40× magnification with the AxioVision software package (Carl Zeiss). Three-dimensional images were created of sample cells from a 25-layer (0.4 μm/layer) z-stack in order to demonstrate colocalization of fluorescent signal. No photosubtraction or processing of artifact was performed.
Statistics
Data were analyzed using the Student's t-test as there were six or fewer measurements within all parent groups. We considered results to be statistically significant only when P < 0.05 (two-sided). Data were reported as the mean value of a group and the standard error of the mean, unless otherwise specified.
Results
Effect of whole bone marrow infusion and total body irradiation on pulmonary hemodynamics and vascular remodeling in saline-treated mice
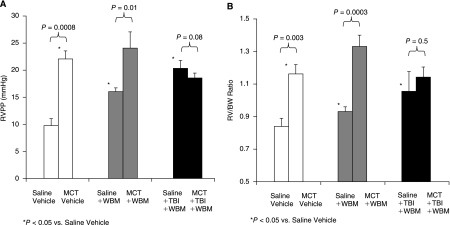
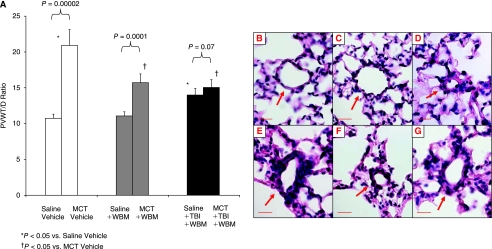
Mice in the saline/WBM and in the saline/TBI/WBM groups had higher RVPP and RV/BW values compared with saline/vehicle mice (Fig. 2A and 2B). Saline/TBI/WBM mice had significantly higher RVPP compared with saline/WBM mice (P = 0.005, this is not shown in the figure), and there was a trend toward higher RV/BW ratio in the saline/TBI/WBM than in the saline/WBM mice, but the difference was not statistically significant (P = 0.17, Fig. 2A and 2B). Pulmonary vascular remodeling, as assessed by PVWT/D, was greater in saline/TBI/WBM mice than in saline/vehicle and saline/WBM mice (P = 0.0005, 0.0002, respectively, Fig. 3A).
FIG. 2.
Right ventricular peak pressure (RVPP) (A) and right ventricular free wall-to-body weight (RV/WB) ratios (B) of saline- and MCT-treated mice, with or without marrow cell infusion or total body irradiation.
FIG. 3.
Pulmonary vessel wall thickness-to-blood vessel diameter (PVWT/D) ratios and lung histology. PVWT/D ratios of saline- and MCT-treated mice, with or without marrow cell infusion or total body irradiation (A). Pulmonary blood vessel from saline/vehicle (B), saline/WBM (C), saline/TBI/WBM (D), MCT/vehicle (E), MCT/WBM (F), and MCT/TBI/WBM (G) groups. Red bar = 20 μm.
Effect of whole bone marrow infusion and total body irradiation on mice with monocrotaline-induced pulmonary hypertension
Mice treated with MCT followed by vehicle alone (MCT/vehicle) developed PH as illustrated by elevations in RVPP and RV/BW ratios compared with saline/vehicle control mice (P = 0.0008, 0.003, respectively, Fig. 2A and 2B). MCT treatment also caused pulmonary vascular remodeling as evidenced by muscularization of peripheral, normally nonmuscularized pulmonary vessels, hypertrophy of the medial layer of blood vessels 25–100 μm in diameter, and increased PWVT/D (Fig. 3A–3G). Treatment with WBM infusion alone following MCT (MCT/WBM mice) did not prevent the MCT-induced increases in RVPP or RV/BW (Fig. 2A and 2B). However, treatment with TBI/WBM following MCT resulted in no change in RVPP and RV/BW compared to saline mice treated with TBI/WBM, suggesting that TBI followed by WBM infusion ameliorated MCT-induced PH (Fig. 2A and 2B). There was a strong trend toward lower RVPP in MCT/TBI/WBM than in MCT/vehicle mice, but the difference did not reach statistical significance (P = 0.082). The lack of a difference in RV pressure and mass between MCT/vehicle and MCT/TBI/WBM mice may have been due to the pulmonary hypertensive effect of WBM and TBI as demonstrated by their effects on saline-treated mice.
Pulmonary vascular remodeling, as measured by PWVT/D, was greater in MCT/vehicle versus saline/vehicle mice as well as MCT/WBM versus saline/WBM mice (Fig. 3A). However, no differences in PWVT/D were seen between MCT/TBI/WBM and saline/TBI/WBM mice. Furthermore, PWVT/D was less in MCT/WBM and MCT/TBI/WBM mice than in MCT/vehicle mice, suggesting that WBM infusion with or without TBI attenuated MCT-induced changes in pulmonary vascular remodeling.
Marrow cell engraftment of the lung: correlates with effects on pulmonary hypertension
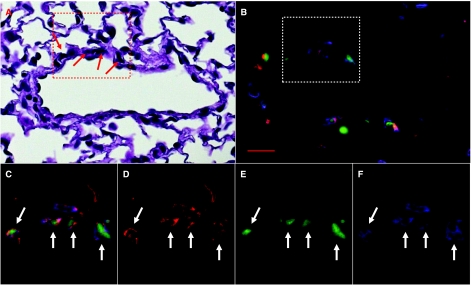
Infusion of WBM alone did not result in any detectable bone marrow chimerism in saline or MCT-treated mice. However, both saline and MCT-treated mice infused with WBM after TBI showed fully chimeric bone marrow. In mice treated with WBM infusion after TBI, there were significantly more GFP+ cells seen in the lungs of MCT-injured mice than in saline controls (P = 0.04, Table 1). Mice treated with MCT and given WBM infusion after TBI had a 170% increase in the number of GFP+ nucleated cells in the lung. The majority of these GFP+ cells were hematopoietic cells, as they also expressed the panleukocyte marker CD45 (GFP+/CD45+, 153% increase). The majority of these bone marrow-derived hematopoietic cells appeared to be of myeloid lineage (63.5% ± 1.65%) as they were both CD11b+ and Gr-1+. Fewer (19.4% ± 1.55%) were of lymphoid lineage (CD3+ and B220+). However, the percentage of myeloid and lymphoid cells was not significantly different in the MCT/TBI/WBM and saline/TBI/WBM groups (P = 0.42, 0.86, respectively). A small percentage of the bone marrow-derived hematopoietic cells clustered around pulmonary blood vessels were also vimentin+ (GFP+/CD45+ and vimentin+, Fig. 4). It is not clear if these cells represent donor marrow-derived macrophages, monocytes, mast cells, or fibroblasts. However, there was no significant difference in the number of these cells in saline versus MCT-treated mice. More nonhematopoietic bone marrow-derived cells (GFP+ and CD45−) were seen in the lungs of MCT-injured mice infused with WBM (MCT/WBM) after irradiation compared with saline mice infused with WBM (saline/WBM) after irradiation (192% increase, Table 1). No structural cells of the pulmonary blood vessels in these groups appeared to be bone marrow-derived, including endothelial cells (GFP+/CD45−/von Willebrand+, Fig. 5) and smooth muscle cells (GFP+/CD45−/actin α-smooth muscle).
Table 1.
Donor Marrow Cells in the Lung
| |
|
|
Expressed as a % DAPI+ cells |
||
|---|---|---|---|---|---|
| TBI | WBM | GFP+ total | GFP+/CD45+ | GFP+/CD45− | |
| Saline | − | − | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| − | + | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| + | + | 23.35 ± 4.05 | 20.92 ± 3.91 | 2.43 ± 0.16 | |
| MCT | − | − | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| − | + | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| + | + | 39.65 ± 2.61a (+170%)b | 34.99 ± 2.29a (+167%)b | 4.66 ± 0.42a (+192%)b | |
Values are mean ± SD. n = 3 in each group.
Statistically significant by Student's t-test, MCT/TBI/WBM vs. saline/TBI/WBM, P ≤ 0.05.
Percent increase compared to saline/TBI/WBM group.
FIG. 4.
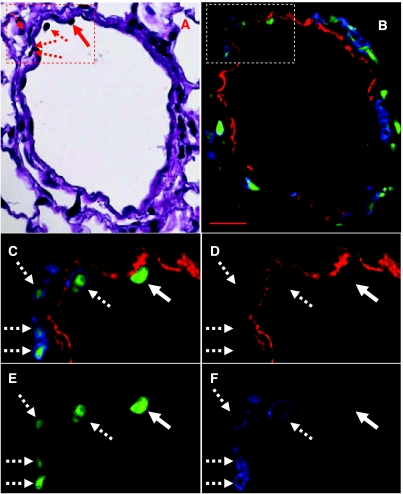
Marrow cell-derived vimentin+ cells. A pulmonary blood vessel from a MCT-injured mouse treated with marrow cell infusion and total body irradiation. Area (red box) and cells (red arrows) of interest (A) from an H&E stained lung section. Same area of interest (white box), fluorescent image (B and C, all filters), showing the same cells (white arrows), which are GFP+ (green), CD45+ (blue), and vimentin+ (red). Texas Red (D), FITC (E), and DAPI (F) filters. Red bar = 20 μm.
FIG. 5.
Marrow cell-derived lung cells are not pulmonary vascular endothelial cells. Pulmonary blood vessel from a MCT-injured mouse treated with marrow cell infusion and total body irradiation. Area (red box) and cells (red arrows) of interest (A) from an H&E stained lung section. Same area of interest (white box), fluorescent image (B and C, all filters), showing the same cells (white dashed arrows), which are GFP+ (green), CD45+ (blue), and von Willebrand factor− (red), representing donor marrow cell-derived hematopoietic cells. Texas Red (D), FITC (E), and DAPI (F) filters. The other cells (solid white arrows) are only GFP+, representing a marrow-derived nonhematopoietic cells. Red bar = 20 μm.
Discussion
In the present study, we hypothesized that infusion of WBM would ameliorate MCT-induced PH by replacing injured pulmonary vascular cells with healthy bone marrow-derived progenitor cells. We also hypothesized that myeloablative irradiation given prior to WBM infusion would increase bone marrow chimerism and increase the number of transplanted cells that remain viable, thereby enhancing the ameliorative effects of WBM infusion on MCT-induced PH.
Our findings suggest that WBM infusion alone attenuates to some degree MCT-induced pulmonary vascular remodeling as demonstrated by a reduction in PVWT/D compared to MCT mice treated with vehicle alone, but that this effect was not enough to prevent MCT-induced increase in RVPP and RV/BW. However, when given after TBI, WBM prevented MCT-induced changes in RV pressure, RV mass, and pulmonary vascular remodeling. Compared to saline alone, mice treated with 4 weeks of MCT had a doubling of right ventricular peak pressure and pulmonary arterial medial wall thickness and a nearly 50% increase in RV mass normalized to body weight. WBM infusion, either alone or following TBI in MCT-treated mice, resulted in lower PVWT/D than in MCT-treated mice given vehicle alone. The effects of WBM infusion with or without TBI on RV pressure and mass are more difficult to evaluate due to the unexpected pulmonary hypertensive effect of WBM and TBI/WBM in mice that did not receive MCT. Mice that received WBM infusion after 4 weeks of saline developed significant increases in both RVPP and RV/BW. Thus, administration of WBM following 4 weeks treatment with MCT should have resulted in a greater degree of PH than in mice given MCT followed by saline infusion. In fact, RVPP and RV/BW were slightly higher in MCT/WBM mice than in MCT controls, although the increase was not statistically significant. However, in mice given TBI and WBM after MCT treatment, there was no increase in RVPP or RV/BW compared to MCT controls. In fact, there was strong trend toward lower RVPP in the MCT/TBI/WBM mice than in MCT/controls (P = 0.083). Furthermore, in mice given vehicle alone for the last 4 weeks of the experiment, RVPP and RV/BW were significantly higher in mice that had received MCT as compared to those given saline during the first 4 weeks. However, in mice given TBI/WBM for the last 4 weeks, there was no difference in RVPP or RV/BW between those treated with MCT and those given saline. Combined with the significant reduction in PVWT/D in the MCT mice treated with TBI/WBM compared to MCT mice treated with vehicle, our findings suggest that WBM infusion combined with TBI can ameliorate pulmonary hypertensive changes in mice with MCT-induced PH.
Our findings are in agreement with those of other investigators who reported their results from similar, although different experimental designs. Zhao et al. [20] first demonstrated that infusion of ELPC could blunt the development of MCT-induced PH in rats. They also found that when given 4 weeks after MCT injection, ELPCs prevented the progression of MCT-induced PH. This study differed from ours primarily in the use of rats, an animal model known to develop considerably greater PH in response to MCT than mice. Zhao et al. also used mononuclear cells that had been isolated from WBM and cultured for 7–10 days to select out a population of enriched ELPCs as opposed to the WBM infusion employed in our study. In a more recent study by Raoul et al. [24], experiments were done in mice and WBM cells were used. However, bone marrow donors were treated with 5-flourouracil, again in an attempt to select for ELPCs by depleting mature cells and allowing proliferation of progenitor cells. Also, Raoul et al. administered cell infusion 3 days after a single IV dose of MCT, and hence their results are limited to the ability of bone marrow-derived cells to prevent the development of PH as opposed to reversing the more established PH that occurred after 4 weeks of MCT treatment in our study. Finally, neither of the previous studies used TBI to enhance bone marrow engraftment, a technique that increased chimerism and increased the antihypertensive effect of WBM infusion.
It is unclear why WBM infusion combined with TBI appeared to ameliorate the pulmonary hypertensive effects of MCT in mice. The mechanism of this observation was not directly studied in these experiments. Only TBI-treated mice had bone marrow engraftment of transplanted cells and a small number of donor marrow-derived lung cells. None of these donor marrow-derived lung cells appeared to be cells of the pulmonary vasculature, arguing against direct cell replacement as a potential mechanism. It is possible that paracrine effects of donor marrow-derived hematopoietic cells, which were most abundant in the MCT/TBI/WBM group, may be responsible for this beneficial outcome. The anti-inflammatory and immunomodulatory properties of certain marrow cell populations, in particular MSC, have been well described. Systemic infusion of MSC into bleomycin-injured mice resulted in less pulmonary fibrosis and this was seen independent of significant donor cell lung engraftment [6]. More recently, investigators have demonstrated that intratracheal administration of MSC may also attenuate certain lung injuries. After endotoxin injury, mice given MSC had a lower mortality rate, less pulmonary edema, and lower bronchoalveolar lavage fluid levels of the pro-inflammatory cytokines TNF-α and MIP-1β with little evidence of donor cell lung engraftment [25]. Although these levels were not checked in this study, we speculate a similar mechanism may explain the ameliorative effects of TBI and WBM in the setting of MCT injury.
Unlike earlier studies, we also examined the effect of WBM infusion with or without TBI on pulmonary hemodynamics and remodeling in saline controls. Previous studies have found that circulating bone marrow-derived cells may contribute to experimental models of PH in animals [26] and to pulmonary vascular remodeling in patients with COPD [27]. However, the effect of bone marrow infusion on pulmonary hemodynamics and vascular remodeling in healthy animals has not been well studied. In the present study, we found that WBM infusion caused a significant increase in RV pressure and mass and pulmonary vascular remodeling, an effect that was enhanced by TBI. The mechanism(s) responsible for these effects was not readily apparent from histological analysis of lung sections. It is possible that the large number of cells infused (1 × 107) caused microembolization of the distal pulmonary vascular bed. However, the number of cells we infused was slightly less than the maximum number of cells used to blunt MCT-induced pulmonary by Raoul et al. [24] and we saw little evidence of GFP+ cells in the lungs of WBM-infused mice. Furthermore, the pulmonary hypertensive effect of WBM appeared to be enhanced by TBI, suggesting that it was caused by increased marrow engraftment of transfused WBM cells as opposed to physical obstruction of the pulmonary circulation. Although future studies will be needed to further elucidate the etiology of PH caused by WBM infusion, our findings have important ramifications for patients undergoing potential stem cell infusion therapies.
The observations that marrow infusion, per se, with or without whole body irradiation may induce aspects of PH in the mouse are of significant interest. In the field of clinical marrow transplantation, lung disorders are ubiquitous and difficult to diagnose definitively, with possible causative factors being infections (viral, bacterial, and fungal), chemoradiotherapy toxicity, and immunologic reactivity. Toxicity related simply to the infused marrow cells adds a new element to considerations of lung problems in the transplant setting. In our experiments, immunologic reactions based on HY male:female differences in C57BL/6 mice may have contributed to this toxicity. The potential interaction of TBI and marrow cell infusion (with engraftment) further complicates the picture. The observation that these changes can occur without engraftment indicates an upfront abscopal effect on pulmonary hemodynamics, presumably through various paracrine effects on the pulmonary vasculature. The effect of irradiation separates from marrow engraftment cannot be ruled out in these studies, but appears unlikely. Similarly, work by Zhao et al. [20], as well as studies of the effects of marrow cells infusions on ischemic myocardium in both animals and patients, has also shown functional effects which did not seem to be explained by marrow cells converting to differentiated organ-specific cells [23,28–30].
We have recently described the delivery of lung-specific mRNA in microvesicles from radiation-injured lung to marrow cells with a functional change in the phenotype of the marrow cells [31]. In addition, Deregibus et al. [32] have shown that microvesicles from endothelial progenitor cells could stimulate both in vitro and in vivo vasculogenesis. It is possible that cross communication between marrow and pulmonary vascular cells via microvesicular genetic information transfer may have attenuated some of the changes seen with MCT injury. This could occur without the vascular cells showing specific donor marrow cell markers such as GFP. Regardless, it appears that marrow cell infusions can significantly ameliorate features of MCT-induced PH in mice, suggesting that marrow infusion strategies could be developed for the treatment of PH in humans.
Acknowledgment
This publication was made possible by support from the following grants: 1 K08 HL086868-01A1 (J.M.A.), R01 HL088328-01 (J.R.K.), 7R01HL073749-05 (P.J.Q.), 1 P20 RR018757-02.
Author Disclosure Statement
No competing financial interests exist for any of the authors of this article.
References
- 1.Aliotta JM. Keaney P. Passero M. Dooner MS. Pimentel J. Greer D. Demers D. Foster B. Peterson A. Dooner G. Theise ND. Abedi M. Colvin GA. Quesenberry PJ. Bone marrow production of lung cells: the impact of G-CSF, cardiotoxin, graded doses of irradiation and subpopulation phenotype. Exp Hematol. 2008;34:230–241. doi: 10.1016/j.exphem.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theise ND. Henegariu O. Grove J. Jagirdar J. Kao PN. Crawford JM. Badve S. Saxena R. Krause DS. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol. 2008;30:1333–1338. doi: 10.1016/s0301-472x(02)00931-1. [DOI] [PubMed] [Google Scholar]
- 3.Herzog E. Van Arnam J. Hu B. Krause DS. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells. 2008;24:1986–1992. doi: 10.1634/stemcells.2005-0579. [DOI] [PubMed] [Google Scholar]
- 4.Abe S. Lauby G. Boyer C. Rennard SI. Sharp JG. Transplanted BM and BM side population cells contribute progeny to the lung and liver in irradiated mice. Cytotherapy. 2008;5:623–633. doi: 10.1080/14653240310003576. [DOI] [PubMed] [Google Scholar]
- 5.Grove JE. Lutzko C. Priller J. Henegariu O. Theise ND. Kohn DB. Krause DS. Marrow-derived cells as vehicles for delivery of gene therapy to pulmonary epithelium. Am J Respir Cell Mol Biol. 2008;27:645–651. doi: 10.1165/rcmb.2002-0056RC. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz LA. Gambelli F. McBride C. Gaupp D. Baddoo M. Kaminski N. Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2008;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotton DN. Ma BY. Cordoso WW. Sanderson EA. Summer RS. Williams MC. Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2008;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto N. Jin H. Liu T. Chensue SW. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishizawa K. Kubo H. Yamada M. Kobayashi S. Numasaki M. Ueda S. Suzuki T. Sasaki H. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett. 2008;566:249–252. doi: 10.1016/s0014-5793(03)01399-1. [DOI] [PubMed] [Google Scholar]
- 10.Baber SR. Deng W. Master RG. Bunnell BA. Taylor BK. Murthy SN. Hyman AL. Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;291:H1378–H1383. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- 11.Beckett T. Loi R. Prenovitz P. Poynter M. Goncz KK. Suratt BT. Weiss DJ. Acute lung injury with endotoxin or NO2 does not enhance development of airway epithelium from bone marrow. Mol Ther. 2008;12:680–686. doi: 10.1016/j.ymthe.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Loi R. Beckett T. Goncz KK. Suratt BT. Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2008;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Y. Jahagirdar JN. Reinhardt RL. Schwartz RE. Keene CD. Ortiz-Gonzalez XR. Reyes M. Lenvik T. Lund T. Blackstad M. Du J. Aldrich S. Lisberg A. Low WC. Largaespada DA. Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2008;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 14.Adachi Y. Imagawa J. Suzuki Y. Yogo K. Fukazawa M. Kuromaru O. Saito Y. Treatment and transfer of emphysema by a new bone marrow transplantation method from normal mice to Tsk mice and vice versa. Stem Cells. 2008;24:2071–2077. doi: 10.1634/stemcells.2005-0575. [DOI] [PubMed] [Google Scholar]
- 15.Abe S. Boyer C. Liu X. Wen FQ. Kobayashi T. Fang Q. Wang X. Hashimoto M. Sharp JG. Rennard SI. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med. 2008;170:1158–1163. doi: 10.1164/rccm.200307-908OC. [DOI] [PubMed] [Google Scholar]
- 16.Bruscia EM. Ziegler EC. Price JE. Weiner S. Egan ME. Krause DS. Engraftment of donor-derived epithelial cells in multiple organs following bone marrow transplantation into newborn mice. Stem Cells. 2008;24:2299–2308. doi: 10.1634/stemcells.2006-0166. [DOI] [PubMed] [Google Scholar]
- 17.Krause DS. Theise ND. Collector MI. Henegariu O. Hwang S. Gardner R. Neutzel S. Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2008;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 18.Macpherson H. Keir P. Webb S. Samuel K. Boyle S. Bickmore W. Forrester L. Dorin J. Bone marrow-derived SP cells can contribute to the respiratory tract of mice in vivo. J Cell Sci. 2008;118:2441–2450. doi: 10.1242/jcs.02375. [DOI] [PubMed] [Google Scholar]
- 19.Dooner M. Cerny J. Colvin G. Demers D. Pimentel J. Greer D. Abedi M. McAuliffe C. Quesenberry PJ. Homing and conversion of murine hematopoietic stem cells to lung. Blood Cells Mol Dis. 2008;32:47–51. doi: 10.1016/j.bcmd.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Zhao YD. Courtman DW. Deng Y. Kugathasan L. Zhang Q. Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2008;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 21.Fagan KA. Fouty BW. Tyler RC. Morris KG. Hepler LK. Sato K. LeCras TD. Abman SH. Weinberger HD. Huang PL. McMurtry IF. Rodman DM. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest. 2008;103:291–299. doi: 10.1172/JCI3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klinger JR. Warburton LA RR. Pietras Smithies O. Swift R. Hill NS. Genetic disruption of atrial natriuretic peptide causes pulmonary hypertension in normoxic and hypoxic mice. Am J Physiol Lung Cell Mol Physiol. 2008;276:L868–L874. doi: 10.1152/ajplung.1999.276.5.L868. [DOI] [PubMed] [Google Scholar]
- 23.Engelmann MG. Theiss HD. Theiss C. Huber A. Wintersperger BJ. Werle-Ruedinger AE. Schoenberg SO. Steinbeck G. Franz WM. G-CSF in patients suffering from late revascularized ST elevation myocardial infarction: Analysis on the timing of G-CSF administration. Exp Hematol. 2008;36(6):703–709. doi: 10.1016/j.exphem.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Raoul W. Wagner-Ballon O. Saber G. Hulin A. Marcos E. Giraudier S. Vainchenker W. Adnot S. Eddahibi S. Maitre B. Effects of bone marrow-derived cells on monocrotaline-and hypoxia-induced pulmonary hypertension in mice. Respir Res. 2008;8:8. doi: 10.1186/1465-9921-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta N. Su X. Popov B. Lee JW. Serikov V. Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2008;179(3):1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 26.Hayashida K. Fujita J. Miyake Y. Kawada H. Ando K. Ogawa S. Fukuda K. Bone marrow-derived cells contribute to pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension. Chest. 2008;127(5):1793–1798. doi: 10.1378/chest.127.5.1793. [DOI] [PubMed] [Google Scholar]
- 27.Peinado VI. Ramírez J. Roca J. Rodriguez-Roisin R. Barberà JA. Identification of vascular progenitor cells in pulmonary arteries of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2006;34(3):257–263. doi: 10.1165/rcmb.2005-0255OC. [DOI] [PubMed] [Google Scholar]
- 28.Charwat S. Gyöngyösi M. Lang I. Graf S. Beran G. Hemetsberger R. Nyolczas N. Sochor H. Glogar D. Role of adult bone marrow stem cells in the repair of ischemic myocardium: Current state of the art. Exp Hematol. 2008;36(6):672–680. doi: 10.1016/j.exphem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Ripa RS. Kastrup J. G-CSF therapy with mobilization of bone marrow stem cells for myocardial recovery after acute myocardial infarction—A relevant treatment? Exp Hematol. 2008;36(6):681–686. doi: 10.1016/j.exphem.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Brunner S. Huber BC. Fischer R. Groebner M. Hacker M. David R. Zaruba MM. Vallaster M. Rischpler C. Wilke A. Gerbitz A. Franz WM. G-CSF treatment after myocardial infarction: Impact on bone marrow-derived vs. cardiac progenitor cells. Exp Hematol. 2008;36(6):695–702. doi: 10.1016/j.exphem.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Aliotta JM. Sanchez-Guijo FM. Dooner GJ. Johnson KW. Dooner MS. Greer KA. Greer D. Pimentel J. Kolankiewicz LM. Puente N. Faradyan S. Ferland P. Bearer EL. Passero MA. Adedi M. Colvin GA. Quesenberry PJ. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells. 2008;25(9):2245–2256. doi: 10.1634/stemcells.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deregibus MC. Cantaluppi V. Calogero R. Lo Iacono M. Tetta C. Biancone L. Bruno S. Bussolati B. Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2008;110(7):2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]