Abstract
Context:
Selective adenomectomy via transsphenoidal surgery induces remission of Cushing's disease (CD) in most patients. Although an undetectable postoperative serum cortisol (<2 μg/dl) has been advocated as an index of remission, there is no consensus on predictors of recurrence.
Objective:
We hypothesized that patients with subnormal cortisol (2–4.9 μg/dl) might achieve long-term remission and that postoperative responses to CRH might predict recurrence.
Design, Setting, and Participants:
We prospectively studied CD patients with initial remission after adenomectomy or hemihypophysectomy (n = 14). Long-term recurrence (n = 39) or remission (n = 293) was assigned by laboratory results, glucocorticoid dependence, or patient survey at a mean of 10.6 yr after surgery.
Intervention and Main Outcome Measures:
Postoperatively, morning cortisol was measured on d 3–5, and cortisol and ACTH responses to ovine CRH were assessed around d 10.
Results:
Follow-up duration was median 11 yr (range 1–22.8 yr). Fewer patients achieved a cortisol nadir below 2 μg/dl (87%) than below 5 μg/dl (98%), yet recurrence rates were similar (<2 μg/dl, 9.5%; <5 μg/dl, 10.4%; 2–4.9 μg/dl, 20%; not significant). CRH-stimulated cortisol (P < 0.002) and ACTH (P = 0.04) values were higher for the recurrence than the remission group. However, no basal or stimulated ACTH or serum or urine cortisol cutoff value predicted all who later recurred.
Conclusions:
A postoperative cortisol below 2 μg/dl predicts long-term remission after transsphenoidal surgery in CD. Remission in those with intermediate d 3–5 postoperative cortisol values (2–4.9 μg/dl) suggests that these patients do not require immediate reoperation. However, because no single cortisol cutoff value excludes all patients with recurrence, all require long-term clinical follow-up.
Transsphenoidal surgery (TSS) is recognized as the optimal treatment strategy for Cushing's disease and offers the potential of clinical and biochemical remission from disease (1). Whereas persistent hypercortisolism is associated with high mortality rates and impaired quality of life, successful remission from hypercortisolism confers reduced morbidity and mortality (2). Although TSS is safe and effective, rates of remission and recurrence from Cushing's disease vary between centers (3). Some of the variation in clinical outcome may be accounted for by differing surgical expertise. However, because there is no consensus on the optimal strategy for determination of immediate postoperative remission, direct comparisons between centers remains challenging (1).
Several groups have attempted to define criteria for prediction of relapse of hypercortisolism using various postoperative testing protocols. Like others, we hypothesized that the postoperative degree of hypocortisolism and the response to CRH testing during the first postoperative week after TSS might predict long-term remission from hypercortisolism in a large group of patients with Cushing's disease.
Subjects and Methods
Subjects
All patients were enrolled in protocols approved by the institutional review board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and provided informed consent. Patients were studied at the National Institutes of Health (NIH) Warren Grant Magnuson Clinical Center in Bethesda, MD, between July 1982 and January 2004 and had follow-up until December 2008.
Cushing's syndrome was confirmed on the basis of elevated late-night salivary or serum cortisol values and/or elevated 24-h urine cortisol or 17-hydroxysteroid (17OHCS) excretion. A presumptive diagnosis of Cushing's disease was assigned to patients with ACTH-dependent hypercortisolism based on a combination of suppression of serum and/or urine cortisol during high-dose dexamethasone suppression testing, stimulation of serum cortisol or ACTH during CRH stimulation testing, a central-to-peripheral ACTH gradient during bilateral inferior petrosal sinus sampling, and/or pituitary magnetic resonance imaging, as previously described (4–7).
Inclusion criteria included TSS with hemi-hypophysectomy and ACTH-staining pituitary adenoma or adenomectomy, low urinary free cortisol (UFC) or serum cortisol within 1 month of surgery, and at least 1 yr of disease-free follow-up. Patients with persistent hypercortisolism or radiation treatment within 1 yr (n = 34) were excluded.
Glucocorticoid replacement regimen and basal hormone tests
Patients received dexamethasone 0.5 mg, given iv or orally every 6 h for five or six doses, beginning on the day of surgery. Some had a sixth dose of 1 mg given at 2300 h on the first postoperative day. Serum cortisol and UFC were obtained daily from postoperative d 3, until hypocortisolism [cortisol < 5 μg/dl (138 nmol/liter) and UFC < 20 μg/d (55 nmol/d)] was achieved in at least one sample. Patients with hypocortisolism then received hydrocortisone 12–15 mg/m2 by mouth in one or two daily doses.
Ovine CRH stimulation testing
Those patients considered in remission in the immediate postoperative period received synthetic ovine CRH (1 μg/kg iv) at around 10 d after surgery at 0800 and/or 2000 h; hydrocortisone was withheld on that day until after the test. An iv catheter was inserted at least 1 h before injection and was kept patent with normal saline. Cortisol and ACTH were measured at −15, 0, 5, 15, 30, 60, 90, 120, 150, and 180 min. CRH was purchased from Bachem Inc. (Torrance, CA) and prepared as previously described (8) or from Ferring Pharmaceuticals Inc. (Parsippany, NJ). The results of 29 of the current adult patients (9) and 14 of the pediatric patients (10) were reported previously.
Evaluation of long-term remission status
Recurrence or remission was confirmed via survey of patients, physicians, and charts. Patients were asked to indicate whether they had experienced a recurrence, whether they had subsequent treatments with surgery, medical therapy, or pituitary irradiation or were currently cured of Cushing's syndrome. They were asked to indicate whether they were currently taking hydrocortisone, prednisone, dexamethasone, mitotane, or ketoconazole. All patients were requested to provide copies of the most recent laboratory screening for objective assessment of remission status.
Long-term remission was defined as the need for glucocorticoid therapy or clinical or biochemical evidence of eucortisolism. Biochemical eucortisolism was defined as a morning serum cortisol from 5–25 μg/dl (138–690 nmol/liter) or normal 24-h urine cortisol or 17OHCS excretion or suppression of serum cortisol after 1 mg dexamethasone (<2.4 μg/dl). Biochemical testing was performed locally for each patient, and reports were submitted to NIH for assessment.
Assays
Serum cortisol was originally measured by RIA (11) and later by fluorescence polarization immunoassay (Abbott Laboratories, Abbott Park, IL) with an intra- and interassay coefficient of variation (CV) of 2.1 and 4.1%, respectively; the functional detection limit was 1–2 μg/dl (12). UFC was measured initially by RIA (SmithKline Bioscience Laboratories, King of Prussia, PA), and subsequently by immunoradiometric assay (Mayo Laboratories, Rochester MN, and Clinical Center Department of Laboratory Medicine). The functional detection limit was <5 μg/dl. Urine 17OHCS were measured by the Porter-Silber method (intra- and interassay CV were <6 and <14%, respectively) (6). ACTH was originally assayed by RIA at Hazleton/Covance Laboratories (Vienna, VA) as previously described (13). Subsequently, the method was revised to use Nichols ACTH immunoradiometric assay kit (Nichols Institute, San Clemente, CA) at Hazleton/Covance Laboratories. After September 2000, ACTH was measured by Nichols Advantage chemiluminescent kit (Nichols Institute, San Clemente, CA) in the Department of Laboratory Medicine at the NIH Clinical Center as previously described (14). The intraassay CV was 4.2% at 6.4 pg/ml (1.4 pmol/liter), and the intraassay variation was 8.4% at 5.8 pg/ml (1.3 pmol/liter). The reported assay range is 0–1500 pg/ml (0–330 pmol/liter).
Statistical analysis
Descriptive statistics were calculated for continuous variables. Frequencies were generated for other variables. Significance was assumed if P < 0.05. All data are expressed as mean ± sem, except where noted. Categorical variables were generated and analyzed by identifying nadir serum cortisol and UFC values for each patient during d 3–5 postoperatively, defined as the lowest postoperative level during this period. The predictive value of a positive and negative test was calculated. These values represent the proportion of true results among the apparent test results. Student's t test and Fisher's exact test were used to examine differences between recurrence and remission groups. Confidence intervals were calculated for proportions. Testing was performed using the statistical package for social science (SPSS version 12 for Windows, release 09.04, 2003; SPSS Inc., Chicago, IL) and JMP version 7 (SAS Institute, Cary NC).
Results
Follow-up data were available from 418 patients and 450 surgical procedures. Of these, 331 patients fulfilled the inclusion criteria (263 females, 68 males; 84% Caucasian, 7% African-American, 5% Hispanic, 4% Asian/other/unknown; age at TSS 36.0 ± 0.8 yr, range 5–71 yr). Overall, 56 patients had had previous TSS. Data for both procedures were included for six women tested after initial surgery and after surgery for recurrence. Three hundred thirty tumors were visualized at surgery. Fourteen patients had hemi-hypophysectomy, whereas the remainder had selective adenomectomy.
Early postoperative assessment
The mean serum cortisol levels on d 3, 4, and 5 postoperatively were 2.4 ± 0.2 (66.2 ± 5.5), 2.2 ± 0.2 (60.7 ± 5.5), and 2.3 ± 0.2 μg/dl (63.5 ± 5.5 nmol/liter), respectively. Serum cortisol levels were below 2 μg/dl (55 nmol/liter) in 79% (252 of 318), 77% (247 of 321), and 74% (206 of 279) and were below 5 μg/dl (138 nmol/liter) in 93% (296 of 318), 92% (297 of 321), and 91% (255 of 279) on postoperative d 3, 4, and 5, respectively. Many of the patients without a d 5 value had received hydrocortisone replacement after blood was drawn on d 4 because of symptoms of adrenal insufficiency; all but two had previous values below 5 μg/dl. Mean UFC concentrations on postoperative d 3–5 were 50.2 ± 31.9 (138.6 ± 88.0), 22.0 ± 8.1 (60.7 ± 22.4), and 20.6 ± 6.5 (56.9 ± 17.9) μg/d (nmol/d), respectively. Mean nadir serum and UFC concentrations were 1.6 ± 0.1 μg/dl (44 ± 2.8 nmol/liter) and 10.3 ± 0.8 μg/d (28.4 ± 2.2 nmol/d). Urine cortisol data were less complete (postoperative d 3, n = 135; postoperative d 4, n = 292; postoperative d 5, n = 270). Two hundred ninety-five achieved stringent postoperative nadir d 3–5 serum cortisol below 2 μg/dl (55 nmol/liter); an additional 30 achieved higher nadir values between 2 and 5 μg/dl (138 nmol/liter).
Long-term remission status
Forty patients (12%) had confirmed recurrence at 4.2 ± 0.5 yr after surgery (median 3.2 yr, range 1–13 yr). Among these patients, 15 had cavernous sinus invasion, eight had dural invasion, 12 had macroadenomas, one had hemihypophysectomy, and nine had previous surgery. Overall, 29 (74%) of these patients had one or more of these risk factors for recurrence. The 291 others achieved apparent long-term remission. Mean follow-up duration was 10.5 ± 0.3 yr (median 11.0 yr, range 1.1–22.8 yr). Of these, 128 supplied biochemistry results to support their self-reported remission status, and 50 reported glucocorticoid use. The self-reported remission status of the remaining patients was identified by chart review and questionnaire responses.
Early postoperative data for remission and recurrence groups
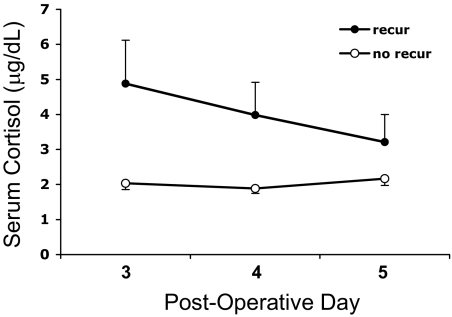
Serum cortisol results for the remission and recurrence groups during the first postoperative week are shown in Fig. 1. Mean postoperative d 3–5 nadir serum cortisol levels were lower for the remission group compared with those who later recurred [1.5 ± 0.1 vs. 2.9 ± 0.7 μg/dl (41.4 ± 2.8 vs. 80.0 ± 19.3 nmol/liter), P = 0.042] (Fig. 2). Similarly, d 3–5 nadir UFC concentrations were lower for those with continued remission [9.9 ± 1.0 μg/d (27.3 ± 2.8 nmol/d)] than for those who subsequently recurred [15.4 ± 2.6 μg/d (42.5 ± 7.2 nmol/d), respectively, P = 0.045].
Fig. 1.
Serial serum cortisol levels, stratified according to long-term remission status, on d 3, 4, and 5 after TSS. (To convert serum values to nanomoles per liter, multiply by 27.6; to convert urine values, multiply by 2.7.)
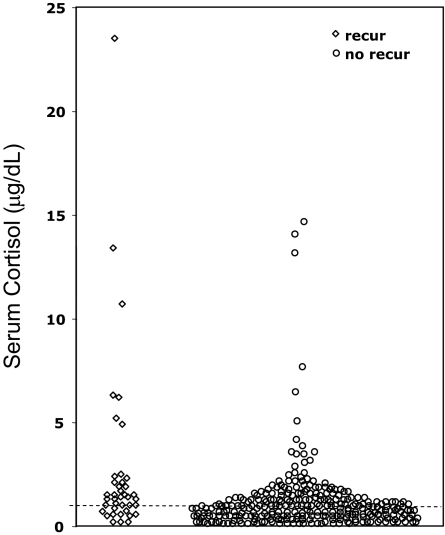
Fig. 2.
Individual nadir serum cortisol on d 3, 4, and 5 after TSS, stratified according to long-term follow-up remission status (♢, recurrence; ○, remission). (To convert to nanomoles per liter, multiply by 27.6.) Cortisol values of 1 μg/dl or undetectable are shown at or below the dashed line at 1 μg/dl.
Relationship between postoperative d 3–5 serum cortisol values and remission status
Of 295 patients who achieved a nadir serum cortisol below 2 μg/dl (55 nmol/liter), 267 remained in long-term remission (predictive value for remission 90.5%; 95% CI 87–93%), whereas 28 (9.5%) later recurred (Table 1). Eighty percent of patients (24 of 30) with higher serum cortisol values [2–4.9 μg/dl (55–138 nmol/liter)] still achieved long-term remission compared with six in this group who later recurred (predictive value for remission 80% (95% CI 66–94%). Overall, 10.9% of patients with values below 5 μg/dl recurred.
Table 1.
Relationship between nadir postoperative serum cortisol values on d 3-5 after TSS and long-term remission
| Serum cortisol (μg/dl) | Remission status |
Total | |
|---|---|---|---|
| + | − | ||
| <2 | 267 | 28 | 295 |
| 2–4.9 | 24 | 6 | 30 |
| Total | 291 | 34 | 325 |
Thirteen patients had nadir cortisol on d 3–5 greater than 5 μg/dl but later had cortisol values < 5 μg/dl and/or normal UFC. Of these, 6 recurred (46%, 95% CI 19–73%). Among those with a nadir cortisol below 2 μg/dl, 9.5% recurred; those with a nadir below 1 μg/dl had a 7% recurrence rate.
No single postoperative nadir serum cortisol cutoff value excluded all patients who subsequently recurred (Fig. 2).
Postoperative CRH stimulation test for prediction of self-reported remission status
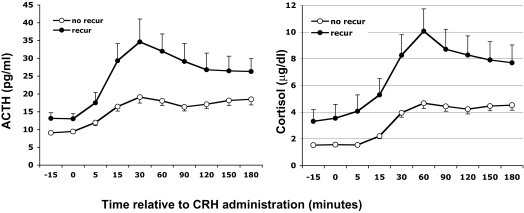
Results were available from 303 CRH stimulation tests performed at a median of 10 d after surgery (5–24 d). Mean basal and stimulated ACTH (except at 5, 120, 150, and 180 min) and stimulated cortisol values were significantly lower for those in long-term remission compared with those who later recurred (P < 0.007–0.02, Fig. 3). The mean peak serum cortisol [5.4 ± 0.4 vs. 10.3 ± 1.7 μg/dl (149.0 ± 11.0 vs. 284.3 ± 46.9 nmol/liter), P = 0.007] and ACTH [23.5 ± 1.8 vs. 44.8 ± 8.5 pg/ml (5.2 ± 0.4 vs. 9.9 ± 1.9 pmol/liter), P < 0.03] concentrations after CRH were significantly lower for the remission than the recurrence groups, respectively (Fig. 4).
Fig. 3.
Postoperative plasma ACTH and serum cortisol responses to CRH stimulation testing (1 μg/kg iv) 4–24 d after surgery at 0800 and/or 2000 h, according to long-term follow-up remission status (●, recurrence; ○, remission). Values are mean ± sem. (To convert ACTH to picomoles per liter, multiply by 0.22; to convert cortisol to nanomoles per liter, multiply by 27.6.)
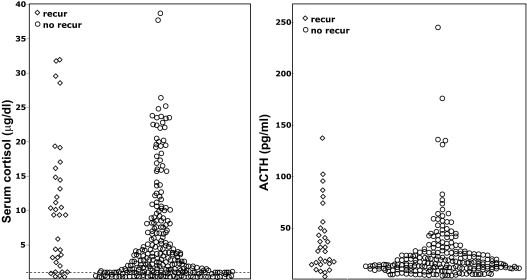
Fig. 4.
Individual peak plasma ACTH and serum cortisol values during CRH stimulation testing, according to long-term follow-up remission status (♢, recurrence; ○, remission). Cortisol values of 1 μg/dl or undetectable are shown at or below the dashed line at 1 μg/dl. (To convert ACTH to picomoles per liter, multiply by 0.22; to convert cortisol to nanomoles per liter, multiply by 27.6.)
Similar to the findings from postoperative d 3–5, 90% (223 of 248) of those with basal serum cortisol below 2 μg/dl (<55 nmol/liter) achieved long-term remission. For those with higher serum cortisol values [2–4.9 μg/dl (55–138 nmol/liter)], 89% (31 of 35) had persistent remission (P = 0.5). The lowest basal cortisol levels before CRH administration correlated with d 3–5 basal nadir cortisol (ρ = 0.60; P < 0.001) and peak serum cortisol (ρ = 0.73; P < 0.001) and peak plasma ACTH values (ρ = 0.31; P < 0.001) after CRH administration.
Among patients with d 3–5 cortisol values less than 2 μg/dl, neither the peak nor any post-CRH ACTH value improved the ability of d 3–5 values to predict recurrence. However, among those with d 3–5 cortisol values over 2 μg/dl, the ACTH value at 90 min after CRH increased the ability to predict recurrence. Thirty-eight patients had a value of 18 pg/ml or more; among this group, 12 patients recurred, for a predictive value of 32% (95% CI 17–46%) (predictive value for remission, 68%; 95% CI 54–83%).
We examined maximal stimulated serum cortisol (area = 0.6) and ACTH (area = 0.6) values by receiver operator characteristic curves to identify cutoff values for prediction of subsequent recurrence. Because there was a wide range of overlap between both groups, no single cutoff value provided sufficient sensitivity or specificity for identification of all cases.
Discussion
Transsphenoidal resection of ACTH-secreting pituitary tumors is the optimal initial treatment for Cushing's disease and offers the potential for remission in most cases. Despite its success in reversing the morbidity and mortality associated with Cushing's disease (15, 16), long-term relapse rates range from 3–46% depending the method of assessment and duration of follow-up (17, 18). Determination of factors predictive of remission or recurrence might influence early postoperative management and surveillance (19).
Testing for postoperative remission relies upon the concept that hypercortisolism suppresses the normal corticotropes. Successful excision of an ACTH-secreting pituitary adenoma unmasks this suppression and results in postoperative hypocortisolism. By contrast, residual tumor remnant results in continued (tumoral) ACTH-dependent cortisol production.
Many strategies have been evaluated as possible predictors of long-term remission. These include assessment of the degree of postoperative hypocortisolism by measurement of serum cortisol in the basal state (19, 20) or after suppression by dexamethasone (21) or loperamide (22, 23) or after stimulation with desmopressin (22, 24), CRH (9, 22, 25–27), or metyrapone (28). The variety of testing protocols and test times complicate the comparison of these tests as does the potential effects of variable glucocorticoid replacement regimens.
The concept that an undetectable postoperative 0900-h serum cortisol best predicts long-term disease remission gained acceptance after a study by Trainer et al. (19) of forty-eight patients undergoing TSS for Cushing's disease. After initial surgery, postoperative 0900-h serum cortisol was undetectable [<1.8 μg/dl (<50 nmol/liter)] in 20 of 48 patients (19). Cushing's syndrome did not recur clinically or biochemically in these patients at a median follow-up interval of 40 months (19). Based upon the premise that detectable serum cortisol levels immediately postoperatively suggests the presence of residual tumor, immediate reoperation for TSS for Cushing's disease has been suggested (29).
The current study was designed to evaluate whether the degree of postoperative hypocortisolism or the response to postoperative CRH testing might predict long-term remission from hypercortisolism. Although 87% of our patients had sustained long-term remission, not all of these occurred among the 90.5% of patients who attained postoperative serum cortisol values below 2 μg/dl. Indeed, 7% of those with undetectable cortisol levels (<1 μg/dl) recurred. Thus, although this most stringent criterion for postoperative remission was highly predictive for remission, no single nadir serum cortisol cutoff value identified all cases that relapsed.
In our study, the proportion of patients with recurrence was increased in those with nadir intermediate cortisol values (2–4.9 μg/dl) on postoperative d 3–5 compared with values below the 2 μg/dl threshold, but confidence intervals overlapped. Using a serum cortisol below 5 μg/dl (<140 nmol/liter) during the first two postoperative days as a criterion for remission, Esposito et al. (30) reported a 97% long-term remission over 32 months. Applying this same criterion to patients reported by Vignati et al. (26) shows a recurrence rate of 12%. Indeed, two of five patients with normal serum cortisol levels remained in remission over 1.5–5 yr of follow-up, suggesting that even higher cutoff criteria are compatible with remission. Taken together, these data suggest that immediate reoperation would not be appropriate for all patients with hypocortisolemic, but not undetectable, postoperative serum cortisol values.
In addition to the absolute threshold of hypocortisolism, other factors may predict the duration of remission. Estrada et al. (23) showed that complete normalization of the hypothalamic-pituitary-adrenal axis after TSS, including the rhythm and stress response in addition to postsurgical hypocortisolism, was associated with a very low recurrence risk. Similarly, demonstration of a normal negative feedback response to low-dose dexamethasone (1 or 2 mg) is associated with long-term remission (20, 21).
The timing of assessment and differing steroid regimens may influence the predictive value of postoperative testing. In one study, serum cortisol measured postoperatively at 3 months from surgery had better predictive value than early values measured during the first 2 wk (31). We studied both early (d 3–5) and late (around d 10) immediate postoperative basal cortisol values and found a similar predictive value for each, suggesting that such testing may be conducted any time within the first 2 wk provided that exogenous corticosteroids are withdrawn for at least 24 h. Our study design did not allow us to evaluate whether any exposure to glucocorticoids might mask abnormal values.
In general, CRH-stimulated cortisol and ACTH values had no added benefit for determination of remission status over that of early basal cortisol testing. Our observations are in contrast to an earlier series from our institution that examined the efficacy of CRH stimulation testing for prediction of long-term remission in a smaller group with Cushing's disease. All of the 23 patients in that series with a subnormal response to CRH remained in remission at 6–42 months of follow-up (9). Vignati et al. (26) later reported recurrence in only one of 21 patients with subnormal or normal CRH responses, whereas five of nine with supranormal ACTH responses relapsed 9 months to 10 yr later. These observations were later confirmed by others using a range of protocols that included human CRH and a range of diagnostic cutoff points and duration of clinical follow-up (32–34). However, interpretation of these studies is limited by the short duration of follow-up and small numbers of patients with an even smaller proportion of recurrences (25, 34, 35). In particular, the length of follow-up may be very important in determining the recurrence rate, because we found recurrence as late as 13 yr after TSS.
We found that the ACTH response to CRH predicted a higher rate of recurrence in patients with postoperative cortisol levels of 2–5 μg/dl in patients with a 90-min value over 18 pg/ml. However, the confidence intervals around this recurrence rate overlapped with those using the postoperative basal cortisol levels. Additional studies would be needed to determine whether CRH testing has any additional value in this group.
The limitations of this study include the use of self-reported status to assess remission in about 35% of patients. Most of our patients who recurred had contacted us independently of the survey, suggesting that we may have underdetected those with continued remission. Also, our findings may be limited to series with similar inclusion criteria (treatment with adenomectomy or hemihypophysectomy with ACTH-staining pituitary adenoma, low UFC or serum cortisol, and at least 1 yr of follow-up from TSS). The strengths of the study are the large patient numbers and long duration of follow-up.
In conclusion, although postoperative serum cortisol below 2 μg/dl had high predictive value for remission, no value excluded all patients with recurrence. The long-term remission rates in those with postoperative serum cortisol from 2–4.9 μg/dl suggests that these patients do not require immediate reoperation. CRH-stimulated cortisol or ACTH values may predict recurrence but do not improve upon the basal cortisol results. Thus, all patients should be followed closely for recurrence.
Acknowledgments
We acknowledge the attending physicians, apart from the authors, who admitted these patients to the Clinical Center, including Drs. D. Lynn Loriaux, Gordon Cutler, and George Chrousos. We thank our neurosurgery colleagues Dr. Zvi Ram, Hetty DeVroom, and Rene Smith for assistance with data collection and establishing long-term recurrence status. Finally, we thank the patients and families who responded to our survey.
The intramural programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Neurologic Disorders and Stroke, National Institutes of Health, supported this work.
Disclosure Summary: None of the authors has a conflict of interest to disclose.
Footnotes
- CV
- Coefficient of variation
- 17OHCS
- 17-hydroxysteroid
- TSS
- transsphenoidal surgery
- UFC
- urinary free cortisol.
References
- 1. Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M. 2003. Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab 88:5593–5602 [DOI] [PubMed] [Google Scholar]
- 2. Hammer GD, Tyrrell JB, Lamborn KR, Applebury CB, Hannegan ET, Bell S, Rahl R, Lu A, Wilson CB. 2004. Transsphenoidal microsurgery for Cushing's disease: initial outcome and long-term results. J Clin Endocrinol Metab 89:6348–6357 [DOI] [PubMed] [Google Scholar]
- 3. Swearingen B, Biller BM, Barker FG, 2nd, Katznelson L, Grinspoon S, Klibanski A, Zervas NT. 1999. Long-term mortality after transsphenoidal surgery for Cushing disease. Ann Intern Med 130:821–824 [DOI] [PubMed] [Google Scholar]
- 4. Nieman LK, Oldfield EH, Wesley R, Chrousos GP, Loriaux DL, Cutler GB., Jr 1993. A simplified morning ovine corticotropin-releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab 77:1308–1312 [DOI] [PubMed] [Google Scholar]
- 5. Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, Cutler GB, Jr, Loriaux DL. 1991. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing's syndrome. N Engl J Med 325:897–905 [DOI] [PubMed] [Google Scholar]
- 6. Flack MR, Oldfield EH, Cutler GB, Jr, Zweig MH, Malley JD, Chrousos GP, Loriaux DL, Nieman LK. 1992. Urine free cortisol in the high-dose dexamethasone suppression test for the differential diagnosis of the Cushing syndrome. Ann Intern Med 116:211–217 [DOI] [PubMed] [Google Scholar]
- 7. Patronas N, Bulakbasi N, Stratakis CA, Lafferty A, Oldfield EH, Doppman J, Nieman LK. 2003. Spoiled gradient recalled acquisition in the steady state technique is superior to conventional postcontrast spin echo technique for magnetic resonance imaging detection of adrenocorticotropin-secreting pituitary tumors. J Clin Endocrinol Metab 88:1565–1569 [DOI] [PubMed] [Google Scholar]
- 8. Chrousos GP, Schulte HM, Oldfield EH, Gold PW, Cutler GB, Jr, Loriaux DL. 1984. The corticotropin-releasing factor stimulation test. An aid in the evaluation of patients with Cushing's syndrome. N Engl J Med 310:622–626 [DOI] [PubMed] [Google Scholar]
- 9. Avgerinos PC, Chrousos GP, Nieman LK, Oldfield EH, Loriaux DL, Cutler GB., Jr 1987. The corticotropin-releasing hormone test in the postoperative evaluation of patients with Cushing's syndrome. J Clin Endocrinol Metab 65:906–913 [DOI] [PubMed] [Google Scholar]
- 10. Batista DL, Oldfield EH, Keil MF, Stratakis CA. 2009. Postoperative testing to predict recurrent Cushing disease in children. J Clin Endocrinol Metab 94:2757–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kao M, Voina S, Nichols A, Horton R. 1975. Parallel radioimmunoassay for plasma cortisol and 11-deoxycortisol. Clin Chem 21:1644–1647 [PubMed] [Google Scholar]
- 12. Papanicolaou DA, Mullen N, Kyrou I, Nieman LK. 2002. Nighttime salivary cortisol: a useful test for the diagnosis of Cushing's syndrome. J Clin Endocrinol Metab 87:4515–4521 [DOI] [PubMed] [Google Scholar]
- 13. Orth D. 1979. Adrenocorticotropic hormone (ACTH). In: Jaffe BM, Berhman HR, eds. Methods of hormone radioimmunoassay. New York: Academic Press; 245 [Google Scholar]
- 14. Lindsay JR, Shanmugam VK, Oldfield EH, Remaley AT, Nieman LK. 2006. A comparison of immunometric and radioimmunoassay measurement of ACTH for the differential diagnosis of Cushing's syndrome. J Endocrinol Invest 29:983–988 [DOI] [PubMed] [Google Scholar]
- 15. Etxabe J, Vazquez JA. 1994. Morbidity and mortality in Cushing's disease: an epidemiological approach. Clin Endocrinol (Oxf) 40:479–484 [DOI] [PubMed] [Google Scholar]
- 16. Faggiano A, Pivonello R, Spiezia S, De Martino MC, Filippella M, Di Somma C, Lombardi G, Colao A. 2003. Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing's disease during active disease and 1 year after disease remission. J Clin Endocrinol Metab 88:2527–2533 [DOI] [PubMed] [Google Scholar]
- 17. Utz AL, Swearingen B, Biller BM. 2005. Pituitary surgery and postoperative management in Cushing's disease. Endocrinol Metab Clin North Am 34:459–478, xi [DOI] [PubMed] [Google Scholar]
- 18. Patil CG, Prevedello DM, Lad SP, Vance ML, Thorner MO, Katznelson L, Laws ER., Jr 2008. Late recurrences of Cushing's disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab 93:358–362 [DOI] [PubMed] [Google Scholar]
- 19. Trainer PJ, Lawrie HS, Verhelst J, Howlett TA, Lowe DG, Grossman AB, Savage MO, Afshar F, Besser GM. 1993. Transsphenoidal resection in Cushing's disease: undetectable serum cortisol as the definition of successful treatment. Clin Endocrinol (Oxf) 38:73–78 [DOI] [PubMed] [Google Scholar]
- 20. McCance DR, Gordon DS, Fannin TF, Hadden DR, Kennedy L, Sheridan B, Atkinson AB. 1993. Assessment of endocrine function after transsphenoidal surgery for Cushing's disease. Clin Endocrinol (Oxf) 38:79–86 [DOI] [PubMed] [Google Scholar]
- 21. Chen JC, Amar AP, Choi S, Singer P, Couldwell WT, Weiss MH. 2003. Transsphenoidal microsurgical treatment of Cushing disease: postoperative assessment of surgical efficacy by application of an overnight low-dose dexamethasone suppression test. J Neurosurg 98:967–973 [DOI] [PubMed] [Google Scholar]
- 22. Barbetta L, Dall'Asta C, Tomei G, Locatelli M, Giovanelli M, Ambrosi B. 2001. Assessment of cure and recurrence after pituitary surgery for Cushing's disease. Acta Neurochir (Wien) 143:477–481; discussion 481–472 [DOI] [PubMed] [Google Scholar]
- 23. Estrada J, García-Uría J, Lamas C, Alfaro J, Lucas T, Diez S, Salto L, Barceló B. 2001. The complete normalization of the adrenocortical function as the criterion of cure after transsphenoidal surgery for Cushing's disease. J Clin Endocrinol Metab 86:5695–5699 [DOI] [PubMed] [Google Scholar]
- 24. Losa M, Mortini P, Dylgjeri S, Barzaghi R, Franzin A, Mandelli C, Giovanelli M. 2001. Desmopressin stimulation test before and after pituitary surgery in patients with Cushing's disease. Clin Endocrinol (Oxf) 55:61–68 [DOI] [PubMed] [Google Scholar]
- 25. Invitti C, Pecori Giraldi F, de Martin M, Cavagnini F. 1999. Diagnosis and management of Cushing's syndrome: results of an Italian multicentre study. Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis. J Clin Endocrinol Metab 84:440–448 [DOI] [PubMed] [Google Scholar]
- 26. Vignati F, Berselli ME, Loi P. 1994. Early postoperative evaluation in patients with Cushing's disease: usefulness of ovine corticotropin-releasing hormone test in the prediction of recurrence of disease. Eur J Endocrinol 130:235–241 [DOI] [PubMed] [Google Scholar]
- 27. Nishizawa S, Oki Y, Ohta S, Yokota N, Yokoyama T, Uemura K. 1999. What can predict postoperative “endocrinological cure” in Cushing's disease? Neurosurgery 45:239–244 [DOI] [PubMed] [Google Scholar]
- 28. van Aken MO, de Herder WW, van der Lely AJ, de Jong FH, Lamberts SW. 1997. Postoperative metyrapone test in the early assessment of outcome of pituitary surgery for Cushing's disease. Clin Endocrinol (Oxf) 47:145–149 [DOI] [PubMed] [Google Scholar]
- 29. Locatelli M, Vance ML, Laws ER. 2005. Clinical review: the strategy of immediate reoperation for transsphenoidal surgery for Cushing's disease. J Clin Endocrinol Metab 90:5478–5482 [DOI] [PubMed] [Google Scholar]
- 30. Esposito F, Dusick JR, Cohan P, Moftakhar P, McArthur D, Wang C, Swerdloff RS, Kelly DF. 2006. Early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing's disease. J Clin Endocrinol Metab 91:7–13 [DOI] [PubMed] [Google Scholar]
- 31. Pereira AM, van Aken MO, van Dulken H, Schutte PJ, Biermasz NR, Smit JW, Roelfsema F, Romijn JA. 2003. Long-term predictive value of postsurgical cortisol concentrations for cure and risk of recurrence in Cushing's disease. J Clin Endocrinol Metab 88:5858–5864 [DOI] [PubMed] [Google Scholar]
- 32. Pieters GF, Hermus AR, Meijer E, Smals AG, Kloppenborg PW. 1989. Predictive factors for initial cure and relapse rate after pituitary surgery for Cushing's disease. J Clin Endocrinol Metab 69:1122–1126 [DOI] [PubMed] [Google Scholar]
- 33. Schrell U, Fahlbusch R, Buchfelder M, Riedl S, Stalla GK, Müller OA. 1987. Corticotropin-releasing hormone stimulation test before and after transsphenoidal selective microadenomectomy in 30 patients with Cushing's disease. J Clin Endocrinol Metab 64:1150–1159 [DOI] [PubMed] [Google Scholar]
- 34. Bochicchio D, Losa M, Buchfelder M. 1995. Factors influencing the immediate and late outcome of Cushing's disease treated by transsphenoidal surgery: a retrospective study by the European Cushing's Disease Survey Group. J Clin Endocrinol Metab 80:3114–3120 [DOI] [PubMed] [Google Scholar]
- 35. Sonino N, Zielezny M, Fava GA, Fallo F, Boscaro M. 1996. Risk factors and long-term outcome in pituitary-dependent Cushing's disease. J Clin Endocrinol Metab 81:2647–2652 [DOI] [PubMed] [Google Scholar]