Abstract
Context:
Consumptive hypothyroidism is a rare syndrome resulting from increased catabolism of T4 and T3 by increased type 3 iodothyronine deiodinase (D3) activity. Consumptive hypothyroidism has primarily been described as a paraneoplastic syndrome in infants as well as in two adults with D3-expressing tumors.
Objective:
The aim of the study was to report the third case of consumptive hypothyroidism in an adult and the first in an athyreotic patient.
Design, Setting, and Patient:
We present a 38-yr-old athyreotic female who was euthyroid on a stable therapeutic dose of thyroid hormone for many years and then developed marked hyperthyrotropinemia, coincident with the discovery of large D3-expressing hepatic vascular tumors. The patient also had low serum T3 and elevated serum rT3. Hyperthyrotropinemia transiently worsened after surgical resection of the vascular tumors and then persisted for 3 wk after the operation, despite further increases in levothyroxine therapy.
Intervention:
The patient's vascular tumor and adjacent normal liver parenchyma were probed with a polyclonal antibody directed against D3.
Main Outcome Measures and Results:
D3 immunostaining of the patient's vascular tumor was positive, with no significant immunoreactivity in the adjacent normal hepatic tissue.
Conclusions:
This is the third case report of consumptive hypothyroidism in an adult and the first in an athyreotic individual. This case demonstrates that hyperthyrotropinemia may persist after partial liver resection, possibly from the hepatic resection itself.
Consumptive hypothyroidism is a rare form of hypothyroidism resulting from increased production of type 3 iodothyronine deiodinase (D3) (1), the major enzyme responsible for the inactivating pathway of thyroid hormone. D3 permits tissue-specific regulation of thyroid hormone signaling (2) by converting T4 to rT3 as well as T3 to diiodothyronine. The end-products of both of these conversions are inactive forms of thyroid hormone. Consequently, D3 can reduce the level of active T3 locally, or even systemically. D3 is highly expressed in fetal tissues, the placenta, and the pregnant uterus, where it helps to tightly regulate thyroid hormone signaling in the developing fetus (3). In healthy adults, D3 activity is generally low. However, recent human and animal studies have revealed its increased expression in states of critical illness, myocardial infarction, and chronic inflammation (4–7). In certain patients, this D3 reactivation is thought to contribute to the aberrations in serum thyroid function tests (TFT) known as the euthyroid sick syndrome. Most case reports of high D3 activity in patients who are not critically ill are the result of hemangiomas in infants (8). Based on the MEDLINE/PubMed database, there are only two case reports of increased D3 activity from tumors in adults with associated consumptive hypothyroidism, one due to a malignant fibrous tumor and the other to a hepatic vascular tumor (9, 10). Thus, our case is the third case report of increased D3 activity from a tumor in an adult, and the first in an athyreotic patient.
Case Report
This is a 38-yr-old female with a distant history (1995) of papillary thyroid cancer (status after near-total thyroidectomy and radioactive iodine remnant ablation). She had several dose changes of levothyroxine (LT4) due to weight fluctuations and compliance issues over the next 10 yr. She was documented 2.5 yr before admission to be clinically stable and to have a TSH level of 1.13 mIU/liter on an oral LT4 dose of 200 μg (2 μg/kg) daily. One year before admission, she had a TSH of 1.65 mIU/liter. Two months before the patient's referral to the University of Colorado Hospital, her primary care physician noted a sudden increase of serum TSH to 37.3 mIU/liter, and she was subsequently referred to an endocrinologist. Her endocrinologist noted the unusual rise in TSH but was more concerned with a palpable right upper quadrant abdominal mass that was nontender but measured about 10 cm in diameter. An abdominal ultrasound demonstrated an 8.5 × 8.1 × 7.8 cm heterogeneous right liver lobe mass. To further characterize the lesion, a computerized tomography scan of the abdomen was performed that revealed two large masses essentially replacing the fifth and seventh segments of the liver. Although there was no change in the dosage of LT4, a preoperative TSH was 7.7 mIU/liter (normal, 0.5–5.0 mIU/liter) the day before the patient underwent a partial hepatectomy (comprising the fifth, sixth, and seventh segments). Her surgery was without complication, and she did not require a blood transfusion. Three days after this procedure, the patient was noted to have an episode of supraventricular tachycardia with a pulse of 200 beats per minute. At the time, the patient was on a ward floor, clinically stable, and without significant symptoms of chest pain or shortness of breath. This led to a recheck of her TFT, which showed an increased TSH of 10.6 mIU/liter, with a free T4 (FT4) of 1.06 ng/dl (normal, 0.58–1.64 ng/dl) and a rT3 of 714 pg/ml (normal, 90–350 pg/ml) (Table 1). Her tachycardia was transient and did not recur in the days after the initial episode, so the issue was not pursued further. One week after surgery, and based on her TFT, the patient was discharged home on an increased dose of LT4 of 250 μg/d.
Table 1.
Thyroid function test results (as related to surgical date)
| Postoperative day | −60 | −1 | +3 | +4 | +16 | +17 | +18 | +19 | +21 | +42 | +76 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TSH (0.5–5.0 mIU/liter) | 37.3 | 7.7 | 10.6 | 33.6 | 15.3 | 58.3 | 12.1 | 3.66 | 0.02 | ||
| FT4 (0.58–1.64 ng/dl) | NR | 1.06 | NR | NR | 1.31 | 1.65 | 1.67 | ||||
| TT3 (90–180 ng/dl) | 83 | NR | NR | NR | 38 | 70 | 126 | ||||
| rT3 (90–350 pg/ml) | NR | 714 | NR | NR | NR | 336 | 286 | ||||
| LT4 dose (μg/d) | 200 | 200 | 250 | 250 | 250 | 300 | 225 | 225 | 225 |
NR, No value recorded that day.
The patient was readmitted to the hospital on postoperative d 16, secondary to a deep vein thrombosis. During this second admission, the patient had another episode of supraventricular tachycardia, at which time her TFT were again assessed and the TSH was found to be almost three times higher (33.6 mIU/liter) than the level observed during her first admission. One day later, a recheck of her TSH was 15.3 mIU/liter, which led to an LT4 increase to 300 μg/d, but 2 d later her thyroid function revealed an even higher TSH of 58.3 mIU/liter, with a FT4 of 1.31 ng/dl and a total T3 (TT3) of 38 ng/dl. Throughout her hospital stay, she did not display clinical signs of hypothyroidism or severe illness. She was ambulatory and mentating well, and her physical exam did not reflect a hypothyroid state. Although there were no clinical signs or symptoms to raise suspicion of a pituitary tumor, a biochemical panel was checked (including prolactin, LH, FSH, IGF-I, and cortisol) and was within normal limits, save for a low LH at less than 1 mIU/ml (the patient's menstrual cycle was not documented before discharge). The patient's LT4 dose was decreased to 225 μg daily based on her rising FT4. Two days later, the patient's TT3 increased to 70 ng/dl, and her TSH decreased to 12.05 mIU/liter. At this time, a very low serum thyroglobulin level of 0.3 ng/ml was documented during hyperthyrotropinemia (TSH, 12.05 mIU/liter), confirming the patient's history of thyroidectomy and that endogenous T4 production was insignificant.
The patient was discharged 2 wk after her second admission, and 2 months later her TSH was suppressed at 0.02 mIU/liter, her FT4 was elevated at 1.67 ng/dl, and her TT3 was 126 ng/dl, whereas her rT3 was down to 286 pg/ml (Table 1). The patient has not reported symptomatic changes consistent with hyper- or hypothyroidism after her second hospital discharge.
Materials and Methods
Pathology
Two specimens fixed in formalin were received for pathology examination, designated “right anterior hemangioma” and “right posterior hemangioma.” The specimens were two segments of liver measuring 15 × 8 × 7 cm and 12 × 12 × 7 cm, respectively. In both specimens, below the serosal surface of the liver, there were rounded soft blood-filled tumors. The surgical margins were inked black and the specimens were serially sectioned, revealing hemorrhagic tumors of 14 × 9 × 5.5 cm and 12 × 10 × 4.5 cm. The central portions of these tumors had prominent myxoid changes. The first tumor did not grossly involve the surgical margin, but the second extended to the surgical margins. Representative sections were submitted for paraffin embedding and histological examination. Staining with hematoxylin and eosin of 5-μm sections were performed by conventional procedure.
Immunohistochemistry
Immunohistochemistry was performed as previously described (10), using the D3–18 primary D3 antibody and 5-μm sections cut from formalin-fixed, paraffin-embedded tissue. Sections were incubated with D3–18 at 1:1000 dilution and then processed with the Vecastain Elite ABC immunoperoxidase kit (Vector Laboratories, Inc., Burlingame, CA). Preimmune rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) was used for isotype controls.
Results
Review of the medical literature in a MEDLINE/PubMed database search (1950 to February 2010), using the key words “hypothyroidism” and “type 3 iodothyronine deiodinase” and limiting the search to “adults (19 plus years),” revealed only two case reports of consumptive hypothyroidism (9, 10).
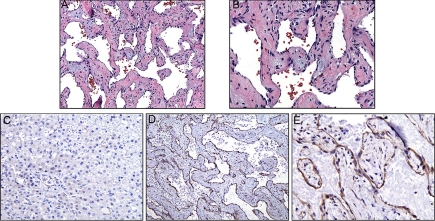
Histopathology examination revealed a well-circumscribed vascular tumor embedded in the hepatic parenchyma. The tumors consisted of irregular blood vessels with cavernous vascular channels lined by flattened endothelial cells and separated by thick fibrous septae (Fig. 1, A and B). Focally, the connective tissue of the vascular walls showed a myxoid appearance. No evidence of atypia or malignancy was observed. Tissue samples were also immunostained for D3, which shows little or no reactivity in the normal, adjacent liver parenchyma (Fig. 1C) but strong, positive staining in the tumor endothelium and focally positive staining in the stromal cells of the tumor vessel walls (Fig. 1, D and E). Unfortunately, fresh tissue was not available to measure deiodinase activity in the vascular malformation or the normal liver.
Fig. 1.
A and B, Histopathology of the vascular tumor (magnification, 10× in A; 20× in B) shows a mesh of irregular blood vessels with cavernous vascular channels lined by flattened endothelial cells and separated by thick fibrous septae. Focally, the fibroconnective tissue of the vascular walls shows a myxoid appearance. There is no evidence of malignancy. C, Immunohistochemistry staining for D3 presence in liver tissue adjacent to vascular malformation. No significant staining is seen in the endothelial or liver cells. D and E, Immunostaining of vascular malformation for presence of D3. Staining is strong in the tumor endothelium and less so in stromal cells (magnification, 10× in D; 20× in E).
Discussion
This report describes the first case of an athyreotic adult who was affected by consumptive hypothyroidism. The large majority of consumptive hypothyroidism cases are reported in infants as a result of hemangiomas (8); however, two case reports in adults have associated this disorder with a hepatic hemangioendothelioma in one case (10) and with an abdominal malignant solitary fibrous tumor in the other (9). Because our patient was solely dependent on exogenous LT4, her alterations in TFT more accurately reflect the presence of consumptive hypothyroidism.
An elevated TSH alone certainly does not confirm the diagnosis of consumptive hypothyroidism. A rising TSH can, of course, be a result of noncompliance to the specific thyroid replacement therapy. This is unlikely in this case because many of our patient's LT4 doses were given while hospitalized with directly observed therapy and were documented as normal over 2 yr before hospitalization. Additionally, her FT4 was rising with increased LT4 doses, suggesting that she was both ingesting and absorbing the drug. In contrast, her increasing TSH in conjunction with a rising FT4 and dropping TT3 suggests a process that primarily decreased the T3, implicating robust D3 activity.
Perhaps the most interesting aspect of this case is the persistence of altered TFT after resection of the D3 overexpressing vascular tumors. Our patient's TSH was elevated on an otherwise stable dose of LT4 before surgery, suggesting increased D3 activity preoperatively. However, her highest TSH and lowest TT3 levels were recorded over 3 wk after her surgery. This suggests the presence of other D3-stimulating factors besides overproduction by the tumors.
One possible explanation stems from the effect of hepatic resection itself. It has been shown that euthyroidism is restored rapidly (within 2 d) after complete liver transplantation in a pediatric patient with consumptive hypothyroidism (11). However, our patient had only a partial resection, and regenerative changes in the liver significantly alter type 1 iodothyronine selenodeiodinase (D1) and D3 activity, as shown by Kester et al. (12) in 2009. They found that after partial hepatectomy in rodents, there was an increase in D3 activity (as high as 40-fold in posthepatectomized mice) in addition to a decrease in D1 activity. This is consistent with the concept that that T3 stimulates cellular differentiation, whereas D3 activity is associated with low intracellular T3 concentrations during cellular proliferation (6, 9). Although the enhanced D3 activity in mice and rats, as reported by Kester et al. (12) was limited to several days, this could potentially translate into a longer time course in humans, as in our patient where a persistently elevated TSH, despite an increased dose of LT4, was present weeks after hepatic surgery, suggesting persistent or increased D3 activity. The idea that humans may have a longer time course of D1 and D3 alterations after hepatic surgery is supported by Pomfret et al. (13), who found that liver regeneration continues throughout the first postoperative year in liver donors. Interestingly, in the Pomfret study, regeneration was significantly slower in females. One would expect that, compared with a complete liver transplant, regenerative changes would be much more significant after a partial hepatectomy. In the pediatric patient who received a complete liver transplant, such changes would be masked by rapid hypothalamic-pituitary-thyroid feedback and compensatory endogenous thyroid hormone secretion.
Although our hypothesis is as yet untested, we posit that the patient's initial consumptive hypothyroidism was a result of her hepatic endothelial tumors, which is supported by overt D3 expression in the immunohistochemistry staining. The condition was exacerbated by her hepatectomy, which led to stimulation of D3 activity by the regenerating liver and a subsequent persistence of her thyroid disorder for at least 3 wk after her surgery. Future studies of serum thyroid function and analysis of D3 expression and activity in human liver parenchyma after injury and/or during repair (such as after partial hepatectomy for other conditions) may help confirm this hypothesis or provide other clues as to the etiology of our patient's persistently elevated TSH in the setting of increasing LT4 dosage and serum thyroid hormone levels.
The patient's most recent TFT suggest that she is slightly overreplaced on a dose of 225 μg daily of LT4. This suggests that she would be euthyroid on her previous dose of 200 μg daily, on which she had been stable for at least 2 yr. Unfortunately, more recent patient follow-up data are not available.
In summary, this represents the first known case of an athyreotic adult with consumptive hypothyroidism. Notably, her hypothyroidism continued for several weeks after resection of the lesion that initially induced her hypothyroidism. The persistence of her altered TFT after surgery may reflect a number of contributing factors, including the effect of hepatic surgery on D1 and D3 activity.
Acknowledgments
This work was supported by Grant DK76099 from the National Institutes of Health.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- D1
- Type 1 iodothyronine selenodeiodinase
- D3
- type 3 iodothyronine deiodinase
- FT4
- free T4
- LT4
- levothyroxine
- TFT
- thyroid function test
- TT3
- total T3.
References
- 1. Huang SA. 2005. Physiology and pathophysiology of type 3 deiodinase in humans. Thyroid 15:875–881 [DOI] [PubMed] [Google Scholar]
- 2. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang SA, Dorfman DM, Genest DR, Salvatore D, Larsen PR. 2003. Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab 88:1384–1388 [DOI] [PubMed] [Google Scholar]
- 4. Peeters RP, Debaveye Y, Fliers E, Visser TJ. 2006. Changes within the thyroid axis during critical illness. Crit Care Clin 22:41–55, vi [DOI] [PubMed] [Google Scholar]
- 5. Boelen A, Mikita J, Boiziau C, Chassande O, Fliers E, Petry KG. 2009. Type 3 deiodinase expression in inflammatory spinal cord lesions in rat experimental autoimmune encephalomyelitis. Thyroid 19:1401–1406 [DOI] [PubMed] [Google Scholar]
- 6. Olivares EL, Marassi MP, Fortunato RS, da Silva AC, Costa-e-Sousa RH, Araújo IG, Mattos EC, Masuda MO, Mulcahey MA, Huang SA, Bianco AC, Carvalho DP. 2007. Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in rats: a time course study. Endocrinology 148:4786–4792 [DOI] [PubMed] [Google Scholar]
- 7. Boelen A, Kwakkel J, Alkemade A, Renckens R, Kaptein E, Kuiper G, Wiersinga WM, Visser TJ. 2005. Induction of type 3 deiodinase activity in inflammatory cells of mice with chronic local inflammation. Endocrinology 146:5128–5134 [DOI] [PubMed] [Google Scholar]
- 8. Huang SA, Tu HM, Harney JW, Venihaki M, Butte AJ, Kozakewich HP, Fishman SJ, Larsen PR. 2000. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med 343:185–189 [DOI] [PubMed] [Google Scholar]
- 9. Ruppe MD, Huang SA, Jan de Beur SM. 2005. Consumptive hypothyroidism caused by paraneoplastic production of type 3 iodothyronine deiodinase. Thyroid 15:1369–1372 [DOI] [PubMed] [Google Scholar]
- 10. Huang SA, Fish SA, Dorfman DM, Salvatore D, Kozakewich HP, Mandel SJ, Larsen PR. 2002. A 21-year-old woman with consumptive hypothyroidism due to a vascular tumor expressing type 3 iodothyronine deiodinase. J Clin Endocrinol Metab 87:4457–4461 [DOI] [PubMed] [Google Scholar]
- 11. Balazs AE, Athanassaki I, Gunn SK, Tatevian N, Huang SA, Haymond MW, Karaviti LP. 2007. Rapid resolution of consumptive hypothyroidism in a child with hepatic hemangioendothelioma following liver transplantation. Ann Clin Lab Sci 37:280–284 [PubMed] [Google Scholar]
- 12. Kester MH, Toussaint MJ, Punt CA, Matondo R, Aarnio AM, Darras VM, Everts ME, de Bruin A, Visser TJ. 2009. Large induction of type III deiodinase expression after partial hepatectomy in the regenerating mouse and rat liver. Endocrinology 150:540–545 [DOI] [PubMed] [Google Scholar]
- 13. Pomfret EA, Pomposelli JJ, Gordon FD, Erbay N, Lyn Price L, Lewis WD, Jenkins RL. 2003. Liver regeneration and surgical outcome in donors of right-lobe liver grafts. Transplantation 76:5–10 [DOI] [PubMed] [Google Scholar]