Abstract
Background:
Ankle fractures are not typically considered osteoporotic fractures. However, bone quality in patients with low trauma ankle fractures has not been explored.
Methods:
Women with (n = 17) and without (n = 112) a history of low trauma ankle fracture after menopause had areal bone mineral density measured by dual-energy x-ray absorptiometry, trabecular (Tb) and cortical volumetric bone mineral density, and Tb microarchitecture measured by high-resolution peripheral computed tomography of the radius and tibia. Finite element analysis was performed to estimate bone stiffness.
Results:
Women with fractures were older (72 ± 2 vs. 68 ± 1 yr; P < 0.02) but similar with respect to race and body mass index. Mean T-scores by dual-energy x-ray absorptiometry of fracture subjects were above the osteoporotic range and did not differ from controls. By high-resolution peripheral computed tomography at the radius, fracture subjects had preferentially lower central trabecular bone density, lower Tb number, and increased separation compared with controls (P < 0.0001–0.04). At the tibia, fracture subjects had lower total and Tb density, lower Tb number, and increased Tb separation and network heterogeneity (P < 0.02). Whole-bone stiffness was 13–17% lower at the radius and tibia in fracture subjects (P < 0.003–0.01).
Conclusions:
Postmenopausal women with ankle fractures have disrupted microarchitecture and decreased stiffness compared with women with no fracture history, suggesting that low trauma ankle fractures should be considered similarly to other classical osteoporotic fractures.
Osteoporosis, a skeletal disorder characterized by compromised bone strength and susceptibility to fracture (1), is increasing in prevalence as the population ages. It has been estimated that 10.5 million women and 3.3 million men will be affected by osteoporosis by the year 2020 (2). More than 2 million osteoporotic fractures occur each year (3); these fractures are associated with significant morbidity, mortality, and health care costs (4–6). Many structural and material properties contribute to overall bone strength and fracture susceptibility (7). Bone strength is governed in large part by the amount of bone present, which can be assessed by dual-energy x-ray absorptiometry (DXA) measurements of bone mineral density (BMD). However, half of all postmenopausal fractures occur in women with BMD values above the World Health Organization threshold for osteoporosis (8, 9), highlighting the need for other modalities to assess fracture risk.
Ankle fractures are not typically considered to be osteoporotic fractures, based on observations that areal BMD (aBMD) by DXA does not differ in patients with and without fractures (10, 11). However, skeletal microarchitecture and other measures of bone quality have not been explored in patients with low trauma ankle fractures. Recently several novel tools for evaluation of bone quality have emerged. High-resolution peripheral quantitative computed tomography (HR-pQCT; Xtreme CT; Scanco Medical, Bassersdorf, Switzerland) is a noninvasive, three-dimensional imaging technique, which provides a measurement of true volumetric BMD (vBMD) of the distal radius and tibia. The high resolution of this technique, with its isotropic voxel size of about 82 μm, permits it to detect microarchitectural changes that are associated with increased bone fragility (12, 13). HR-pQCT can distinguish between cortical and cancellous bone and visualize fine details of trabecular microarchitecture. Computed tomography data sets from individual scans can be computationally modeled by microstructural finite element analysis to assess bone mechanical competence (stiffness), a surrogate for strength.
Several studies have used HR-pQCT to detail differences in microarchitecture and stiffness in subjects with a history of osteoporotic fracture (14–19). Microarchitectural deterioration has been described in postmenopausal women with fractures at the wrist (15, 17, 19), spine (20), hip (17), and heterogeneous fracture locations (14, 16, 18). In this study, we hypothesized that women with postmenopausal ankle fractures have abnormal bone quality and that HR-pQCT would reveal differences in microarchitecture and strength when compared with women with no history of fracture. If this hypothesis is correct, ankle fractures should be investigated further for their significance as an early indicator of bone fragility and increased risk of future fracture.
Patients and Methods
Patients
Postmenopausal women, over age 60 yr or more than 10 yr past menopause, were recruited at Columbia University Medical Center (New York, NY) or Helen Hayes Hospital (West Haverstraw, NY) by advertisement, self- or physician referral. Subjects were eligible for inclusion as fracture cases if they had a documented history of a low trauma ankle fracture that occurred after menopause. Low trauma was defined as equivalent to a fall from a standing height or less. Fractures were confirmed by review of radiographs or radiograph report when possible. Control subjects had no history of low trauma fractures at any site and no vertebral deformity on lateral radiograph, as dictated by prespecified exclusion criteria. There were no BMD requirements for inclusion. Potential cases and controls were excluded if they had endocrinopathies (e.g. untreated hyperthyroidism, Cushing's syndrome, prolactinoma); celiac or other gastrointestinal diseases; abnormal mineral metabolism (e.g. osteomalacia, primary hyperparathyroidism); malignancy except for skin cancer; and drug exposures that could affect bone metabolism (e.g. glucocorticoids, anticonvulsants, anticoagulants, methotrexate, aromatase inhibitors, thiazolidinediones). Women using hormone replacement therapy or raloxifene were permitted to participate. Women who had ever used teriparatide or who had taken bisphosphonates for more than 1 yr were excluded. All subjects provided written informed consent, and the Institutional Review Board of Columbia University Medical Center approved this study. At the study visit, past medical history, reproductive history, and medication use were assessed. A physical examination was performed including height by Harpenden stadiometer and weight. Dietary intake of calcium and vitamin D was assessed using a modified version of the Block food frequency questionnaire (21).
Of 261 women screened, 129 were eligible and agreed to participate. The most common reasons for exclusion were bisphosphonate use for longer than 1 yr (18%), subject preference not to participate (7%), age younger than 60 yr (5%), glucocorticoid use (3%), primary hyperparathyroidism (2%), or inability to be properly positioned in the HR-pQCT scanner (2%).
Areal bone mineral density
aBMD was measured by DXA (QDR-4500; Hologic Inc., Walton, MA, at Columbia University Medical Center; Lunar Prodigy; GE, Madison, WI, at Helen Hayes Hospital) of the lumbar spine (LS), total hip (TH), femoral neck (FN), one third radius (1/3R), and ultradistal radius (UDR). T-scores compared subjects and controls with young-normal populations of the same race and sex, as provided by the manufacturer.
HR-pQCT of the distal radius and tibia
HR-pQCT (XtremeCT; Scanco Medical) was performed by immobilizing the nondominant forearm and distal tibia (or nonfractured leg in subjects with prior ankle fracture) in a carbon fiber shell and scanning as described (14, 16, 22). The region of interest was defined on a scout film by manual placement of a reference line at the end plate of the radius or tibia, with the first slice 9.5 and 22.5 mm proximal to the reference line at the radius and tibia, respectively. A stack of 110 parallel computed tomography slices was acquired at the distal end of both sites using an effective energy of 40 keV, image matrix size 1024 × 1024, with a nominal voxel size of 82 μm. This provided a three-dimensional image of approximately 9 mm in the axial direction. Attenuation data were converted to equivalent hydroxyapatite (HA) densities. The European Forearm Phantom was scanned regularly for quality control.
The analysis methods have been described, validated (23–25), and applied in several recent clinical studies (14–17, 19, 20, 26, 27). Briefly, the volume of interest was automatically separated into cortical and trabecular regions using a threshold-based algorithm set to one third the apparent cortical bone density (Dcort). Mean cortical thickness was defined as the mean cortical volume divided by the outer bone surface. Trabecular bone density (Dtrab) was defined as the average bone density within the trabecular volume of interest, and bone volume fraction (BV/TVd) (percent) derived from Dtrab, assuming the density of fully mineralized bone was 1.2 g hydroxyapatite per cubic centimeter (BV/TVd = 100 × Dtrab per 1200 mg hydroxyapatite per cubic centimeter). Inner trabecular density (Dinn) was defined as the inner 60%, and the metatrabecular density (Dmeta) as the outer 40% of the trabecular region. Because measurements of trabecular microstructure are limited by the resolution of the XtremeCT (Scanco Medical), which approximates the width of individual trabeculae, trabecular structure was assessed using a semiderived algorithm (12, 23). Trabeculae were identified by a medial-axis transformation method and the distance between them assessed by the distance-transform method (28, 29). Trabecular number (Tb.N*) was defined as the inverse of the mean spacing of the medial axes. Trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp) were then derived from BV/TVd and Tb.N* using formulae from traditional quantitative histomorphometry: Tb.Th = (BV/TVd)/Tb.N* and Tb.Sp = (1 − BV/TVd)/Tb.N*.
HR-pQCT image-based microstructural finite element analysis
HR-pQCT data were used to calculate whole bone stiffness and trabecular bone stiffness, surrogate measures of bone's resistance to force, as we have previously described (24). First, the mineralized phase was thresholded automatically by using a Laplace-Hamming filter followed by global threshold using a fixed value of 40% of maximal gray-scale value of the images. Then each thresholded HR-pQCT image of the distal radius or distal tibia was converted to a microstructural finite element model by directly converting bone voxels to eight-node elastic brick elements with an element size of 82 × 82 × 82 μm3. Bone tissue was modeled as an isotropic, linearly elastic material with a Young's modulus (Es) of 15 GPa and a Poisson's ratio of 0.3 (30). A uniaxial displacement equaling 1% of the bone segment height was applied perpendicularly to the distal surface of the radius or tibia, whereas the proximal surface was imposed with zero displacement along the same direction. Both ends of the tibia were allowed to expand freely in the transverse plane. The total reaction force was calculated from the linear microstructural finite element analysis, and the axial stiffness was calculated as the reaction force divided by the imposed displacement. Similarly, trabecular bone stiffness was calculated for the trabecular bone compartment after removing the cortex.
Statistical methods
Analyses were conducted with STATA version 9.0 (Stata Corp, College Station, TX) and SAS version 9.1 (SAS Institute Inc., Cary, NC). Two-sided P < 0.05 was considered to indicate statistical significance. Descriptive data are presented as mean ± sd and group comparisons as mean ± sem. Differences between fracture and nonfracture subjects were assessed by Student's t test or χ2. Normality testing (Kolmogorov-Smirnov) was performed, and for variables that were not normally distributed, a nonparametric one-way analysis of ranks (Wilcoxon) was used to assess differences between the fracture and nonfracture groups. analysis of covariance was used to evaluate differences in HR-pQCT parameters at the radius or tibia after adjustment for age, body mass index (BMI), and aBMD T-score at the ultradistal radius for radial HR-pQCT comparisons or total hip for tibial HR-pQCT comparisons.
Results
Of 129 women enrolled, 17 had a history of low trauma ankle fracture after menopause. Women enrolled were ambulatory and generally in good health. Women with fractures were on average 4 yr older (72 ± 2 vs. 68 ± 1 yr; P < 0.02) and thus had a greater number of years since menopause (24 ± 2 vs. 19 ± 1; P < 0.03) but were similar to controls with respect to race, BMI, and medical conditions including hypertension, heart disease, and thyroid disease. None of the fracture subjects were diabetic, compared with 18% of controls (P < 0.08). Mean duration of diabetes in these subjects was 11 ± 7 yr. Family history of osteoporosis and fractures, alcohol and tobacco use, and medication and supplement use, notably use of calcium and vitamin D supplements, T4, hormone replacement therapy, raloxifene, and bisphosphonates, did not differ between the groups (Table 1).
Table 1.
Subject characteristics
| Fracture (n = 17) | Control (n = 112) | P value | |
|---|---|---|---|
| Age (yr) | 72 ± 2 | 68 ± 1 | 0.02 |
| BMI | 25.4 ± 1.1 | 26.4 ± 0.5 | 0.46 |
| Race (percent Caucasian) | 82 | 83 | 0.94 |
| African American (%) | 6 | 7 | |
| Other (%) | 12 | 10 | |
| Ethnicity (percent Hispanic) | 24 | 18 | 0.54 |
| Non-Hispanic (%) | 76 | 81 | |
| Height (cm) | 160 ± 2 | 161 ± 1 | 0.9 |
| Weight (kg) | 65 ± 3 | 68 ± 1 | 0.44 |
| Years since menopause | 24 ± 2 | 19 ± 1 | 0.03 |
| Oophorectomy (%) | 13 | 17 | 0.71 |
| Family history of osteoporosis (%) | 36 | 43 | 0.77 |
| Family history of fracture (%) | 40 | 37 | 0.81 |
| Tobacco use (pack-years) | 22 ± 9 | 22 ± 3 | 0.97 |
| Never (%) | 47 | 43 | 0.79 |
| Former (%) | 53 | 55 | |
| Current (%) | 0 | 1 | |
| Alcohol use (beverages per day) | 1 ± 0 | 1 ± 0 | 0.98 |
| Medication use | |||
| Calcium supplements [total daily dose (mg)] | 533 ± 145 | 610 ± 57 | 0.61 |
| Vitamin D supplements [total daily dose (IU)] | 725 ± 212 | 975 ± 176 | 0.37 |
| Hormone replacement therapy [never (%)] | 59 | 62 | 0.72 |
| Past (%) | 29 | 32 | |
| Current (%) | 12 | 6 | |
| Bisphosphonates [never (%)] | 82 | 93 | 0.14 |
| Past (%) | 12 | 6 | |
| Current (%) | 6 | 1 | |
| Raloxifene (%) | 6 | 2 | 0.38 |
| T4 (%) | 27 | 20 | 0.52 |
| aBMD by DXA | |||
| LS (g/cm2) | 0.911 ± 0.021 | 0.927 ± 0.014 | 0.53 |
| TH (g/cm2) | 0.809 ± 0.028 | 0.809 ± 0.012 | 0.99 |
| FN (g/cm2) | 0.674 ± 0.022 | 0.684 ± 0.009 | 0.69 |
| 1/3R (g/cm2) | 0.607 ± 0.024 | 0.613 ± 0.007 | 0.77 |
| UDR (g/cm2) | 0.354 ± 0.016 | 0.379 ± 0.006 | 0.15 |
Almost all fractures occurred in the setting of a fall (90%). Falls occurred when subjects tripped on the street (60%) or on the stairs (40%); two subjects slipped on ice and one caught her foot between seats at the theater. The average time between fracture and study evaluation was 9 ± 2 yr. The majority of fractures were distal fibular fractures (41%). Five fracture subjects (29%) also had sustained fractures at other sites, including forearm, ribs, spine, and metatarsals. In these subjects, ankle fractures preceded those at other sites by 11 yr on average.
Only 35% of women with fractures and 28% of controls had osteoporosis by DXA at any site.
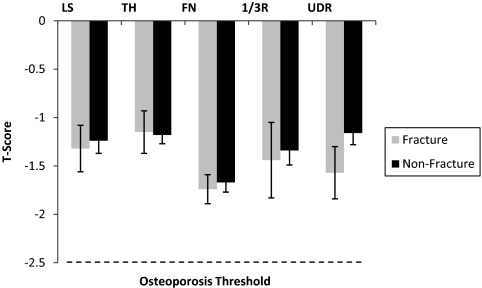
Mean T-scores of women with fractures were well above the osteoporotic range (LS: −1.4 ± 0.2; TH: −1.2 ± 0.2; FN: −1.8 ± 0.2; 1/3R: −1.4 ± 0.3; UDR: −1.6 ± 0.3) and did not differ from controls (Fig. 1). In contrast to DXA measurements, HR-pQCT and finite element analysis measurements at both the radius and tibia differed significantly between women with and without ankle fractures (Table 2). At the radius, after adjustment for age, BMI, and T-score at the ultradistal radius, women with fractures had preferentially fewer central (inner) trabeculae relative to outer subcortical trabeculae (Dmeta/Dinn) compared with those without fractures (Fig. 2). Women with fractures also had lower trabecular number and greater trabecular separation and network heterogeneity.
Fig. 1.
Comparison of T-scores by DXA at the (LS), TH, FN, 1/3R, and UDR in women with (gray bars) and without (black bars) postmenopausal ankle fractures. Mean values ± se are shown. T-scores were well above −2.5 the osteoporosis threshold (dashed line) and did not differ between the groups.
Table 2.
Microarchitecture and stiffness by fracture history (mean ± se)
| Fracture | Nonfracture | P value | Adjusted Pa | |
|---|---|---|---|---|
| Radius | ||||
| Total density (mg HA/cm3) | 272 ± 13 | 299 ± 7 | 0.17 | 0.31 |
| Dcort (mg HA/cm3) | 828 ± 18 | 853 ± 7 | 0.2 | 0.78 |
| Ct.Th (μm) | 632 ± 3 | 724 ± 2 | 0.07 | 0.17 |
| Trabecular density (mg HA/cm3) | 118 ± 11 | 131 ± 4 | 0.23 | 0.17 |
| Outer/inner trabecular density | 18.4 ± 11.1 | 2.6 ± 0.1 | 0.17 | 0.0007 |
| Tb.N* (1/mm) | 1.56 ± 0.11 | 1.77 ± 0.03 | 0.04 | 0.03 |
| Tb.Sp (μm) | 664 ± 82 | 538 ± 19 | 0.15 | 0.04 |
| Tb.Th (μm) | 63 ± 3 | 61 ± 1 | 0.49 | 0.44 |
| Network heterogeneity (μm) | 417 ± 103 | 273 ± 23 | 0.19 | 0.052 |
| Whole-bone stiffness (kN/mm) | 58.5 ± 3.1 | 69.9 ± 1.7 | 0.02 | 0.01 |
| Trabecular bone stiffness (kN/mm) | 11.5 ± 2.1 | 16.3 ± 0.9 | 0.07 | 0.07 |
| Tibia | ||||
| Total density (mg HA/cm3) | 216 ± 12 | 244 ± 5 | 0.04 | 0.02 |
| Dcort (mg HA/cm3) | 743 ± 23 | 785 ± 6 | 0.03 | 0.07 |
| Ct.Th (μm) | 711 ± 69 | 860 ± 26 | 0.04 | 0.07 |
| Trabecular density (mg HA/cm3) | 132 ± 9 | 146 ± 3 | 0.11 | 0.02 |
| Outer/inner trabecular density | 3.4 ± 0.8 | 2.5 ± 0.2 | 0.29 | 0.07 |
| Tb.N* (1/mm) | 1.58 ± 0.08 | 1.74 ± 0.03 | 0.07 | 0.01 |
| Tb.Sp (μm) | 588 ± 33 | 527 ± 12 | 0.05 | 0.01 |
| Tb.Th (μm) | 69 ± 4 | 71 ± 1 | 0.6 | 0.52 |
| Network heterogeneity (μm) | 320 ± 44 | 255 ± 12 | 0.07 | 0.02 |
| Whole-bone stiffness (kN/mm) | 176.4 ± 10.0 | 204.9 ± 4.2 | 0.01 | 0.003 |
| Trabecular bone stiffness (kN/mm) | 83.0 ± 7.2 | 100.3 ± 3.3 | 0.053 | 0.02 |
Adjusted for age, BMI, and aBMD.
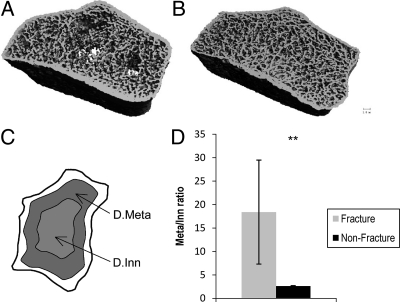
Fig. 2.
Loss of central trabecular bone at the radius in women with ankle fractures. Radius image from a fracture subject (A) shows preferential loss of inner trabecular bone compared with a control (B). Demarcation of inner and outer trabecular bone compartments in radial image (C) are shown. Significantly lower ratio of outer (Dmeta) to inner (Dinn) trabecular bone in women with and without fractures (D). Mean values ± se are shown. **, P < 0.01.
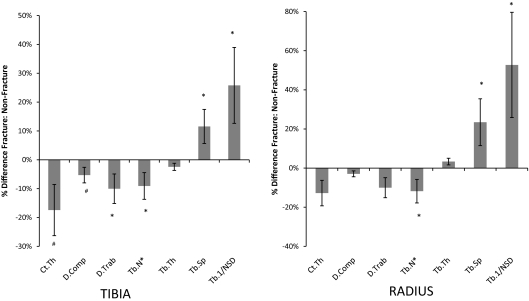
At the tibia, after adjustment for age, BMI, and T-score at the total hip, women with fractures had lower total density and trabecular density and a trend toward lower cortical density. Women with fractures had lower central (inner) trabecular density relative to subcortical (outer) trabecular density compared with those without fractures, but interestingly, this difference was less pronounced than at the radius. Cortical thickness tended to be lower. Trabecular number was lower, and trabecular separation and network heterogeneity were greater in women with fractures (Fig. 3).
Fig. 3.
Comparison of the percentage difference (±se) in HR-pQCT measurements between fracture and nonfracture subjects at the tibia (left panel) and radius (right panel). *, P < 0.05; #, P < 0.1. D.comp, Cortical density; Tb.1/NSD, network heterogeneity.
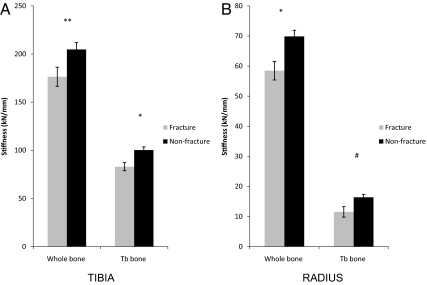
Bone stiffness, estimated by finite element analysis, was reduced in fracture subjects at both sites. At the radius, fracture subjects had lower whole-bone stiffness and tended to have lower trabecular bone stiffness. At the tibia, both whole-bone and trabecular bone stiffness were lower in fracture subjects (Fig. 4). Cortical load share did not differ between fracture subjects and controls at either site.
Fig. 4.
Comparison of whole bone and trabecular (Tb) bone stiffness in women with and without fractures at the tibia (A) and radius (B). #, P < 0.1; *, P < 0.05; **, P < 0.01.
Discussion
In this study, we found that women with postmenopausal ankle fractures had lower vBMD, disrupted trabecular microarchitecture, and decreased stiffness compared with women without fractures. That we found changes in microstructure and stiffness at the radius as well as the tibia suggests that ankle fractures are evidence of a generalized deficit in bone strength and quality and provide support for considering them to be indicative of fragility. In contrast to the changes detected by HR-pQCT, aBMD in women with ankle fractures did not differ from BMD in women with no fracture history.
Other authors have described similar microarchitectural differences in groups of subjects with other fractures classically associated with osteoporosis. Studies examining postmenopausal women with fragility fractures at multiple sites have reported lower vBMD, thinner cortices, and fewer, thinner, more widely, and unevenly spaced trabeculae in fracture subjects compared with controls (16, 18). Similar differences have been seen in studies of single fracture types. Postmenopausal women with wrist fractures had lower vBMD, microstructural deterioration, and reduced estimated bone strength (15, 19, 26). Women with vertebral fractures had altered cortical and trabecular microarchitecture at both the radius and tibia; the number and severity of vertebral fractures was associated with differences in cortical microarchitecture (20). Women with hip fractures had lower vBMD and worse microarchitecture, particularly of cortical bone parameters compared with controls (17).
Although we found that differences between women with ankle fractures and controls were most pronounced at the tibia in this study, there was evidence of microarchitectural deterioration and decreased strength at the radius as well. In studies that included subjects with various types of fragility fractures, most (18, 20) but not all (14) have found pronounced differences at both radius and tibia. In the majority of studies that have examined microstructure and strength in patients with wrist fractures; however, tibial properties have not been examined (19, 26, 31). In patients with hip (17) and vertebral (20) fractures, differences at both sites have been found.
The observed microarchitectural abnormalities provide a mechanism for the reductions in whole-bone and trabecular bone stiffness found in women with ankle fractures. Decreased stiffness has been reported in postmenopausal women with other types of osteoporotic fractures, including cohorts with multiple fracture types (18, 32) and specifically among wrist fracture patients (19, 26). The reductions in stiffness that we observed at both the radius and tibia, albeit with a small sample size, again suggest a generalized decrease in bone quality and imply that such women may prove to be susceptible to fracture at other sites.
Several previous studies have suggested that ankle fractures are not osteoporotic fractures. This opinion was largely based on the observation that BMD was similar in subjects with and without ankle fractures (11, 33, 34). In agreement with prior studies, we did not find an association between aBMD and ankle fractures. Microarchitecture and stiffness, which we found to be substantially different in women with and without fractures, were not assessed in these other studies. Our results suggest that women with postmenopausal ankle fractures have underlying bone fragility despite relatively normal BMD. Recent work has shown that ankle fractures increase the risk of subsequent fractures at multiple sites (35). Although we did not have longitudinal data necessary to evaluate prediction of other types of fracture, we did find that almost one third of patients with ankle fractures had fractures at other sites, primarily fractures of the appendicular skeleton. It is notable that in all of these subjects, ankle fractures preceded fractures at other sites by several years.
Clinical risk factors for ankle fractures that have been reported by other authors include diabetes (35–37), high BMI (10), falls (35), and tobacco use (38). Increased physical activity is associated with risk of ankle fracture, with higher rates described with strenuous activity and increased time spent outdoors (11). We did not find differences in these factors, possibly because of the small number of subjects with ankle fractures that we evaluated compared with the populations in these epidemiological studies. It has also been shown that although men have higher rates of ankle fractures than young adults, women have higher rates between the ages of 50 and 70 yr (39–41). If, as our results suggest, these fractures reflect fragility, the question arises as to why incidence of ankle fractures does not continue to rise with advancing age. It is conceivable that because ankle fractures are increased in subjects with greater levels of physical activity their rates decline as patients become less mobile with age. Further support for the idea that ankle fractures are associated with activity is the finding that the risk of ankle fracture is lower after a hip fracture (35). Due to constraints of our study design, we were unable to assess physical activity at the time of fracture occurrence.
Limitations of this work include the cross-sectional design, which precluded the assessment of microstructure and strength at the time of fracture occurrence. Many of the fractures took place several years before study evaluation. Other limitations are the small number of ankle fractures and our method for subject selection, which was not random, both of which may restrict the generalizability of our results. Despite the long duration between time of fracture and evaluation for some subjects and small number of subjects, we detected many significant differences in microstructure and strength between groups. The majority of fractures involved the fibula, and HR-pQCT does not assess microstructure at this site. However, we and other authors have reported that changes at the distal radius and tibia discriminate subjects with fragility fractures at multiple central and peripheral sites (16–18, 20). We have also recently reported that stiffness at the radius and tibia correlates well with stiffness at central sites (42). Furthermore, our finding of differences at both the radius and tibia suggest global skeletal fragility and provides evidence that these fractures are in fact indicative of osteoporosis.
In conclusion, we found that postmenopausal women with ankle fractures have microarchitectural deterioration and decreased stiffness compared with women with no history of fracture. The presence of these changes at both the radius and tibia provides evidence for a generalized decrease in bone quality in women with ankle fractures. These findings bring into question the current perception that ankle fractures are not related to osteoporosis and call for further, larger studies that investigate whether ankle fractures predict future osteoporotic fractures. They suggest that low trauma ankle fractures after menopause should prompt the same evaluation and treatment as classic osteoporotic fractures.
Acknowledgments
This work was supported by National Institutes of Health Grants U01 AR055968 (to E.S.), K23 DK084337 (to E.M.S.), and R01 AR051376 (to X.E.G.) and the Thomas L. Kempner and Katheryn C. Patterson Foundation.
Disclosure Summary: The authors have no conflict of interest.
Footnotes
- aBMD
- Areal BMD
- BMD
- bone mineral density
- BMI
- body mass index
- BV/TVd
- bone volume fraction
- Ct.Th
- cortical thickness
- Dinn
- inner trabecular density
- Dmeta
- metatrabecular density
- Dtrab
- trabecular bone density
- DXA
- dual-energy x-ray absorptiometry
- FN
- femoral neck
- HA
- hydroxyapatite
- HR-pQCT
- high-resolution peripheral quantitative computed tomography
- LS
- lumbar spine
- 1/3R
- one third radius
- Tb. Th
- trabecular thickness
- Tb.N*
- trabecular number
- Tb.Sp
- trabecular separation
- TH
- total hip
- UDR
- ultradistal radius
- vBMD
- volumetric BMD.
References
- 1. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy 2001. Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–79511176917 [Google Scholar]
- 2. National Osteoporosis Foundation 2002. America's bone health: the state of osteoporosis and low bone mass in our nation. Washington, DC: National Osteoporosis Foundation; 1–55 [Google Scholar]
- 3. U.S. Department of Health and Human Services 2004. Bone health and osteoporosis: a report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General [Google Scholar]
- 4. Hannan EL, Magaziner J, Wang JJ, Eastwood EA, Silberzweig SB, Gilbert M, Morrison RS, McLaughlin MA, Orosz GM, Siu AL. 2001. Mortality and locomotion 6 months after hospitalization for hip fracture: risk factors and risk-adjusted hospital outcomes. JAMA 285:2736–2742 [DOI] [PubMed] [Google Scholar]
- 5. Cummings SR, Melton LJ. 2002. Epidemiology and outcomes of osteoporotic fractures. Lancet 359:1761–1767 [DOI] [PubMed] [Google Scholar]
- 6. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. 2007. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22:465–475 [DOI] [PubMed] [Google Scholar]
- 7. Cheung AM, Detsky AS. 2008. Osteoporosis and fractures: missing the bridge? JAMA 299:1468–1470 [DOI] [PubMed] [Google Scholar]
- 8. Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR. 2003. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 18:1947–1954 [DOI] [PubMed] [Google Scholar]
- 9. Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JP, Pols HA. 2004. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 34:195–202 [DOI] [PubMed] [Google Scholar]
- 10. Greenfield DM, Eastell R. 2001. Risk factors for ankle fracture. Osteoporos Int 12:97–103 [DOI] [PubMed] [Google Scholar]
- 11. Seeley DG, Kelsey J, Jergas M, Nevitt MC. 1996. Predictors of ankle and foot fractures in older women. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 11:1347–1355 [DOI] [PubMed] [Google Scholar]
- 12. Laib A, Häuselmann HJ, Rüegsegger P. 1998. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care 6:329–337 [PubMed] [Google Scholar]
- 13. Laib A, Rüegsegger P. 1999. Comparison of structure extraction methods for in vivo trabecular bone measurements. Comput Med Imaging Graph 23:69–74 [DOI] [PubMed] [Google Scholar]
- 14. Boutroy S, Bouxsein ML, Munoz F, Delmas PD. 2005. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90:6508–6515 [DOI] [PubMed] [Google Scholar]
- 15. Melton LJ, 3rd, Riggs BL, van Lenthe GH, Achenbach SJ, Müller R, Bouxsein ML, Amin S, Atkinson EJ, Khosla S. 2007. Contribution of in vivo structural measurements and load/strength ratios to the determination of forearm fracture risk in postmenopausal women. J Bone Miner Res 22:1442–1448 [DOI] [PubMed] [Google Scholar]
- 16. Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. 2007. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res 22:425–433 [DOI] [PubMed] [Google Scholar]
- 17. Vico L, Zouch M, Amirouche A, Frère D, Laroche N, Koller B, Laib A, Thomas T, Alexandre C. 2008. High-resolution pQCT analysis at the distal radius and tibia discriminates patients with recent wrist and femoral neck fractures. J Bone Miner Res 23:1741–1750 [DOI] [PubMed] [Google Scholar]
- 18. Stein EM, Liu XS, Nickolas TL, Cohen A, Thomas V, McMahon DJ, Zhang C, Yin PT, Cosman F, Nieves J, Guo XE, Shane E. 2010. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res 25:2296–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. 2008. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res 23:392–399 [DOI] [PubMed] [Google Scholar]
- 20. Sornay-Rendu E, Cabrera-Bravo JL, Boutroy S, Munoz F, Delmas PD. 2009. Severity of vertebral fractures is associated with alterations of cortical architecture in postmenopausal women. J Bone Miner Res 24:737–743 [DOI] [PubMed] [Google Scholar]
- 21. Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. 2001. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America's Table Study. Am J Epidemiol 154:1089–1099 [DOI] [PubMed] [Google Scholar]
- 22. Cohen A, Dempster DW, Müller R, Guo XE, Nickolas TL, Liu XS, Zhang XH, Wirth AJ, van Lenthe GH, Kohler T, McMahon DJ, Zhou H, Rubin MR, Bilezikian JP, Lappe JM, Recker RR, Shane E. 2010. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos Int 21:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laib A, Rüegsegger P. 1999. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone 24:35–39 [DOI] [PubMed] [Google Scholar]
- 24. Liu XS, Zhang XH, Sekhon KK, Adam MF, McMahon DJ, Shane E, Bilezikian JP, Guo XE. 2010. High-resolution peripheral quantitative tomography can assess micorstructural and mechanical properties of human distal tibial bone. J Bone Miner Res 25:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. MacNeil JA, Boyd SK. 2007. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys 29:1096–1105 [DOI] [PubMed] [Google Scholar]
- 26. Melton LJ, 3rd, Christen D, Riggs BL, Achenbach SJ, Müller R, van Lenthe GH, Amin S, Atkinson EJ, Khosla S. 2009. Assessing forearm fracture risk in postmenopausal women. Osteoporos Int 21:1161–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S. 2008. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res 23:205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hildebrand T, Ruegsegger P. 1997. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc 185:67–75 [Google Scholar]
- 29. Laib A, Hildebrand T, Häuselmann HJ, Rüegsegger P. 1997. Ridge number density: a new parameter for in vivo bone structure analysis. Bone 21:541–546 [DOI] [PubMed] [Google Scholar]
- 30. Guo XE, Goldstein SA. 1997. Is trabecular bone tissue different from cortical bone tissue? Forma 12:185–196 [Google Scholar]
- 31. Melton LJ, 3rd, Riggs BL, Keaveny TM, Achenbach SJ, Hoffmann PF, Camp JJ, Rouleau PA, Bouxsein ML, Amin S, Atkinson EJ, Robb RA, Khosla S. 2007. Structural determinants of vertebral fracture risk. J Bone Miner Res 22:1885–1892 [DOI] [PubMed] [Google Scholar]
- 32. Vilayphiou N, Boutroy S, Sornay-Rendu E, Van Rietbergen B, Munoz F, Delmas PD, Chapurlat R. 2010. Finite element analysis performed on radius and tibia HR-pQCT images and fragility fractures at all sites in postmenopausal women. Bone 46:1030–1037 [DOI] [PubMed] [Google Scholar]
- 33. Guggenbuhl P, Meadeb J, Chalès G. 2005. Osteoporotic fractures of the proximal humerus, pelvis, and ankle: epidemiology and diagnosis. Joint Bone Spine 72:372–375 [DOI] [PubMed] [Google Scholar]
- 34. Hasselman CT, Vogt MT, Stone KL, Cauley JA, Conti SF. 2003. Foot and ankle fractures in elderly white women. Incidence and risk factors. J Bone Joint Surg Am 85-A:820–824 [DOI] [PubMed] [Google Scholar]
- 35. Taylor AJ, Gary LC, Arora T, Becker DJ, Curtis JR, Kilgore ML, Morrisey MA, Saag KG, Matthews R, Yun H, Smith W, Delzell E. 2011. Clinical and demographic factors associated with fractures among older Americans. Osteoporos Int 22:1263–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holmberg AH, Johnell O, Nilsson PM, Nilsson J, Berglund G, Akesson K. 2006. Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women. Osteoporos Int 17:1065–1077 [DOI] [PubMed] [Google Scholar]
- 37. Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL. 2006. Risk of fracture in women with type 2 diabetes: the Women's Health Initiative Observational Study. J Clin Endocrinol Metab 91:3404–3410 [DOI] [PubMed] [Google Scholar]
- 38. Valtola A, Honkanen R, Kröger H, Tuppurainen M, Saarikoski S, Alhava E. 2002. Lifestyle and other factors predict ankle fractures in perimenopausal women: a population-based prospective cohort study. Bone 30:238–242 [DOI] [PubMed] [Google Scholar]
- 39. Court-Brown CM, McBirnie J, Wilson G. 1998. Adult ankle fractures—an increasing problem? Acta Orthop Scand 69:43–47 [DOI] [PubMed] [Google Scholar]
- 40. Daly PJ, Fitzgerald RH, Jr, Melton LJ, Ilstrup DM. 1987. Epidemiology of ankle fractures in Rochester, Minnesota. Acta Orthop Scand 58:539–544 [DOI] [PubMed] [Google Scholar]
- 41. Jensen SL, Andresen BK, Mencke S, Nielsen PT. 1998. Epidemiology of ankle fractures. A prospective population-based study of 212 cases in Aalborg, Denmark. Acta Orthop Scand 69:48–50 [DOI] [PubMed] [Google Scholar]
- 42. Liu XS, Cohen A, Shane E, Yin PT, Stein EM, Rogers H, Kokolus SL, McMahon DJ, Lappe JM, Recker RR, Lang T, Guo XE. 2010. Bone density, geometry, microstructure, and stiffness: relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res 25:2229–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]