Abstract
Context and Objective:
Estrogen sulfotransferase (EST) catalyzes the inactivation of estrone and estradiol in numerous tissues. Animal studies suggest that EST modulates glucose and lipid metabolism in adipose tissue, but it is unknown whether EST is expressed in human adipose tissue and, if so, how its expression relates to features of the metabolic syndrome.
Design and Participants:
Cross-sectional data from 16 obese men and women with metabolic dysregulation were collected as part of a larger randomized trial at an academic medical center.
Outcome Measures:
Participants underwent assessment of body composition, oral glucose tolerance testing, measurement of serum hormones and inflammatory markers, and sc fat biopsy to assess adipose expression of TNF-α, suppressor of cytokine signaling 3 (SOCS3), leptin, adiponectin, and EST.
Results:
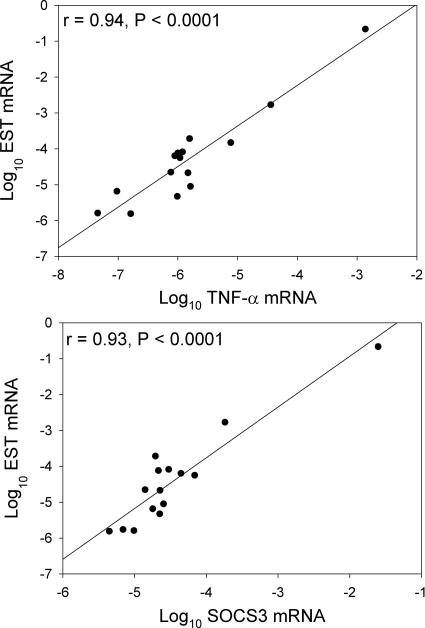
EST expression was detectable in sc adipose tissue from both men and women. Log10 EST mRNA was not significantly associated with age, race, sex or menopausal status, or circulating levels of estrogen or testosterone. In univariate analysis, log10 EST mRNA was significantly associated with visceral adipose tissue area (r = 0.57, P = 0.02) as well as adipose tissue expression of TNF-α (r = 0.94, P < 0.0001) and SOCS3 mRNA (r = 0.93, P < 0.0001). The associations between EST expression and TNF-α and SOCS3 held in multivariate modeling controlling for age, race, sex and menopausal status, and visceral adiposity. EST expression was not significantly associated with the adipose tissue levels of leptin or adiponectin expression.
Conclusions:
EST is expressed in abdominal sc adipose tissue of both obese males and females in association with expression of TNF-α and SOCS3, suggesting potential roles in inflammation. Further studies are needed to determine the specific metabolic roles of EST expression in human adipose tissue.
Estrogen plays an important role in glucose homeostasis and lipid handling in numerous tissues, including adipocytes (reviewed in Ref. 1). Estrogen sulfotransferase (EST; SULT1E1) is an enzyme that catalyzes transfer of a sulfuryl moiety to estrone and estradiol, rendering them biologically inactive. EST is known to be expressed in numerous human tissues, including the mammary gland, adrenal gland, vasculature, liver, testis, endometrium, and placenta (2). EST protects male reproductive tissues from estrogen effects (3), and loss of EST activity is thought to play a role in the pathogenesis of breast, endometrial, and possibly colon cancer (4–6). It remains unknown, however, whether EST has biological relevance in human adipose tissue. Khor et al. (7) have reported that EST is expressed in murine adipose tissue and that testosterone induces EST expression in adipose tissue from both male and female mice. Moreover, a transgenic model demonstrates that EST overexpression in adipocytes and macrophages of female mice reduces adipocyte differentiation, decreases expression of adipogenic enzymes, and causes adipocyte insulin resistance (8). These data illustrate the potential metabolic importance of EST, but, to our knowledge, EST expression has not been described in human adipose tissue, and the regulation of EST expression is not well understood. In the current analysis, we examined EST expression in sc adipose tissue obtained at the baseline visit during a study of the effects of TNF-α antagonism in patients with obesity (9). To investigate a possible association between EST and metabolic and inflammatory factors, we examined relationships between EST expression and expression of adiponectin, TNF-α, and suppressor of cytokine signaling-3 (SOCS3), a downstream target of TNF-α and well-characterized biomarker of inflammation, cytokine action, and insulin resistance in adipose tissue (10, 11). We demonstrate that EST is expressed in human sc abdominal adipose tissue from both men and women and that its expression is closely associated with the expression of TNF-α and SOCS3.
Materials and Methods
EST mRNA expression was examined in a subset of men and women with obesity and metabolic syndrome (12) who were recruited from December 2006 to March 2009 to participate in a larger study of the effects of TNF-α antagonism on metabolic and inflammatory parameters (9). Eighteen subjects underwent elective sc fat biopsy for this protocol; of these, one subject was excluded from the analysis due to the use of hormonal contraceptives, and another was excluded due to incomplete data. Baseline data from the remaining 16 subjects, before treatment with etanercept, are presented.
Written, informed consent was obtained from each subject. The study was approved by the Massachusetts General Hospital and Massachusetts Institute of Technology Institutional Review Boards. Study entry criteria have been previously described (9) and included age 18–60 yr, body mass index (BMI) greater than 30 kg/m2, and metabolic syndrome, defined using modified World Health Organization criteria: either fasting insulin 10 μU/ml or greater or fasting glucose 110–125 mg/dl, and at least one of the following: systolic blood pressure 140 mm Hg or greater, diastolic blood pressure 90 mm Hg or greater, triglycerides greater than 150 mg/dl, or high-density lipoprotein less than 35 mg/dl (males) or less than 39 mg/dl (females). These modified criteria were used to ensure the selection of subjects with impaired glucose homeostasis as part of their metabolic syndrome.
After a screening visit, eligible subjects returned after an overnight 12 h fast for a baseline visit that included blood sampling, standard 2-h, 75 g oral glucose tolerance test, single-slice abdominal computed tomography scan (9), and abdominal sc fat biopsy. Subcutaneous abdominal fat was obtained using a 4-mm punch biopsy and was immediately frozen for future analysis. The expression of adipose tissue genes was measured using real-time PCR as previously described (9). RNA was extracted by mechanical homogenization using TRIzol (Invitrogen, Carlsbad, CA). Cleared lysates were mixed with 1 volume of 70% ethanol and applied to RNeasy minicolumns for RNA isolation according to the manufacturer's protocol (QIAGEN, Valencia, CA). RNA was treated with deoxyribonuclease I according to the manufacturer's protocol (Invitrogen). The RNA was reverse transcribed using Superscript First Strand (Invitrogen). cDNA was treated with ribonuclease H according to the manufacturer's protocol (Invitrogen). cDNA was amplified using Taqman gene expression assays for EST, adiponectin, leptin, TNF-α, and SOCS3. Variability of cDNA levels was normalized by subtracting the cycle threshold (Ct) value of 18S from the target Ct value. The range of Ct values for EST in human adipose tissue is similar to ranges for leptin and adiponectin. Relative levels were quantified by measuring the 2ΔCt. Expression data that were not normally distributed were logarithmically transformed using log10.
Glucose was measured using standard methodology. Insulin, total testosterone, and estradiol were measured by a paramagnetic-particle chemiluminescence immunoassay (Beckman Access immunoassay; Beckman Coulter, Inc., Brea, CA). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as a measure of insulin resistance (13). The high-sensitivity C-reactive protein was measured using a kit from Genzyme Diagnostics (Cambridge, MA). The circulating concentrations of TNF receptor 1, TNF receptor 2, and adiponectin were measured in plasma by ELISA (R&D Systems, Minneapolis, MN). IL-6 was also measured in plasma by an ELISA (Biosource, Camarillo, CA).
Baseline variables that were not normally distributed were log10 transformed. Univariate analyses were performed using Pearson correlation coefficient or, for assessment of differences between groups, Tukey-Kramer honestly significant difference test. Multivariate analyses were subsequently performed using standard least squares. All multivariate analyses controlled for age, race, sex, and menopausal status. Analyses were performed using SAS JMP (SAS Institute, Cary, NC). Data are presented as mean ± sem unless otherwise specified. Statistical significance was defined as P < 0.05.
Results
Baseline characteristics are described in Table 1. The mean age of the subjects was 47 ± 2 yr, and mean BMI was 38.6 ± 1.4 kg/m2. Among female subjects, three women were premenopausal with regular menses, and three women were postmenopausal, not receiving hormone replacement therapy. The cohort was, on average, insulin resistant, with a mean HOMA-IR of 2.5 ± 0.5.
Table 1.
Characteristics of study subjects
| All subjects (n = 16) | Males (n = 10) | Females (n = 6) | |
|---|---|---|---|
| Race (n) | |||
| White | 9 | 5 | 4 |
| Black/African-American | 5 | 3 | 2 |
| Other | 2 | 2 | 0 |
| Age (yr) | 47 ± 2 | 43 ± 2a | 54 ± 1a |
| BMI (kg/m2) | 38.6 ± 1.4 | 38.3 ± 1.9 | 39.0 ± 1.9 |
| VAT (cm2) | 201 ± 17 | 215 ± 25 | 177 ± 19 |
| Fasting glucose (mg/dl) | 95 ± 4 | 96 ± 4 | 95 ± 9 |
| Fasting insulin (μU/ml) | 10.2 ± 1.5 | 11.3 ± 2.2 | 8.4 ± 2.0 |
| HDL cholesterol (mg/dl) | 45 ± 2 | 45 ± 3 | 46 ± 2 |
| Triglycerides (mg/dl) | 138 ± 17 | 139 ± 26 | 136 ± 17 |
| Systolic blood pressure (mm Hg) | 128 ± 4 | 124 ± 4 | 136 ± 9 |
| Diastolic blood pressure (mm Hg) | 77 ± 3 | 77 ± 3 | 76 ± 4 |
| Total testosterone (ng/dl)b | (Not applicable) | 364 ± 45a | 18 ± 4a |
| Log10 estradiol (pg/ml)c | (Not applicable) | 1.7 ± 0.1 | 1.7 ± 0.2 |
| Log10 adiponectin mRNA | −4.7 ± 0.4 | −5.4 ± 0.4a | −3.7 ± 0.5a |
| Log10 leptin mRNA | −5.3 ± 0.5 | −6.0 ± 0.4 | −4.5 ± 0.8 |
| Log10 SOCS3 mRNA | −4.5 ± 0.2 | −4.3 ± 0.3 | −4.7 ± 0.2 |
| Log10 TNF-α mRNA | −5.8 ± 0.3 | −5.8 ± 0.4 | −5.8 ± 0.4 |
| Log10 EST mRNA | −4.4 ± 0.3 | −4.1 ± 0.4 | −4.7 ± 0.5 |
Values are mean ± sem unless otherwise specified. HDL, High-density lipoprotein.
P < 0.05 for comparison between males and females.
Total testosterone levels were below the limit of detection for three females.
Estradiol levels were below the limit of detection for one male and two females.
In univariate analysis, log10 EST mRNA was not significantly associated with sex (P = 0.40, see Table 1), menopausal status (P = 0.84), age (P = 0.94), or race (no significant differences between groups by Tukey-Kramer honestly significant difference test). There were also no significant associations between log10 EST mRNA and circulating levels of testosterone (P = 0.61) or log10 estradiol (P = 0.55), and this remained the case in multivariate modeling controlling for age, race, sex, and menopausal status. Log10 EST mRNA was not significantly associated with sc adipose tissue (SAT; P = 0.60) or BMI (P = 0.61), but it was significantly positively associated with visceral adipose tissue (VAT; r = 0.57, P = 0.02) in univariate analysis. This association did not persist when controlling for age, race, sex, and menopausal status in multivariate modeling (data not shown). Log10 EST mRNA was not significantly associated with fasting glucose (P = 0.83), fasting insulin (P = 0.25), or HOMA-IR (P = 0.34).
Log10 EST mRNA showed strong positive associations with log10 TNF-α mRNA (r = 0.94, P < 0.0001) and log10 SOCS3 mRNA (r = 0.93, P < 0.0001) (Fig. 1), whereas there were no associations between log10 EST mRNA and sc adipose mRNA expression of leptin (P = 0.95) or adiponectin (P = 0.81). Log10 EST mRNA was not significantly associated with serum concentrations of inflammatory markers, including soluble TNF receptor 1, IL-6, and adiponectin (data not shown), although there were possible trends toward association between log10 EST mRNA and soluble TNF receptor 2 (r = 0.34, P = 0.20) and log10 high-sensitivity C-reactive protein (r = 0.32, P = 0.23) in this small cohort.
Fig. 1.
Univariate associations between sc adipose mRNA levels of EST and TNF-α (top panel) and SOCS3 (bottom panel).
Neither log10 TNF-α mRNA nor log10 SOCS3 mRNA was significantly associated with sex, age, race, menstrual status, BMI, SAT, fasting glucose, HOMA, fasting insulin, testosterone, or log10 estradiol. There was a trend toward association between VAT and both log10 TNF-α mRNA (r = 0.48, P = 0.07) and log10 SOCS3 mRNA (r = 0.49, P = 0.06).
In multivariate analysis, controlling for age, race, sex, and menopausal status, log10 TNF-α mRNA remained a significant predictor of log10 EST mRNA (P < 0.0001; R2 = 0.95, P < 0.0001 for model). This strong association between log10 TNF-α mRNA and log10 EST mRNA persisted (P < 0.0001) when VAT was also added to the model. Similarly, log10 SOCS3 mRNA was a significant predictor of log10 EST mRNA in multivariate modeling controlling for age, race, sex, and menopausal status (P = 0.0002; R2 = 0.89, P = 0.002 for model) and remained a significant predictor (P = 0.0007), with VAT added to the model. In these models, age, race, sex, and menopausal status were not significantly associated with the log10 EST mRNA (data not shown).
Discussion
Our data demonstrate that EST is expressed in human SAT from both men and women and that its expression is strongly related to TNF-α and SOCS3 expression. To our knowledge this is the first report that EST is expressed in human adipose tissue. Estrogen receptor-α and -β are known to be expressed in both sc and visceral human adipocytes (14), and estrogen is emerging as an important regulator of glucose and lipid metabolism in adipose tissue (15–17). in vitro studies demonstrate that physiological concentrations of estradiol stimulate, whereas the supraphysiological concentrations inhibit, insulin-stimulated glucose uptake in 3T3-L1 adipocytes (16). With regard to lipid metabolism, animal studies demonstrate that estradiol treatment down-regulates adipose expression of lipogenic genes, including sterol regulatory element-binding protein 1c, lipoprotein lipase, acetyl-CoA carboxylase-1, and fatty acid synthase (15, 17). In conjunction with these observations, estrogen-treated, ovariectomized mice have reduced adiposity, compared with pair-fed controls (15), and estradiol attenuates the gain in fat mass seen in mice fed a high-fat diet (17). These data, along with the well-described changes in adiposity and insulin sensitivity that accompany the menopause, suggest that physiological estrogen concentrations contribute to glucose and lipid homeostasis in human adipose tissue. Thus, the identification of EST mRNA in human adipose tissue adds an important element to our understanding of the role of estrogen and its numerous regulators in adipose tissue metabolism.
In the current study, EST expression in sc adipocytes was not different in males vs. females or in menopausal vs. premenopausal women. Moreover, there was no association between adipocyte expression of EST and circulating levels of estradiol or testosterone. It is important to highlight, however, that our population was selected for obesity and metabolic dysregulation. Consequently, many or all participants may have had some degree of abnormality in sex steroid dynamics due to obesity, and this may have affected EST expression. Moreover, half of our female subjects were menopausal, which may explain the finding of similar levels of estradiol in males and females in this study. Larger studies of healthy men and women will be needed to investigate sex-specific differences between EST expression in adipocytes and whether EST expression changes with the menopause.
Our data do not show a relationship between sc EST expression and BMI or SAT, although there was a significant univariate association between EST expression and VAT, which did not persist when controlling for age, race, sex, and menopausal status. EST expression was not significantly related to measures of glucose homeostasis. In these analyses, sample size may have significantly limited our ability to detect associations, and larger studies will be needed to better elucidate relationships between adipocyte EST expression and body composition parameters and insulin sensitivity.
The close relationship that we demonstrate between adipose mRNA expression of TNF-α and SOCS3 has not previously been reported. Although testosterone is known to increase EST (7), the regulation of EST expression has not been comprehensively described. Hepatic EST expression is significantly upregulated in db/db mice compared with lean mice, suggesting that diabetes, hyperlipidemia, and other metabolic abnormalities accompanying obesity may alter EST expression (18). In human vascular smooth muscle cells, treatment with IL-1β in vitro significantly increases EST mRNA, also pointing to potential inflammatory regulation of this enzyme (19). In the same report, Nakamura et al. (19) demonstrate that aortic specimens from women with severe atherosclerosis have increased expression of EST mRNA compared with those with mild atherosclerosis, suggesting a possible interaction between EST expression and inflammation in humans. Further studies are needed to investigate the interrelationships between inflammatory cytokines, gonadal steroids, and EST expression in humans.
The current analysis has a number of limitations. First, the findings should be interpreted in the context of the small sample size. In particular, any negative findings of the study cannot be taken to be definitive. In addition, as this is a cross-sectional evaluation, we cannot determine causality of the relationship between EST and TNF-α and SOCS3. Samples were not available to determine EST expression in healthy lean subjects for comparison. Moreover, our measurements of sex steroids and glucose homeostasis are systemic, and we do not have data on adipose-specific insulin sensitivity, nor do we know the local concentrations of sex steroids in adipose tissue. This is an important limitation because the regulation and actions of EST may be primarily local rather than systemic. For instance, although EST expression in adipose tissue may affect local insulin sensitivity, it may not similarly affect measures of systemic glucose homeostasis, as was seen in the murine model of EST overexpression (8). Furthermore, levels of EST expression may depend on local concentrations of sex steroids rather than circulating levels. Finally, we do not have tissue from the visceral depot, and further studies will be needed to investigate EST expression in visceral adipocytes. We also do not have data on adipocyte size, which may be affected by estrogen (15) and thus would be interesting to investigate in relation to EST expression. Further studies will also be needed to investigate the regulation of EST in other tissues including muscle and hypothalamus. Overall, our data establish that EST is expressed in human adipose tissue and show a strong relationship between EST expression and expression of inflammatory cytokines, pointing to the need for future research into the interrelationship between EST expression and inflammation as well as the potential physiological relevance of EST to body composition, insulin sensitivity, lipid metabolism, and atherogenesis.
Acknowledgments
We gratefully acknowledge the Massachusetts General Hospital and Massachusetts Institute of Technoplogy bionutrition and nursing staffs and the research volunteers for their participation in the study.
This work was supported by National Institutes of Health Grants M01-RR-01066 and 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center, and the National Center for Research Resources. National Institutes of Health funding was also provided through Grants F32 DK080642-02 and K23 DK089910-01 (to T.L.S.); Grant K24 DK064545-06 (to S.K.G.); Grant PO1-DK049210 (to R.S.A.); and Grant F32 DK085969-01 (to M.V.Z.).
Disclosure Summary: R.S.A., T.L.S., V.K.K., and M.V.Z., have nothing to disclose. S.K.G. received funding for this project from Amgen in the form of an investigator-initiated research grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Footnotes
- BMI
- Body mass index
- Ct
- cycle threshold
- EST
- estrogen sulfotransferase
- HOMA-IR
- homeostasis model assessment of insulin resistance
- SAT
- sc adipose tissue
- SOCS3
- suppressor of cytokine signaling-3
- VAT
- visceral adipose tissue.
References
- 1. Foryst-Ludwig A, Kintscher U. 2010. Metabolic impact of estrogen signalling through ERα and ERβ. J Steroid Biochem Mol Biol 122:74–81 [DOI] [PubMed] [Google Scholar]
- 2. Miki Y, Nakata T, Suzuki T, Darnel AD, Moriya T, Kaneko C, Hidaka K, Shiotsu Y, Kusaka H, Sasano H. 2002. Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. J Clin Endocrinol Metab 87:5760–5768 [DOI] [PubMed] [Google Scholar]
- 3. Tong MH, Song WC. 2002. Estrogen sulfotransferase: discrete and androgen-dependent expression in the male reproductive tract and demonstration of an in vivo function in the mouse epididymis. Endocrinology 143:3144–3151 [DOI] [PubMed] [Google Scholar]
- 4. Suzuki T, Miki Y, Nakata T, Shiotsu Y, Akinaga S, Inoue K, Ishida T, Kimura M, Moriya T, Sasano H. 2003. Steroid sulfatase and estrogen sulfotransferase in normal human tissue and breast carcinoma. J Steroid Biochem Mol Biol 86:449–454 [DOI] [PubMed] [Google Scholar]
- 5. Utsunomiya H, Ito K, Suzuki T, Kitamura T, Kaneko C, Nakata T, Niikura H, Okamura K, Yaegashi N, Sasano H. 2004. Steroid sulfatase and estrogen sulfotransferase in human endometrial carcinoma. Clin Cancer Res 10:5850–5856 [DOI] [PubMed] [Google Scholar]
- 6. Sato R, Suzuki T, Katayose Y, Miura K, Shiiba K, Tateno H, Miki Y, Akahira J, Kamogawa Y, Nagasaki S, Yamamoto K, Ii T, Egawa S, Evans DB, Unno M, Sasano H. 2009. Steroid sulfatase and estrogen sulfotransferase in colon carcinoma: regulators of intratumoral estrogen concentrations and potent prognostic factors. Cancer Res 69:914–922 [DOI] [PubMed] [Google Scholar]
- 7. Khor VK, Tong MH, Qian Y, Song WC. 2008. Gender-specific expression and mechanism of regulation of estrogen sulfotransferase in adipose tissues of the mouse. Endocrinology 149:5440–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khor VK, Dhir R, Yin X, Ahima RS, Song WC. 2010. Estrogen sulfotransferase regulates body fat and glucose homeostasis in female mice. Am J Physiol Endocrinol Metab 299:E657–E664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H, Khor VK, Ahima RS, Grinspoon SK. 2011. TNF-α antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab 96:E146–E150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGillicuddy FC, Chiquoine EH, Hinkle CC, Kim RJ, Shah R, Roche HM, Smyth EM, Reilly MP. 2009. Interferon γ attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J Biol Chem 284:31936–31944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, Shah R, Pruscino L, Tabita-Martinez J, Sellers KF, Rickels MR, Reilly MP. 2010. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 59:172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization 1999. Definitions, diagnosis, and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1. Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization [Google Scholar]
- 13. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 14. Dieudonné MN, Leneveu MC, Giudicelli Y, Pecquery R. 2004. Evidence for functional estrogen receptors α and β in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol 286:C655–C661 [DOI] [PubMed] [Google Scholar]
- 15. D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. 2005. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 280:35983–35991 [DOI] [PubMed] [Google Scholar]
- 16. Nagira K, Sasaoka T, Wada T, Fukui K, Ikubo M, Hori S, Tsuneki H, Saito S, Kobayashi M. 2006. Altered subcellular distribution of estrogen receptor α is implicated in estradiol-induced dual regulation of insulin signaling in 3T3-L1 adipocytes. Endocrinology 147:1020–1028 [DOI] [PubMed] [Google Scholar]
- 17. Bryzgalova G, Lundholm L, Portwood N, Gustafsson JA, Khan A, Efendic S, Dahlman-Wright K. 2008. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab 295:E904–E912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leiter EH, Chapman HD. 1994. Obesity-induced diabetes (diabesity) in C57BL/KsJ mice produces aberrant trans-regulation of sex steroid sulfotransferase genes. J Clin Invest 93:2007–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakamura Y, Miki Y, Suzuki T, Nakata T, Darnel AD, Moriya T, Tazawa C, Saito H, Ishibashi T, Takahashi S, Yamada S, Sasano H. 2003. Steroid sulfatase and estrogen sulfotransferase in the atherosclerotic human aorta. Am J Pathol 163:1329–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]