Abstract
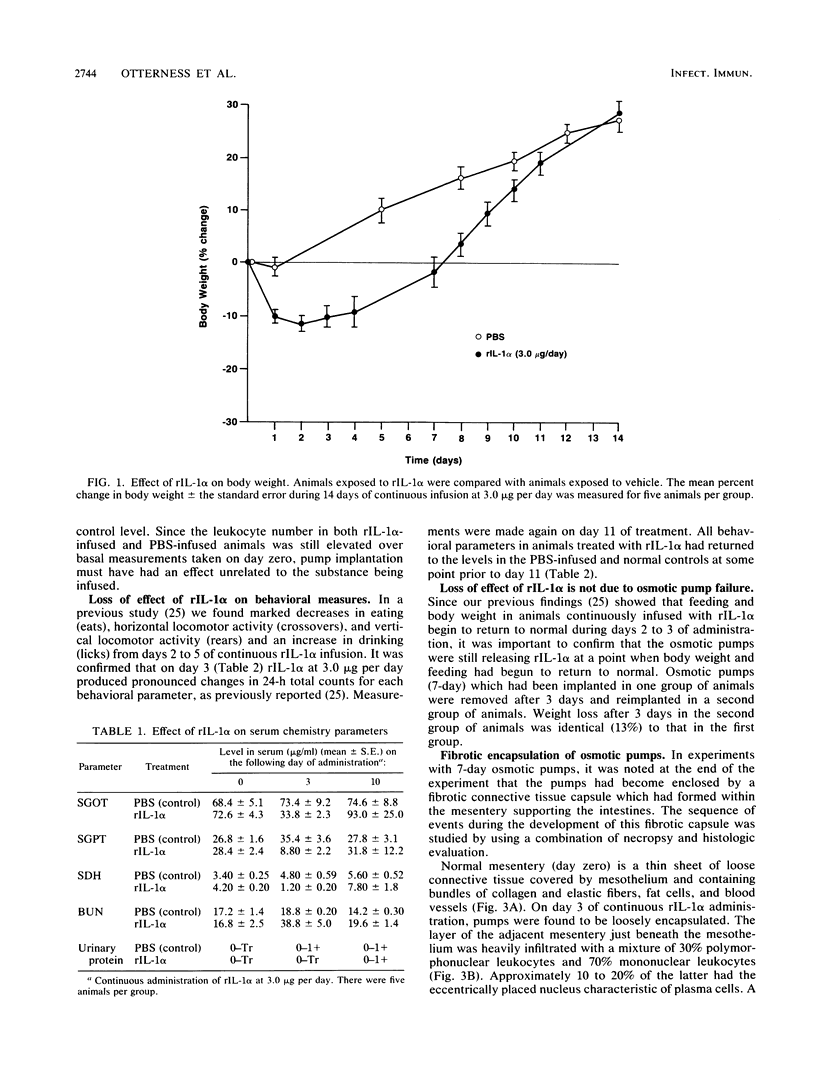
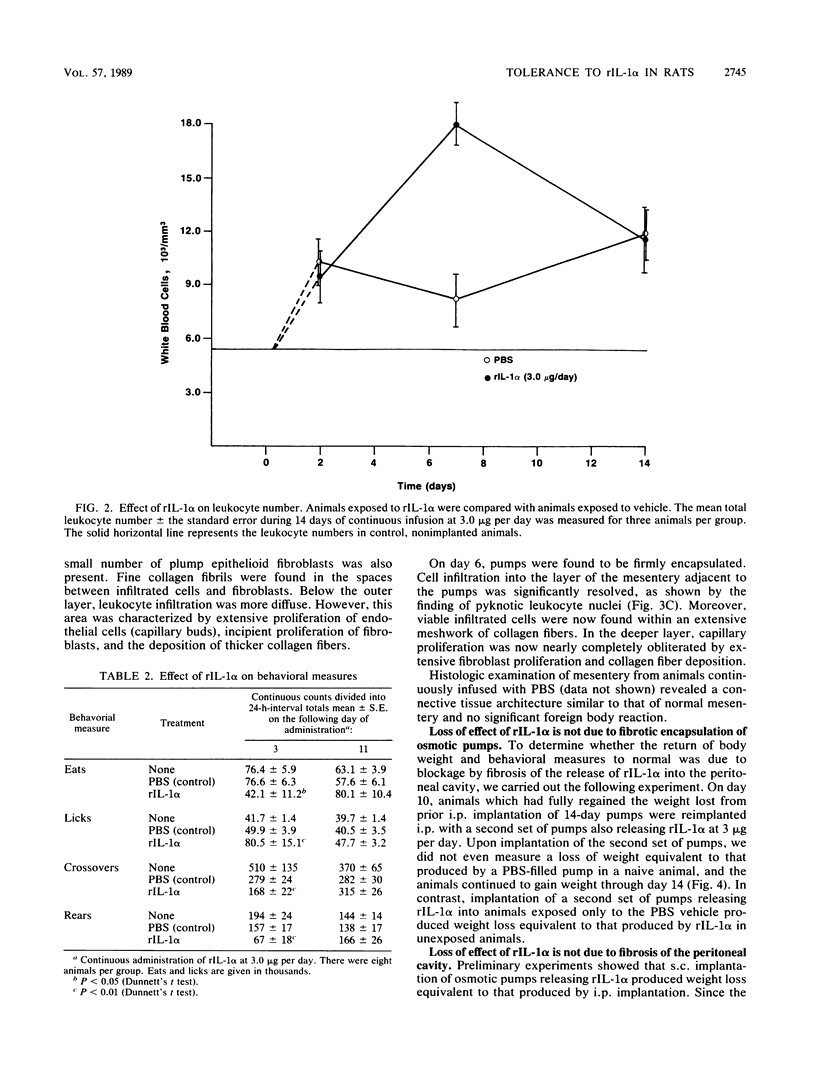
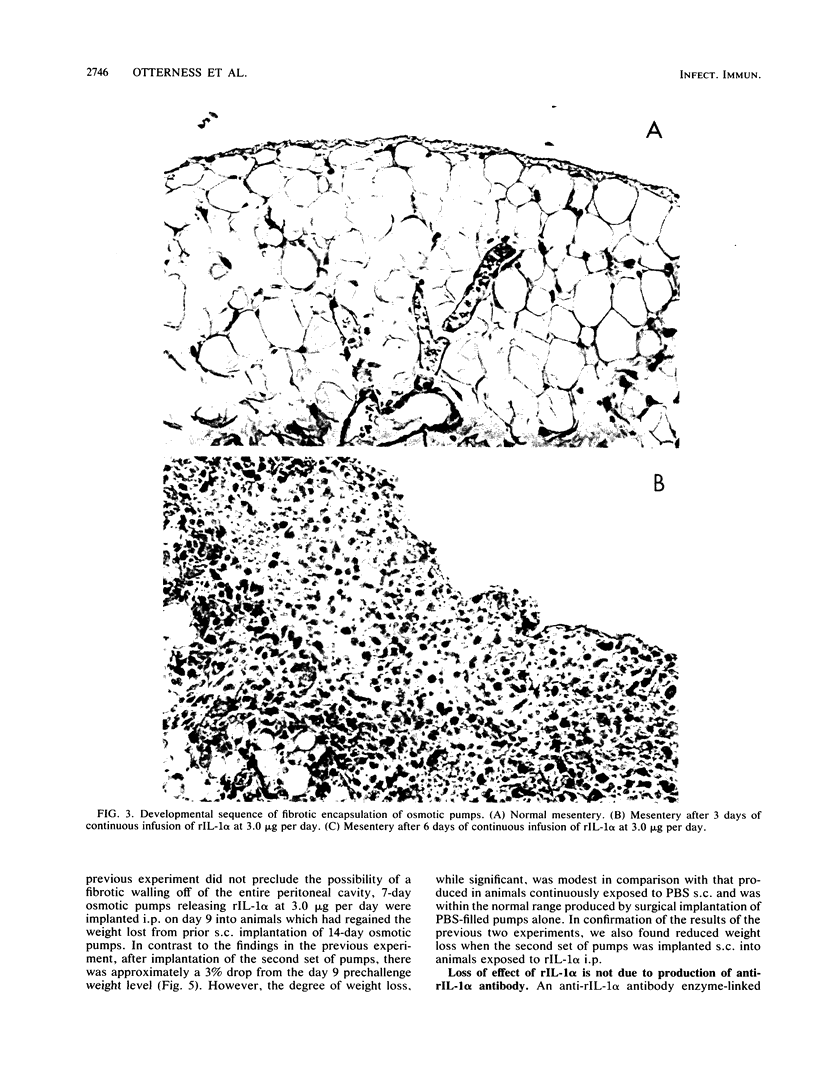
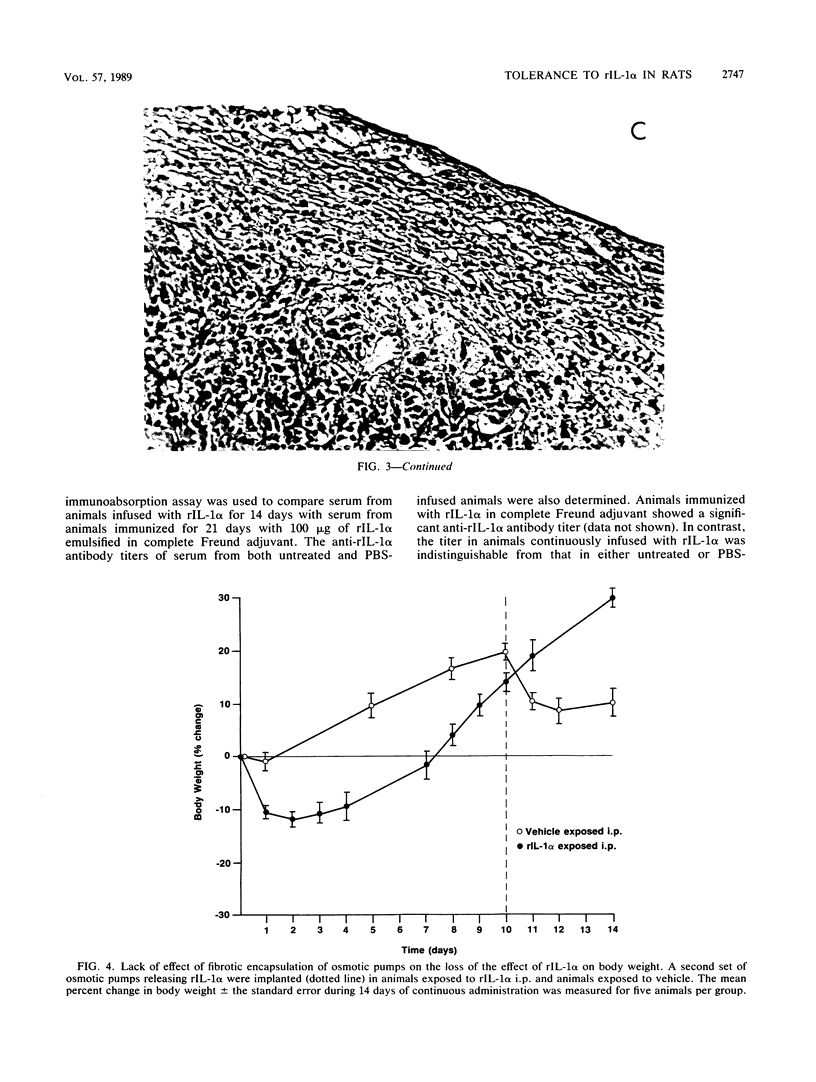
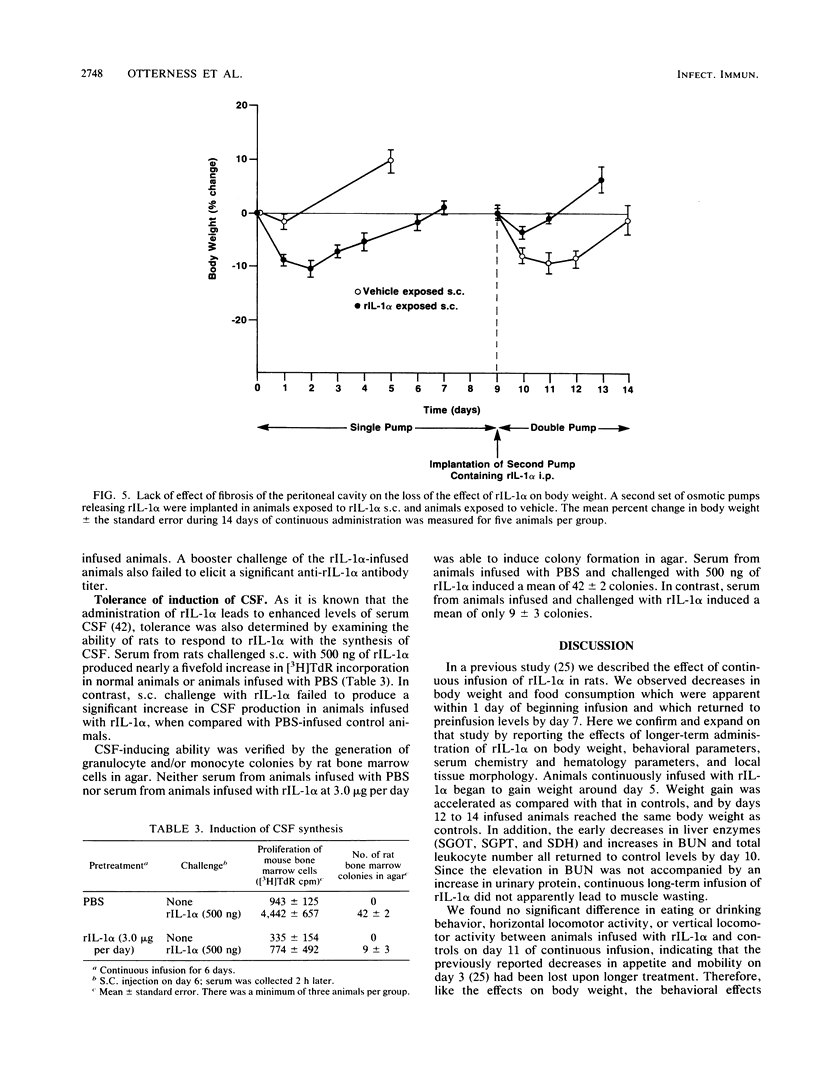
Continuous infusion of murine recombinant interleukin-1 alpha (rIL-1 alpha) into rats by using intraperitoneally implanted osmotic pumps led to marked decreases in body weight, liver enzymes (serum glutamic oxalacetic transaminase, serum glutamic pyruvic transaminase, and sorbitol dehydrogenase), appetite, and mobility and increases in drinking, blood urea nitrogen, and total peripheral blood leukocytes within 3 days. Granuloma formation was found in the local area of rIL-1 alpha release. As early as day 3, a focal infiltrate of polymorphonuclear leukocytes, mononuclear leukocytes, and plasma cells filled the area; by day 6, extensive fibrosis was found. A loss of rIL-1 alpha-induced changes, with the exception of granuloma formation, occurred by day 10. A marked decrease in the response to rIL-1 alpha was also observed when animals were challenged by implantation of new pumps containing rIL-1 alpha, with monitoring of body weight, or by subcutaneous injection of rIL-1 alpha, with monitoring of serum colony-stimulating factor production. We propose that, even in the continuous presence of interleukin-1, replacement of the acute responses to interleukin-1 by restoration of more normal physiology may be advantageous upon acquisition of specific immunity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisel W. R. Metabolic response to infection. Annu Rev Med. 1975;26:9–20. doi: 10.1146/annurev.me.26.020175.000301. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol. 1985 Dec;121(3):394–403. [PMC free article] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Otterness I. G., Higashi G. I., Forsch C. S., Kunkel S. L. Monokine production by hypersensitivity (Schistosoma mansoni egg) and foreign body (Sephadex bead)-type granuloma macrophages. Evidence for sequential production of IL-1 and tumor necrosis factor. J Immunol. 1989 Feb 15;142(4):1281–1286. [PubMed] [Google Scholar]
- Dascombe M. J., Rothwell N. J., Sagay B. O., Stock M. J. Pyrogenic and thermogenic effects of interleukin 1 beta in the rat. Am J Physiol. 1989 Jan;256(1 Pt 1):E7–11. doi: 10.1152/ajpendo.1989.256.1.E7. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984 Nov 29;311(22):1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Interleukins. Annu Rev Med. 1986;37:173–178. doi: 10.1146/annurev.me.37.020186.001133. [DOI] [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Gladue R., Girard A., Newborg M. Enhanced antibacterial resistance in neutropenic mice treated with human recombinant interleukin-1 beta. Agents Actions. 1988 Jun;24(1-2):130–136. doi: 10.1007/BF01968091. [DOI] [PubMed] [Google Scholar]
- Granstein R. D., Margolis R., Mizel S. B., Sauder D. N. In vivo inflammatory activity of epidermal cell-derived thymocyte activating factor and recombinant interleukin 1 in the mouse. J Clin Invest. 1986 Mar;77(3):1020–1027. doi: 10.1172/JCI112354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Madonna G. S., Peterson J. E., Ribi E. E., Vogel S. N. Early-phase endotoxin tolerance: induction by a detoxified lipid A derivative, monophosphoryl lipid A. Infect Immun. 1986 Apr;52(1):6–11. doi: 10.1128/iai.52.1.6-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone D. G., Wahl S. M., Tsokos M., Cattell H., Decker J. L., Wilder R. L. Immune function in severe, active rheumatoid arthritis. A relationship between peripheral blood mononuclear cell proliferation to soluble antigens and synovial tissue immunohistologic characteristics. J Clin Invest. 1984 Oct;74(4):1173–1185. doi: 10.1172/JCI111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Yodoi J., Tagaya Y., Oppenheim J. J. Down-regulation of interleukin 1 (IL 1) receptor expression by IL 1 and fate of internalized 125I-labeled IL 1 beta in a human large granular lymphocyte cell line. J Immunol. 1986 Nov 15;137(10):3183–3188. [PubMed] [Google Scholar]
- McCarthy D. O., Kluger M. J., Vander A. J. Suppression of food intake during infection: is interleukin-1 involved? Am J Clin Nutr. 1985 Dec;42(6):1179–1182. doi: 10.1093/ajcn/42.6.1179. [DOI] [PubMed] [Google Scholar]
- Miossec P., Yu C. L., Ziff M. Lymphocyte chemotactic activity of human interleukin 1. J Immunol. 1984 Oct;133(4):2007–2011. [PubMed] [Google Scholar]
- Miyasaka N., Sato K., Goto M., Sasano M., Natsuyama M., Inoue K., Nishioka K. Augmented interleukin-1 production and HLA-DR expression in the synovium of rheumatoid arthritis patients. Possible involvement in joint destruction. Arthritis Rheum. 1988 Apr;31(4):480–486. doi: 10.1002/art.1780310404. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Kilian P. L., Lewis J. C., Paganelli K. A., Chizzonite R. A. The interleukin 1 receptor. Dynamics of interleukin 1 binding and internalization in T cells and fibroblasts. J Immunol. 1987 May 1;138(9):2906–2912. [PubMed] [Google Scholar]
- Movat H. Z., Cybulsky M. I., Colditz I. G. Emigration and accumulation of PMN-leukocytes induced by endotoxin, interleukin 1 and other chemotactic substances. Folia Histochem Cytobiol. 1986;24(2):75–88. [PubMed] [Google Scholar]
- Neta R., Sztein M. B., Oppenheim J. J., Gillis S., Douches S. D. The in vivo effects of interleukin 1. I. Bone marrow cells are induced to cycle after administration of interleukin 1. J Immunol. 1987 Sep 15;139(6):1861–1866. [PubMed] [Google Scholar]
- Otterness I. G., Seymour P. A., Golden H. W., Reynolds J. A., Daumy G. O. The effects of continuous administration of murine interleukin-1 alpha in the rat. Physiol Behav. 1988;43(6):797–804. doi: 10.1016/0031-9384(88)90379-4. [DOI] [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Lachman L. B., Mainardi C. L., Kang A. H. Interleukin 1 stimulation of collagenase production by cultured fibroblasts. J Exp Med. 1983 Feb 1;157(2):801–806. doi: 10.1084/jem.157.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powanda M. C., Beisel W. R. Hypothesis: leukocyte endogenous mediator/endogenous pyrogen/lymphocyte-activating factor modulates the development of nonspecific and specific immunity and affects nutritional status. Am J Clin Nutr. 1982 Apr;35(4):762–768. doi: 10.1093/ajcn/35.4.762. [DOI] [PubMed] [Google Scholar]
- Prystowsky M. B., Naujokas M. F., Ihle J. N., Goldwasser E., Fitch F. W. A microassay for colony-stimulating factor based on thymidine incorporation. Am J Pathol. 1984 Jan;114(1):149–156. [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Sauder D. N., Mounessa N. L., Katz S. I., Dinarello C. A., Gallin J. I. Chemotactic cytokines: the role of leukocytic pyrogen and epidermal cell thymocyte-activating factor in neutrophil chemotaxis. J Immunol. 1984 Feb;132(2):828–832. [PubMed] [Google Scholar]
- Schwarz T., Urbanska A., Gschnait F., Luger T. A. UV-irradiated epidermal cells produce a specific inhibitor of interleukin 1 activity. J Immunol. 1987 Mar 1;138(5):1457–1463. [PubMed] [Google Scholar]
- Seckinger P., Lowenthal J. W., Williamson K., Dayer J. M., MacDonald H. R. A urine inhibitor of interleukin 1 activity that blocks ligand binding. J Immunol. 1987 Sep 1;139(5):1546–1549. [PubMed] [Google Scholar]
- Shore A., Jaglal S., Keystone E. C. Enhanced interleukin 1 generation by monocytes in vitro is temporally linked to an early event in the onset or exacerbation of rheumatoid arthritis. Clin Exp Immunol. 1986 Aug;65(2):293–302. [PMC free article] [PubMed] [Google Scholar]
- Socher S. H., Friedman A., Martinez D. Recombinant human tumor necrosis factor induces acute reductions in food intake and body weight in mice. J Exp Med. 1988 Jun 1;167(6):1957–1962. doi: 10.1084/jem.167.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Dalldorf F. G., Otterness I. G., Schwab J. H. Exacerbation of arthritis by IL-1 in rat joints previously injured by peptidoglycan-polysaccharide. J Immunol. 1988 May 1;140(9):2964–2969. [PubMed] [Google Scholar]
- Tartakovsky B., Finnegan A., Muegge K., Brody D. T., Kovacs E. J., Smith M. R., Berzofsky J. A., Young H. A., Durum S. K. IL-1 is an autocrine growth factor for T cell clones. J Immunol. 1988 Dec 1;141(11):3863–3867. [PubMed] [Google Scholar]
- Tracey K. J., Wei H., Manogue K. R., Fong Y., Hesse D. G., Nguyen H. T., Kuo G. C., Beutler B., Cotran R. S., Cerami A. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988 Mar 1;167(3):1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Douches S. D., Kaufman E. N., Neta R. Induction of colony stimulating factor in vivo by recombinant interleukin 1 alpha and recombinant tumor necrosis factor alpha 1. J Immunol. 1987 Apr 1;138(7):2143–2148. [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B. Lymphocyte-mediated activation of fibroblast proliferation and collagen production. J Immunol. 1978 Sep;121(3):942–946. [PubMed] [Google Scholar]
- Wallach D., Holtmann H., Engelmann H., Nophar Y. Sensitization and desensitization to lethal effects of tumor necrosis factor and IL-1. J Immunol. 1988 May 1;140(9):2994–2999. [PubMed] [Google Scholar]
- Wood D. D., Ihrie E. J., Dinarello C. A., Cohen P. L. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983 Aug;26(8):975–983. doi: 10.1002/art.1780260806. [DOI] [PubMed] [Google Scholar]
- van der Meer J. W., Barza M., Wolff S. M., Dinarello C. A. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]




