Abstract
Context:
Maternal obesity, gestational diabetes (GDM), or type 2 diabetes (T2DM) is associated with altered lipid metabolism and fetal overgrowth.
Objective:
The objective of the study was to test the hypothesis that hyperlipidemia and hyperinsulinemia regulate lipid content and expression of lipid-trafficking proteins in human placental trophoblasts.
Study Design:
Pregnant women were prospectively enrolled for clinical specimens collection, and cultured human trophoblasts were used for experiments.
Setting:
This was a translational study conducted at an academic biomedical research center.
Patients or Other Participants:
Normal weight, obese, or obese with gestational diabetes or type 2 diabetes pregnant women (n = 10 in each group) undergoing scheduled cesarean delivery at term were enrolled.
Interventions:
Cultured primary human trophoblasts, exposed to insulin (10 nm) and/or fatty acids mix (1200 μm) in the absence or presence of an fatty acid binding protein 4 (FABP4) inhibitor or after small interfering RNA-mediated knockdown of FABP4.
Main Outcome Measures:
Serum lipid levels were analyzed in the maternal venous and fetal cord blood. Placental biopsies and cultured trophoblasts were analyzed for FABP expression and lipid accumulation.
Results:
Obese diabetic women and their fetuses had elevated serum triglyceride levels. Nonesterified fatty acids were elevated and triglycerides were reduced in placental villi from obese diabetic women, and this was accompanied by a 2.6-fold increase in FABP4 expression (P < 0.05). In primary human trophoblasts, fatty acids markedly increased the expression of FABP4 (20- to 40-fold, P < 0.05) and cellular triglyceride content (4-fold, P < 0.05), and this effect was attenuated by small interfering RNA-mediated knockdown of FABP4 or the selective FABP4 inhibitor BMS309403.
Conclusions:
Hyperlipidemia alters lipid content and increases the expression of FABP4 in trophoblasts. The reduced triglyceride content after FABP4 inhibition suggests that FABP4 is essential for trophoblast lipid accumulation.
Normal fetal development depends on placental transport of fatty acids (1). Supporting increasing fetal needs, maternal serum lipids rise throughout pregnancy (2). The supply of lipids is particularly important during the second half of human pregnancy, when the fetus more than doubles in size (3). Obesity is common during pregnancy and is frequently accompanied by gestational diabetes mellitus (GDM) or type 2 diabetes mellitus (T2DM) (4, 5). Obesity and diabetes increase the risk of maternal and fetal complications during pregnancy, especially fetal macrosomia (4, 6, 7). Diabetes, independent of obesity, is associated with maternal dyslipidemia (8), which manifests as high plasma triglyceride concentrations, low levels of high-density lipoprotein cholesterol, and increased concentrations of low-density lipoprotein cholesterol particles (9). Maternal diabetes also affects lipid levels in umbilical cord blood, with elevated concentrations of nonesterified fatty acids (NEFA), total cholesterol, triglycerides, and phospholipids in pregnancies complicated by maternal type 1 diabetes mellitus (10, 11). The level of lipids in cord blood from nonobese women with GDM is similar to that in to nondiabetic controls (12), yet data on the influence of GDM or T2DM on cord blood lipids in the setting of maternal obesity are limited. In well-controlled GDM pregnancies, maternal lipids are strong predictors of fetal growth, supporting a role for placental lipid transport in fetal overgrowth (13).
T2DM is associated with ectopic lipid accumulation in the heart, liver, and skeletal muscle (14). This accumulation is characterized by an increase in the concentration of cellular lipid droplets, which store triglycerides and other neutral lipids, and provide a source for metabolic fuels (15, 16). Perilipins 1–4, formerly referred to as perilipin, adipophilin, TIP47, and S312, respectively (17), are lipid droplet-associated proteins that are expressed in human and murine placentas (15, 18, 19). Lipid droplet formation is enhanced in cultured trophoblasts exposed to fatty acids combined with insulin, demonstrating that trophoblasts are capable of packaging lipids for further storage (20). The placenta in women with obesity, diabetes, or both is likely exposed to hyperlipidemia, hyperinsulinemia, and an overall increased supply of nutrients. Little is known about the impact of hyperlipidemia and hyperinsulinemia on placental lipid trafficking and storage.
Cells involved in active lipid trafficking, such as hepatocytes, intestinal epithelial cells, and cardiac myocytes express discrete types of fatty acid binding proteins (FABP) (21). These proteins are implicated in cellular uptake and transport of fatty acids as well as coordination of metabolic and inflammatory pathways (22). We previously found that FABP1, FABP3, FABP4, FABP5, and FABP plasma membrane (FABPpm) are expressed in human trophoblasts (23). Importantly, hypoxia and peroxisome proliferator-activated receptor (PPAR)-γ agonists increase the expression of selected FABP and fatty acid transport proteins (23), suggesting that these proteins are regulated by, and likely play a role in, placental lipid uptake, metabolism, and storage.
We surmised that the expression of genes related to fatty acid binding and lipid storage in placentas from pregnancies complicated by diabetes may be different from their expression in placentas from pregnancies complicated by obesity without diabetes or from healthy nondiabetic normal weight controls. We tested the hypothesis that placentas from pregnancies complicated by obesity plus diabetes exhibit altered lipid content and lipid-trafficking proteins when compared with placentas from pregnancies complicated by obesity alone or from nonobese, nondiabetic pregnancies. We also assessed the influence of fatty acids, insulin, or a combination of the two on the expression of FABPs and the intracellular accumulation of lipids in cultured primary human trophoblasts.
Patients and Methods
Patient enrollment and placental sampling
Institutional review board approval was obtained and informed consent provided by all participants. We enrolled women to each of the following three groups: 1) normal-weight women with a prepregnancy body mass index (BMI) less than 25 kg/m2 without T2DM or GDM (n = 10); 2) obese women, defined as a prepregnancy BMI greater than 30 kg/m2 without T2DM or GDM (n = 10); and 3) obese women with either T2DM or GDM (n = 10). All participants without diabetes underwent glucose challenge screening at 24–28 wk. For the diabetic women, fasting and 1-h postprandial values from breakfast, lunch, and dinner were collected at gestational wk 30, 34, and 37 to assess blood glucose control. All women fasted for 8 h before scheduled cesarean delivery without labor at 37–40 wk. Maternal venous blood was collected before delivery and umbilical venous blood samples were collected at delivery. Within 30 min after delivery, placentas without umbilical cord or membranes were weighed and placental fragments approximately 0.125 cm3 in size were sharply dissected at a site midway between the cord insertion and the lateral placental margin and midway between the chorionic and basal plates as we previously described (24) and either fixed in formalin for 24 h and embedded in paraffin or stored for assessment of lipids, RNA, and protein.
Serum lipid analysis
Serum samples were assayed for the concentration of triglyceride, total cholesterol, NEFA, and very long-chain fatty acid (VLCFA). The assays for triglycerides and total cholesterol were performed by the clinical chemistry laboratory at Barnes-Jewish Hospital (St. Louis, MO), and serum samples for NEFA and VLCFA were frozen in −80 C within 1 h of collection and transported to the Mayo Laboratory (Rochester, MN) for analysis.
Isolation and culture of term primary human trophoblasts (PHT)
Placentas were collected from uncomplicated singleton pregnancies delivered by repeat cesarean section between 37 and 40 wk, and PHT were isolated by the modified trypsin-deoxyribonuclease, Percoll gradient centrifugation method (25, 26). Cultures were plated at a density of 300,000 cells/cm2 and maintained as we previously described (27, 28). Cells were then cultured in serum-free DMEM in the presence of 0.1% fatty acid-free BSA (Sigma, St. Louis, MO), 800 μm linoleic acid, and 400 μm oleic acid (Sigma), 10 nm insulin (Sigma), both, or neither for an additional 6–24 h. Where indicated, cells were also cultured in either high-glucose DMEM (4.5 g/liter glucose) or low-glucose DMEM (1 g/liter) in the absence or presence of 800 μm linoleic acid and 400 μm oleic acid (Sigma). The selective FABP4 inhibitor BMS309403 (12.5–50 μm; Calbiochem, San Diego, CA) (29, 30) was added in some of the experimental paradigms 1 h before addition of the fatty acid mixture.
Small interfering RNA (siRNA)-mediated knockdown of FABP4 in PHT
Cells were plated at a density of 300,000 cells/cm2 in six-well plates and maintained overnight in DMEM containing 10% fetal bovine serum. Cells were then incubated in 2 ml OPTI-MEM per well plus 5 μl DharmaFECT 1 (Dharmacon, Lafayette, CO) and either 50 nm FABP4 Silencer Select siRNA (no. s4964; Ambion, Norwalk, CT) or 50 nm Silencer Select control siRNA for 24 h and then exposed to fatty acids as described above for an additional 24 h. FABP4 knockdown was confirmed by Western immunolotting.
RNA isolation, reverse transcription (RT), and quantitative PCR (RT-qPCR)
RNA was purified from villous tissues or PHT using TriReagent (Molecular Research Center, Cincinnati, OH) according to the manufacturer's instructions. Purified RNA was treated for 20 min at 37 C with deoxyribonuclease I (DNA-free; Ambion, Austin, TX) to remove contaminating DNA. The RT reaction was performed as we have previously described (23). The RT product (3 μl) was used for quantitative PCR along with 300 nm of each of forward and reverse primers and SYBRgreen PCR master mix (Applied Biosystems, Carlsbad, CA) in a total reaction volume of 25 μl. Sequences for the PCR primers (Table 1) were checked for specificity using BLAST (31). Reactions were run in duplicate and analyzed using an Applied Biosystems Geneamp 7300 sequence detection system. Dissociation curves were run on all reactions to ensure amplification of a single product with the appropriate melting temperature. Samples were normalized to parallel reactions using primers specific for 18S RNA. The fold increase relative to control was determined by the 2-ΔΔCT method (32).
Table 1.
Primers used for RT-qPCR assays
| Transcript | Accession No. | Forward Reverse | Sequence |
|---|---|---|---|
| FABPpm | NM_002080 | F (2221-41) | TCT GCC CGG TTG GAC CT |
| R (2294-2276) | GCC GCT GGA CTC ACA GTG T | ||
| FABP3 | NM_004102 | F (42-61) | TCA GCC TAG CCC AGC ATC AC |
| R (108-87) | TCT TGC TGT CCA CTA GCT TCC A | ||
| FABP4 | NM_001442 | F (388-406) | TGG TGG TGG AAT GCG TCA T |
| R (463-444) | GGT CAA CGT CCC TTG GCT TA | ||
| FABP5 | NM_00144 | F (93-115) | CCA CAG TTC AGC AGC TGG AA |
| R (293-273) | ACT CCT AGC TCC TTC ATG TAT TCA TCA | ||
| Plin 1 | NM_002666 | F (2099-2122) | GAT TCT GCC TCT GCG GAT AAA TAT |
| R (2196-2171) | TCA GTG CTA AGA ATG TGT CAA AAC CT | ||
| Plin 2 | NM_001122 | F (326-346) | GGC AGA GAA CGG TGT GAA GAC |
| R (390-372) | TCT GGA TGA TGG GCA GAG C | ||
| Plin 3 | NM_005817 | F (209-229) | TAT GCC TCC ACC AAG GAG AG |
| R (356-335) | ATT CGC TGG CTG ATG CAA TCT | ||
| Plin 4 | NM_001080400 | F (5658-5678) | TAC TGG GAC GGA GGC AAC TC |
| R (5740-5720) | CGT GCT CCG AAG TTG CTC AT | ||
| 18S | NR_003286 | F (68-86) | CAC GGC CGG TAC AGT GAA A |
| R (137-121) | AGA GGA GCG AGC GAC CAA |
F, forward; R, reverse; Plin, perilipin.
Western immunoblotting
Tissue or PHT cells were rinsed with PBS, lysed with 120 μl of cold lysis buffer (1% Nonidet P-40, 0.5% deoxycholate, and 0.1% SDS in PBS), plates were scraped, and the lysates were sonicated three times for 10 sec each time on ice with a Sonic Dismembrator 50 (Fisher Scientific, Pittsburgh, PA). The lysates were centrifuged, protein levels were determined, and 30 μg of protein/lane was resolved using 12% SDS-PAGE at 95 V for 2.5 h before protein transfer to polyvinylidene difluoride membranes (Immobilon-P; Millipore Corp., Bedford, MA) overnight at 200 mA. Blots were blocked with 5% nonfat dry milk in 1× PBS with 0.05% Tween 20) for 1 h, incubated overnight at 4 C with either goat polyclonal anti-FABP4 (final concentration 0.4 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) or goat polyclonal antiactin antibodies (0.2 μg/ml; Santa Cruz Biotechnology), washed, and incubated overnight at 4 C with horseradish peroxidase-conjugated donkey antigoat IgG (0.8 μg/ml; Santa Cruz Biotechnology). The blots were processed for chemiluminescence using an Amersham Biosciences enhanced chemiluminescence kit (Amersham Biosciences, Piscataway, NJ) and chemiluminescence quantified by densitometry using Epichemi-3 software (UVP BioImaging Systems, Upland, CA).
Immunofluorescence
Immunofluorescence of FABP4 was performed on paraffin-embedded sections using polyclonal antibody to FABP4 (Santa Cruz Biotechnology). After deparaffination and rehydration, the sections were incubated in 10 mm sodium citrate buffer and microwaved for 20 min. After blocking with 10% nonimmune serum, the sections were incubated with antibody to FABP4 (1 μg/ml; Santa Cruz Biotechnology) for 2 h at room temperature. Negative controls were exposed to primary antibody (1 μg/ml) that was preincubated with a blocking peptide (1 μg/ml; both from Santa Cruz Biotechnology). The slides were then incubated with Alexa Fluor 488 donkey antigoat antibodies (2 μg/ml; Invitrogen, Carlsbad, CA) for 1 h at room temperature. Nuclei were counterstained using Hoescht 33342 (Invitrogen). The immunofluorescence was visualized using an E400 Nikon microscope (Nikon Instruments, Melville, NY).
Lipid accumulation assay
Cultured cells were washed in PBS, fixed in 2% paraformaldehyde for 20 min at room temperature, permeabilized for 5 min with 1 ml 0.1% Triton X-100 in PBS, and stained for lipid droplets by a 15-min exposure to dipyrromethene boron difluoride (BODIPY 493/503, 10 μg/ml; Invitrogen/Molecular Probes) diluted in PBS. The cells were washed four times with PBS, nuclei were stained with 4′-6-diamidino-2-phenylindole (DAPI) 358/461 (Invitrogen/Molecular Probes), and lipid droplets were visualized as above. The ratio of BODIPY to DAPI signal was quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Quantification of lipid content
Lipids were extracted from human placental villous fragments or cultured trophoblasts using a modified Folch method (33). Tissues were first dried on blot paper and weighed. Cultures or tissues were homogenized in water and then vortexed in a 2:1 chloroform-methanol mixture with 0.1% sulfuric acid. For the assessment of NEFA levels, 1% butylated hydroxytoluene (Sigma) was added. The lipid phase was removed, chloroform was added, and the samples were dried under nitrogen. The samples were dissolved in isopropanol for assays of cholesterol and triglycerides. Samples for NEFA assays were dissolved in PBS containing 1% lipid-free BSA (Sigma). All assays were performed in duplicate using commercial kits (Wako Chemicals, Richmond, VA).
Statistical analysis
Data are presented as mean and sd, where appropriate. All experiments were repeated at least three times. Some of the figures depict a representative experiment, with analysis of all replicates in the corresponding text. Statistical analyses were performed using ANOVA with post hoc Bonferroni correction (version 10.0; STATA, College Station, TX). A P < 0.05 was considered significant.
Results
Information on women and their newborns is shown in Table 2. Women in the obese diabetic group were older than women in the normal or obese groups. As expected, prepregnancy weight and prepregnancy BMI were lower in the normal-weight group. There were no significant differences in maternal weight gain during pregnancy. In addition, the infants of the women with obesity and diabetes weighed more than those from normal weight or obese women, and placental weights from the obese diabetes mellitus patients were higher than those from control women. The mean blood sugar values for the diabetic women indicate that control of blood sugar was adequate through the third trimester of pregnancy.
Table 2.
Maternal and neonatal characteristics (mean ± sd)
| Control | Obese | Obese DM | |
|---|---|---|---|
| Maternal age (yr) | 24.0 (±2.4) | 28.1 (±3.0) | 32.8 (±6.3)a |
| Gravidity | 3.6 (±1.3) | 3.4 (±1.1) | 3.4 (±1.1) |
| Prepregnancy weight (kg) | 57.7 (±9.6)b | 99.5 (±16.2) | 111.2 (±20.4) |
| Prepregnancy BMI (kg/m2) | 22.1 (±1.4)b | 37.0 (±5.3) | 41.5 (±7.1) |
| Gestational weight gain (kg) | 15.5 (±4.9) | 12.5 (±4.4) | 16.0 (±7.9) |
| Neonatal weight (g) | 3141 (±342) | 3461 (±617) | 3867 (±413)a |
| Placental weight (g) | 570 (±130) | 616 (±134) | 746 (±50)c |
| Triglycerides (mg/dl) | |||
| Maternal | 143.9 (±39.6) | 197.7 (±68.8) | 244.9 (±97.3)c |
| Neonatal | 16.1 (±5.8) | 14.4 (±5.0) | 30.4 (±14.8)a |
| Cholesterol (mg/dl) | |||
| Maternal | 256.1 (±96.8) | 231.2 (±96.8) | 194.9 (±35.3) |
| Neonatal | 53.5 (±14.4) | 47.4 (±10.7) | 52.4 (±10.2) |
| NEFA (mEq/liter) | |||
| Maternal | 0.58 (±0.08) | 0.63 (±0.16) | 0.64 (±0.08) |
| Neonatal | 0.15 (±0.06) | 0.14 (±0.06) | 0.13 (±0.13) |
| VLCFA (μmol/liter) | |||
| Maternal | 125.7 (±40.9) | 155.5 (±32.8) | 147.5 (±51.1) |
| Neonatal | 58.4 (±21.7) | 60.4 (±13.9) | 70.1 (±11.0) |
| 1 h GCT result (mg/dl) | 100.7 (±18.7) | 102.4 (±19.6) | 189.7 (±24.9)a |
| Fasting glucose (mg/dl) | 85.7 (±14.0) | ||
| Glucose 1 h after breakfast (mg/dl) | 130.4 (±20.1) | ||
| Glucose 1 h after lunch (mg/dl) | 131.0 (±15.5) | ||
| Glucose 1 h after dinner (mg/dl) | 137.5 (±14.5) |
GCT, Glucose challenge test.
P < 0.05 vs. control and obese.
P < 0.05 vs. obese and obese diabetic.
P < 0.05 vs. control.
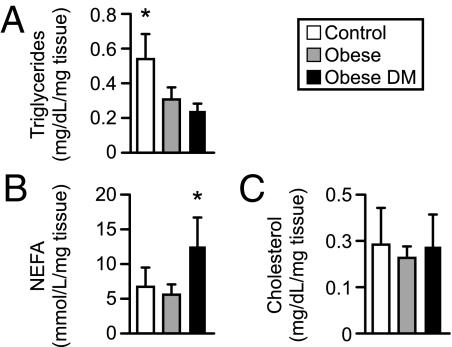
We first analyzed the maternal and umbilical venous serum levels of triglycerides, cholesterol, NEFA, and VLCFA. As shown in Table 2, maternal triglycerides were significantly higher in the obese diabetic group than controls. In addition, serum triglycerides were approximately 2-fold higher in the neonates of obese diabetic women than those from either normal-weight or obese women. There were no differences in total cholesterol, NEFA, or VLCFA among the three groups. To examine whether these differences are associated with alterations in placental lipid content, we measured the levels of lipids in placental tissue from the three groups. As shown in Fig. 1, obesity or obesity with diabetes was associated with decreased placental triglyceride content, whereas NEFA content in placentas from the obese diabetic women was approximately 2-fold higher than either the normal-weight or obese placentas. There were no differences in placental cholesterol content. Together, these data indicate that the concentrations of triglycerides in maternal and fetal serum, as well as in the placenta, are altered in pregnancies complicated by obesity and diabetes, and obesity with diabetes is associated with increased placental NEFA concentration.
Fig. 1.
Lipid content in placental tissues from normal-weight, obese, and obese diabetic women (n = 8 for each group). Results were normalized to tissue weight. A, Triglycerides. B, NEFA. C, Cholesterol. DM, Diabetes mellitus. *, P < 0.05 vs. the other two groups.
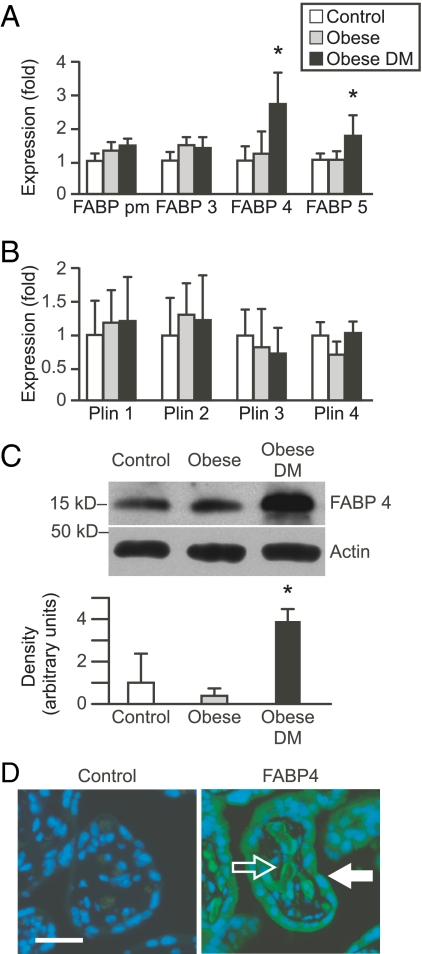
Because FABP mediate fatty acid trafficking (34), we surmised that changes in intracellular triglycerides and NEFA content might be associated with altered expression of placental FABP. We examined the expression of FABPs that we previously found to be expressed in trophoblasts (23), except for FABP1, which was barely detectable in the intact placental samples (not shown). We found that placentas from obese diabetic women exhibited a 2.6-fold increase in FABP4 mRNA expression when compared with placentas from obese, nondiabetic, or normal-weight women (Fig. 2A). The expression of placental FABP5 was also mildly increased (1.7-fold) in these women. There was no difference in FABPpm or FABP3 expression among groups. The expression of the lipid droplet associated proteins perilipin 1–4 was not significantly different among the three experimental groups (Fig. 2B). The increase in FABP4 mRNA was associated with enhanced expression of FABP4 protein (Fig. 2C). Given the potential importance of FABP4 in maternal-fetal lipid trafficking, we used immunofluorescence to localize placental FABP4 and found this protein to be expressed in the trophoblast layer as well as in villous endothelial cells (Fig. 2D).
Fig. 2.
The expression of FABP and lipid droplet-associated perilipin proteins in placental samples from normal-weight, obese, or obese diabetic women. A, RT-qPCR assessment of FABP expression in placental tissue (n = 10 per group). DM, Diabetes mellitus. B, RT-qPCR assessment of perilipin (Plin) proteins expression in placental tissues. C, A representative Western blot of FABP4 expression in placental biopsies. The bands were quantified using densitometry and normalized to β-actin, as described in Patients and Methods. The graph depicts densitometry from all subjects in each group (n = 10 per group). D, Immunofluorescence of placental villous tissue (n = 5). Cells were stained with anti-FABP4 and Alexa Fluor 488-linked secondary antibody (Invitrogen) for FABP4 (green) and Hoechst 33342 for nuclei (blue). A primary antibody that was preincubated with blocking peptide was used as negative control. The open arrow indicates fetal endothelial cells, and the closed arrow indicates syncytiotrophoblast. Bar, 10 μm. *, P < 0.05 vs. the other two groups.
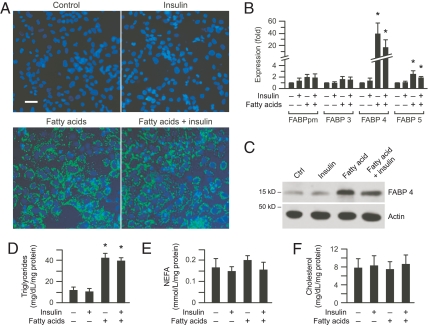
To further our understanding of the role of FABP in cellular trafficking of fatty acids, we examined the effect of insulin, fatty acids, or fatty acids plus insulin on lipid accumulation in cultured term human trophoblasts. Exposure of PHT to fatty acids or fatty acids plus insulin, but not insulin alone, caused marked accumulation of lipid droplets (Fig. 3A). We observed a similar increase in cellular lipid droplets when the culture medium contained either high glucose or low glucose concentration (data not shown). This increase in cellular lipid droplets was associated with a 20- to 40-fold increase in the expression of FABP4 mRNA and a 3.2- to 3.3-fold increase in protein (P < 0.01) from cells exposed to fatty acids or fatty acids plus insulin (Fig. 3, B and C). Similar to changes found in placental samples in vivo, we also detected a small increase in FABP5 expression (Fig. 3B). The levels of cellular triglycerides were approximately 4-fold higher in cells exposed to fatty acids or fatty acids plus insulin (Fig. 3D). There was no significant change in cellular levels of either NEFA or cholesterol (Fig. 3, E and F).
Fig. 3.
Expression of FABP and lipid accumulation in cultured PHT. Cells were cultured for 24 h with vehicle control, insulin (10 nm), linoleic acid (800 μm)/oleic acid (400 μm), or a combination of insulin plus fatty acids as described in Patients and Methods. A, Representative photomicrographs of PHT (n = 5) stained with the BODIPY fluorophore 493/503 for lipid droplets (green) and DAPI for nuclei (blue). Bar, 10 μm. DM, Diabetes mellitus. B, The expression of FABP, determined using RT-qPCR. C, A representative Western blot of FABP4 from PHT from the four experimental groups (n = 4). The bands were quantified using densitometry and normalized to β-actin, as described in Patients and Methods. D–F, Lipid content in cultured PHT including triglycerides (D), NEFA (E), and cholesterol (F). Lipid levels were normalized to total cellular protein (n = 3). *, P < 0.05 vs. the other two groups.
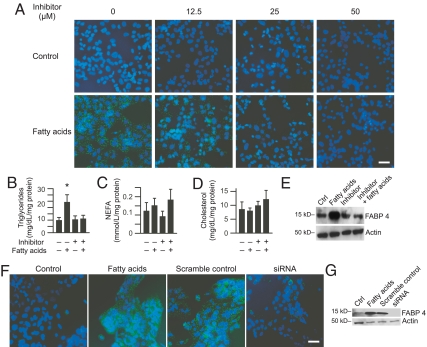
To elucidate the function of FABP4 in cellular lipid accumulation, we exposed cultured PHT to fatty acids in the presence or absence of the selective FABP4 inhibitor BMS309403 (30). As shown in Fig. 4, addition of BMS309403 to cultured PHT exposed to fatty acids caused a concentration-dependent reduction in the accumulation of lipid droplets, with a loss of detectable lipid droplet formation at a BMS309403 concentration of 50 μm (Fig. 4A). Consistent with this finding, BMS309403 abolished the fatty acid-induced increase in triglycerides in cultured trophoblasts, with no effect on NEFA or cholesterol accumulation (Fig. 4, B–D). BMS309403 also attenuated the fatty acid-induced increase in FABP4 protein expression (Fig. 4E). We confirmed these results using siRNA-mediated knockdown of FABP4, which attenuated lipid droplet buildup in PHT cells that were loaded with fatty acids (Fig. 4, F and G). Together these data confirmed that FABP4 is essential for fatty acid-induced lipid droplet accumulation in PHT cells.
Fig. 4.
The influence of the FABP4 inhibitor BMS309403 on intracellular lipid accumulation. Cells were cultured for 6 h with vehicle control or linoleic acid (800 μm)/oleic acid (400 μm) in the absence or presence of BMS309403 (12.5–50 μm) as described in Patients and Methods. A, Representative photomicrographs of PHT (n = 4). Cells were stained with BODIPY fluorophore 493/503 for lipid droplets (green) and DAPI for nuclei (blue) in the presence of increasing concentrations of BMS309403. Bar, 10 μm. B–D, Lipid content in cultured PHT exposed to fatty acids as in A or to 50 μm BMS309403. Lipid levels were normalized to total cellular protein (n = 3). *, P < 0.05 vs. the other three groups. E, A representative Western blot of FABP4 expression in PHT from each of the four experimental groups (BMS309403 concentration 50 μm, n = 4). Ctrl, Control. F, siRNA-medicated knockdown of FABP4, performed as described in Patients and Methods. Shown are representative photomicrographs of PHT (n = 3, bar, 10 μm). G, A representative Western blot of siRNA-mediated FABP4 knockdown (n = 3).
Discussion
FABP4 affects intracellular lipid metabolism by transporting fatty acids or fatty acid substrates to the nucleus for transcriptional regulation, to mitochondria for β-oxidation, and to lipid droplets for storage as triglycerides (35). We found that the expression of FABP4 in placentas of women with pregnancies complicated by obesity and diabetes is increased compared to placentas from nondiabetic obese women or nonobese controls. Our data reveal that FABP4 is expressed in both the trophoblast layer and fetal capillaries of placental villi. Consistent with the transplacental transport of lipids from the maternal blood through the syncytiotrophoblast and capillary endothelial cells into the fetal circulation (36), the expression pattern of FABP4 suggests that it plays a key role in this transport function.
Exposure of cultured human trophoblasts to fatty acids in vitro and in vivo markedly increased the expression of FABP4, suggesting that fatty acids play a dominant role regulating FABP4 expression in PHT. This effect is enhanced by ligand-activated PPARγ (23, 37). Notably, the cross talk between PPARγ and FABP4 might be more complex because FABP4 also binds the PPARγ agonist troglitazone (38). Nonetheless, augmented FABP4 expression by fatty acid-bound PPARγ may explain, at least in part, why inhibition of FABP4 by its potent and selective inhibitor BMS309403, which lowers cellular fatty acid levels, results in compromised ability of PPARγ to up-regulate FABP4 expression and therefore reduced FABP4 levels (29). BMS309403 interacts with the fatty-acid-binding pocket inside FABP4 and competitively inhibits the binding of fatty acids (30). BMS309403 has inhibitory constant values of less than 2 nm for FABP4, compared with inhibitory constant of 250 nm for FABP3 or 350 nm for FAPB5 (30).
As expected, inhibition of FABP4 in PHT cells attenuated lipid droplet formation and triglyceride accumulation. These results are consistent with prior studies, which demonstrated that oral administration of BMS309403 to nonpregnant obese diabetic mice ameliorated hepatic triglyceride accumulation (29). The lack of accumulation of NEFA suggests that inhibition of FABP4 results in decreased uptake or increased efflux of fatty acids and not simply a failure of lipid droplet accumulation. We buttressed our results obtained with FABP4 inhibitor using siRNA-mediated knockdown of FABP4, which resulted in reduced accumulation of lipid droplets in PHT cells, as predicted. Taken together, our results indicate that FABP4 plays a pivotal role in the uptake and accumulation of lipids in human trophoblasts and suggest that inhibition of fatty acid binding to FABP4 attenuates the fatty acid-induced up-regulation of FABP4 expression.
FABP5 is also up-regulated in placentas from obese diabetic women and cells exposed to fatty acids, albeit to a lesser degree than FABP4. FABP4 and FABP5 are expressed concurrently in tissues such as macrophages and adipose tissue, in which the two proteins bind fatty acids with similar selectivity and affinity. In FABP4-null mice, adipose tissue morphology and ability to accumulate triglycerides are relatively unchanged, likely secondary to a compensatory increase in FABP5 expression. FABP4 and FABP5 double knockout mice exhibit a reduction in high-fat-diet-induced obesity as well as improved insulin sensitivity compared with deficiency of either protein alone (39). Although we found that pharmacological inhibition of FABP4 is sufficient to attenuate accumulation of excess lipids in cultured trophoblasts, we cannot rule out the possibility that this inhibitory effect might have been potentiated by concurrent inhibition of FABP5.
Despite elevated levels of maternal serum triglycerides in obese-diabetic patients, we did not detect lipid droplet formation or accumulation of triglycerides within placental biopsies from these patients. Notably, the placenta is different from liver, skeletal muscle, or adipose tissue, which accumulates triglycerides when supply is excessive (14, 40). Our data support the notion that when pregnancy is complicated by obesity and diabetes, increased availability of maternal lipid substrates promotes FABP4-mediated transplacental fatty acid trafficking into the fetal circulation without accumulation of triglycerides in the placenta, resulting in elevated fetal triglycerides and possibly macrosomia (13). Indeed, newborns of obese diabetic women weigh more and had higher blood levels of triglycerides. We note that lipid accumulation in cultured PHT cells was unaffected by the glucose concentration in the culture medium, consistent with findings by others (41, 42). These data, along with the stable blood sugar in the obese diabetes mellitus patients, support the notion that maternal hyperlipidemia is the primary factor leading to increased placental lipid transfer. The reason for elevated placental NEFA in women with diabetes plus obesity is unclear and may suggest reduced esterification and triglyceride formation within these placentas. This observation, as well as the accumulation of lipids in PHT cells in vitro, cannot be explained by the expression levels of diacylglycerol acyltransferase 1, which catalyzes the final committed step in triglyceride synthesis, or adipose triglyceride lipase, a triglyceride lipase that catalyzes the initial step in triglyceride hydrolysis and is expressed in human trophoblasts. With the exception of 2.4-fold increase in diacylglycerol acyltransferase 1 levels in placentas of obese diabetic women, which does not explain our results, the level of these enzymes was largely unchanged in our experimental paradigms (Scifres C., and Y. Sadovsky, unpublished observations).
Although the use of cultured primary trophoblasts provides valuable information regarding the role of FABP4 in lipid uptake and accumulation, these cells do not capture the directional flux of fatty acids that is germane for transplacental lipid transport. We are currently testing experimental systems designed to elucidate the mechanistic role of maternal hyperlipidemia, FABP4, and excess fat transport in the development of fetal macrosomia in vivo. Our studies may have direct clinical implications because they illuminate critical pathways that contribute to fetal macrosomia in pregnancies complicated by maternal diabetes and obesity, in which regulation of placental fatty acid and trafficking play a critical role. Our findings may also suggest new pathways in fetal hyponutrition and subsequent growth restriction.
Acknowledgments
We thank Lori Rideout for assistance during preparation of the manuscript.
This work was supported by the American College of Obstetricians and Gynecologists/Ross Products Division Abbott Research Award on Nutrition in Pregnancy and National Institutes of Health Grants K12-HD063087 (to C.M.S), R01-HD29190 (to D.M.N.), R01-ES011597 and R01-HD045675 (to Y.S.).
Results from this work were presented, in part, at the 29th Annual Meeting of the Society for Maternal-Fetal Medicine, San Diego California, 2009.
Disclosure Summary: The authors declare that there are no conflicts of interest in relation to this study.
Footnotes
- BMI
- Body mass index
- DAPI
- 4′-6-diamidino-2-phenylindole
- FABP
- fatty acid binding protein
- FABPpm
- FABP plasma membrane
- GDM
- gestational diabetes mellitus
- NEFA
- nonesterified fatty acid
- PHT
- primary human trophoblast
- PPAR
- peroxisome proliferator-activated receptor
- RT
- reverse transcription
- RT-qPCR
- RT quantitative PCR
- siRNA
- small interfering RNA
- T2DM
- type 2 diabetes mellitus
- VLCFA
- very long-chain fatty acid.
References
- 1. Innis SM. 2008. Dietary omega 3 fatty acids and the developing brain. Brain Res 1237:35–43 [DOI] [PubMed] [Google Scholar]
- 2. Wiznitzer A, Mayer A, Novack V, Sheiner E, Gilutz H, Malhotra A, Novack L. 2009. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol 201:482.e1–482.-e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haggarty P. 2002. Placental regulation of fatty acid delivery and its effect on fetal growth—a review. Placenta 23(Suppl A):S28–S38 [DOI] [PubMed] [Google Scholar]
- 4. Ehrenberg HM, Dierker L, Milluzzi C, Mercer BM. 2002. Prevalence of maternal obesity in an urban center. Am J Obstet Gynecol 187:1189–1193 [DOI] [PubMed] [Google Scholar]
- 5. Lawrence JM, Contreras R, Chen W, Sacks DA. 2008. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care 31:899–904 [DOI] [PubMed] [Google Scholar]
- 6. Cedergren MI. 2004. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol 103:219–224 [DOI] [PubMed] [Google Scholar]
- 7. Reece EA, Leguizamón G, Wiznitzer A. 2009. Gestational diabetes: the need for a common ground. Lancet 373:1789–1797 [DOI] [PubMed] [Google Scholar]
- 8. Kissebah AH, Alfarsi S, Adams PW, Wynn V. 1976. Role of insulin resistance in adipose tissue and liver in the pathogenesis of endogenous hypertriglyceridaemia in man. Diabetologia 12:563–571 [DOI] [PubMed] [Google Scholar]
- 9. Mooradian AD. 2009. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 5:150–159 [DOI] [PubMed] [Google Scholar]
- 10. Kilby MD, Neary RH, Mackness MI, Durrington PN. 1998. Fetal and maternal lipoprotein metabolism in human pregnancy complicated by type I diabetes mellitus. J Clin Endocrinol Metab 83:1736–1741 [DOI] [PubMed] [Google Scholar]
- 11. Merzouk H, Madani S, Korso N, Bouchenak M, Prost J, Belleville J. 2000. Maternal and fetal serum lipid and lipoprotein concentrations and compositions in type 1 diabetic pregnancy: relationship with maternal glycemic control. J Lab Clin Med 136:441–448 [DOI] [PubMed] [Google Scholar]
- 12. Couch SC, Philipson EH, Bendel RB, Wijendran V, Lammi-Keefe CJ. 1998. Maternal and cord plasma lipid and lipoprotein concentrations in women with and without gestational diabetes mellitus. Predictors of birth weight? J Reprod Med 43:816–822 [PubMed] [Google Scholar]
- 13. Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, Herrera E. 2008. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 31:1858–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Szendroedi J, Roden M. 2009. Ectopic lipids and organ function. Curr Opin Lipidol 20:50–56 [DOI] [PubMed] [Google Scholar]
- 15. Ducharme NA, Bickel PE. 2008. Lipid droplets in lipogenesis and lipolysis. Endocrinology 149:942–949 [DOI] [PubMed] [Google Scholar]
- 16. Farese RV, Jr, Walther TC. 2009. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139:855–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. 2010. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res 51:468–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schaiff WT, Knapp FF, Jr, Barak Y, Biron-Shental T, Nelson DM, Sadovsky Y. 2007. Ligand-activated peroxisome proliferator activated receptor γ alters placental morphology and placental fatty acid uptake in mice. Endocrinology 148:3625–3634 [DOI] [PubMed] [Google Scholar]
- 19. Bildirici I, Roh CR, Schaiff WT, Lewkowski BM, Nelson DM, Sadovsky Y. 2003. The lipid droplet-associated protein adipophilin is expressed in human trophoblasts and is regulated by peroxisomal proliferator-activated receptor-γ/retinoid X receptor. J Clin Endocrinol Metab 88:6056–6062 [DOI] [PubMed] [Google Scholar]
- 20. Elchalal U, Schaiff WT, Smith SD, Rimon E, Bildirici I, Nelson DM, Sadovsky Y. 2005. Insulin and fatty acids regulate the expression of the fat droplet-associated protein adipophilin in primary human trophoblasts. Am J Obstet Gynecol 193:1716–1723 [DOI] [PubMed] [Google Scholar]
- 21. Storch J, Corsico B. 2008. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu Rev Nutr 28:73–95 [DOI] [PubMed] [Google Scholar]
- 22. Furuhashi M, Hotamisligil GS. 2008. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7:489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Biron-Shental T, Schaiff WT, Ratajczak CK, Bildirici I, Nelson DM, Sadovsky Y. 2007. Hypoxia regulates the expression of fatty acid-binding proteins in primary term human trophoblasts. Am J Obstet Gynecol 197:516.e1–516.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wyatt SM, Kraus FT, Roh CR, Elchalal U, Nelson DM, Sadovsky Y. 2005. The correlation between sampling site and gene expression in the term human placenta. Placenta 26:372–379 [DOI] [PubMed] [Google Scholar]
- 25. Nelson DM, Johnson RD, Smith SD, Anteby EY, Sadovsky Y. 1999. Hypoxia limits differentiation and up-regulates expression and activity of prostaglandin H synthase 2 in cultured trophoblast from term human placenta. Am J Obstet Gynecol 180:896–902 [DOI] [PubMed] [Google Scholar]
- 26. Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., 3rd 1986. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118:1567–1582 [DOI] [PubMed] [Google Scholar]
- 27. Chen B, Nelson DM, Sadovsky Y. 2006. N-myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury. J Biol Chem 281:2764–2772 [DOI] [PubMed] [Google Scholar]
- 28. Rimon E, Chen B, Shanks AL, Nelson DM, Sadovsky Y. 2008. Hypoxia in human trophoblasts stimulates the expression and secretion of connective tissue growth factor. Endocrinology 149:2952–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Furuhashi M, Tuncman G, Görgun CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, Sulsky R, Robl JA, Parker RA, Hotamisligil GS. 2007. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 447:959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sulsky R, Magnin DR, Huang Y, Simpkins L, Taunk P, Patel M, Zhu Y, Stouch TR, Bassolino-Klimas D, Parker R, Harrity T, Stoffel R, Taylor DS, Lavoie TB, Kish K, Jacobson BL, Sheriff S, Adam LP, Ewing WR, Robl JA. 2007. Potent and selective biphenyl azole inhibitors of adipocyte fatty acid binding protein (aFABP). Bioorg Med Chem Lett 17:3511–3515 [DOI] [PubMed] [Google Scholar]
- 31. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-ΔΔC(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 33. Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509 [PubMed] [Google Scholar]
- 34. Storch J, McDermott L. 2009. Structural and functional analysis of fatty acid-binding proteins. J Lipid Res 50(Suppl):S126–S131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Storch J, Thumser AE. 2000. The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta 1486:28–44 [DOI] [PubMed] [Google Scholar]
- 36. Duttaroy AK. 2009. Transport of fatty acids across the human placenta: a review. Prog Lipid Res 48:52–61 [DOI] [PubMed] [Google Scholar]
- 37. Schaiff WT, Bildirici I, Cheong M, Chern PL, Nelson DM, Sadovsky Y. 2005. Peroxisome proliferator-activated receptor-γ and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J Clin Endocrinol Metab 90:4267–4275 [DOI] [PubMed] [Google Scholar]
- 38. Gillilan RE, Ayers SD, Noy N. 2007. Structural basis for activation of fatty acid-binding protein 4. J Mol Biol 372:1246–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, Minokoshi Y, Kahn BB, Parker RA, Hotamisligil GS. 2005. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab 1:107–119 [DOI] [PubMed] [Google Scholar]
- 40. Thompson BR, Lobo S, Bernlohr DA. 2010. Fatty acid flux in adipocytes: the in's and out's of fat cell lipid trafficking. Mol Cell Endocrinol 318:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Radaelli T, Lepercq J, Varastehpour A, Basu S, Catalano PM, Hauguel-De Mouzon S. 2009. Differential regulation of genes for fetoplacental lipid pathways in pregnancy with gestational and type 1 diabetes mellitus. Am J Obstet Gynecol 201:209.e1—209.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pathmaperuma AN, Maña P, Cheung SN, Kugathas K, Josiah A, Koina ME, Broomfield A, Delghingaro-Augusto V, Ellwood DA, Dahlstrom JE, Nolan CJ. 2010. Fatty acids alter glycerolipid metabolism and induce lipid droplet formation, syncytialisation and cytokine production in human trophoblasts with minimal glucose effect or interaction. Placenta 31:230–239 [DOI] [PubMed] [Google Scholar]