Abstract
Objective:
The aim was to describe the clinical presentation and to characterize the genetic mutation present in a child with congenital malabsorptive diarrhea and neonatal diabetes.
Research Design and Methods:
Clinical data were obtained from chart review. Histopathological characterization of intestinal samples and neurogenin-3 (NEUROG3) sequencing were performed. Expression and function of the mutated NEUROG3 protein were assessed by Western blot analysis and luciferase reporter assay.
Results:
At birth, the proband was small for gestational age. She presented for evaluation with persistent diarrhea and a poor postnatal growth pattern. Although the pancreas was present, serum amylase and fecal elastase levels were decreased, and blood glucose levels were persistently elevated by 5 months of age. Immunostaining of a small intestine biopsy for chromogranin A demonstrated complete absence of neuroendocrine cells. Genetic analysis revealed a nonsense mutation (E123X) in the region encoding helix II of the NEUROG3 gene, leading to premature termination at amino acid 123. The mutated truncated NEUROG3 protein was identified by Western blot analysis. Reporter assays show decreased transactivation of the NEUROD1 promoter by mutant NEUROG3 protein as compared to wild type.
Conclusions:
This report describes a newly identified nonsense mutation in human NEUROG3 that in the homozygous state is associated with neonatal diabetes and malabsorptive diarrhea.
Neurogenin-3 (NEUROG3) is a basic helix loop helix transcription factor that is first detected in the mouse embryonic pancreatic epithelium at d 9.5, peaking by embryonic d 15.5 (1). After birth, expression of NEUROG3 begins to decline, and little if any expression is present in the adult mouse (2, 3). NEUROG3 is an essential factor in the development of enteroendocrine, Paneth, goblet, and enterocyte cells in the intestine (2, 4–6), and lineage tracing studies have determined that NEUROG3+ cells are early endocrine progenitors (7). NEUROG3−/− mice are completely devoid of endocrine cells in both the pancreas and intestine and die shortly after birth of diabetes (2). Transcriptional targets of NEUROG3 that mediate its role include NeuroD/Beta2, Pax4, Nkx2.2, IA-1 and NEUROG3 itself, among others (1).
In humans, homozygous mutations of NEUROG3 (8) manifest with congenital malabsorptive diarrhea and childhood-onset diabetes. The intestinal mucosa of these children is devoid of enteroendocrine cells. Herein, we describe an infant with congenital malabsorptive diarrhea and neonatal diabetes, who we hypothesized had a homozygous NEUROG3 mutation resulting in her severe phenotype, affecting both enteroendocrine cell function and insulin production of the pancreatic β-cell with clinical manifestations presenting within the first 6 months of life. The proband was subsequently found to have a homozygous mutation in NEUROG3, encoding an early stop codon in the helix II region of NEUROG3.
Subject and Methods
Clinical data
Clinical information was obtained by chart review. This study was approved by the Children's Hospital of Philadelphia Institutional Review Board.
Pathological analysis of the intestine
Formalin-fixed, paraffin-embedded intestinal biopsy samples were stained with hematoxylin and eosin, chromogranin A, periodic acid-Schiff, lysozyme, and Ki-67.
DNA mutation analysis
NEUROG3 mutation analysis was performed on genomic DNA isolated from the proband and her mother. Exon 1 (noncoding sequence) and exon 2 (coding sequence and 3′ untranslated region) of NEUROG3 were amplified by PCR. Sequences generated were compared with published sequence NM_020999 (http://www.ncbi.nlm.nih.gov/). For details, see Supplemental Data published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Expression studies in 293T cells
A pCMV-XL5 expression vector containing human NEUROG3 was obtained from OriGene Technologies Inc. (Rockville, MD). Site-directed mutagenesis was performed using a QuickChange II Site-Directed Mutagenesis Kit (Stratagene, Santa Clara, CA). Transfections of wild-type and mutant NEUROG3 into 293T cells were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with 0.5 μg luciferase reporter plasmid (NEUROD1 E1-E3-pGL3 basic), 0.1 μg CMV-βgal internal control plasmid, and 0.25 μg expression plasmids [pCMV6-XL5-Ngn3 [Ngn3 wild type (WT)], pCMV6-XL5-Ngn3 mutant (Ngn3 mut), or pCMV6-XL5 empty vector (EV)], plus 0.25 μg of CMV-E47 expression plasmid. Cells were harvested after 48 h and assayed for luciferase activity (Promega, Madison, WI). All transfection assays were performed in triplicate. Western blot analysis from whole cell extracts was performed using neurogenin-3 antibody (Abcam, Cambridge, MA). For the Western blot, loading protein quantity was standardized by equivalent β-actin bands. For the luciferase assay, cells were normalized to β-galactosidase activity and expressed as fold change over EV. The luciferase assay and Western blot transfections were performed in parallel. Data were analyzed by one-way ANOVA.
Results
Clinical case summary
A 23-d-old female infant presented with persistent diarrhea, increased sleepiness, irritability, and abdominal distention. At birth, the infant was small for gestational age weighing 1960 g at 37 wk gestation. At presentation, she was afebrile with stable vital signs but cachectic with a weight of 1825 g. The diarrhea had been present continuously since birth, with six to eight nonbloody, loose stools daily. Initial laboratory findings revealed hyperchloremic acidosis and a plasma glucose of 5.9 mmol/liter (106 mg/dl). The newborn screen and other metabolic studies were normal. After admission, the proband's volume of stool reached 260 ml/kg · d (normal stool volume for children is 20 ml/kg · d).
Consultations by gastroenterology, immunology, and infectious disease specialists initially suspected an infectious or allergic etiology, but no infectious agent or allergen was isolated. Sweat chloride testing was negative. Immunological evaluation revealed no abnormalities; T-cell functional studies were normal, and HIV-1 and HIV-2 antibody testing was negative. IPEX (immunodeficiency, polyendocrinopathy, and enteropathy, X-linked syndrome) was suspected, but FOXP3 mutation analysis was negative. Stool-reducing substances were mildly elevated (1+/4), indicating impairment of carbohydrate absorption. Stool volume decreased dramatically when enteral feeds were held, but as feedings were reintroduced, stool output increased. The infant was maintained on trophic enteral feeds with total parental nutrition.
Amylase levels were low (<30 U/liter; normal, 30–100 U/liter), and fecal elastase levels were decreased (79 mg/g; normal, 200–500 mg/g), indicating impaired pancreatic exocrine function. The proband was treated with supplemental pancreatic enzymes with limited improvement. An abdominal computed tomography scan demonstrated a normal pancreas. The infant had persistent hyperglycemia at 5½ months of age and was started on insulin. Before 20 wk of age, blood sugars were rarely over 8.3 mmol/liter (150 mg/dl), but at 20 wk blood sugars were regularly greater than 11.1 mmol/liter (200 mg/dl). C-peptide level before insulin initiation was present [0.6 nmol/liter (1.9 ng/ml)] but low for an infant with hyperglycemia.
Neonatal diabetes testing (KCNJ11, GCK, and IPF-1 mutation analysis) and GAD-65, INS, and ICA-512 antibody testing were negative, providing no evidence for autoimmune diabetes or known genetic forms of neonatal diabetes.
Eventually, the proband's chronic diarrhea was treated with corticosteroids and tacrolimus, exacerbating the hyperglycemia, and subsequently her insulin requirement increased to approximately 1.5 U/kg · d at age 10 months. Postnatal growth continued to track less than the 5th percentile, with failure of catch-up growth. The child expired at 10 months of age from chronic liver failure secondary to cholestasis caused by total parenteral nutrition. An autopsy was declined by the family.
Family history
The proband's mother had diet-controlled gestational diabetes and a history of polycystic ovarian syndrome. The proband's father had three additional healthy male children from another relationship. His DNA was not available for genetic testing, but he suffered from chronic diarrhea of unknown etiology. The parents were not known to be consanguineous but were from the same region of southern Ecuador.
Histopathological analysis of the proband's intestine
Immunohistochemical analyses of small intestine specimens taken at 5 months revealed moderate to severe villous blunting and atrophy with mild crypt hyperplasia (Fig. 1A). Epithelial replication rates assessed by Ki-67 staining were decreased compared with age-matched control (9 vs. 52%) (Supplemental Fig. 1). Goblet cells and Paneth cells are present in a normal pattern (Fig. 1, B and C). Staining of the small intestine with chomogranin A, a neuroendocrine marker, shows the complete absence of neuroendocrine cells (Fig. 1E).
Fig. 1.
Small intestine biopsy samples show rare enteroendocrine cell staining. Endoscopic biopsy samples were obtained at 5 months of age. Immunohistochemical analysis was carried out on paraffin-embedded intestinal biopsies. A, Hematoxylin and eosin stain shows small intestinal mucosa with villous atrophy and mild crypt hyperplasia. B, Periodic acid-Schiff stain shows goblet cells. C, Lysozyme stain shows Paneth cells. D, Normal control with chromogranin A stain shows neuroendocrine cells (NE) within the glands. E, Chromogranin A stain shows complete absence of neuroendocrine cells within the glands.
Sequencing of NEUROG3
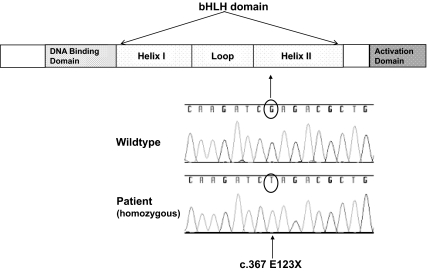
We hypothesized that the paucity of enteroendocrine cells in the intestine along with the clinical symptoms of malabsorptive diarrhea and diabetes were caused by a mutation in the NEUROG3 gene and therefore sequenced NEUROG3 in the proband. The proband was homozygous for a guanine to thymidine mutation leading to an early stop codon at amino acid 123 in the region encoding helix II of the NEUROG3 gene (Fig. 2). The proband's mother was heterozygous for the same mutation. Two other human NEUROG3 homozygous mutations have been reported, both in the region of helix I (8). Both the helix I and helix II regions of NEUROG3 are highly conserved, indicating a critical function for these regions.
Fig. 2.
Homozygous mutation in helix II of NEUROG3 encodes an early stop codon. NEUROG3 mutation analysis was performed in genomic DNA from blood samples obtained from the patient and her mother. The two exons of NEUROG3 were amplified by PCR, isolated, and sequenced using standard methods. The patient was found to have a G→T mutation encoding an early stop codon in helix II. The patient's mother was found to be heterozygous for this same mutation.
Identification of mutant NEUROG3 protein
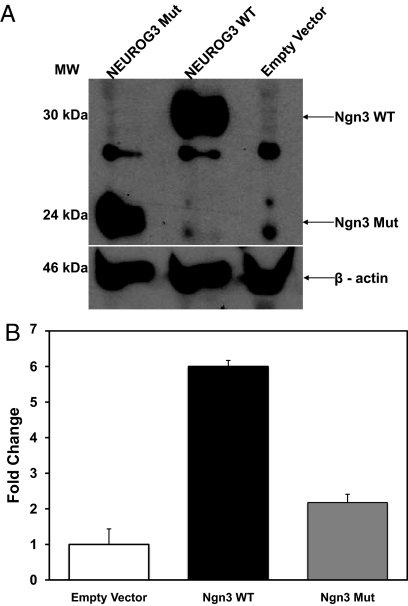
Site-directed mutagenesis was performed to recreate the mutation in a wild-type human NEUROG3 expression plasmid. Mutant and wild-type human NEUROG3 were expressed in 293T cells, and protein levels were evaluated by Western blot analysis. Expression levels were similar for mutant and wild-type protein (Fig. 3A). As expected, the mutant NEUROG3 protein showed increased mobility on SDS-PAGE with an apparent molecular mass of 24 kDa, compared with 30 kDa for wild-type NEUROG3.
Fig. 3.
Functional analysis of mutated NEUROG3 protein. A, Identification of mutant NEUROG3 protein. Wild-type (WT) and mutant forms of Homo sapiens NEUROG3 protein expression levels in 293T cells were measured by Western blot analysis. NEUROG3 WT protein is visualized at 30 kDa, and a truncated NEUROG3 mut protein is visualized at 24 kDa. This visualized gel is a representative image from three replicates. B, Mutant NEUROG3 protein fails to activate the NEUROD1 promoter. 293T cells were transfected with luciferase reporter plasmid, internal control plasmid, and expression plasmids (Ngn3 WT, Ngn3 mut, or EV), plus E47 expression plasmid. Values are expressed as means ± se of three independent transfections; P = 0.006 between groups.
Transactivation of the NEUROD1 promoter
To characterize the functional impact of the proband's NEUROG3 mutation, a luciferase reporter assay was developed to define the ability of mutant NEUROG3 to activate a target gene promoter. 293T cells were transfected with a NEUROD1 luciferase reporter plasmid and with one of three expression plasmids [EV, neurogenin-3 wild-type (Ngn3 WT), or neurogenin-3 mutant (Ngn3 mut) plasmid] in conjunction with a plasmid expressing E47. Cells were harvested 48 h after transfection and assayed for luciferase activity. The Ngn3 WT plasmid had six times greater luciferase activity than EV, whereas the Ngn3 mut showed only 2.1 times the luciferase activity of EV (P = 0.006), indicating that the mutation significantly impacted NEUROG3 ability to activate the NEUROD1 promoter (Fig. 3B).
Discussion
Here we report a novel human NEUROG3 mutation resulting in a premature stop codon at amino acid 123 in helix II, which in the homozygous state is associated with congenital malabsorptive diarrhea, neonatal diabetes, and impaired pancreatic exocrine function.
The proband's intestine shows severe villous blunting and atrophy and mild crypt hyperplasia, with a paucity of neuroendocrine cells, consistent with mouse studies indicating that enteroendocrine cells are derived from NEUROG3-expressing cells (4, 9–12). We did not observe any effect on Paneth and goblet cell populations, which is not surprising given the small percentage of these cells that are derived from NEUROG3-expressing cells (11). Epithelial replication rates were markedly decreased, possibly due to disuse atrophy. Previously identified human NEUROG3 mutations (R107S, R93L) led to congenital malabsorptive diarrhea in part due to a reduction of intestinal endocrine cells without affecting the number of Paneth and goblet cells (8). Rates of replication were not reported for these cases, but in a transgenic model with intestinal-specific ablation of NEUROG3, there was enlargement of the proliferative crypt compartment, accompanied by an accelerated cell turnover and shorter villi (12). The previously described patients with NEUROG3 mutations (R107S, R93L) did not have gross changes in intestinal villus architecture, suggesting that: 1) human E123X is a more severe mutation and NEUROG3 affects intestinal development by influencing formation of nonendocrine cells directly or via a noncell autonomous mechanism; or 2) the abnormality in villous architecture is a consequence of the disease process, i.e. malabsorptive diarrhea; and/or 3) patients with R107S and R93L mutations were able to compensate for defects in intestinal development and remodel with age.
All three human cases of NEUROG3 mutations presented with chronic malabsorptive diarrhea (8). In the mouse model of the intestinal-specific NEUROG3 ablation, the authors attribute the yellowish stools to lipid malabsorption, impaired bile acid function, and decreased intestinal transit time (12). In the present human case, the etiology of the malabsorptive diarrhea is likely multifactorial and could be secondary to pancreatic exocrine deficiencies and/or changes in the intestinal epithelium, given that the NEUROG3 deficiency is global.
In contrast to intestinal development, the role of NEUROG3 in mouse pancreas development has been more extensively studied. NEUROG3 is expressed in pancreatic endocrine progenitors during development and is required for formation of all islet endocrine cells in mice (2, 11, 13–15). Thus, NEUROG3 null newborn mice are severely diabetic (2). Similarly, the proband with E123X NEUROG3 mutation developed diabetes by 5 months of age. Furthermore, her low birth weight suggests insulin deficiency during fetal development. The child's mother had a history of gestational diabetes, although we were unable to confirm her phenotype with glucose tolerance testing. Furthermore, the two previously described individuals with R93L mutations developed hyperglycemia after 8 yr of age, implying an important role for NEUROG3 in islet development and/or function in humans.
The difference in severity between the gastrointestinal and islet phenotypes of human NEUROG3 mutations suggests either that the threshold level of NEUROG3 required for islet formation/function may be less than for intestinal endocrine formation or that binding of NEUROG3 to putative partners is differentially affected. Moreover, the difference in the level of hyperglycemia between the patients with NEUROG3 mutations may also reflect differences in the ability to bind to target sequences or partners (16). Surprisingly, R107S and R93L (8) as well as E123X NEUROG3 mutants all show marked blunting in transactivation of the NEUROD1 promoter in vitro, suggesting that additional factors influence endocrine development and/or function in vivo. Activation of other NEUROG3 targets involved in endocrine development and function, including IA-1, Pax4, Nkx2.2, Rfx6, and NEUROG3 itself (17–21), may also be affected by these mutations and will require further characterization.
Few reports suggest that NEUROG3 may influence pancreatic acinar morphogenesis, possibly through its activation of NEUROD1 (2, 11). In addition, NEUROG3 may influence exocrine function by regulating formation of cholecystokinin and secretin-expressing enteroendocrine cells through NEUROD1 (22). Unlike previous reports of human NEUROG3 mutations where pancreatic exocrine function was unchanged (8), we observed a reduction in serum amylase and fecal elastase levels in the proband, indicating impairment in pancreatic exocrine function and implicating a role for NEUROG3 in acinar development and/or function in humans. Alternatively, this finding may be secondary to the primary pathology and not directly related to the NEUROG3 mutation.
Currently, the literature is inconsistent regarding attempts to define the contribution of NEUROG3 to the development of diabetes (23–29). This case indicates that homozygous NEUROG3 mutations can cause neonatal diabetes and may cause late-onset diabetes in the heterozygous state.
In summary, we have identified a novel NEUROG3 mutation associated with neonatal diabetes, pancreatic exocrine deficiency, and malabsorptive diarrhea. Our findings confirm that NEUROG3 plays an essential role in the development of enteroendocrine and pancreatic β-cells in humans and suggest a potential role of NEUROG3 in acinar cell development and/or function. NEUROG3 may be responsible for monogenic forms of diabetes, and further identification and analysis of larger cohorts of families with NEUROG3 mutations is required to definitely establish whether NEUROG3 is a novel familial monogenic diabetes gene and to determine its association with type 2 diabetes in the heterozygous state.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant 1K23 DK073663-01 (to D.D.D.L.), the Lawson Wilkins Pediatric Endocrine Society Fellowship Award (to S.E.P.), and the NIH Child Health Research Program Career Development Award (K12) (to S.E.P.). The project described was supported by Grant no. UL1RR024134 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the NIH.
Author Contributions: S.E.P. researched data, contributed to discussion, wrote manuscript, and reviewed/edited manuscript. J.O.-K. researched data, contributed to discussion, wrote manuscript, and reviewed/edited manuscript. L.E. researched data and reviewed/edited manuscript. N.H. researched data. P.P. researched data and reviewed/edited manuscript. D.A.S. reviewed/edited manuscript. P.R. researched data and reviewed/edited manuscript. D.D.D.L. researched data, contributed to discussion, and reviewed/edited manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- EV
- Empty vector
- mut
- mutant
- NEUROG3
- neurogenin-3
- WT
- wild type.
References
- 1. Oliver-Krasinski JM, Stoffers DA. 2008. On the origin of the β cell. Genes Dev 22:1998–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gradwohl G, Dierich A, LeMeur M, Guillemot F. 2000. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA 97:1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gu G, Wells JM, Dombkowski D, Preffer F, Aronow B, Melton DA. 2004. Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development 131:165–179 [DOI] [PubMed] [Google Scholar]
- 4. Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. 2002. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J 21:6338–6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee CS, Perreault N, Brestelli JE, Kaestner KH. 2002. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev 16:1488–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. 2001. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294:2155–2158 [DOI] [PubMed] [Google Scholar]
- 7. Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. 2000. Control of endodermal endocrine development by Hes-1. Nat Genet 24:36–44 [DOI] [PubMed] [Google Scholar]
- 8. Wang J, Cortina G, Wu SV, Tran R, Cho JH, Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, Hill ID, Vargas JH, Gershman G, Farmer DG, Reyen L, Martín MG. 2006. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med 355:270–280 [DOI] [PubMed] [Google Scholar]
- 9. López-Díaz L, Jain RN, Keeley TM, VanDussen KL, Brunkan CS, Gumucio DL, Samuelson LC. 2007. Intestinal neurogenin 3 directs differentiation of a bipotential secretory progenitor to endocrine cell rather than goblet cell fate. Dev Biol 309:298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bjerknes M, Cheng H. 2006. Neurogenin 3 and the enteroendocrine cell lineage in the adult mouse small intestinal epithelium. Dev Biol 300:722–735 [DOI] [PubMed] [Google Scholar]
- 11. Schonhoff SE, Giel-Moloney M, Leiter AB. 2004. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol 270:443–454 [DOI] [PubMed] [Google Scholar]
- 12. Mellitzer G, Beucher A, Lobstein V, Michel P, Robine S, Kedinger M, Gradwohl G. 2010. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J Clin Invest 120:1708–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. 2000. Expression of neurogenin 3 reveals an islet cell precursor population in the pancreas. Development 127:3533–3542 [DOI] [PubMed] [Google Scholar]
- 14. Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. 2000. Independent development of pancreatic α- and β-cells from neurogenin 3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes 49:163–176 [DOI] [PubMed] [Google Scholar]
- 15. Gu G, Dubauskaite J, Melton DA. 2002. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129:2447–2457 [DOI] [PubMed] [Google Scholar]
- 16. Huang HP, Liu M, El-Hodiri HM, Chu K, Jamrich M, Tsai MJ. 2000. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol Cell Biol 20:3292–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith SB, Gasa R, Watada H, Wang J, Griffen SC, German MS. 2003. Neurogenin 3 and hepatic nuclear factor 1 cooperate in activating pancreatic expression of Pax4. J Biol Chem 278:38254–38259 [DOI] [PubMed] [Google Scholar]
- 18. Watada H, Scheel DW, Leung J, German MS. 2003. Distinct gene expression programs function in progenitor and mature islet cells. J Biol Chem 278:17130–17140 [DOI] [PubMed] [Google Scholar]
- 19. Smith SB, Watada H, German MS. 2004. Neurogenin 3 activates the islet differentiation program while repressing its own expression. Mol Endocrinol 18:142–149 [DOI] [PubMed] [Google Scholar]
- 20. Mellitzer G, Bonné S, Luco RF, Van De Casteele M, Lenne-Samuel N, Collombat P, Mansouri A, Lee J, Lan M, Pipeleers D, Nielsen FC, Ferrer J, Gradwohl G, Heimberg H. 2006. IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. EMBO J 25:1344–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith SB, Qu HQ, Taleb N, Kishimoto NY, Scheel DW, Lu Y, Patch AM, Grabs R, Wang J, Lynn FC, Miyatsuka T, Mitchell J, Seerke R, Désir J, Eijnden SV, Abramowicz M, Kacet N, Weill J, Renard ME, Gentile M, Hansen I, Dewar K, Hattersley AT, Wang R, Wilson ME, Johnson JD, Polychronakos C, German MS. 2010. Rfx6 directs islet formation and insulin production in mice and humans. Nature 463:775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. 1997. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev 11:2323–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jackson AE, Cassell PG, North BV, Vijayaraghavan S, Gelding SV, Ramachandran A, Snehalatha C, Hitman GA. 2004. Polymorphic variations in the neurogenic differentiation-1, neurogenin-3, and hepatocyte nuclear factor-l alpha genes contribute to glucose intolerance in a South Indian population. Diabetes 53:2122–2125 [DOI] [PubMed] [Google Scholar]
- 24. Li J, Bergmann A, Reimann M, Schulze J, Bornstein SR, Schwarz PE. 2008. Genetic variation of neurogenin 3 is slightly associated with hyperproinsulinaemia and progression toward type 2 diabetes. Exp Clin Endocrinol Diabetes 116:178–183 [DOI] [PubMed] [Google Scholar]
- 25. Milord E, Gragnoli C. 2006. NEUROG3 variants and type 2 diabetes in Italians Minerva Med 97:373–378 [PubMed] [Google Scholar]
- 26. Jensen JN, Hansen L, Ekstrom CT, Pociot F, Nerup J, Hansen T, Pedersen O. 2001. Polymorphisms in the neurogenin 3 gene (NEUROG) and their relation to altered insulin secretion and diabetes in the Danish Caucasian population. Diabetologia 44:123–126 [DOI] [PubMed] [Google Scholar]
- 27. Kim SH, Warram JH, Krolewski AS, Doria A. 2001. Mutation screening of the neurogenin-3 gene in autosomal dominant diabetes. J Clin Endocrinol Metab 86:2320–2322 [DOI] [PubMed] [Google Scholar]
- 28. del Bosque-Plata L, Lin J, Horikawa Y, Schwarz PE, Cox NJ, Iwasaki N, Ogata M, Iwamoto Y, German MS, Bell GI. 2001. Mutations in the coding region of the neurogenin 3 gene (NEUROG3) are not a common cause of maturity-onset diabetes of the young in Japanese subjects. Diabetes 50:694–696 [DOI] [PubMed] [Google Scholar]
- 29. Okada T, Tobe K, Hara K, Yasuda K, Kawaguchi Y, Ikegami H, Ito C, Kadowaki T. 2001. Variants of neurogenin 3 gene are not associated with Type II diabetes in Japanese subjects. Diabetologia 44:241–244 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.