Abstract
Context:
Albright hereditary osteodystrophy (AHO) is a rare genetic disorder characterized by phenotypic abnormalities including brachydactyly/brachymetacarpia, short stature, and sc ossifications. Carpal tunnel syndrome (CTS) is a chief complaint in many patients with AHO.
Objective:
The objective of the study was to investigate the prevalence of CTS in patients with AHO.
Design:
This was a cross-sectional study.
Setting:
The study was conducted at the Clinical Research Center (Institute of Clinical and Translational Medicine), Johns Hopkins University School of Medicine and Albright Clinic, Kennedy Krieger Institute.
Participants:
Thirty-three subjects with a diagnosis of AHO participated in the study.
Main Outcome Measures:
We assessed for the presence and location of hand tingling, numbness, pain, weakness, flick sign, difficulty with fine motor skills, severe hand or nail biting, and nocturnal symptoms in the setting of normocalcemia and a euthyroid state. Patients were considered to have CTS if they were positive for three of these symptoms. All subjects were analyzed for mutations in the GNAS gene.
Results:
Twenty-two subjects (67%) had a clinical diagnosis of CTS (95% confidence interval 0.48, 0.82). Twenty-eight of 33 subjects were confirmed to have mutations in GNAS, of whom 68% had CTS (95% confidence interval 0.48, 0.84). There were 14 children in this study; 36% had a clinical diagnosis of CTS. Body mass index, brachydactyly/brachymetacarpia, prior GH treatment, and specific GNAS mutations were not associated with CTS.
Conclusions:
We report a high prevalence of CTS in both adults and children with AHO. The diagnosis of CTS should be considered when evaluating a patient with AHO because the intervention for CTS could improve overall function and quality of life in these patients.
Albright hereditary osteodystrophy (AHO) is a rare genetic disorder characterized by phenotypic abnormalities including brachydactyly, brachymetacarpia, short stature, and sc ossifications. This disorder is caused by heterozygous inactivating mutations in GNAS, the gene encoding the α-chain of the heterotrimeric G protein, Gs, that couples receptors for many hormones and neurotransmitters to activation of adenylyl cyclase. Transcripts encoding Gαs are preferentially expressed from the maternally inherited allele in the renal proximal tubule, thyroid, gonad, and pituitary (1–6). AHO patients who have GNAS mutations on maternally inherited alleles manifest resistance to multiple hormones (including PTH, TSH, gonadotropins, and GHRH) as well as obesity and cognitive deficits (1–4, 7–10). This condition is termed pseudohypoparathyroidism type 1a (PHP 1a) and is due to tissue-specific paternal imprinting (for review, see Ref. 11–14). Conversely, patients with AHO and GNAS mutations on their paternally inherited alleles have no evidence of hormonal resistance, are typically not obese (9), and cognitive function may be normal (10). This condition is termed pseudopseudohypoparathyroidism (PPHP) (11–14).
During our evaluation of a large population of patients with AHO followed up by us at the Albright Clinic at Kennedy Krieger Institute as well as the Clinical Research Center at the Johns Hopkins Hospital, it became evident that carpal tunnel syndrome (CTS) caused significant morbidity in many of these patients. There are no reports documenting CTS in adults or children with AHO. Therefore, we evaluated the prevalence of CTS in these subjects.
CTS is caused by compression of the median nerve in the wrist. It is common in adults, with a prevalence of 3.8% when diagnosed by clinical findings and a prevalence of 2.7% when neurophysiologic testing is used to confirm symptoms (15). CTS is more common in patients older than 40 yr old, and there is a female predominance (15–19). Risk factors include inflammatory arthritis, diabetes, hypothyroidism, hemodialysis, acromegaly, corticosteroids, and trauma (20–22).
CTS is exceedingly rare in children and is usually associated with lysosomal storage diseases (21, 23, 24). Other causes include trauma, hemangiomas, stenosing tenosynovitis, median nerve tumors, familial syndromes, scleroderma, and diabetes (23–28). Diagnosis in children can be difficult because they may present with nonspecific symptoms such as poor fine motor skills, weakness, clumsiness, and biting of their hands and fingers (24, 25, 27, 28).
The purpose of this study was to determine the prevalence of CTS in subjects with AHO. Additionally, we sought to investigate a potential role for body mass index (BMI), brachydactyly/brachymetacarpia, prior GH treatment, and specific GNAS mutations in the development of CTS in AHO.
Subjects and Methods
Subjects
This study was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions (which includes the Kennedy Krieger Institute). Informed consent was obtained from each subject or a parent of each subject before participation. Thirty-three subjects who had been diagnosed with PHP 1a or PPHP in the Clinical Research Center of the Johns Hopkins Medical Institutions and/or the Albright Clinic, Kennedy Krieger Institute (Baltimore, MD) were examined by a single endocrinologist for consistency (E.L.G.-L.), and extensive past medical histories were obtained (Table 1). These patients were diagnosed based on clinical criteria including phenotype for PHP 1a/PPHP, hormonal resistance (for PHP1a), and mutation analyses. Because hypocalcemia can cause symptoms that are similar to those of CTS, we restricted participation in our study to those PHP 1a patients who had a normal calcium status in the 12 months preceding the assessment for CTS. (All PPHP patients, by definition, had no evidence of hormonal resistance, and normal TSH, PTH, and calcium levels were verified at their initial presentation to us.) At the time of the study, all subjects were under medical management by an endocrinologist at our institution (E.L.G.-L.), often in conjunction with the subjects' local endocrinologists. Through our own laboratory studies and/or through correspondence with the patients' local laboratories and/or endocrinologists, all subjects were known to be euthyroid and have normal serum calcium and phosphate levels in the 12 months before the study (Table 2). [Three subjects had very minimally elevated phosphate levels (mean of 0.08 mmol/liter above normal) and one had a minimally elevated TSH but normal free T4].
Table 1.
Clinical characteristics and demographics of study subjects at time of CTS symptoms
| Subject | CTS Dx | AHO Dx | Age | Sex | GH | BMI Z-score | CTS EMG | Brachymetacarpia/brachydactyly | CTS-related signs and symptoms |
Weakness | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain | Tingling | Numbness | Bilateral | Flick sign | Nocturnal Sx | Fine motor | ||||||||||
| 1a | + | PHP 1a | 8.3 | F | Y | 2.64 | N/A | Y | Y | U | N | Y | Y | N | Y | Y |
| 2 | + | PHP 1a | 8.5 | F | N | 3.21 | N/A | Y | Y | U | U | Y | Y | Y | Y | N |
| 3b | + | PHP 1a | 11.0 | M | N | 1.61 | N/A | Y | Y | Y | Y | Y | Y | N | Y | Y |
| 4c | + | PHP 1a | 14.0 | F | P | 2.64 | N/A | Y | Y | Y | N | N | Y | N | Y | N |
| 5 | + | PHP 1a | 14.1 | F | P | 1.02 | N/A | Y | N | Y | Y | Y | N | Y | Y | N |
| 6 | + | PHP 1a | 25.0 | F | N | 1.44 | N/A | Y | Y | Y | Y | Y | Y | N | N | N |
| 7d | + | PHP 1a | 25.2 | F | P | 2.10 | Y | Y | Y | Y | N | Y | Y | N | Y | Y |
| 8 | + | PHP 1a | 25.8 | F | P | 2.30 | Y | Y | Y | Y | Y | Y | Y | Y | N | N |
| 9d | + | PHP 1a | 28.1 | M | P | 2.24 | N/A | Y | Y | Y | Y | N | Y | N | Y | Y |
| 10 | + | PHP 1a | 38.9 | F | N | 1.9 | N/A | Y | Y | Y | Y | Y | Y | N | N | Y |
| 11 | + | PHP 1a | 41.9 | F | N | 1.78 | N/A | N | Y | Y | Y | Y | Y | N | N | Y |
| 12 | + | PHP 1a | 63.6 | F | N | 2.01 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 13 | + | PPHP | 25.4 | F | N | 1.28 | N/A | Y | Y | Y | Y | Y | Y | N | Y | Y |
| 14 | + | PPHP | 30.0 | F | N | 1.2 | N/A | Y | Y | Y | N | Y | Y | N | Y | Y |
| 15e | + | PPHP | 32.6 | F | N | 1.92 | N/A | Y | N | Y | Y | Y | Y | Y | N | Y |
| 16 | + | PPHP | 33.6 | F | N | −1.03 | Y | N | Y | Y | Y | Y | Y | Y | Y | N |
| 17f,h | + | PPHP | 38.2 | F | N | 1.82 | N/A | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 18a | + | PPHP | 39.2 | F | N | 0.64 | N/A | Y | Y | Y | Y | Y | Y | Y | N | N |
| 19g | + | PPHP | 44.8 | F | N | −1.15 | N/A | Y | N | Y | Y | Y | Y | Y | N | N |
| 20c | + | PPHP | 47.8 | F | N | 1.00 | N/A | Y | Y | Y | N | Y | Y | N | N | N |
| 21b | + | PPHP | 47.9 | F | N | 0.78 | N/A | N | Y | N | Y | Y | Y | Y | N | Y |
| 22d | + | PPHP | 56.4 | F | N | 1.02 | N/A | Y | Y | Y | N | N | Y | N | N | Y |
| 23f | − | PHP 1a | 11.1 | F | Y | 1.06 | N/A | Y | N | N | N | N | N | N | N | N |
| 24 | − | PHP 1a | 12.0 | F | N | 2.31 | N/A | Y | N | N | N | U | U | U | U | U |
| 25 | − | PHP 1a | 13.5 | F | P | 1.16 | N/A | Y | N | N | N | N | N | N | N | N |
| 26e | − | PHP 1a | 13.5 | F | P | 2.39 | N/A | Y | N | N | N | N | N | N | N | N |
| 27 | − | PHP 1a | 13.6 | M | Y | 1.05 | N/A | Y | Y | N | N | N | N | N | N | N |
| 28 | − | PHP 1a | 15.0 | F | N | 2.39 | N/A | Y | N | N | N | N | N | N | N | N |
| 29 | − | PHP 1a | 15.4 | M | P | 2.33 | N/A | Y | N | N | N | N | N | N | N | N |
| 30 | − | PHP 1a | 17.0 | F | P | 1.43 | N/A | Y | N | N | N | N | N | N | N | N |
| 31 | − | PHP 1a | 18.0 | M | N | −0.02 | N/A | Y | N | N | N | N | N | N | N | N |
| 32 | − | PHP 1a | 19.7 | M | P | 1.17 | N/A | Y | N | N | N | N | N | N | N | N |
| 33g | − | PHP 1a | 21.7 | F | N | 1.83 | N/A | Y | N | N | Y | N | N | N | N | N |
CTS diagnosis, AHO subtype, age, sex, GH therapy status, BMI Z-score, CTS EMG study result, brachymetacarpia/brachydactyly, and CTS-related symptoms/characteristics (pain, tingling, numbness, bilateral symptoms, flick sign, nocturnal symptoms, fine motor problems, weakness) are listed. Y, Yes; N, no; U, unsure/unknown; N/A, unavailable or not performed; P, previous history of GH treatment; Dx, diagnosis; EMG, electromyogram; F, female; M, male; Sx, symptoms.
Subjects of the same kindred.
Weight for this subject is prior to recent weight gain secondary to pregnancy.
Table 2.
Serum calcium, phosphate, PTH, TSH, and free T4 for PHP 1a subjects
| Subject | AHO Dx | sCa (mmol/liter) | sPhos (mmol/liter) | PTH (ng/liter) | TSH (mIU/liter) | Free T4 (ng/dl) |
|---|---|---|---|---|---|---|
| 1 | PHP 1a | 2.50 | 2.07 | 27 | 0.73 | 0.76 |
| 2 | PHP 1a | 2.43 | 1.74 | 114 | 2.11 | 1.13 |
| 3 | PHP 1a | 2.40 | 1.62 | 62 | 1.53 | 1.22 |
| 4 | PHP 1a | 2.20 | 1.65 | 73 | 0.40 | 1.14 |
| 5 | PHP 1a | 2.28 | 1.58 | 84 | 3.26 | 0.99 |
| 6 | PHP 1a | 2.55 | 1.55 | 53 | 2.24 | 1.04 |
| 7 | PHP 1a | 2.53 | 1.39 | 39 | 2.88 | 1.46 |
| 8 | PHP 1a | 2.18 | 1.71 | 112 | 1.07 | 1.70 |
| 9 | PHP 1a | 2.35 | N/A | 75 | 4.10 | 0.91 |
| 10 | PHP 1a | 2.38 | 1.55 | 128 | 7.69 | 1.00 |
| 11 | PHP 1a | 2.33 | 1.26 | 104 | 0.34 | 1.05 |
| 12 | PHP 1a | 2.40 | 1.39 | 65 | 3.90 | 1.40 |
| 23 | PHP 1a | 2.43 | 1.55 | 61 | 4.19 | 1.14 |
| 24 | PHP 1a | 2.30 | 1.91 | 113 | 3.05 | 0.90 |
| 25 | PHP 1a | 2.40 | 1.65 | 40 | 2.91 | 1.30 |
| 26 | PHP 1a | 2.45 | 1.74 | 61 | 4.49 | 0.90 |
| 27 | PHP 1a | 2.33 | 1.62 | 51 | 1.57 | 1.32 |
| 28 | PHP 1a | 2.43 | 1.52 | 42 | 2.38 | 1.07 |
| 29 | PHP 1a | 2.35 | 1.39 | 56 | 2.60 | 1.42 |
| 30 | PHP 1a | 2.60 | 1.49 | 35 | 0.18 | 8.9a |
| 31 | PHP 1a | 2.63 | 1.45 | 24 | 3.98 | 1.38 |
| 32 | PHP 1a | 2.53 | 1.32 | 47 | 2.84 | 1.13 |
| 33 | PHP 1a | 2.40 | 1.32 | 33 | 2.16 | 1.40 |
| Normal range | 2.18–2.63 | Adults: 0.74–1.52; Children: 1.29–2.26 |
10–65 | 0.5–4.5 | 0.7–1.8 |
All values were measured within 12 months prior to assessment for CTS. sCa, Serum calcium; sPhos, serum phosphate; N/A, unavailable or not performed.
Subject 30 has the total T4 noted.
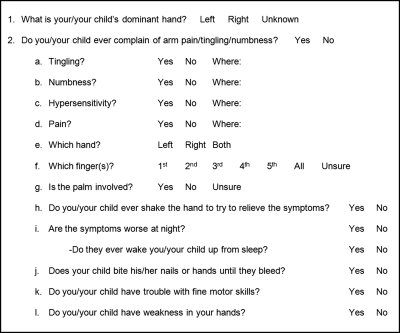
The subjects were asked a series of questions to assess for current symptoms of CTS: location and presence of hand tingling, numbness, pain or weakness, flick sign, difficulty with fine motor skills, or severe hand or nail biting as well as the presence of nocturnal symptoms (Fig. 1). Subjects were considered to have CTS if they were positive for at least three of the aforementioned symptoms and the anatomical distribution of symptoms was consistent with CTS. Subjects were asked about other medical diagnoses that could contribute to their symptoms. Electrodiagnostic studies were performed on four subjects before our current assessment for CTS.
Fig. 1.
Subjects and/or parents of subjects completed this series of questions during their interview.
Mutation analyses
GNAS mutation analyses were performed from peripheral blood on all subjects in the Johns Hopkins DNA Diagnostics Laboratory (Clinical Laboratory Improvement Amendments approved laboratory) or a research laboratory at the Johns Hopkins University School of Medicine as described previously (7, 9).
Anthropometric and radiographic evaluations
Standing height and weight measurements were obtained in clinic using a Harpendon stadiometer (Holtian Ltd., Crymych, Dysfed, UK) and Detecto scale (Detecto Scale Co., Webb City, MO). For subjects who had not been evaluated in our clinic within 3 months before this study, measurements from recent physician visits were used. BMI Z-scores were computed from anthropometric data using http://www.bcm.edu/bodycomplab/Applications/bmirefcalc.htm (Baylor College of Medicine, Houston, TX; 2006). In all children, bone age evaluations were performed based on hand/wrist radiographs, and brachydactyly/brachymetacarpia were confirmed by a pediatric radiologist on the faculty at the Johns Hopkins University School of Medicine and a pediatric endocrinologist (E.L.G.-L.).
Statistical analyses
Statistical analyses were conducted using Statistica version 7.0 (Statsoft, Inc., Tulsa, OK). Continuous variables (BMI Z-scores, age) were analyzed by using the unpaired Student t test. Fisher's exact test was used to compare categorical variables. All tests were two tailed and significance was denoted by P < 0.05.
Results
Patient population
Twenty-three PHP 1a (17 female, six male) and 10 PPHP (all female) subjects were assigned to a CTS diagnostic group based on reported symptoms (Table 1). The female preponderance of PPHP is consistent with the diagnosis of this condition typically being made at the time that a mother with PPHP has a child diagnosed with PHP 1a (13). At the time of this study, all PHP 1a subjects were under medical management and, in the preceding 12 months, had normal serum calcium, phosphate, and free T4 levels (Table 2) [Three subjects had very minimally elevated phosphate levels (mean of 0.08 mmol/liter above normal) and one had a minimally elevated TSH but normal free T4].
Diagnosis of CTS
Twenty-two subjects (67%; 95% confidence interval 0.48, 0.82) had a clinical diagnosis of CTS, and all had at least three signs/symptoms of the condition, with a large proportion (19 of 22) reporting bilateral symptoms. Of the GNAS mutation-confirmed subjects, 68% had a clinical diagnosis of CTS (Table 3). Forty-two percent of all subjects were children, 36% of whom had CTS. Ten of 22 subjects in the CTS group (45%) [eight of 19 (42%) in those who were mutation confirmed] reported nocturnal symptoms.
Table 3.
GNAS mutation analysis
| Subject | Mutation |
|---|---|
| 1 | Exon 1: c.85C>T (Q29X) |
| 2 | Exon 10: c.814C>T (L272F)a |
| 3 | Intron 4: c.312 + 5G>Aa |
| 4 | Exon 7: c.565–568delGACT |
| 5 | Exon 13: c.1083insC |
| 6 | Exon 13: c.1174G>A (E392K) |
| 7 | Exon 1: c.21insT |
| 8 | Exon 1: c.103C>T (Q35X) |
| 9 | Exon 1: c.21insT |
| 10 | Exon 13: c.1096G>A (A366T)a |
| 11 | None found exons 1–13 |
| 12 | None found exons 1–13 |
| 13 | Exon 1: c.34C>T(Q12X) |
| 14 | Exon 9: c.701G>A (W234X) |
| 15 | Exon 1: c.85C>T (Q29X) |
| 16 | Exon 10: c.730A>T (I244F) |
| 17 | Exon 13: c.1174G>A (E392K) |
| 18 | Exon 1: c.85C>T (Q29X) |
| 19 | None found exons 1–13 |
| 20 | Exon 7: c.565–568delGACT |
| 21 | Intron 4: c.312 + 5G>Aa |
| 22 | Exon 1: c.21insT |
| 23 | Exon 13: c.1174G>A (E392K) |
| 24 | Exon 12: c.1006C>T (R336W) |
| 25 | None found exons 1–13 |
| 26 | Exon 1: c.85C>T (Q29X) |
| 27 | Exon 7: c.565–568delGACT |
| 28 | Exon 7: c.565–568delGACT |
| 29 | Exon 7: c.565–568delGACT |
| 30 | Exon 1: c.85C>T (Q29X) |
| 31 | Exon 7: c.565–568delGACT |
| 32 | Exon 7: c.565–568delGACT |
| 33 | None found exons 1–13 |
del, Deletion; ins, insertion.
Unpublished mutation.
Before the study, four subjects underwent electrodiagnostic testing for CTS (Table 1). All four subjects met electrodiagnostic criteria for CTS and were CTS positive by the methods outlined here, and three of the four had documented mutations in GNAS. Two of these subjects with electrodiagnostic evidence of CTS (subjects 7 and 8 in Table 1) have undergone carpal tunnel release surgery.
Clinical characteristics
Subject characteristics and symptoms are shown in Table 1. The ages of subjects in our study population ranged from 8 to 63 yr (mean 26.4 yr). The mean age (M) and sd in the CTS group was 31.8 yr (sd 15.3) vs. 15.5 yr (sd 3.3) in the asymptomatic subjects (P = 0.002). Similar differences in ages were observed when considering mutation confirmed subjects in each CTS group (P < 0.001). There was also a significantly higher age (P < 0.001) in PPHP subjects (M 39.6, sd 9.6) compared with PHP 1a subjects (M 20.6, sd 12.8). This is consistent with the older age of presentation of PPHP, given their lack of hormonal abnormalities as well as their diagnosis typically occurring at the time of their children being diagnosed with PHP 1a (13). When PPHP subjects were excluded, the mean age of the CTS group (M 25.4, sd 4) remained greater than the asymptomatic group (M 15.5, sd 3.3), but this was not significant (P = 0.064). Finally, the proportion of PPHP subjects with CTS (1.00) was compared with the proportion of PHP 1a subjects who had CTS (0.52) and was significantly larger (P = 0.013). Similarly, when examining proportions of subjects with GNAS mutations who have CTS (1.00 for PPHP and 0.50 for PHP 1a), this again was significantly different (P = 0.009). In fact, 100% of PPHP subjects in this study had CTS. Additionally, as previously stated, the PPHP subjects as a group were older.
The children in our study with CTS had medical histories involving extensive occupational therapy to help with writing skills, whereas those without CTS did not have this intervention. This history of the initiation of occupational therapy occurred at approximately the same time as the onset of the CTS symptoms. For adults, we were unable to examine the relationship of occupational therapy with CTS because occupational therapy either had not been involved or its temporal relationship to the CTS was unclear.
Relationship of CTS to clinical parameters
There was no significant difference in the BMI Z-scores (P = 0.82), proportion with brachymetacarpia/brachydactly (P = 0.53), or proportion actively receiving GH (P = 0.25) in subjects with CTS vs. those without. There were also no differences in these variables when examining only those subjects with mutations. The mean BMI Z-score and sem of the subjects with PHP 1a was 1.83 (sem 0.15) vs. 0.75 (sem 0.33) in the subjects with PPHP. This difference was statistically significant (P < 0.002) and consistent with previous findings (9).
In 28 of 33 subjects (85%), we confirmed the diagnosis by mutation analyses within the 13 coding exons and intron-exon boundaries of GNAS (Table 3). We were unable to identify mutations in five subjects. This is consistent with the frequency of identified mutations that have been reported by others for AHO (29). The most frequent mutations in our patients occurred in exons 1 and 7. There was no difference (P = 0.06) in the occurrence of CTS between subjects who possessed exon 1 mutations vs. those who had exon 7 mutations. Statistical calculations were not performed for mutations that occurred in other exons secondary to the low number of subjects with those mutations. Upon examination of the group with CTS, 14 of 19 (74%) had mutations that led to premature stop codons, and the remaining five patients had missense mutations (26%). This is very similar to the group without CTS in whom seven of 9 (78%) had mutations leading to premature termination codons vs. two of 9 (22%) with missense mutations.
Relationship of CTS to biochemical status
Specific biochemical parameters (serum calcium, phosphate, PTH, TSH, and free T4 or total T4) for all PHP 1a subjects are shown in Table 2. (PPHP subjects, by definition, are normal biochemically.) Serum calcium and free T4/T4 levels were normal in all cases. Phosphate levels were also normal with the exception of three adults who had values minimally above the normal range (mean of 0.08 mmol/liter above normal). The PTH levels were appropriate for patients with PHP 1a on treatment (mean 65 ng/liter, sd 31). Only one subject had a minimally elevated TSH, although the free T4 was normal.
Discussion
To our knowledge, this is the first report of CTS in AHO. Our study revealed an unusually high prevalence of symptomatic subjects. It is estimated that 15% of the adult population have symptoms of CTS and less than 3% have medically confirmed CTS (15, 19). In comparison, 67% of our subjects reported symptoms of CTS and 100% (four of four) of subjects who had previously undergone electrodiagnostic studies had a confirmed diagnosis of CTS (Table 1). In mutation-confirmed subjects alone, the prevalence was 68%.
The overall prevalence of CTS in our sample is even more striking, considering the young age of our subjects. In the general population, most cases of CTS are in patients older than 40 yr (15–19). In contrast, the mean age in our CTS group was 31.8 yr (sd 15.3), and five of the subjects were children 14 yr of age and younger. The greater prevalence of CTS among the PPHP population compared with the PHP 1a population is likely due to the much older mean age in the PPHP group and not secondary to differences in the two conditions themselves. Furthermore, in light of the young age of the asymptomatic subject group (M 15.5, sd 3.3) with AHO, it seems quite possible that these individuals could develop CTS as they grow older.
Another finding is that the AHO children with CTS symptoms all had extensive involvement with occupational therapy soon after the onset of CTS symptoms. The occupational therapy involvement in 100% of cases was secondary to impairment of fine motor skills, most notably writing. The children without CTS symptoms did not have a history of impairment with fine motor skills or a history of occupational therapy involvement. For the adults, we were unable to determine any correlation because occupational therapy either had not been involved or its temporal relationship to the CTS was unclear.
It is possible that the impairment of fine motor skills and the need for occupational therapy intervention in children is a warning flag for CTS. We are now involving occupational therapy to initiate early interventional techniques to help mitigate the symptoms of CTS. It is possible that earlier attention to CTS could lead to improved functionality and quality of life in children with AHO.
There are several potential etiologies for symptoms of CTS in patients with AHO. CTS is a known side effect in adult patients actively receiving GH therapy, although symptoms are reversible upon completion of therapy (30–32). A systematic review by Liu et al. (32) reported that an average of 19% of elderly patients treated with GH had symptoms of CTS compared with 1% of controls. It is known that PHP 1a patients have a high risk of GH deficiency secondary to GHRH resistance (7, 8, 33). Although we have treated 56% of our PHP 1a subjects in this study with GH, only 13% (three subjects) were actively receiving treatment at the time of our study. Only one of these subjects had CTS, and symptoms were present before the start of GH therapy. GH therapy is not clearly associated with CTS in children and may be related to the lower incidence of edema with treatment (34).
Another potential cause of CTS symptoms may be related to hypothyroidism. PHP 1a often presents with hypothyroidism, especially in young children (13, 35). These patients' hypothyroidism is a result of TSH resistance due to partial paternal imprinting of GαS in the thyroid (1, 3, 4). Hypothyroidism is a known risk factor for CTS but only when hypothyroidism is not adequately treated (22, 36, 37). All of our subjects were receiving treatment and were euthyroid at the time of our evaluation. Therefore, undetected hypothyroidism is very unlikely to be responsible for the increased prevalence of CTS in our subjects. Furthermore, 100% of patients with PPHP had CTS, and, because patients with PPHP have no hormonal resistance, hypothyroidism alone would not explain the high prevalence in these subjects.
In the general population, there is a known association between obesity and CTS, especially in younger patients (15, 38), although it is not clear whether there is a causal relationship. Severe obesity is considered part of the PHP 1a phenotype specifically and is not part of the PPHP phenotype (9). In this population, we found that the subjects with PHP 1a had a significantly greater BMI Z-score (M 1.83, sem 0.15) compared with subjects with PPHP (M 0.75, sem 0.33), consistent with our previous findings (9). However, all subjects with PPHP had CTS. There were no significant differences in the BMI Z-scores in subjects with CTS vs. those without (P =0.82).
Brachydactyly/brachymetacarpia could potentially cause compression of the carpal tunnel and predispose patients to CTS. Brachydactyly/brachymetacarpia alone could be the etiology of the poor fine motor skills reported by our subjects, but all of the subjects without CTS symptoms also had brachydactyly/brachymetarcarpia, and none of the subjects in the asymptomatic group reported difficulty with fine motor skills. We were unable to detect a significant difference in the prevalence of CTS in subjects with brachydactyly/brachymetacarpia and those without, although there was a very high prevalence of this phenotype in our study (91% of all subjects). It is possible that more specific measures of hand and/or wrist anthropometrics (e.g. wrist index, shape index, digit index) could help to explain the etiology of CTS in this population because they have been varyingly associated with CTS in the general population (39–41). However, previously reported effect sizes have been relatively modest (40, 41) and may be difficult to detect in AHO study populations because they often have limited power. Nonetheless, carefully conducted case-control studies may provide evidence of an association and represent opportunities for future investigations.
In all children in this study, the bone ages were advanced as is characteristic of patients with AHO (7, 8, 13). Biallelic expression of GNAS has been demonstrated in human bone (42) and mouse chondrocytes (43), and several studies have implicated haploinsufficiency of Gαs as being responsible for the premature epiphyseal fusion observed in patients with AHO (13, 43–45). We hypothesized that mutations in GNAS leading to premature stop codons may be more deleterious than missense mutations and result in more significantly advanced bone ages in the children examined. However, all bone ages were advanced to approximately the same degree relative to the chronologic age in the prepubertal subjects, and the degree of advancement did not correlate with the degree of brachydactyly/brachymetacarpia or with specific mutations (data not shown). In addition, we did not observe any statistically significant association between the type of mutation and CTS. Moreover, the proportions of missense mutations and mutations resulting in premature stop codons were similar in those with and without CTS.
One limitation to this study is related to the difficulty in diagnosing CTS. Median nerve conduction study is the gold standard for diagnosis with a sensitivity of greater than 85% and specificity of greater than 95% according to the American Academy of Neurology (46). However, approximately 13% of patients with CTS confirmed by surgery have normal electrodiagnostic studies (47), and other studies have revealed that 10–20% of patients can have positive electrodiagnostic examinations without symptoms of CTS. In addition, it is not clear that electrodiagnostic studies correlate with the severity of patient symptoms or functional impairment (15, 18, 48).
In our study, we relied on patient symptoms and medical history to elicit cases of CTS. Numerous studies have examined the reliability of history and physical examination findings for diagnosing CTS. The utility of these studies is limited by the weaknesses of nerve conduction studies as the comparative gold standard (49). Although the clinical presentation of CTS is varied, clinical signs and symptoms may have moderate sensitivities and specificities (17, 18, 48, 50, 51). Specificity is an important aspect of screening for CTS in the setting of PHP 1a because hypocalcemia may manifest similarly. Although the symptom of hand tingling could be attributable to hypocalcemia in PHP 1a subjects, inclusion in our study was restricted to subjects who were on medical management and were normocalcemic. Moreover, hypocalcemia would not explain the CTS symptoms in the PPHP subjects who have an absence of hormonal resistance. Finally, serum calcium levels were similar in each CTS group.
The diagnosis of CTS is even more difficult in children because they often present with nonclassic symptoms including clumsiness, difficulty with fine motor tasks, weakness, nocturnal waking, and severe hand gnawing or nail biting (23, 24, 26–28). However, we attempted to minimize measurement error by including these atypical symptoms in our assessment for CTS.
Conclusion
Symptoms of CTS are considerably more common in patients with AHO than in the general population, occurring in 67% of our study population. The etiology of CTS in patients with AHO remains unclear, although it does not appear to be related to hormonal resistance, biochemical disturbances, obesity, prior/current GH treatment, or specific mutations in GNAS. Early detection and intervention for CTS could improve overall function and quality of life in patients with AHO.
Acknowledgments
We thank all of the patients and their families who made this research possible.
This work was supported by National Institutes of Health Grant T35 HD007446 to the American Pediatric Society/Society for Pediatric Research Student Research Program (to A.W.J.), U.S. Food and Drug Administration Orphan Products Development Grant R01 FD002568 (to E.L.G.-L.), and National Institutes of Health/National Center for Research Resources Grant UL1 RR025005 (a component of the National Institutes of Health Roadmap for Medical Research). (The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources/National Institutes of Health).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- AHO
- Albright hereditary osteodystrophy
- BMI
- body mass index
- CTS
- carpal tunnel syndrome
- M
- mean age
- PHP 1a
- pseudohypoparathyroidism type 1a
- PPHP
- pseudopseudohypoparathyroidism.
References
- 1. Germain-Lee EL, Ding CL, Deng Z, Crane JL, Saji M, Ringel MD, Levine MA. 2002. Paternal imprinting of Gα(s) in the human thyroid as the basis of TSH resistance in pseudohypoparathyroidism type 1a. Biochem Biophys Res Commun 296:67–72 [DOI] [PubMed] [Google Scholar]
- 2. Hayward BE, Barlier A, Korbonits M, Grossman AB, Jacquet P, Enjalbert A, Bonthron DT. 2001. Imprinting of the G(s)α gene GNAS1 in the pathogenesis of acromegaly. J Clin Invest 107:R31–R36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu J, Erlichman B, Weinstein LS. 2003. The stimulatory G protein α-subunit Gsα is imprinted in human thyroid glands: implications for thyroid function in pseudohypoparathyroidism types 1A and 1B. J Clin Endocrinol Metab 88:4336–4341 [DOI] [PubMed] [Google Scholar]
- 4. Mantovani G, Ballare E, Giammona E, Beck-Peccoz P, Spada A. 2002. The gsα gene: predominant maternal origin of transcription in human thyroid gland and gonads. J Clin Endocrinol Metab 87:4736–4740 [DOI] [PubMed] [Google Scholar]
- 5. Chen M, Gavrilova O, Liu J, Xie T, Deng C, Nguyen AT, Nackers LM, Lorenzo J, Shen L, Weinstein LS. 2005. Alternative Gnas gene products have opposite effects on glucose and lipid metabolism. Proc Natl Acad Sci USA 102:7386–7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Germain-Lee EL, Schwindinger W, Crane JL, Zewdu R, Zweifel LS, Wand G, Huso DL, Saji M, Ringel MD, Levine MA. 2005. A mouse model of Albright hereditary osteodystrophy generated by targeted disruption of Exon 1 of the Gnas gene. Endocrinology 146:4697–4709 [DOI] [PubMed] [Google Scholar]
- 7. Germain-Lee EL, Groman J, Crane JL, Jan de Beur SM, Levine MA. 2003. Growth hormone deficiency in pseudohypoparathyroidism type 1a: another manifestation of multihormone resistance. J Clin Endocrinol Metab 88:4059–4069 [DOI] [PubMed] [Google Scholar]
- 8. Germain-Lee EL. 2006. Short stature, obesity, and growth hormone deficiency in pseudohypoparathyroidism type 1a. Pediatr Endocrinol Rev 3(Suppl 2):318–327 [PubMed] [Google Scholar]
- 9. Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL. 2007. Body mass index differences in pseudohypoparathyroidism type 1a versus pseudopseudohypoparathyroidism may implicate paternal imprinting of Gα(s) in the development of human obesity. J Clin Endocrinol Metab 92:1073–1079 [DOI] [PubMed] [Google Scholar]
- 10. Mouallem M, Shaharabany M, Weintrob N, Shalitin S, Nagelberg N, Shapira H, Zadik Z, Farfel Z. 2008. Cognitive impairment is prevalent in pseudohypoparathyroidism type Ia, but not in pseudopseudohypoparathyroidism: possible cerebral imprinting of Gs α. Clinical Endocrinology 68:233–239 [DOI] [PubMed] [Google Scholar]
- 11. Bastepe M, Jüppner H. 2003. Pseudohypoparathyroidism and mechanisms of resistance toward multiple hormones: molecular evidence to clinical presentation. J Clin Endocrinol Metab 88:4055–4058 [DOI] [PubMed] [Google Scholar]
- 12. Levine MA. 2002. Pseudohypoparathyroidism. In: Bilezikian JP, Raisz LG, Rodan GA, eds. Principles of bone biology. 2nd ed San Diego: Academic Press; 1137–1163 [Google Scholar]
- 13. Plagge A, Kelsey G, Germain-Lee EL. 2008. Physiological functions of the imprinted Gnas locus and its protein variants Gα(s) and XLα(s) in human and mouse. J Endocrinol 196:193–214 [DOI] [PubMed] [Google Scholar]
- 14. Weinstein LS, Yu S, Warner DR, Liu J. 2001. Endocrine manifestations of stimulatory G protein α-subunit mutations and the role of genomic imprinting. Endocr Rev 22:675–705 [DOI] [PubMed] [Google Scholar]
- 15. Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. 1999. Prevalence of carpal tunnel syndrome in a general population. JAMA 282:153–158 [DOI] [PubMed] [Google Scholar]
- 16. Bland JD, Rudolfer SM. 2003. Clinical surveillance of carpal tunnel syndrome in two areas of the United Kingdom, 1991–2001. J Neurol Neurosurg Psychiatry 74:1674–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katz JN, Larson MG, Sabra A, Krarup C, Stirrat CR, Sethi R, Eaton HM, Fossel AH, Liang MH. 1990. The carpal-tunnel syndrome—diagnostic utility of the history and physical-examination findings. Ann Intern Med 112:321–327 [DOI] [PubMed] [Google Scholar]
- 18. Nora DB, Becker J, Ehlers JA, Gomes IN. 2004. Clinical features of 1039 patients with neurophysiological diagnosis of carpal tunnel syndrome. Clin Neurol Neurosurg 107:64–69 [DOI] [PubMed] [Google Scholar]
- 19. Tanaka S, Wild DK, Seligman PJ, Behrens V, Cameron L, Putz-Anderson V. 1994. The U.S. prevalence of self-reported carpal tunnel syndrome: 1988 National Health Interview Survey data. Am J Public Health 84:1846–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holdaway IM, Rajasoorya C. 1999. Epidemiology of acromegaly. Pituitary 2:29–41 [DOI] [PubMed] [Google Scholar]
- 21. O'Duffy JD, Randall RV, MacCarty CS. 1973. Median neuropathy (carpal-tunnel syndrome) in acromegaly. A sign of endocrine overactivity. Ann Intern Med 78:379–383 [DOI] [PubMed] [Google Scholar]
- 22. Solomon DH, Katz JN, Bohn R, Mogun H, Avorn J. 1999. Nonoccupational risk factors for carpal tunnel syndrome. J Gen Intern Med 14:310–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poilvache P, Carlier A, Rombouts JJ, Partoune E, Lejeune G. 1989. Carpal tunnel syndrome in childhood: report of five new cases. J Pediatr Orthop 9:687–690 [DOI] [PubMed] [Google Scholar]
- 24. Yuen A, Dowling G, Johnstone B, Kornberg A, Coombs C. 2007. Carpal tunnel syndrome in children with mucopolysaccaridoses. J Child Neurol 22:260–263 [DOI] [PubMed] [Google Scholar]
- 25. Deymeer F, Jones HR., Jr 1994. Pediatric median mononeuropathies—a clinical and electromyographic study. Muscle Nerve 17:755–762 [DOI] [PubMed] [Google Scholar]
- 26. Kayali H, Kahraman S, Sirin S, Bedük A, Timurkaynak E. 2003. Bilateral carpal tunnel syndrome with type 1 diabetes mellitus in childhood. Pediatr Neurosurg 38:262–264 [DOI] [PubMed] [Google Scholar]
- 27. Swoboda KJ, Engle EC, Scheindlin B, Anthony DC, Jones HR. 1998. Mutilating hand syndrome in an infant with familial carpal tunnel syndrome. Muscle Nerve 21:104–111 [DOI] [PubMed] [Google Scholar]
- 28. Van Meir N, De Smet L. 2005. Carpal tunnel syndrome in children. J Pediatr Orthop B 14:42–45 [DOI] [PubMed] [Google Scholar]
- 29. Aldred MA, Trembath RC. 2000. Activating and inactivating mutations in the human GNAS1 gene. Hum Mutat 16:183–189 [DOI] [PubMed] [Google Scholar]
- 30. Cohn L, Feller AG, Draper MW, Rudman IW, Rudman D. 1993. Carpal tunnel syndrome and gynaecomastia during growth hormone treatment of elderly men with low circulating IGF-I concentrations. Clin Endocrinol (Oxf) 39:417–425 [DOI] [PubMed] [Google Scholar]
- 31. de Boer H, Blok GJ, Van der Veen EA. 1995. Clinical aspects of growth-hormone deficiency in adults. Endocr Rev 16:63–86 [DOI] [PubMed] [Google Scholar]
- 32. Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR. 2007. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med 146:104–115 [DOI] [PubMed] [Google Scholar]
- 33. Mantovani G, Maghnie M, Weber G, De Menis E, Brunelli V, Cappa M, Loli P, Beck-Peccoz P, Spada A. 2003. Growth hormone-releasing hormone resistance in pseudohypoparathyroidism type Ia: new evidence for imprinting of the Gs alpha gene. J Clin Endocrinol Metab 88:4070–4074 [DOI] [PubMed] [Google Scholar]
- 34. Blethen SL, Allen DB, Graves D, August G, Moshang T, Rosenfeld R. 1996. Safety of recombinant deoxyribonucleic acid-derived growth hormone: the national cooperative growth study experience. J Clin Endocrinol Metab 81:1704–1710 [DOI] [PubMed] [Google Scholar]
- 35. Gelfand IM, Eugster EA, DiMeglio LA. 2006. Presentation and clinical progression of pseudohypoparathyroidism with multi-hormone resistance and Albright hereditary osteodystrophy: a case series. J Pediatr 149:877–880 [DOI] [PubMed] [Google Scholar]
- 36. Kececi H, Degirmenci Y. 2006. Hormone replacement therapy in hypothyroidism and nerve conduction study. Neurophysiol Clin 36:79–83 [DOI] [PubMed] [Google Scholar]
- 37. Soy M, Guldiken S, Arikan E, Altun BU, Tugrul A. 2007. Frequency of rheumatic diseases in patients with autoimmune thyroid disease. Rheumatol Int 27:575–577 [DOI] [PubMed] [Google Scholar]
- 38. Bland JD. 2005. The relationship of obesity, age, and carpal tunnel syndrome: more complex than was thought? Muscle Nerve 32:527–532 [DOI] [PubMed] [Google Scholar]
- 39. Kouyoumdjian JA, Zanetta DM, Morita MP. 2002. Evaluation of age, body mass index, and wrist index as risk factors for carpal tunnel syndrome severity. Muscle Nerve 25:93–97 [DOI] [PubMed] [Google Scholar]
- 40. Boz C, Ozmenoglu M, Altunayoglu V, Velioglu S, Alioglu Z. 2004. Individual risk factors for carpal tunnel syndrome: an evaluation of body mass index, wrist index and hand anthropometric measurements. Clin Neurol Neurosurg 106:294–299 [DOI] [PubMed] [Google Scholar]
- 41. Moghtaderi A, Izadi S, Sharafadinzadeh N. 2005. An evaluation of gender, body mass index, wrist circumference and wrist ratio as independent risk factors for carpal tunnel syndrome. Acta Neurol Scand 112:375–379 [DOI] [PubMed] [Google Scholar]
- 42. Mantovani G, Bondioni S, Locatelli M, Pedroni C, Lania AG, Ferrante E, Filopanti M, Beck-Peccoz P, Spada A. 2004. Biallelic expression of the Gsα gene in human bone and adipose tissue. J Clin Endocrinol Metab 89:6316–6319 [DOI] [PubMed] [Google Scholar]
- 43. Bastepe M, Weinstein LS, Ogata N, Kawaguchi H, Jüppner H, Kronenberg HM, Chung UI. 2004. Stimulatory G protein directly regulates hypertrophic differentiation of growth plate cartilage in vivo. Proc Natl Acad Sci USA 101:14794–14799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sakamoto A, Chen M, Kobayashi T, Kronenberg HM, Weinstein LS. 2005. Chondrocyte-specific knockout of the G protein G(s)α leads to epiphyseal and growth plate abnormalities and ectopic chondrocyte formation. J Bone Miner Res 20:663–671 [DOI] [PubMed] [Google Scholar]
- 45. Sakamoto A, Chen M, Nakamura T, Xie T, Karsenty G, Weinstein LS. 2005. Deficiency of the G-protein α-subunit G(s)α in osteoblasts leads to differential effects on trabecular and cortical bone. J Biol Chem 280:21369–21375 [DOI] [PubMed] [Google Scholar]
- 46. Jablecki CK, Andary MT, Floeter MK, Miller RG, Quartly CA, Vennix MJ, Wilson JR. 2002. Practice parameter: electrodiagnostic studies in carpal tunnel syndrome—report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology 58:1589–1592 [DOI] [PubMed] [Google Scholar]
- 47. Concannon MJ, Gainor B, Petroski GF, Puckett CL. 1997. The predictive value of electrodiagnostic studies in carpal tunnel syndrome. Plast Reconstr Surg 100:1452–1458 [DOI] [PubMed] [Google Scholar]
- 48. Ferry S, Silman AJ, Pritchard T, Keenan J, Croft P. 1998. The association between different patterns of hand symptoms and objective evidence of median nerve compression—a community-based survey. Arthritis Rheum 41:720–724 [DOI] [PubMed] [Google Scholar]
- 49. D'Arcy CA, McGee S. 2000. The rational clinical examination. Does this patient have carpal tunnel syndrome? JAMA 283:3110–3117 [DOI] [PubMed] [Google Scholar]
- 50. Hansen PA, Micklesen P, Robinson LR. 2004. Clinical utility of the flick maneuver in diagnosing carpal tunnel syndrome. Am J Phys Med Rehabil 83:363–367 [DOI] [PubMed] [Google Scholar]
- 51. Pryse-Phillips WE. 1984. Validation of a diagnostic sign in carpal tunnel syndrome. J Neurol Neurosurg Psychiatry 47:870–872 [DOI] [PMC free article] [PubMed] [Google Scholar]