Abstract
Human adipose stromal cells (ASCs) reside within the stromal-vascular fraction (SVF) in fat tissue, can be readily isolated, and include stem-like cells that may be useful for therapy. An important consideration for clinical application and functional studies of stem/progenitor cells is their capacity to maintain chromosome stability in culture. In this study, cultured ASC populations and ASC clones were evaluated at intervals for maintenance of chromosome stability. Uncultured SVF (uSVF) cells were included for comparison. G-banded chromosome analysis demonstrated that ASCs are diploid and have a normal karyotype. However since only ~20 cells are examined, low levels of chromosome instability would not be detected. To increase detection sensitivity, fluorescence in situ hybridization was employed, to permit chromosome enumeration in larger numbers of interphase cells. Seven cultured ASC populations, two ASC clones and four uSVF samples were examined. Chromosome X and 17 probes identified diploid, tetraploid, and aneuploid interphase cells. Both cultured ASC populations [up to ~35 Population Doublings (PDs)] and uSVF cells exhibited a similar level of diploidy (97.8% n = 6,355 and 98.83% n = 1,197, respectively) and numerical abnormalities, suggesting that cultured ASCs are genomically stable and supporting their suitability for transplantation applications. In comparison, cultured primary human chorionic villus cells exhibited marked genomic instability resulting in an 11.6% tetraploidy rate after 8–10 PD. Thus effects of culture on genomic stability may be cell type dependent and should be tested by appropriately scaled interphase fluorescence in situ hybridization analysis in any ex vivo expanded cell population destined for transplantation.
Introduction
Cells with stem or progenitor-like properties have been isolated from a range of human tissues [1–7] and offer significant clinical promise. With regard to ischemic disease, bone marrow–derived mesenchymal cells and endothelial progenitor cells are being evaluated for their capacity to promote new vessel formation both in animal models and in clinical trials. A further cell type with potential utility in treatment of ischemia is the adipose stromal cell (ASC) population that resides within fat tissue, is readily obtained by liposuction or abdominoplasty, and possesses progenitor-like properties [8]. Our recent studies have demonstrated that ASCs have cell surface markers and in vivo location consistent with a pericyte cell type [9]. In vitro, ASCs substantially improve endothelial network formation in cooperation with endothelial cells suggesting that they may be useful for treatment of vascular disease [9]. Furthermore, since they can be harvested in relatively large numbers (6.6 × 107 cells/L of adipose tissue), expanded for up to ~40 population doublings (PDs) and differentiated into cells of mesenchymal, hepatocyte, neuronal, endothelial, and cardiomyocyte lineages [8,10–19], ASCs are being considered for a range of therapeutic applications.
A primary consideration regarding the safety of transplanting cultured stem/progenitor cells and for their application in functional studies, is their ability to maintain chromosome stability in culture. Although genomic instability can be associated with loss of growth inhibition and transformation both in vitro and in vivo, maintenance of chromosome stability is an area of adult stem cell research that has received relatively sparse attention. Where chromosome stability has been examined, the method typically employed has been preparation of chromosome spreads followed by G-banding which permits detection of chromosome numerical and structural abnormalities. This procedure is routinely employed in screening chorionic villus samples (CVS) and amniocentesis samples for prenatal diagnosis. Reflecting its labor intensive nature, typically only around 20 cells are analyzed by the G-banding technique, making it unsuited to detection of low frequency abnormalities. A recent study of three ASC populations and three ASC clonal lines using G-banding detected no deviation from a diploid chromosome content [20] and our own G-banding analysis confirmed this finding.
Clearly, even a small percentage of chromosomally abnormal cells within a population could pose a risk upon transplantation. To increase sensitivity in this study, we employed fluorescence in situ hybridization (FISH) using chromosome-specific probes to assess ploidy in greater than 6,000 interphase cells representing cultured ASCs from eight donors and in 1,197 cells prepared from the uncultured stromal-vascular fractions (SVFs) of four donors.
Materials and Methods
Preparation of SVF and ASC culture
The SVF was harvested from 11 independent female donors (samples 1–11) and one male donor (sample 12) undergoing abdominal lipoaspiration procedures. Donors were 22–59 years of age with body mass indices in the normal range. Samples were collected according to institutional guidelines (Indiana University IRB 0305-59). Cells from the SVF were dispersed by collagenase digestion, filtration and centrifugation followed by a final step to remove red blood cells as described previously [9]. The final pellet was suspended in EGM2-MV medium (Lonza, Walkersville, MD). To permit enrichment of ASCs, the SVF was plated into uncoated plastic T75 cm2 tissue culture flasks containing EGM2-MV medium. ASCs grow as attached cells and were typically grown to ~60–70 % confluency prior to passaging and re-seeded at ~20–30% confluency. To estimate ASC PD time, ASC cell number in ASC samples 3 and 4 were counted each time cells were passaged (Fig. 2A). Over this time course (measured over 25–30 days), average PD time was 0.76/day which approximates to 1 PD every 31 h. ASC samples 5, 6, and 7 (Fig. 2B) were continuously passaged until they stopped dividing, which took ~50 days (PD37-38). The SVF from sample 8 was cultured for three days in EGM2-MV medium to enrich for ASCs, harvested and plated at limiting dilution (approximately 1 cell/well) into 96-well plates to permit development of clonal lines. Approximately 24 h after plating, wells containing >1 cell were eliminated. Clones 8-E1 and 8-F6 (Fig. 2C) represent descendent cells of a single ASC from ASC sample 8. 8-E1 and 8-F6 were highly proliferative (average PD = 0.86/day), undergoing 36 and 39 PD, respectively, before reaching senescence. The uncultured SVF (Fig. 3) from four independent donors (samples 9–12) was suspended in EGM2-MV medium as described above (typically within 6h after surgery), pelleted and resuspended in 3:1 w/v methanol:glacial acetic acid to fix cells prior to fluorescence in situ hybridization.
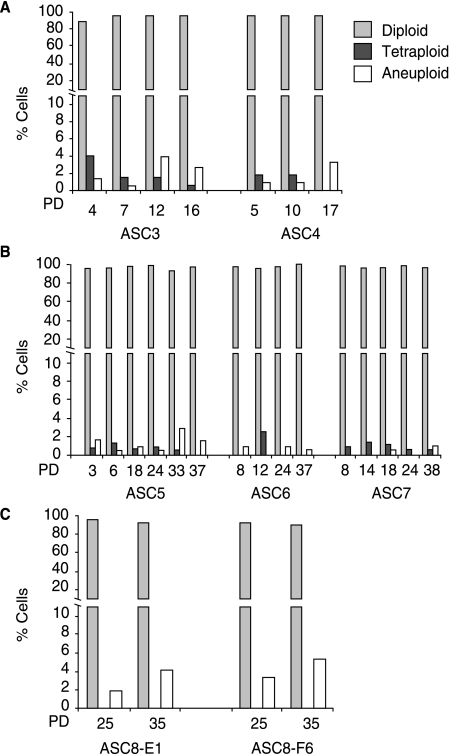
FIG. 2.
Analysis of chromosome stability in freshly cultured ASCs and in high proliferative potential clones. FISH analysis of ≥200 interphase cells/ASC sample with chromosome 17 and X probes (see Fig. 1B) was used to determine the percentage of diploid, tetraploid, and aneuploid cells. Detailed FISH results for each sample are provided in Supplementary Table S1. (A) ASC samples 3 and 4 were cultured to permit determination of population doubling (PD) which was estimated to be 0.79/day (see Materials and Methods) and to provide information on chromosome stability in culture using FISH analysis. (B) ASCs were prepared from an additional three female donors (ASC samples 5, 6, and 7) and cultured for the indicated PD before harvesting for FISH analysis. At ~PD37–38, cells stopped dividing and became senescent. (C) ASC sample 8 was plated at early passage at limiting dilution to generate clonal ASC lines 8-E1 and 8-F6. Mid (25 PD) and late (35 PD) passage cells were analyzed by FISH as described above.
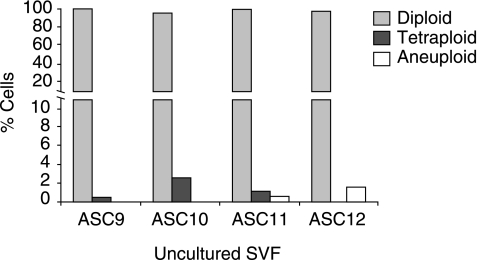
FIG. 3.
Detection of chromosomally aberrant cells in the uncultured stromal-vascular fraction (SVF). Dissociated cells from the SVF from lipoaspirates (samples 9–12) were resuspended in EGM2-MV medium then immediately processed for FISH analysis of ≥200 interphase cells using chromosome X and 17 probes as described in Fig. 1B and Fig. 2. Detailed FISH results are presented in Supplementary Table S2. Data are presented for 3 independent female donors (samples 9–11) and 1 male donor (sample 12). The graph illustrates the percentage of diploid, tetraploid, and aneuploid cells.
CVS preparation and culture
Twenty three CVS were obtained for prenatal diagnosis (Fig. 4). Specimens were dissected under a dissecting microscope and tissue was digested with a collagenase/dispase solution. CVS preparations were grown as attached monolayers on coverslips in Amniomax complete medium with a final concentration of 12% serum (Gibco-Invitrogen, Rockville, MD). Standard methods were used to harvest CVS preparations [21].
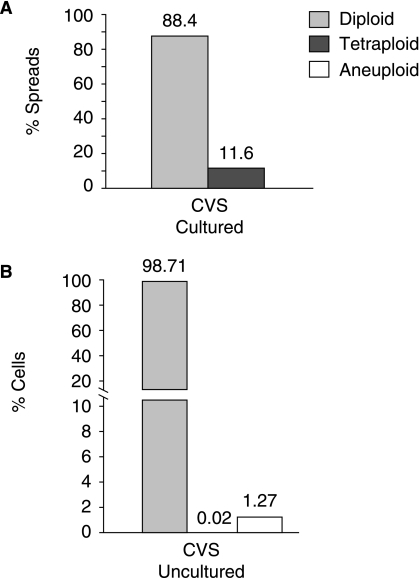
FIG. 4.
Chromosome stability in prenatal diagnostic samples. (A) Twenty three independent chorionic villus samples (C1–C23) were cultured for prenatal diagnosis. Chromosome spreads were prepared following 7–8 days (~PD 8–10) in culture to determine if numerical or structural abnormalities were detectable using the G-banding technique described in Fig. 1A. Twenty to thirty six chromosome spreads were analyzed in each CVS preparation (details are provided in Supplementary Table S3). Structural abnormalities were not detected (data not shown). The average percentages of diploid and tetraploid cells across all 23 CVS preparations are indicated. The tetraploid category includes spreads with close to double the normal chromosome number and may therefore encompass an aneuploid subset. (B) Uncultured cells from CVS preparations C1–C23 were dropped onto microscope slides. Each sample was hybridized with probes that detect chromosomes 13 and 21 (set 1) and also independently with a probe mix targeting chromosomes 18, X, and Y (set 2). A total of 200–400 interphase cells were scored in each sample. Half of the cells scored per sample were hybridized with each probe set and the frequency of diploid, tetraploid, and aneuploid cells recorded. A total of 4,900 cells were scored across the 23 CVS preparations. The average percentage of diploid, tetraploid, and aneuploid cells is presented. The average diploid rate was 98.71%. Tetraploidy was only observed in only one cell (in CVS sample C23 using probe set 1) giving a frequency of 0.02%. Cells in the aneuploid class averaged 1.27%.
Preparation of mitotic chromosome spreads and G-banding
ASC sample 4 was cultured in EGM2-SV. At ~ PD10, colcemid (Invitrogen) was added to ASC sample 4 at a final concentration of 0.02 μg/mL for 4 h at 37°C to block dividing cells in mitosis. Cells were harvested and resuspended in a hypotonic solution (1:1:1 mixture of 0.8% w/v sodium citrate, 0.8% w/v potassium chloride and sterile water) at 37°C for 10 min after which they were pelleted again and resuspended in fixative (3:1 w/v methanol: glacial acetic acid) and dropped onto microscope slides to generate chromosome spreads and stained using G-banding techniques (Fig. 1A). Standard procedures were used to harvest mitotic cells from CVS preparations to permit chromosome preparation and G-banded chromosome staining (Fig. 4A) [21]. Chromosome spreads were analyzed using CytoVision software (Applied Imaging, Santa Clara, CA).
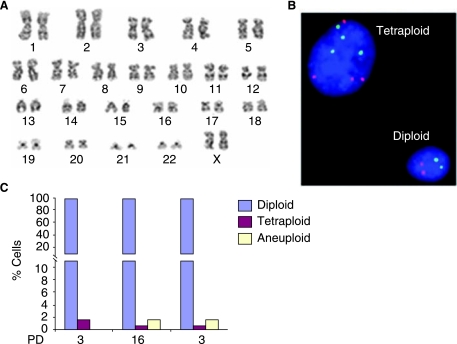
FIG. 1.
Genomic stability in cultured adipose stromal cells (ASCs). (A) Mitotic chromosomes were harvested from ASC sample 4 at ~10 population doublings (PD) and examined using G-banding techniques. Twenty metaphase chromosome spreads were analyzed and found to be normal (46,XX). (B) Fluorescence in situ hybridization (FISH) analysis of cultured ASCs using centromere probes specific for the X chromosome (red) and chromosome 17 (green). Nuclei are stained with DAPI (blue). In normal (diploid) female cells there are two X chromosomes (red spots) and two chromosome 17s (green spots). A tetraploid cell exhibits whole genome duplication resulting in an approximate doubling of the nuclear volume and detection of double the normal chromosome number. In a tetraploid female cell there are 4 X chromosomes (4 red spots) and 4 chromosome 17s (4 green spots). (C) FISH analysis of ≥200 cells from ASC samples 1 and 2 at the indicated PD times revealed a high diploid content. Chromosomally aberrant cells (classed as tetraploid or aneuploid) were also detectable at a frequency up to 1.5% (see details in Supplementary Table S1). Split-scale Y axes were used as indicated for better illustration.
FISH analysis of interphase cells
ASCs were collected, resuspended in hypotonic solution followed by a fixation step and dropped onto microscope slides, as described above. Uncultured SVF cells were also dropped onto slides following fixation. Probes specific to the centromeres of chromosome 17 (CEP 17, Spectrum Green) and the X (CEP X, Spectrum Orange) were purchased from Abbott Molecular Inc. (Des Plaines, IL). Following probe hybridization and washes, cells were counterstained with 4′, 6 diamidino-2-phenylindole (DAPI; Vector Labs, Burlingame, CA) which stains nuclei blue. The number of signals obtained with the 17 and X probes on at least 200 interphase nuclei per experiment were counted using a Leica DM5000B fluorescence microscope (Leica Microsystems, Bannockburn, IL). Images were captured with a Spot RTKE camera (Diagnostic Instruments, Sterling Heights, MI). Uncultured cells from CVS preparations C1–C23 (prepared as described above) were further digested with a trypsin/EDTA solution, subjected to hypotonic treatment, fixed in 3:1 w/v methanol acetic acid and dropped onto glass slides. CVS interphase cells were denatured and hybridized with AneuVysion probe sets that detected chromosome 13 and 21 (probe set 1) and chromosomes X, Y and 18 (probe set 2) (Abbott Molecular Inc. Des Plaines, IL) and counterstained with DAPI. For each probe set the number of signals was assessed in at least a total of 100 interphase cells by two observers (Fig. 4B, Supplementary Table S4; Supplementary information is available online at http://www.liebertpub.com/scd). CVS cells were viewed with a Leitz DMR fluorescence microscope (Bannockburn, IL) equipped with FISH imaging software (Applied Imaging, Santa Clara, CA). Standard procedures were used for FISH.
Results
Maintenance of chromosome stability in cultured ASCs
To investigate the genomic stability of ASCs, chromosome content was initially assessed at PD10 in cultured ASCs from a single female donor (ASC sample 4) by preparing chromosome spreads and staining using the G-banding technique. Consistent with a previous report [20], analysis of 20 chromosome spreads revealed that this population was diploid with no evidence of chromosomal rearrangements (Fig. 1A). However, since a low level of chromosome instability could escape detection by examination of such a limited cell number, FISH with probes specific to the centromeres of chromosome 17 and the X chromosome was used to screen a larger population of interphase cells. While every chromosome is not sampled in this assay, it does provide an indirect estimate of the frequency of diploid (2n), tetraploid (4n) and aneuploid cells in a large sample set. Tetraploid cells are thought to arise from cytokinesis failure via binucleated intermediates or through endoreduplication and may be prone to aneuploidy and transformation. Aneuploid cells, arising from chromosome segregation errors, exhibit a chromosome number which is not a multiple of n (the haploid number). However the interphase FISH technique does not provide an accurate measure of true aneuploidy as it includes hybridization artifacts and excludes aneuploidy of chromosomes other than X or 17. The significance of the low and uniform level of aneuploid cells detected using interphase FISH in this study (Table 1) is therefore unclear.
Table 1.
Chromosome Stability in Uncultured and Cultured Adipose-Derived Cells and Prenatal Diagnostic Samplesa
| |
# Scored by |
Chromosome content |
|||
|---|---|---|---|---|---|
| Cells | FISH | G-banding | Diploid | Tetraploid | Aneuploid |
| Adipose-derived cells | |||||
| Uncultured | |||||
| SVF | 1197 | 1,183 (98.83) | 10 (0.83) | 4 (0.33) | |
| Cultured | |||||
| ASC182 | 20 | 20 (100) | 0 (0) | 0 (0) | |
| ASC | 6355 | 6,214 (97.8) | 55 (0.9) | 86 (1.3) | |
| Prenatal diagnostic samples | |||||
| Uncultured | |||||
| CVS | 4,900 | 4,837 (98.71) | 1 (0.02) | 62 (1.27) | |
| Cultured | |||||
| CVS | 559 | 494 (88.4) | 65 (11.6) | ||
Chromosome stability was measured by either counting chromosome number in G-banded chromosome preparations from adipose stromal cell (ASC) sample ASC182 Fig. 1A) and chorionic villus sample (CVS) preparations (C1-C23; Fig. 4A) or by FISH analysis of interphase cells using 17 and X specific centromere probes (Figs. 1–3) on cultured ASCs and uncultured stromal vascular fraction (SVF) or 13, 21, 18, X, and Y probes on uncultured CVS preparations (Fig. 4B). The total number of chromosome spreads scored by G-banding or cells scored by FISH analysis in each group are indicated as well as the fraction of diploid, tetraploid, and aneuploid cells. Data for four independent uncultured SVF samples and eight independent cultured ASC samples are summarized (details in Figs. 1–3 and Supplementary Tables S1 and S2). Chromosome stability in 23 independent prenatal CVS preparations was measured in matched uncultured and cultured samples using FISH analysis or chromosome G-banding, respectively (Fig. 4; Supplementary Tables S3 and S4).
At least 200 interphase cells were scored in each FISH experiment. In a normal female cell, two spots are detected with each of the X and 17 probes (Fig. 1B). In a female cell that has become tetraploid, four spots are detected with each probe and the nuclear volume increases approximately two fold (Fig. 1B). A preliminary examination of ASC sample 1 at PD3 and PD16 and ASC sample 2 at PD3 using FISH analysis of interphase cells revealed that cultures were predominantly diploid (98.5%). However, tetraploid nuclei were detectable in up to 1.5% of cells (Fig. 1C; Supplementary Table S1). To extend this analysis, a further five ASC populations were freshly isolated and cultured for either 25–30 days (ASC samples 3, 4) or until senescence at ~ day 50 (ASC samples 5, 6, and 7). Chromosome stability was assessed at intervals by interphase FISH. Cells were counted in ASC samples 3 and 4 at each passage to provide an estimate of PD number in cultured ASCs which was determined to be 0.79/day (see Materials and Methods). In all five samples, the vast majority of ASCs maintained a high diploid content ranging from 94.7–99.1% (average 97.7%) at early passage (PD 3–8) to 96.7–99.5 % (average 98.1%) at mid to late passage (PD 10–38) (Fig 2A, B; Supplementary Table S1). While tetraploid cells were detected, they appeared to decrease in frequency over time in culture, although these differences were not statistically significant (Fig. 2A, B; Table S1).
To determine whether ASCs harbor a subset of cells with high proliferative potential (HPP), ASC sample 8 was harvested after 3 days in culture following initial isolation and plated at limiting dilution in 96-well plates to generate clonal lines. PD time in clones 8-E1 and 8-F6 was estimated to be ~0.86 PD/day. 8-E5 and 8-F6 were harvested for interphase FISH analysis at mid (PD25) and late passage (PD35). These analyses revealed that the HPP clones also maintained a high level of genomic stability with an average diploid frequency of 96.35% (Fig. 2C, Supplementary Table S1). Interestingly, no tetraploid cells were detectable in 868 HPP cells scored in this assay suggesting clonal variation in propensity for tetraploidy.
Uncultured SVFs harbor aberrant cells at a similar low frequency to cultured ASCs
While all seven cultured ASC populations exhibited a high level of normal diploid cells, tetraploid and aneuploid cells were detected. These findings raise the concern that a small sub-population of aberrant cells may arise as a consequence of passage under culture conditions. To address this question, lipoaspirates were harvested from 4 donors (samples 9–12) and single cell suspensions obtained. This cell mixture is referred to as the stromal vascular fraction (SVF). The uncultured SVF was immediately processed for FISH analysis without exposure to growth on plastic dishes. Results of interphase FISH analysis with chromosome 17 and X centromere probes revealed that most cells were normal (Fig 3; Supplementary Table S2). However an average tetraploidy rate of 0.83% (in 1,197 cells scored) and aneuploidy rate of 0.33% was detected which is comparable to cultured ASCs (0.9 and 1.3%, respectively) (Fig. 3, Supplementary Table S2, Table 1).
Chromosome stability in cultured and uncultured prenatal diagnostic samples
Of significant note, data on chromosome stability in cultured human primary cells are not widely reported. In order to compare the ASC results with nondiploid cell frequency in other primary human cells we examined chromosome stability in prenatal diagnostic samples analyzed by the Indiana University School of Medicine Cytogenetics Diagnostic laboratory. Chromosome stability rates were measured in matched cultured and uncultured CVS, comprising mostly fetal-derived cells from the placenta. Chromosome stability was measured using G-banding in cultured CVS cells and by interphase FISH analysis in uncultured CVS preparations. Twenty three CVS preparations (C1–C23; Supplementary Table S3)were collected and cultured for ~8–10PD after which G-banded chromosomes were prepared. A total of 20–36 chromosome spreads were analyzed in each CVS preparation for chromosome number and karyotype stability. Results of chromosome analysis demonstrated that cultured CVS preparations exhibited markedly elevated levels of tetraploid cells that averaged 11.6% across all twenty three samples (Fig. 4A, Supplementary Table S3, Table 1), when compared to cultured ASCs (average 0.9%, Table 1). Tetraploid cells were detected in 18/23 CVS samples (in 3.85–28.6% of spreads) (Supplementary Table S3).
To determine whether the elevated tetraploidy rate in cultured CVS preparations is a consequence of growth in culture, chromosome stability rates were determined using FISH analysis in uncultured cells from the same 23 CVS preparations (C1–C23) used for G-banding analysis (Fig. 4A). Prior to plating samples C1–C23 in culture, a small fraction of the cells were fixed, dropped onto microscope slides and analyzed using FISH. In the clinical lab, probes to chromosomes 13, 18, 21, X and Y are used on uncultured prenatal samples to detect the most frequently occurring chromosomal aneuploidies. A total of 4,900 cells (a minimum of 200 cells/sample) were scored for the presence of signals consistent with diploidy, tetraploidy, or aneuploidy (see materials and methods, Fig. 4B). In comparison to the diploid rate of 88.4% measured by G-banding in cultured CVS preparations (Fig. 4A), uncultured CVS preparations have a substantially higher proportion of diploid cells averaging 98.71% among the twenty three samples (Fig. 4B, Table 1, Supplementary Table S4). Only one chorionic villus–derived cell out of the 4,900 evaluated, exhibited a pattern consistent with a tetraploid chromosome content suggesting that tetraploid cells are rare in this tissue (0.02% frequency, Fig. 4B, Table 1, Supplementary Table S4)compared to uncultured adult adipose-derived cells from the SVF, in which 10 (0.83%) of the 1,197 cells analyzed were tetraploid (Fig. 3; Table 1). The proportion of uncultured CVS-derived cells scored as aneuploid averaged 1.27 % (Table 1). All prenatal CVS were reported as normal based on G-banding of cultured cells.
Discussion
The transplantation of adult stem or progenitor cells is currently being explored as a means of tissue regeneration or correction of genetic deficiency in a range of human diseases [22]. The isolation and propagation of stem/progenitor cell populations typically involves a period of in vitro culture under nonphysiological conditions. Given the weight of evidence linking chromosome gain or loss with cancer initiation or progression, an essential prerequisite for the safe application of stem/progenitor cell transplantation is to ensure maintenance of chromosome stability in culture.
A long-term goal is to exploit adipose tissue obtained by liposuction or abdominoplasty as an abundant source of ASCs for therapeutic transplantation. While ASCs have the capacity for multi-lineage differentiation, they have recently been determined to exhibit properties of pericytes in vivo and substantially improve microvessel formation in vitro, suggesting that combinations of transplanted cell types may improve treatment of ischemic disease [9]. Production of ASCs in the quantity required for transplantation, can involve culture for at least a few PDs. This study was aimed at assessing chromosome stability in ASCs during an extended culture period.
As a point of reference we examined data from the inhouse prenatal cytogenetic diagnostic laboratory on chromosome stability from cultured CVS, obtained by the G-banding technique. This analysis revealed a level of tetraploidy averaging 11.6% (Fig. 4A) which far exceeds the tetraploid cell detection rate in uncultured CVS preparations using FISH analysis with chromosome 13, 21, 18, X and Y probes (0.02%; Fig. 4B) and is evidently a consequence of growth in culture. Such a high frequency of chromosomal abnormality may be undesirable in cells destined for transplantation. In contrast, previously reported karyotype analyses of ASCs by G-banding detected no numerical chromosome abnormalities upon extended growth in culture [20] and our own G-banding analysis replicated these findings (Fig. 1A).
In comparison to the prenatal diagnostic samples, these data point to a relatively high level of chromosome stability in cultured ASCs. Notably however, the constrained cell number included in G-banding analysis makes this technique unsuitable for the detection of low frequency chromosome number alterations. To increase the rigor of this study, FISH analysis was performed on more than 6,000 interphase ASC cells representing seven cultured ASC populations and two sub-clones sampled at regular intervals between PD 3–35 with probes to chromosomes 17 and X. In this enlarged sample tetraploid cells were detectable at a frequency of approximately 0.9% (Table 1). However, the frequency of chromosomal abnormality appeared to be independent of PD number and a comparison with the uncultured SVF obtained directly from lipoaspirates revealed a similar proportion of tetraploid cells (Table 1). Notably the existence of tetraploid cells has been reported at a higher level in other human tissues including liver and cardiac muscle where it has been suggested to contribute to tissue survival [23,24]. In this study, no evidence of highly proliferating cells was observed in any sample after 50 days in culture suggesting that ASCs are not prone to spontaneous transformation. In the two highly proliferative ASC sub-clones analyzed, no tetraploid cells were detected suggesting that sub-cloning could achieve the elimination of tetraploid cells from ASC cultures. While a low level of aneuploid cells was detected in all samples (average 1.33% in cultured ASCs, 0.33% in uncultured SVF and 1.27% in uncultured CVS samples, Table 1) this is probably within the range of hybridization artifacts.
The finding that ASC culture under the conditions employed does not elevate the level of chromosomal abnormalities above that observed in vivo is supportive of the use of this particular cell population in clinical and functional studies. However the results with CVS preparations do highlight the potential risk of culture-induced chromosomal abnormalities in artificially propagated primary cells. Furthermore, the disparity between results obtained in this study by G-banding and FISH analysis of CVS argues for the inclusion of appropriately scaled FISH analysis of any cultured cell population to be used for transplantation.
In cell types susceptible to genomic instability in culture, growth conditions may prove critical to maintenance of a diploid karyotype. For example, serum concentration was shown to affect ploidy of glioma cells such that growth in defined (serum free) medium maintained a largely diploid karyotype and primary cell morphology whilst standard medium supplemented with 10% serum led to elevated levels of tetraploidy and loss of the primary cell phenotype [25]. Thus, modification of culture conditions may be an effective way to manage problems of tetraploidy or chromosome instability should they occur in some stem/progenitor cell types. Notably, serum concentrations in this study were considerably lower for ASCs (2%) than CVS (12%). However another study has found that ASCs also maintain chromosome stability, as measured by G-banding, when grown in 20% serum [20], pointing again to cell-type dependence in the effects of growth conditions on genomic instability.
In summary, this study has determined that cultured ASCs exhibit no loss of genomic stability under the conditions employed, thus satisfying a potentially important requirement of any cell destined for human transplantation. In contrast, fetal-derived cells of the chorionic villus are prone to a degree of tetraploidization when cultured under standard conditions, which is likely to be unacceptable in clinical application. These findings highlight the risk of culture-induced alteration of chromosome content in primary human cells and the need for appropriate screening before transplantation. Furthermore, the higher sensitivity of FISH analysis in comparison to conventional G-banding suggests that it should be used in parallel with G-banding to detect low level chromosomal changes induced in culture prior to clinical application.
Supplementary Material
Acknowledgments
Support for this study in the Grimes lab was generously provided by the Indiana Genomics Initiative (INGEN), Indiana University School of Medicine Cytogenetics Division, and the Ralph W. and Grace M. Showalter Trust. INGEN is supported in part by Lilly Endowment. Research in the March lab is supported by an NIH RO1 grant (HL77688-01), a Veterans Affairs Merit Review Grant, and an American Heart Association Postdoctoral Fellowship awarded to D.O.T. A portion of the results included in this article were presented at the International Federation of Adipose Therapeutics and Science (IFATS) International Meeting in October 2007, Indianapolis, IN, USA.
References
- 1.Broxmeyer HE. Srour E. Orschell C. Ingram DA. Cooper S. Plett PA. Mead LE. Yoder MC. Cord blood stem and progenitor cells. Methods Enzymol. 2008;419:439–473. doi: 10.1016/S0076-6879(06)19018-7. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 2008;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Kawamoto A. Gwon HC. Iwaguro H. Yamaguchi JI. Uchida S. Masuda H. Silver M. Ma H. Kearney M. Isner JM. Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2008;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 4.Iwami Y. Masuda H. Asahara T. Endothelial progenitor cells: past, state of the art, and future. J Cell Mol Med. 2008;8:488–497. doi: 10.1111/j.1582-4934.2004.tb00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingram DA. Mead LE. Tanaka H. Meade V. Fenoglio A. Mortell K. Pollok K. Ferkowicz MJ. Gilley D. Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2008;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 6.Perin EC. Dohmann HF. Borojevic R. Silva SA. Sousa AL. Mesquita CT. Rossi MI. Carvalho AC. Dutra HS. Dohmann HJ. Silva GV. Belem L. Vivacqua R. Rangel FO. Esporcatte R. Geng YJ. Vaughn WK. Assad JA. Mesquita ET. Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2008;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 7.Prockop DJ. Azizi SA. Colter D. Digirolamo C. Kopen G. Phinney DG. Potential use of stem cells from bone marrow to repair the extracellular matrix and the central nervous system. Biochem Soc Trans. 2008;28:341–345. [PubMed] [Google Scholar]
- 8.Gronthos S. Franklin DM. Leddy HA. Robey PG. Storms RW. Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2008;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 9.Traktuev DO. Merfeld-Clauss S. Li J. Kolonin M. Arap W. Pasqualini R. Johnstone BH. March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 10.Wickham MQ. Erickson GR. Gimble JM. Vail TP. Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2008:196–212. doi: 10.1097/01.blo.0000072467.53786.ca. [DOI] [PubMed] [Google Scholar]
- 11.Mizuno H. Zuk PA. Zhu M. Lorenz HP. Benhaim P. Hedrick MH. Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg. 2008;109:199–209. doi: 10.1097/00006534-200201000-00030. discussion 210–191. [DOI] [PubMed] [Google Scholar]
- 12.Jonasson L. Hansson GK. Bondjers G. Bengtsson G. Olivecrona T. Immunohistochemical localization of lipoprotein lipase in human adipose tissue. Atherosclerosis. 2008;51:313–326. doi: 10.1016/0021-9150(84)90179-5. [DOI] [PubMed] [Google Scholar]
- 13.Case J. Horvath TL. Ballas CB. March KL. Srour EF. In vitro clonal analysis of murine pluripotent stem cells isolated from skeletal muscle and adipose stromal cells. Exp Hematol. 2008;36:224–234. doi: 10.1016/j.exphem.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo MJ. Suh SY. Bae YC. Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2008;328:258–264. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- 15.Safford KM. Hicok KC. Safford SD. Halvorsen YD. Wilkison WO. Gimble JM. Rice HE. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2008;294:371–379. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 16.Miranville A. Heeschen C. Sengenes C. Curat CA. Busse R. Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2008;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y. Sun Z. Liao L. Meng Y. Han Q. Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2008;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 18.Planat-Benard V. Menard C. Andre M. Puceat M. Perez A. Garcia-Verdugo JM. Penicaud L. Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2008;94:223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 19.Strem BM. Zhu M. Alfonso Z. Daniels EJ. Schreiber R. Beygui R. MacLellan WR. Hedrick MH. Fraser JK. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy. 2008;7:282–291. doi: 10.1080/14653240510027226. [DOI] [PubMed] [Google Scholar]
- 20.Meza-Zepeda LA. Noer A. Dahl JA. Micci F. Myklebost O. Collas P. High resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence. J Cell Mol Med. 2008;12:553–563. doi: 10.1111/j.1582-4934.2007.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Dyke DL. Roberson JR. Wiktor A. Prenatal Cytogenetic Diagnosis. In: McClatchey KD, editor. Clinical Laboratory Medicine. 2nd. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 645–647. [Google Scholar]
- 22.Daley GQ. Scadden DT. Prospects for stem cell-based therapy. Cell. 2008;132:544–548. doi: 10.1016/j.cell.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Biesterfeld S. Gerres K. Fischer-Wein G. Bocking A. Polyploidy in non-neoplastic tissues. J Clin Pathol. 2008;47:38–42. doi: 10.1136/jcp.47.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anatskaya OV. Vinogradov AE. Genome multiplication as adaptation to tissue survival: evidence from gene expression in mammalian heart and liver. Genomics. 2007;89:70–80. doi: 10.1016/j.ygeno.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Lee J. Kotliarova S. Kotliarov Y. Li A. Su Q. Donin NM. Pastorino S. Purow BW. Christopher N. Zhang W. Park JK. Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2008;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.