Abstract
Tuberculosis, a List B disease of World Organization for Animal Health, caused by M. avium or M. genavense predominantly affects poultry and pet or captive birds. Clinical manifestations in birds include emaciation, depression and diarrhea along with marked atrophy of breast muscle. Unlike tuberculosis in animals and man, lesions in lungs are rare. Tubercular nodules can be seen in liver, spleen, intestine and bone marrow. Granulomatous lesion without calcification is a prominent feature. The disease is a rarity in organized poultry sector due to improved farm practices, but occurs in zoo aviaries. Molecular techniques like polymerase chain reaction combined with restriction fragment length polymorphism and gene probes aid in rapid identification and characterization of mycobacteria subspecies, and overcome disadvantages of conventional methods which are slow, labour intensive and may at times fail to produce precise results. M. avium subsp. avium with genotype IS901+ and IS1245+ causes infections in animals and human beings too. The bacterium causes sensitivity in cattle to the tuberculin test. The paper discusses in brief the M. avium infection in birds, its importance in a zoonotic perspective, and outlines conventional and novel strategies for its diagnosis, prevention and eradication in domestic/pet birds and humans alike.
1. Introduction
Avian tuberculosis is one of the most important diseases that affect domestic and pet birds. Several mycobacterial species can be involved in the aetiology of avian tuberculosis. The disease is most often caused by Mycobacterium avium belonging to serotypes 1, 2, 3, and 6 (genotype IS901+ and IS1245+) and M. genavense [1–3]. Other species, such as M. intracellulare, M. scrofulaceum, M. fortuitum, M. tuberculosis, and M. bovis can also cause avian tuberculosis, but the incidences are rare [2, 4–6]. M. avium causes avian tuberculosis in probably all avian species, especially in waterfowl, galliformes, columbiformes, passerines, psittacines, raptors, and ratites [1, 7–10]. The disease has a worldwide distribution but is seen most frequently in the North Temperate Zone [11–14]. Susceptibility to disease varies from species to species. Hejlicek and Treml [15] broadly classified bird species into four groups according to their susceptibility to avian tuberculosis as highly susceptible: domestic fowl, sparrows, pheasants, and partridges; less susceptible: guinea fowl and domestic turkeys; moderately resistant: domestic goose and duck, highly resistant: the domestic pigeon. In any avian species, stress factors appear to enhance the development of the disease and this is particularly noteworthy in case of birds living in captivity [4]. Infected birds and contaminated water and soil are the main source of infection as the Mycobacteria can survive for several months in the environment [2, 5]. The disease is more prevalent in places with high population density and poor sanitation and hygienic conditions. The practices of allowing birds to roam freely and keeping the breeders for several years are highly conducive to the spread of tuberculosis [11]. In a flock if once established, TB induces unthriftiness, decreased egg production, and increased mortality, which culminates into severe economical losses.
Mycobacterium avium complex (MAC), comprising M. avium subsp. avium, M. avium subsp. paratuberculosis, M. avium subsp. Silvaticum, and M. intracellulare, may also infect different animal species like swine, cattle, deer, sheep, goat, horses, cats, dogs, and exotic species besides causing infection in immunocompromised human beings [3, 4, 16, 17]. M. genavense has also been reported in a dog and an immunocompromised cat. M. intracellulare is a closely related pathogen of birds with a lower prevalence [18]. Although successful experimental infections with M. a. paratuberculosis in poultry have been reported [19], however, this subspecies, known to cause Johne's disease (paratuberculosis) in ruminants and other mammals, has not been encountered during any of the cases of avian tuberculosis till date. M. avium subsp. avium (MAA) is considered as the most important pathogen causing tuberculosis in domestic birds [2, 20]. On the basis of genetic and phenotypic differences it has been proposed to categorize MAA into two subspecies, namely, M. a. hominissuis for human and porcine isolates and M. a. avium for bird-type isolates [21]. In humans, M. avium is capable of inducing a progressive disease that is refractory to antibiotic treatment and is recognized as localized primary lymphadenitis, pulmonary disease, and a disseminated form of infection [4, 22]. Hence the handling of infected birds in farms or live cultures of M. avium in laboratories should be carried out with adequate care.
2. Etiology
M. avium, the causative agent of avian tuberculosis, considered as “atypical mycobacteria”, comprises aerobic, nonspore-forming and nonmotile rod shaped bacteria that vary in length from 1–3 μm and cords are not formed, unlike M. tuberculosis [2]. They are weakly Gram-positive and stained specifically by acid fast (Ziehl-Neelsen) staining method, due to high levels of lipids in mycobacterial cell wall. M. avium is highly resistant to environmental challenges and can survive in soil for up to 4 years, and this makes eradication of the organism difficult [1, 2, 23]. M. avium is resistant to high and low temperatures, dryness, pH changes, and many commonly used disinfectants. However, the unprotected organism is killed by direct sunlight. In contrast to M. tuberculosis and M. bovis, M. avium grows at temperatures ranging from 25–45°C, the most favorable range being 29–45°C and for primary isolation, growth can be enhanced with 5–10% CO2 tension [3, 23]. Strains of M. avium can be identified by serological procedures. To date, 28 MAC serotypes have been identified from which the serotypes 1–6, 8–11, and 21 belong to M. avium subsp. avium (MAA). Serovars 7, 12–20, and 25 have been ascribed to M. intracellulare. However, no consensus was achieved on other serovars, and some isolates cannot be typed [3, 24]. Serotypes 1, 2, and 3 are considered virulent for chickens (Table 1) [2, 11, 23]. Serotypes 1 and 2 are most commonly isolated from domestic birds, and serovar 3 is recovered sporadically from wild birds. Serotypes 1 and 2 can affect animals, whereas 4–20 are mainly found in humans. Serovar-1 is the most common organism isolated from birds and from human beings. Distinguishing serovars can help provide a means for studying origin and distribution of specific strains. According to the current taxonomy, M. avium contains four subspecies, namely, M. avium subsp. avium; M. avium. hominissuis; M. avium. Paratuberculosis; M. avium. silvaticum, which is diagnosed rarely in birds [3, 21]. It is well established that most M. a. avium isolates from birds have a repetitive sequence IS901 in their genome and also produce a characteristic three band pattern in IS1245 restriction fragment length polymorphism (RFLP) [25]. It has been postulated that the presence of IS901 correlates with pathogenicity in birds [25–27]. Other than M. a. silvaticum, IS901 has only been detected in M. avium strains with serotypes 1, 2, and 3 [5].
Table 1.
Serotypes of M. avium complex (MAC) and their susceptibility to various species of certain birds and mammals [2, 9].
| Species | MAC serotypes | Susceptibility |
|---|---|---|
| Domestic fowl (Gallus domesticus) | 1, 2 | High |
| Turkey | 1, 2 | Moderate |
| Pheasants | 1, 2 | High |
| Wild birds | 2, 3 | High |
| Cattle | 1, 2 | Moderate |
| Swine | 1, 2, 4, 8 | High |
| Rabbit | 1, 2 | High |
| Man | 1, 4 to 20; 23, 25 | Low (in healthy individuals); High (in immunocompromised) |
M. avium is the most significant cause of poultry disease. Disease onset in birds is normally more rapid with M. genavense than with M. avium. In wild birds, though the disease is uncommon, TB may develop when they are in contact with infected chickens. Mycobacterium avium complex and M. intracellulare can also infect an extensive range of different animal species. M. tuberculosis is less commonly the cause of infection in birds, often as a result of transmission from pet bird owners, and also clinical signs differ from those caused by the more commonly occurring species of mycobacteria. In case of psittacine birds, apart from this, tuberculosis due to M. tuberculosis or M. bovis has also been reported. In canaries, tuberculosis may be caused frequently by M. tuberculosis [2].
3. Transmission
The main source of infection is infected birds as they shed large amounts of organism into the environment. The bacilli are exuded from ulcerated lesions of the intestine and are voided in droppings. The most common route of infection for susceptible birds is the alimentary tract [1, 2]. Respiratory tract is also suggested as a potential source of infection. The disease gets transmitted to the susceptible birds by ingestion and inhalation of aerosolized infectious organisms. Persistence within flocks is associated with keeping older stocks without following adequate cleanliness and hygiene [2]. Further, maintaining birds closely confined under stressful conditions provide favorable ways for the spread of the disease. The ability of the organism to persist in the environment for many years, especially in soil and litter favor the disease transmission to a great extent [5]. Litter, pens, equipment, and pasture contaminated with excreta of infected domestic birds and the hands, feet, and clothing of attendants play an important role in disease transmission. Wild birds, pigs, and some mammals may also act as significant reservoirs of infection [2, 11]. Wild birds, such as sparrows, crows, and pigeons may be infected with M. avium and may spread it to poultry flocks [7]. Also, rats and other rodents are known to act as mechanical carriers in transmission of the disease. The agent can also be disseminated by infected carcasses and offals. Occasionally, skin invasion and spread via infected eggs may occur. M. avium has been isolated from eggs of naturally infected chickens, but hatched chicks have not developed the disease [2]. The bacilli does not survive in eggs after proper boiling.
4. The Disease and Manifestations
Avian tuberculosis is a contagious disease which occurs in chickens, pheasants, quail, guinea fowl, turkeys, parrots, budgerigars, ducks, goose, doves, partridges, pigeons, and other captive and wild game birds and has also been reported in ostriches, emus, and rheas in many zoological parks. Tuberculosis in birds is most prevalent in chickens and in wild birds raised in captivity. In poultry, the disease follows a slow course through the flocks. The classical presentation is characterised by chronic and progressive wasting and weakness. Avian tuberculosis in domestic birds is primarily an intestinal and hepatic disease with dissemination to other organs including the lungs, air sacs, spleen, bone marrow, and skin [2, 6, 11, 23]. Similarly, avian tuberculosis reported in free living birds including raptors were presented with the disseminated form involving the digestive tract, liver and spleen [8, 28, 29]. The disease has a long incubation period and a protracted course and if appreciable, the symptoms can prolong for weeks or months. Because of the chances to become established through a longer exposure, the disease is less prevalent in young fowls and lesions are less severe in them when compared to adult birds. Usually the losses are experienced more in older stocks of age group 18–20 months. The disease process can be divided into three phases: latency, lesion development, and period of cachexia [2, 5, 11]. During cachexia, massive tubercles with large numbers of bacilli develop. In the classic form of infection the tubercles or granulomas develop in multiple organs; a second form is manifested with lesions in the intestinal tract; a third type of infection often experienced as a nontuberculous one, mainly seen in finches, canaries, and psittacines [2, 5]. Some birds show respiratory signs and sudden death may occur, dyspnoea is less common, and granulomatous ocular lesions [30] and skin lesions have been reported.
Clinical signs are not pathognomonic in avian TB and vary depending on the organs involved. Birds with the intestinal form of tuberculosis often present with chronic wasting disease. In majority of cases of tuberculosis in birds, especially in the initial phase of infection, clinical signs are not grossly observable. However, in advanced cases, birds may develop symptoms like progressive weight loss, depression, white diarrhea with soiled feathers, increased thirst, respiratory distress, fatigue, and decreased egg production [11, 23, 31–33]. Feathers are often dull or ruffled and comb, wattle, and earlobes often appear pale, thinner and dry. Birds eventually become lethargic and emaciated with marked atrophy of breast muscles manifested as “knife edged” keel [2, 5]. In extreme cases, the body fat disappears, and the face of the bird appears smaller than normal. If a jerky hopping gait is observed due to unilateral lameness then it should be assumed that there could be the presence of tubercular lesions in bone marrow of the leg bones or joints. Some birds may adapt a sitting position. Tuberculous arthritis can even lead to paralysis. Fatal results often occur due to massive hemorrhage caused by ruptured liver or spleen. In this case, occasionally birds may die suddenly in good bodily condition and yet show advanced lesions of tuberculosis. The body temperature of the affected bird remains normal, even in severe cases. In most cases, an infected bird without overt clinical signs may serve as carrier that result in the persistence of infection in flocks. In commercial broiler production units, generally avian tuberculosis is uncommon primarily due to the short life span and in layers and breeders, the infection is a matter of much concern. Mortality over a short period may be insignificant, but the intermittent loss of adult birds in valuable breeding stock and decreased egg production in layers are detrimental. Occasionally, heavy losses may occur in pullets on multiage sites where the infection is endemic and the hygienic standards are poor.
After entering the host, M. avium prevents the fusion of phagosomes with lysosome and the subsequent bacteremia provides a generalized distribution of lesion. The gross lesions are characterized by the presence of epithelioid cells containing large numbers of organisms that may either diffusely infiltrate the organ or form discrete granulomas [6]. There is presence of tubercular nodules in intestine, liver, spleen, ovaries, testes, and bone marrow but the pulmonary lesions, which are a striking feature of tuberculosis in other species, are rarely observed in birds [2, 5]. Pulmonary avian tuberculosis is only seen occasionally as in case of tuberculosis of pigeons and water fowl [1, 2]. The principal lesions of tuberculosis in birds are seen in intestine, where affection often presents with studded greyish-white to greyish-yellow nodules. Before the intestinal tract is opened, the ulcerated areas appear as tumour-like masses attached to the gut wall, but when the intestine is opened, the true nature of the mass becomes evident. The nodules bulge from the serosal surface of the intestine and can be palpated. Due to this, spleen takes irregular “knobbly” appearance. Lesions evident as deep ulcers filled with caseous material discharges the organism into the intestinal lumen and get excreted via the droppings. Typical caseous lesions, without calcification, are always found in the liver and spleen, with considerable enlargement of the organs [2, 5]. Nodules are firm but can be incised easily since mineralization is rare in avian TB (this is in contrast to leucosis, in which lesions cannot be enucleated from the surrounding tissue). The bone marrow of the long bones frequently contains tubercular nodules. Some exotic bird species may have lesions in the liver and spleen without intestinal involvement. Microscopically, lesions consist of granulomas with a central necrosis, either coagulative or caseous, and multinucleate giant cells. Acid fast bacilli are numerous in the central or necrotic zone of the tubercle [2, 11]. Gross and microscopic lesions in spleen of Demoiselle cranes (Anthropoides virgo) are depicted in Figures 1 and 2.
Figure 1.
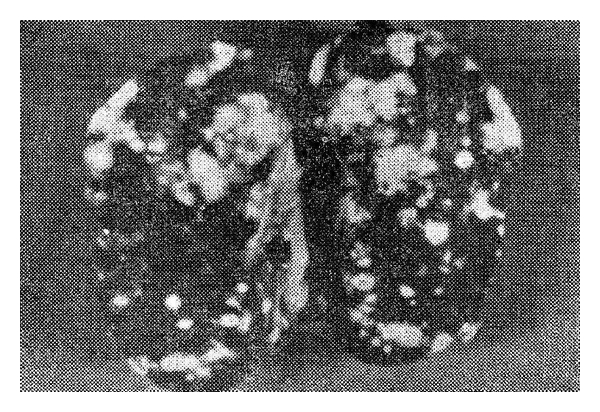
Spleen of Demoiselle cranes (Anthropoides virgo) showing caseous nodules, measuring 1–5 mm in size, on the cut surfaces of the organ.
Figure 2.

Section of spleen showing a granuloma with associated inflammatory cells, H&E × 120.
The incidence of avian tuberculosis in pet birds kept in captivity appears to exceed the prevalence in poultry [22, 34]. Some of the reasons of the incidence of the infection in pet birds are age of the host, population density, and the ability of organism to survive environmental inclemency [2]. Contact with contaminated water, soil, or feed predisposes to infection [22]. In case of pet birds, the etiology of avian tuberculosis is rarely identified due to the difficulty in isolating some mycobacterial species [23]. Weight loss, diarrhea, dyspnea, lameness, and poor feathering are the usual signs in pet birds. Earlier, most cases of infection were assumed to be caused by Mycobacterium avium complex (MAC). However, the use of molecular techniques brought to light the prominent role of fastidious mycobacteria, primarily M. genavense, in avian tuberculosis of pet birds [22]. M genavense is responsible for the majority of avian mycobacterial infections (up to 80%) in pet birds while the MAC was found responsible for 5% to 10% of mycobacterial infections [22]. In pet birds, M. genavense causes a disseminated disease with clinical and histopathological features indistinguishable from infection caused by members of the MAC [34].
Recently, avian tuberculosis in domestic poultry have declined due to changes in poultry husbandry practices, namely, integrated poultry farming, emphasizing all-pullet flocks rather than older hens and maintaining one-age flocks, all in all out farming system, along with better hygiene, disinfection, and biosecurity practices. However, the occurrence of avian TB in birds in zoo aviaries is still an economically important affair since certain species of exotic birds are of high value and most of these birds will be in endangered or near extinction categories. Avian TB is more common in zoological parks, perhaps because of inadequate cleaning and disinfection of pens. Caged birds are reported to soon succumb to avian TB.
M. avium can infect and cause disease in some domesticated mammals but lesions usually are localized and less severe. It multiplies in tissue for a considerable period and induces sensitivity to tuberculin. Swine, rabbit, and mink are readily infected; infection has been reported in cattle and horse; monkey is also susceptible; while goat, guinea pig, rat, and mouse are relatively resistant to infection, cat and dog are highly resistant to M. avium infection [16, 17].
5. Infection and Immunity
The cellular arm of the immune system is more important than the humoral arm in preventing and controlling mycobacterial infections [2, 5]. Delayed type of hypersensitivity (DTH), judged by the thickness of wattle, is evident at 2 days after infection and increases as the disease progresses. The organism after entry when phagocytosed by nonactivated macrophages is able to downregulate its killing mechanism by preventing normal fusion of the phagosome with lysosomes. Macrophages that lack microbicidal components are destroyed by the intracellular growth of the organism, and a lesion develops. Also, during infections, thymus is consistently colonized by M. avium and as the T-cell differentiation depends on the antigens encountered within the thymus, infection of this organ can alter the immune response to infection [35]. However, if activated, the macrophages can readily destroy and degrade phagocytosed mycobacteria [11, 36]. They have usually good killing potential against the invading mycobacterial species [37, 38]. This is augmented by the release of lymphokines like tumor necrosis factor (TNF) and interleukin-2 (IL-2), which helps in killing M. avium. Macrophage activation is also performed by interferon gamma (IFN-γ), which is released by a subset of CD4+ T lymphocytes and natural killer (NK) cells on stimulation by the interleukins released by the macrophage during its encounter with the mycobacteria. The Tlymphocytes also stimulates B cells to produce antibodies against mycobacteria but these antibodies do not appear to have a major protective effect for the host against infection and high antibody titers can be correlated with serious infections [6, 36, 39]. Recently, it has been identified that lipoarabinomannan, an important outer cell wall component of mycobacteria, are highly potent nonpeptidic molecules which can be used to modulate the host immune response [40].
6. Diagnosis of Infection
The diagnosis of M. avium infection is based on clinical signs, postmortem gross lesions, and by demonstrating the acid-fast bacilli in crushed lesions using microscopy, which is sufficient for a positive diagnosis [2–5]. If acid-fast bacilli are not found, but typical signs or lesions are present in the birds, culture of the organism must be attempted. In necropsy, liver or spleen is usually the best organ to use, but if the carcass is decomposed, bone marrow may prove more satisfactory as it could be less contaminated. In live birds, cultural examination using feces or tracheal swabs is necessary to isolate and identify the etiological agent [23, 41]. But usually a definitive diagnosis is performed by culturing the organism in suitable media,namely, Dorset's or Herrold's egg yolk medium, Lowenstein-Jensen medium, Middlebrook 7H10 and 7H11, or Coletsos medium, with 1% sodium pyruvate [3–5, 23]. For growth of M. avium, media containing whole egg or egg yolk is desirable and the incubation temperature should be 37–40°C. Growth may be confined to the edge of the water of condensation. Cultures should be incubated for at least 8 weeks. Typically M. avium produces “smooth” colonies, within 2–4 weeks; rough variants do occur; smooth transparent colonies are virulent for chickens while variants with smooth domed or rough colonies are avirulent. The colonies, observed only after 10–21 days of incubation, are small, slightly raised, discrete, and grayish white in appearance [2, 11]. Colonies are larger if the medium contains glycerin. Shorter incubation times can be achieved using the liquid culture BACTEC system. Some strains of M. avium have been identified to have special requirement of mycobactin as a growth factor.
Recently, comparison of the different methods, namely, the conventional culture method (solid Herrold's and Stonebrink media and liquid Sula medium) and newly developed liquid culture systems, the manual mycobacteria growth indicator tube (M-MGIT), and the fully automated BACTEC MGIT 960 system (A-MGIT), for the detection of M. avium subsp. avium (MAA) in naturally infected hens revealed overall detection rates to be 60, 70, and 76%, with the mean time of mycobacteria detection being 32.6, 17.6, and 14.6 d, respectively [42].
In live birds, during life time, besides the culture and isolation techniques, immunological tests, namely, tuberculin test; whole blood agglutination test and enzyme-linked immunosorbent assay (ELISA) are also valuable diagnostic tools; and nowadays various molecular tools are also being employed for identification of the causative agent at subspecies level and epidemiological studies (Table 2). Using standard purified protein derivative (PPD) of heat-treated culture of M. avium, tuberculin test can be performed in the wattle, which is considered as the test of choice in domestic fowl/poultry. This test is less useful in other species of bird. Birds are tested by intradermal inoculation of 0.05 mL or 0.1 mL tuberculin (2000 IU) and the test is read after 48 hours [2]. A positive reaction is identified as a hot and oedematous swelling at the site or by the presence of a small firm nodule of approximately 5 mm in diameter [5, 11]. It serves as a means of identifying birds infected with or sensitized to the same species of tubercle bacillus. Tuberculin test has 80% accuracy in detecting infective birds relative to gross lesions but in an advanced stage of infection birds may give no reaction. In whole blood agglutination test, a drop of antigen (M. avium stained with 1% malachite green) is mixed with a drop of blood and a positive reaction is indicated by agglutination within few minutes [5, 48]. It is a better test, especially for waterfowl. Advantage of this test is that stock has only to be handled once, but false positive reaction is a disadvantage which makes the test a less specific one. The tuberculin test or the haemagglutination (stained antigen) tests are most frequently used for export testing of poultry. However, neither the tuberculin test nor the agglutination test is likely to be of any value in cases of M. tuberculosis infection in caged birds. ELISA which is reported to be less specific than tuberculin test can detect specific antibodies and thereby help determine exposure to M. avium. However, false positives may be common in ELISA. The identification of immunogenic proteins of M. avium may favor the development of more precise sero-diagnostic tools [49]. Tuberculin test and serological tests are normally used to determine the prevalence of disease in a flock, or to detect infected birds. When used to detect the presence of tuberculosis in a flock they should be supported by the necropsy of any birds that give positive reactions. IFN-γ assay used to diagnose human tuberculosis may also be useful in diagnosing the infection in birds.
Table 2.
| Type of test | Performed in | Time required | Merits | Demerits |
|---|---|---|---|---|
| Observing gross lesions | Dead birds | 1 hour | Easy diagnosis | Only presumptive diagnosis |
| Acid fast staining | Dead birds | 1 hour | Easy definitive diagnosis | Less sensitive, Not able to distinguish amongst species |
| Isolation/Culture | Dead birds | About 4 weeks | Definitive diagnosis | Time consuming |
| Tuberculin test | Live birds | 48 hours | Easy to perform Definitive diagnosis |
Time consuming, Test is not very sensitive, Possibility of false positive and false negative results |
| Agglutination test | Live birds | Few minutes | Can differentiate serotypes. Useful for screening large flocks for immediate culling | Occasionally false positive reactionsNot reliable in caged birds |
| ELISA | Live birds | 2 hours | Definitive diagnosis Can be used for exotic and pet birds |
Less specific than tuberculin test False positives may be there |
| DNA probes | Bacterial cultures | 4–6 hours | Highly sensitive and specific | Probe may react with isolates that genetically or biochemically do not fit within the MAC |
| PCR | Dead/live birds/cultures | 4 hours | Highly sensitive and specific | Requires specialized laboratory and trained personnel |
| RFLP | Bacterial cultures, clinical samples | 1 day | Differentiates mycobacteria to the species level Discriminative for the analysis of strain relatedness | Insufficient quantities of gene makes visualization of digested fragments difficult |
| Multiplex PCR | Bacterial cultures/clinical samples | 5–8 hrs | Rapid and inexpensive technique for subspecies identification and differential diagnosis of the MAC complex | Requires specialized laboratory |
| Sequencing of the 16S rRNA gene | Bacterial cultures | 2 days | Powerful technique for differentiating species | Labor-intensive and difficult to implement in routine diagnosis |
| HPLC | Bacterial cultures | 1 day | Can identify Mycobacteriumisolates to the species level | Uses costly equipment and requires substantial amounts of the test organism. |
| Real-Time PCR | Bacterial cultures/clinical samples | 4–6 h | Low risk of sample contaminationOffers the possibility to quantify bacterial load | Sensitivity could be affected by the initial volume of DNA present |
| MIRU-VNTR/MATR-VNTR typing | Bacterial cultures/clinical samples | 1 day | Improves RFLP discrimination Useful for determination of genotypic diversity of M. avium subspecies | Requires specialized laboratory |
| Pathogenicity tests | Live young birds | 5–6 weeks | Likelihood of the etiological agent can be knownUseful in cases where the typing facilities are not available | Time consuming and concerned to ethical issues |
Species and subspecies level typing of mycobacteria requires a specialised laboratory. Conventional biochemical tests for species identification are lengthy and fail to distinguish between M. avium and M. intracellulare. Classification of MAC organisms into 28 serovars has been made by seroagglutination [3]. The MAC colonies can be identified using high performance liquid chromatography (HPLC) for detecting mycolic acid [4]. HPLC and use of monoclonal antibodies to major serovars in ELISA also facilitates typing of mycobacteria. In the past decade, biotechnological tools, exploiting nucleic acid detection methodology, like DNA probes, polymerase chain reaction (PCR), and PCR-restriction fragment length polymorphism (RFLP) are being widely employed for specific detection of the etiological agent [3, 4, 50, 51]. Commercial nucleic acid hybridisation probes have become a “gold standard” for distinction between M. avium, M. intracellulare, and M. genavense [2, 5, 41, 52]. For intraspecies genotyping, pulsed-field gel electrophoresis of large DNA restriction fragments has proved to be highly sensitive [53]. The PCR approach, using species-specific primers is also capable of specifically detecting DNA fragments of M. avium genome, thus acting as a diagnostic alternative to the conventional procedures [41, 54–57]. Also, a multiplex PCR method has been developed for the determination of the subspecies within M. avium species, and for differentiating M. avium from M. intracellulare and M. tuberculosis complex [56, 58, 59]. Efficient differentiation of MAC species and subspecies by use of five-target multiplex PCR, designed to amplify a 16S rRNA gene target common to all Mycobacterium species, has been proved to be rapid, reliable, and simple [60]. Lappayawichit et al. [61] reported the differentiation of species of mycobacteria by amplifying 16-23S ribosomal DNA and further digesting with restriction enzyme like Hae III, Msp I, and Bst XI.
Likewise, for differentiation of various mycobacterial species, insertion sequences (IS) in DNA molecule have been identified. IS 901 and IS 1245, which are virtually M. avium specific, has been shown to be the most discriminative for the analysis of various strains based on PCR-RFLP [1, 5, 25, 62]. Generally, the PCR-RFLP analysis of suspected tissue samples like liver, spleen, and gonads can be performed targeting 16S-rRNA gene for Mycobacterium spp., IS6110 for M. tuberculosis, IS1245 for MAC, IS901 for M. avium subsp. Avium, and hsp65 for M. genavense [34, 58, 63–66]. Utility of PCR-RFLP of hsp65 has been reported for the identification of M. avium [67]. O'Grady et al. [43] performed RFLP investigation using probes derived from IS901, IS1245 and IS1311 to study the molecular epidemiology of M. avium, and M. intracellulare infection, in particular to gain an understanding of the sources of infection in humans. 16S rRNA and hsp65 sequencing may also be used to differentiate between mycobacterial strains and for distinguishing the M. avium subsets [68–72]. The real-time TaqMan PCR assay targeting the hsp65 gene of M. genavense and MAC subsp. may provide a useful tool for evaluating clinical samples for DNA from mycobacteria species that most commonly infect birds [44]. Slana et al. [45] has recently developed a real-time quantitative PCR for the identification and quantification of Mycobacterium avium subsp. avium and M. a. hominissuis. Other novel tests like IFN-γ assay, GenoType assay, and DNA microarrays that are used to diagnose human tuberculosis may also be useful in diagnosing the infection in birds [22, 73, 74]. More recently, the use of molecular techniques for species identification brought to light the prominent role of nonculturable mycobacteria, primarily M. genavense, in several cases of avian tuberculosis in pet birds [5, 34].
Utilization of new variable-number tandem-repeat markers (VNTRs) of genetic elements called mycobacterial interspersed repetitive units (MIRUs) for typing Mycobacterium avium subsp. paratuberculosis and M. avium strains has been reported to be fast typing method, and which in combination with other methods, might prove to be optimal for PCR-based molecular epidemiological studies [46]. More recently, the usefulness of a MIRU-VNTR typing has been described for determination of genotypic diversity of M. avium subspecies (M. avium subsp. avium, M. avium subsp. hominissuis, and M. avium subsp. Silvaticum) from human and animal origins [47]. Inagaki et al. [75] reported MATR (Mycobacterium avium tandem repeat—MATR)-VNTR typing method (MATR-VNTR) to be having excellent discriminatory power compared with MIRU-VNTR and IS1245-RFLP typing; and its concomitant use with IS1245-RFLP typing increases the discriminatory power. MATR-VNTR typing is inexpensive and easy to perform and thus could be very useful for epidemiological studies.
In case the typing facilities are not available, pathogenicity tests are performed for knowing the likelihood of the etiological agent, which should be carried out on the species of bird being investigated, but failing that, domestic fowl or Japanese quail may be used. Young adult birds are best, and when inoculated intravenously with 1 mL of the suspension (culture at 0.1 mg/mL) the bird will die in 5–6 weeks if the organism is virulent, or, by that time, the bird will have extensive lesions filled with acid-fast bacilli [3].
Avian tuberculosis should be differentially diagnosed from those diseases that are known to develop tumorous or granulomatous lesions in gastrointestinal (GI) tract and other visceral organs. Diseases that are to be differentially diagnosed are pseudotuberculosis (common in ducks and turkeys caused by Yersinia pseudotuberculosis), Coligranuloma (Hjarre's disease-Escherichia coli), neoplasia due to lymphoid leucosis (Retrovirus) or Marek's disease (Herpes virus), fowl cholera (Pasteurella multocida), Pullorum disease (Salmonella Pullorum), and enterohepatitis (Black head, Histomonas meleagridis) [9].
7. Therapy in Avian Tuberculosis
Generally, mycobacterium infections caused by M. tuberculosis and M. bovis are treated with antibiotics such as isoniazid, ethambutol, rifampicin, and pyrazinamide in human beings [6]. Treatment of infected animals is rarely attempted because of the high cost and prolonged time. Moreover it is considered illegal in some countries. M. avium, on the other hand, is resistant to these antituberculosis drugs [2]. Due to this fact and also because of the economical considerations, treatment is not considered a viable option, particularly in poultry sector. However, in case of M. avium infection of exotic pet birds or birds maintained in zoo aviaries, treatment against M. avium has to be considered and therapy duration can go up to 12–18 months. In avian therapeutics related to mycobacterial infections, the major difficulty is that the pharmacokinetics in birds for most of the antimycobacterial drugs is unknown [6]. Also, the relative hydrophobicity of the mycobacterial cell wall acts as a barrier that restricts the activity of many hydrophilic antibiotics like the aminoglycosides, fluoroquinolones, and macrolides [6]. Besides, the slow growth and intracellular location of mycobacteria necessitate the need for extended periods of therapy.
There are clinical reports documenting the apparent successful treatment of parrots with mycobacterial infections, but no studies to date investigate the treatment of mycobacterial infections in birds [6]. M. avium has been reported to respond to trimethoprim-sulfamethoxazole, sulfisoxazole, amikacin, gentamicin, and kanamycin, during in vitro studies [76]. M. avium infections in pet birds have been treated with isoniazid, rifampin, rifabutin, ethambutol, clofazimine, ciprofloxacin, enrofloxacin, streptomycin, and amikacin and successful therapy of M. genavense infections with clarithromycin in humans has been reported [6]. The apparent effectiveness of the newer macrolides like clarithromycin and azithromycin against both M. avium and M. genavense make them suitable for treating mycobacterial infections in birds. However, the initial therapeutic regimen should include rifabutin and ethambutol, and later azithromycin or clarithromycin can be administered concurrently. Birds that respond poorly to therapy should have either a fluoroquinolone or an aminoglycoside added to the regimen. An alternative or additional drug that may prove useful, especially in birds with a marked inflammatory response, would be clofazamine. All these drugs may be curative at a total daily dose of 85 mg/kg for clarithromycin, 43 mg/kg for azithromycin, 56 mg/kg for rifabutin, 56 to 85 mg/kg for ethambutol, and 6 to 12 mg/kg for clofazamine as per the reports of VanDerHeyden [6]. In another study, to augment the potential of existing drugs, a mycobacteriolytic preparation called “stazyme” has been developed from the Staphylococcus strain Clavelis. Stazyme was able to break the permeability barrier of M. avium isolates, significantly enhancing the activity of anti-tuberculous drugs like ethambutol, rifampicin, and amikacin [77].
8. Preventive Measures
The eradication of M. avium infection is difficult due to the chronic carrier state and intermittent shedding of organisms by the infected birds. Measures to eliminate disease and establishing/maintaining TB-free flock should be followed. Gill and Blandy [78] and Dhama et al. [11, 79] described measures like sacrificing the affected flocks, abandoning the equipments and housing materials, removal of litter and contaminated soil, elimination of older flocks, following of strict biosecurity procedures besides regular monitoring with tuberculin and agglutination tests. Stress is a key factor as it causes an increase in the rate of shedding to precipitate outbreaks. The best way to control this disease is to remove infected ones and carriers and also to reduce stress factors by improving the environmental parameters [2, 11]. Prevention is best done by minimizing overcrowding, providing proper ventilation and supplementing adequate amounts of vitamins and minerals in diet. In case of avian TB in a farm, birds in other flocks in the same farm should be quarantined and tested at 6–12 week intervals. Neither the tuberculin nor the agglutination tests can be depended upon for the detection of every infected bird, therefore, as long as one infected bird remains in a flock, dissemination of disease is possible. So entire flock needs to be depopulated and repopulation on noninfected soil and fresh litter may be the best approach. Frequent removal of fecal material is the single most important factor in preventing transmission. Disinfection of the poultry houses should be done frequently. The practice of managing poultry in free-range system should not be followed. Provide proper biosecurity measures to prevent unrestricted movement of chickens, thus preventing exposure from previously infected premises or from wild birds, pigs, and other mammals. For exotic birds prevent contact with infected birds or the premises and housing previously used by them. Monitoring for infection and disposing of positive reactors should be followed along with practicing all reasonable hygienic precautions to prevent entry of the infection. During the import of exotic or domestic birds, tuberculin testing must be mandatory in order to identify the presence of M. avium and the newly introduced birds should be quarantined for a minimum of 2 months [2, 5]. The difficulty of tuberculin testing of all chickens or even a majority of flocks makes it impossible to obtain exact data on the incidence of M. avium infections of chickens. No vaccines are available for use in birds. Experimental vaccines with killed and/or live mycobacteria for protecting chickens against TB have been evaluated. Satisfactory protection was obtained when M. avium serovar 6 was given orally [5, 9, 11]. Combination of inactivated and live M. avium serovars 7 and 19 can also give protection to a limited extent. Nucleic acid-based vaccines may also be experimentally tried using M. avium genes that can generate proteins to elicit cell mediated immunity in birds [80, 81]. Simple, whole cell or lysate vaccines and combinations of vaccine preparations were identified that led to high levels of protection [82].
9. Mycobacterium avium: The Zoonotic Implications
Mycobacterium avium subsp. avium (MAA) represent veterinary and economic risks in birds (mainly poultry) as well as mammals (pigs, etc.). Infected animals and their products (mainly eggs) often come from small household production and pose a risk for human health [83]. Exposure of humans to infected birds with the MAA microorganism may cause a zoonotic infection, particularly in those with immunocompromised diseases such as HIV/AIDS [84]. In addition, the situation worsens due to the spread of HIV infection in developing countries [85]. Unlike M. tuberculosis, human beings are generally resistant to M. avium infection but occasionally they can get infected. Human pulmonary tuberculosis due to avian tubercle bacilli has been reported during the early 1940's [86]. High incidence of sensitivity to avian tuberculin in man has also been identified [87, 88]. It is essential to bear in mind that M. avium, M. intracellulare, and M. genavense are of public health concern mostly in immunocompromised hosts. Infections of humans and animals caused by this M. intracellulare agent are expected to rise. The M. avium infection, seen in many AIDS patients, is a progressive disease that is refractory to treatment [65, 89–91]. This is especially true in cases of exposure to large numbers of organisms [6]. In humans, M. avium is capable of inducing localized primary lymphadenitis, pulmonary disease, and a disseminated form of infection particularly in case of immunosuppressed individuals or patients under transplant therapy [4, 22, 92, 93]. M. avium also causes local wound infections with swelling of regional lymph nodes. In adults, the organism frequently affects the lungs, producing respiratory signs and in children, the cervical lymph nodes are often involved. Eccles and Ptak [93] reported that M. avium causes a serious disseminated bacterial infection in up to 40% of patients with advanced HIV infection. In AIDS patients, the main route for M. avium infection is the gastrointestinal tract and M. avium is naturally tolerant to the low pH that exists in stomach [94]. The transmission occurring via aerosols results in pulmonary infections as the organism frequently affects the lungs with endobronchial lesions [95]. During the infection, M. avium can be demonstrated in vivo in lymph nodes, bone marrow, urine, and sputum [87, 91, 95, 96]. Primarily, the serotype-1 of M. avium subsp. avium has been isolated from such individuals, clearly pointing role of birds in acquiring infection [4]. It should also noteworthy that M. avium is a pathogen that infects several hosts including birds, humans, cattle, and pigs [45, 97, 98]. They are also encountered in environmental sources like soil and water, having considerable ability to overcome adverse and competitive conditions thanks to a major preprotein translocase subunit that is coded by secA gene of the species [99].
During a study from 1953 to 1968, in cattle and swine of Great Britain, 13% of the total tubercle bacilli were typed as M. avium and in pig population it was an astonishing 81%. This should be correlated with the ever increasing number of recorded cases of tuberculosis in man caused by M. avium [64, 100]. Contaminated food originating from pig or other livestock is identified as potential source of human infection. M. avium can infect and cause disease in some domestic mammals but lesions usually are localized and less severe. When domestic farm animals are infected, particularly cattle and pigs, tuberculous lesions are commonly found localized to the head and intestinal lymph nodes [1, 101]. As reported by some workers, M. avium isolates from swine represent the major threat to human beings. The similarity of the IS1245 RFLP patterns of the human and porcine isolates indicates close genetic relatedness, suggesting that M. avium is transmitted between pigs and humans [64, 102].
Regarding the therapeutics, M. avium is of special concern because drug regimens commonly used for treating tuberculosis in humans are not effective [22]. M. avium strains are notorious in being resistant to isoniazid, the most popular anti-TB drug [103, 104]. However the infection was found subsided when treated with azithromycin or clarithromycin together with ethambutol [6, 105]. Further, the M. avium isolates have been demonstrated to get inhibited by sufficient concentrations of sulfamethoxazole in serum [106]. Rifabutin prophylaxis may also help in controlling the disseminated infection [93]. Dunne et al. [107] suggested the use of azithromycin as a safe, effective, and convenient option during disseminated infection in HIV-infected patients. As per the findings of Horgen et al. [108], rifampin-clarithromycin and rifampin-amikacin are the most potent two-drug combinations, while rifampin-amikacin-clarithromycin has been identified as a potent three-drug combination. Likewise, Saito et al. [109] suggested the use of benzoxazinorifamycin in combination with clofazimine to be highly efficacious in the therapy of M. avium infections. In case of infection with M. genavense, clarithromycin is the better choice when compared to azithromycin [6].
Besides therapeutic interventions, there have been numerous attempts to check the M. avium isolates in both environment and in host. Lin et al. [110] suggested that copper-silver ionization of drinking water is a better option for the effective control of M. avium. David et al. [111] proposed the use of synthetic macrocyclic compounds as a new family of compounds that are capable of acting against M. avium infections. The use of recombinant cytokine molecules for the effective killing of M. avium especially interleukin-4 (IL-4) has been well studied [112–114]. The use of adjunctive immunomodulatory therapy by using recombinant granulocyte-macrophage colony-stimulating factor has also been reported [115]. Salem et al. [116] reported that by encapsulating antibiotics like amikacin, streptomycin, ciprofloxacin, sparfloxacin, and clarithromycin, their effect against M. avium can be enhanced. Iron accumulation has been suggested to contribute to an increase of the susceptibility to mycobacterial infections. Iron deprivation, by the use of iron chelators, restricts M. avium growth and this offers a novel approach in controlling infections in man [117].
So it is considered prudent to keep infected birds away from humans, particularly the elderly, and individuals with poor immune status. Hence the handling of infected birds in farms should be carried out with adequate care, and manipulation of material from infected birds or open live cultures of M. avium in laboratories must be performed with appropriate biohazard containment [3]. Healthy individuals with normally functioning immune system have a high resistance to this infection. However, it is recommended to take proper precautions and avoid contact or exposure to infected birds or their carcasses.
Salient features of avian tuberculosis are presented in Figure 3.
Figure 3.
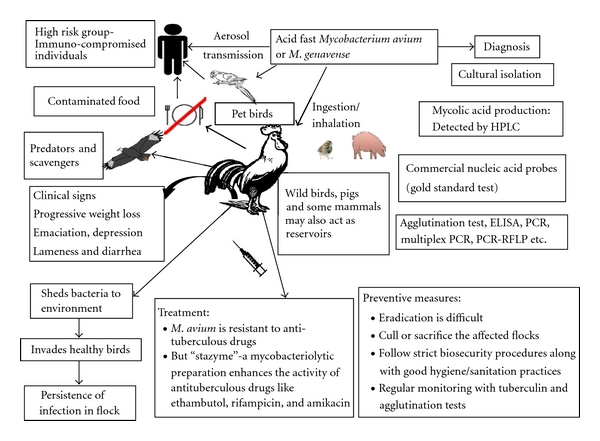
Salient features of avian tuberculosis.
10. Conclusion and Future Perspectives
Members of the Mycobacterium avium complex (MAC) are ubiquitous bacteria that can be found in water, food, and other environmental samples and are considered opportunistic pathogens for numerous animal species, mainly birds and pigs, as well as for humans. Infections caused by the MAC are on the rise in both human and veterinary medicine. Avian tuberculosis is an important disease which affects companion, captive exotic, wild, and domestic bird, and has public health significance too. The most significant cause of poultry disease is M. avium. In recent years, the incidence of avian tuberculosis in domestic poultry have declined due to introduction of novel poultry husbandry practices, namely, maintaining one-age flocks, all in-all outfarming system; provision of better hygiene and sanitation; stringent implementation of biosecurity practices. But the inevitable occasional stress and production demands in the poultry sector could create dynamics similar to those that occur in immune-compromised individuals. Also, M. avium pose a significant threat in layer and breeder farms, where high age groups are maintained. Unless eliminated from the domestic birds, tuberculosis will remain an economic burden on the swine industry too and the role of pigs in transmitting the disease to humans has been well documented. Disseminated form of infections with M. avium is seen in increasing numbers in immunocompromised individuals. M. avium subsp. avium may have wild birds as major reservoirs that are responsible for its shedding into environment and facilitating its spread for years. The diversity of strain types indicates that infections are acquired not from a single reservoir alone. This is in contrary to the belief that existent infected birds are the primary sources of infection through fecal contamination of the environment. Under these perspectives more studies should be performed on identifying avian reservoirs and environmental sources of M. avium. RFLP analysis and multiplex PCR methods can further discriminate between different isolates, which is particularly useful for epidemiological studies. Identification of the MAC members based on culture examination followed by biochemical testing, can take up to several weeks, as opposed to molecular biology methods that provide fast and accurate identification to the species level, which is important in diagnosis and treatment of avian tuberculosis. A means of effectively discriminating among closely related yet pathogenically diverse members of the MAC would enable better diagnosis and treatment as well as further our understanding of the epidemiology of these pathogens. Moreover, viewing the importance, the advanced diagnostic tools and novel prevention strategies that are employed against M. tuberculosis and M. avium in man needs to be standardized for M. avium infections in birds and animals as well.
References
- 1.Dvorska L, Matlova L, Ayele WY, et al. Avian tuberculosis in naturally infected captive water birds of the Ardeideae and Threskiornithidae families studied by serotyping, IS901 RFLP typing, and virulence for poultry. Veterinary Microbiology. 2007;119(2–4):366–374. doi: 10.1016/j.vetmic.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Fulton RM, Thoen CO. Tuberculosis. In: Saif YM, Barnes HJ, Glisson JR, Fadly FM, Mc Dougald LR, Swayne DE, editors. Diseases of Poultry. Ames, IA, USA: Iowa State University Press; 2003. pp. 836–844. [Google Scholar]
- 3.Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Avian tuberculosis. Chapter 2.3.6. pp. 497–508, 2010, http://www.oie.int/en/international-standard-setting/terrestrial-manual/access-online.
- 4.Aranaz A, Liibana E, Mateos A, Domínguez L. Laboratory Diagnosis of Avian Avian tuberculosis. Seminars in Avian and Exotic Pet Medicine. 1997;6(1):9–17. [Google Scholar]
- 5.Tell LA, Woods L, Cromie RL. Avian tuberculosis in birds. Review Science and Technology Office Internationale des Epizooties. 2001;20:180–203. doi: 10.20506/rst.20.1.1273. [DOI] [PubMed] [Google Scholar]
- 6.VanDerHeyden N. Mycobacterial infections: new strategies in the treatment of avian tuberculosis. Seminars in Avian Exotic Pet Medicine. 1997;6(1):25–33. [Google Scholar]
- 7.Dhama K, Mahendran M, Tomar S. Pathogens transmitted by migratory birds: threat perceptions to poultry health and production. International Journal of Poultry Science. 2008;7(6):516–525. [Google Scholar]
- 8.Heatley JJ, Mitchell MM, Roy A, Cho DY, Williams DL, Tully TN., Jr. Disseminated avian tuberculosis in a bald eagle (Haliaeetus leucocephalus) Journal of Avian Medicine and Surgery. 2007;21(3):201–209. doi: 10.1647/1082-6742(2007)21[201:DMIABE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Thoen CO. Tuberculosis. In: Calnek BW, editor. Diseases of Poultry. Mosby-Wolfe: London, UK; 1997. pp. 167–178. [Google Scholar]
- 10.Sah RL, Singh SD, Arya SC. Tuberculosis in some captive zoo birds: case records. Indian Journal of Veterinary Pathology. 1985;9:84–87. [Google Scholar]
- 11.Dhama K, Mahendran M, Tomar S. Avian tuberculosis: an overview. Poultry Punch. 2007;24(3):38–52. [Google Scholar]
- 12.Mallick BB, Chakraborty RL, Chattopadhyay SK. Some observations on the naturally occurring cases of tuberculosis in ducks. Indian Journal of Animal Health. 1970;9:171–173. [Google Scholar]
- 13.Mohanty DN, Patnaik GM. Pathology of tuberculosis in a silver pheasant. Indian Journal of Animal Science. 1971;41:196–198. [Google Scholar]
- 14.Singh G, Joshi TP, Lall JM, Iyer PK. Tuberculosis in ducks. Indian Journal of Veterinary Science. 1968;38:424–430. [Google Scholar]
- 15.Hejlicek K, Treml F. Comparison of pathogenesis and epizootiology signification of avian avian tuberculosis in different sorts of domestic and free living synanthropic fowl. Veterinary Medicine Czechoslovakia. 1995;40:187–194. [Google Scholar]
- 16.Thorel MF, Huchzermeyer H, Weiss R, Fontaine JJ. Mycobacterium avium infections in animals. Veterinary Research. 1997;28(5):439–447. [PubMed] [Google Scholar]
- 17.Thorel MF, Huchzermeyer H, Michel AL. Mycobacterium avium and M. intracellulare infection in mammals. Review Science and Technology Office Internationale des Epizooties. 2001;20:204–218. doi: 10.20506/rst.20.1.1272. [DOI] [PubMed] [Google Scholar]
- 18.Schrenzel M, Nicolas M, Witte C, et al. Molecular epidemiology of Mycobacterium avium subsp. avium and Mycobacterium intracellulare in captive birds. Veterinary Microbiology. 2008;126(1–3):122–131. doi: 10.1016/j.vetmic.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Larsen AB, Moon HW. Experimental Mycobacterium paratuberculosis infection in chickens. American Journal of Veterinary Research. 1972;33(6):1231–1235. [PubMed] [Google Scholar]
- 20.Thorel MF, Krichevsky M, Levy-Frebault VV. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. International Journal of Systematic Bacteriology. 1990;40(3):254–260. doi: 10.1099/00207713-40-3-254. [DOI] [PubMed] [Google Scholar]
- 21.Mijs W, De Haas P, Rossau R, et al. Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium to bird-type isolates and M. avium subsp. hominissuis for the human/porcine type of M. aviumavium. International Journal of Systematic and Evolutionary Microbiology. 2002;52(5):1505–1518. doi: 10.1099/00207713-52-5-1505. [DOI] [PubMed] [Google Scholar]
- 22.Hoop RK. Public health implications of exotic pet avian tuberculosis. Seminars in Avian Exotic Pet Medicine. 1997;6(1):3–8. [Google Scholar]
- 23.Thoen CO. Tuberculosis. In: Swayne DE, Gilson JR, Jackwood MW, Pearson JE, Reed WM, editors. A Laboratory Manual for the Isolation and Identification of Avian Pathogens. Philadelphia, Pa, USA: American Association of Avian Pathologists; 1998. pp. 69–73. [Google Scholar]
- 24.Inderlied CB, Kemper CA, Bermudez LEM. The Mycobacterium avium complex. Clinical Microbiology Review. 1993;6(3):266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritacco V, Kremer K, Van Der Laan T, Pijnenburg JEM, De Haas PEW, Van Soolingen D. Use of IS901 and IS1245 in RFLP typing of Mycobacterium avium complex: relatedness among serovar reference strains, human and animal isolates. International Journal of Tuberculosis and Lung Disease. 1998;2(3):242–251. [PubMed] [Google Scholar]
- 26.Pavlik I, Svastova P, Bartil J, Dvorska L, Rychlik I. Relationship between IS901 in the Mycobacterium avium complex strains isolated from birds, animals, humans, and the environment and virulence for poultry. Clinical and Diagnostic Laboratory Immunology. 2000;7(2):212–217. doi: 10.1128/cdli.7.2.212-217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dvorska L, Bull TJ, Bartos M, et al. A standardised restriction fragment length polymorphism (RFLP) method for typing Mycobacterium avium isolates links IS901 with virulence for birds. Journal of Microbiological Methods. 2003;55(1):11–27. doi: 10.1016/s0167-7012(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 28.Lairmore M, Spraker T, Jones R. Two cases of tuberculosis in raptors in Colorado. Journal of Wildlife Diseases. 1985;21(1):54–57. doi: 10.7589/0090-3558-21.1.54. [DOI] [PubMed] [Google Scholar]
- 29.Millan J, Negre N, Castellanos E, et al. Avian tuberculosis in free-living raptors in majorca Island, Spain. Avian Pathology. 2010;39(1):1–6. doi: 10.1080/03079450903389945. [DOI] [PubMed] [Google Scholar]
- 30.Pocknell AM, Miller BJ, Neufeld JL, Grahn BH. Conjunctival mycobacteriosis in two emus (Dromaius novaehollandiae) Veterinary Pathology. 1996;33(3):346–348. doi: 10.1177/030098589603300314. [DOI] [PubMed] [Google Scholar]
- 31.Boughton E. Tuberculosis caused by Mycobacterium avium. Veterinary Bulletin. 1969;39:457–465. [Google Scholar]
- 32.Chandra R, Rao VDP, Gomez-Villamandos JC, Shukla SK, Banerjee PS. Diseases of Poultry and their Control. Bombay, India: IBD Book Company; 2001. Avian tuberculosis; pp. 93–98. [Google Scholar]
- 33.Kahn CM. Merck Veterinary Manual. Philadelphia, Pa, USA: National Publishing; 2005. Avian tuberculosis; pp. 2267–2268. [Google Scholar]
- 34.Manarolla G, Liandris E, Pisoni G, et al. Avian tuberculosis in companion birds: 20-year survey. Veterinary Microbiology. 2009;133(4):323–327. doi: 10.1016/j.vetmic.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Nobrega C, Cardona PJ, Roque S, Oa PP, Appelberg R, Correia-Neves M. The thymus as a target for mycobacterial infections. Microbes and Infection. 2007;9(14-15):1521–1529. doi: 10.1016/j.micinf.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Collins FM. Antituberculous immunity: new solutions to an old problem. Reviews of Infectious Diseases. 1991;13(5):940–950. doi: 10.1093/clinids/13.5.940. [DOI] [PubMed] [Google Scholar]
- 37.Dhama K, Bansal MP, Ram GC. In vitro evaluation of nitrite production and intracellular killing activities of bovine peripheral blood monocytes pulsed with M. bovis BCG. Indian Veterinary Journal. 1998;75(12):1075–1078. [Google Scholar]
- 38. Dhama K, Bansal MP, Ram GC. In vitro study of the role of vitamin D3 in activating peripheral bovine blood monocytes pulsed with Mycobacterium bovis BCG. Indian Veterinary Medical Journal. 1999;23:225–228. [Google Scholar]
- 39.Cromie RL, Ash NJ, Brown MJ, Stanford JL. Avian immune responses to Mycobacterium avium: the wildfowl example. Developmental and Comparative Immunology. 2000;24(2-3):169–185. doi: 10.1016/s0145-305x(99)00071-3. [DOI] [PubMed] [Google Scholar]
- 40.Colavecchia S, Jolly A, Stempler A, Fernandez E, Mundo S. Lipoarabinomannans from Mycobacterium avium subsp. avium affect bovine immune responses to different antigens. Veterinary Immunology and Immunopathology. 2009;128(1–3):p. 317. [Google Scholar]
- 41.Saito H, Tomioka H, Sato K, Tasaka H, Dawson DJ. Identification of various serovar strains of Mycobacterium avium complex by using DNA probes specific for Mycobacterium avium and Mycobacterium intracellulare. Journal of Clinical Microbiology. 1990;28(8):1694–1697. doi: 10.1128/jcm.28.8.1694-1697.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shitaye EJ, Beran V, Svobodová J, Morávková M, Babák V, Pavlík I. Comparison of the conventional culture, the manual fluorescent MGIT system and the automated fluorescent MGIT 960 culture system for the detection of Mycobacterium avium subsp. avium in tissues of naturally infected hen. Folia Microbiology (Praha) 2009;54(2):137–141. doi: 10.1007/s12223-009-0020-y. [DOI] [PubMed] [Google Scholar]
- 43.O’Grady D, Flynn O, Costello E, et al. Restriction fragment length polymorphism analysis of Mycobacterium avium isolates from animal and human sources. International Journal of Tuberculosis and Lung Disease. 2000;4(3):278–281. [PubMed] [Google Scholar]
- 44.Tell LA, Leutenegger CM, Larsen RS, et al. Real-time polymerase chain reaction testing for the detection of Mycobacterium genavense and Mycobacterium avium complex species in Avian samples. Avian Diseases. 2003;47(4):1406–1415. doi: 10.1637/7063. [DOI] [PubMed] [Google Scholar]
- 45.Slana I, Kaevska M, Kralik P, Horvathova A, Pavlik I. Distribution of Mycobacterium avium subsp. avium and M. a. hominissuis in artificially infected pigs studied by culture and IS901 and IS1245 quantitative real time PCR. Veterinary Microbiology. 2010;144(3-4):437–443. doi: 10.1016/j.vetmic.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 46.Thibault VC, Grayon M, Boschiroli ML, et al. New variable-number tandem-repeat markers for typing Mycobacterium avium subsp. paratuberculosis and M. avium strains: comparison with IS900 and IS1245 restriction fragment length polymorphism typing. Journal of Clinical Microbiology. 2007;45(8):2404–2410. doi: 10.1128/JCM.00476-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radomski N, Thibault VC, Karoui C, et al. Determination of genotypic diversity of Mycobacterium avium subspecies from human and animal origins by mycobacterial interspersed repetitive-unit-variable-number tandemrepeat and IS1311 restriction fragment length polymorphism typing methods. Journal of Clinical Microbiology. 2010;48(4):1026–1034. doi: 10.1128/JCM.01869-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rozanska M. Preparation of antigen for whole blood rapid agglutination test and its specificity for diagnosis of avian tuberculosis. Bulletin of Veterinary Institute Pulawy. 1965;9(1):20–25. [Google Scholar]
- 49.Amador E, Lloret L, Castillo AI, Lopez Y. Identification of immunogenic proteins of Mycobacterium avium with diagnostic potential. International Journal of Infectious Diseases. 2010;14(1):p. 129. [Google Scholar]
- 50.Crawford JT. Development of rapid techniques for identification of M. avium infections. Research in Microbiology. 1994;145(3):177–181. doi: 10.1016/0923-2508(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 51.Fries JWU, Patel RJ, Piessens WF, Wirth DF. Genus- and species-specific DNA probes to identify mycobacteria using the polymerase chain reaction. Molecular and Cellular Probes. 1990;4(2):87–105. doi: 10.1016/0890-8508(90)90011-n. [DOI] [PubMed] [Google Scholar]
- 52.Soini H, Enrola E, Viljanen MK. Genetic diversity among Mycobacterium avium complex AccuProbe-positive isolates. Journal of Clinical Microbiology. 1996;34(1):55–57. doi: 10.1128/jcm.34.1.55-57.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazurek GH, Hartman S, Zhang Y. Large DNA restriction fragment polymorphism in the Mycobacterium avium M. intracellulare complex: a potential epidemiologic tool. Journal of Clinical Microbiology. 1993;31(2):390–394. doi: 10.1128/jcm.31.2.390-394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thierry D, Matsiota-Bernard P, Nauciel C, Guesdon JL. Comparison of polymerase chain reaction and nonradioactive hybridization techniques for the identification of Mycobacterium avium strains. Molecular and Cellular Probes. 1994;8(6):469–471. doi: 10.1006/mcpr.1994.1067. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto T, Shibagaki T, Yamori S, et al. Polymerase chain reaction for the differentiation of Mycobacterium intracellulare and Mycobacterium avium. Tubercle and Lung Disease. 1993;74(5):342–345. doi: 10.1016/0962-8479(93)90110-J. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto T, Shibagaki T, Yamori S, et al. Strategy for the detection and differentiation of Mycobacterium avium species in isolates and heavily infected tissues. Research in Veterinary Science. 2008;85(2):257–264. doi: 10.1016/j.rvsc.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Springer B, Stockman L, Teschner K, Roberts GD, Bottger EC. Two-laboratory collaborative study on identification of mycobacteria: molecular versus phenotypic methods. Journal of Clinical Microbiology. 1996;34(2):296–303. doi: 10.1128/jcm.34.2.296-303.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bartos M, Hlozek P, Svastova P, et al. Identification of members of Mycobacterium avium species by Accu-Probes, serotyping, and single IS900, IS901, IS1245 and IS901-flanking region PCR with internal standards. Journal of Microbiological Methods. 2006;64(3):333–345. doi: 10.1016/j.mimet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Cousins D, Francis B, Dawson D. Multiplex PCR provides a low-cost alternative to DNA probe methods for rapid identification of Mycobacterium avium and Mycobacterium intracellulare. Journal of Clinical Microbiology. 1996;34(9):2331–2333. doi: 10.1128/jcm.34.9.2331-2333.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shin SJSJ, Lee BS, Koh WJ, et al. Efficient differentiation of Mycobacterium avium complex species and subspecies by use of five-target multiplex PCR. Journal of Clinical Microbiology. 2010;48(11):4057–4062. doi: 10.1128/JCM.00904-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lappayawichit P, Rienthong S, Rienthong D, et al. Differentiation of Mycobacterium species by restriction enzyme analysis of amplified 16S-23S ribosomal DNA spacer sequences. Tubercle and Lung Disease. 1996;77(3):257–263. doi: 10.1016/s0962-8479(96)90010-6. [DOI] [PubMed] [Google Scholar]
- 62.O’Grady D, Flynn O, Costello E, et al. Restriction fragment length polymorphism analysis of Mycobacterium avium isolates from animal and human sources. International Journal of Tuberculosis and Lung Disease. 2000;4(3):278–281. [PubMed] [Google Scholar]
- 63.Ikonomopoulos J, Fragkiadaki E, Liandris E, Sotirakoglou K, Xylouri E, Gazouli M. Estimation of the spread of pathogenic mycobacteria in organic broiler farms by the polymerase chain reaction. Veterinary Microbiology. 2009;133(3):278–282. doi: 10.1016/j.vetmic.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 64.Mobius P, Lentzsch P, Moser I, Naumann L, Martin G, Kohler H. Comparative macrorestriction and RFLP analysis of Mycobacterium avium subsp. avium and Mycobacterium avium subsp. hominissuis isolates from man, pig, and cattle. Veterinary Microbiology. 2006;117(2–4):284–291. doi: 10.1016/j.vetmic.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 65.Van Soolingen D, Bauer J, Ritacco V, et al. IS1245 restriction fragment length polymorphism typing of Mycobacterium avium isolates: proposal for standardization. Journal of Clinical Microbiology. 1998;36(10):3051–3054. doi: 10.1128/jcm.36.10.3051-3054.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shitaye JE, Matlova L, Horvathova A, et al. Mycobacterium avium subsp. avium distribution studied in a naturally infected hen flock and in the environment by culture, serotyping and IS901 RFLP methods. Veterinary Microbiology. 2008;127(1-2):155–164. doi: 10.1016/j.vetmic.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 67.Bannalikar AS, Verma R. Detection of Mycobacterium avium & M. tuberculosis from human sputum cultures by PCR-RFLP analysis of hsp65 gene & pncA PCR. Indian Journal of Medical Research. 2006;123(2):165–172. [PubMed] [Google Scholar]
- 68.Devallois A, Picardeau M, Goh KK, Sola C, Vincent V, Rastogi N. Comparative evaluation of PCR and commercial DNA probes for detection and identification to species level of Mycobacterium avium and Mycobacterium intracellulare. Journal of Clinical Microbiology. 1996;34(11):2756–2759. doi: 10.1128/jcm.34.11.2756-2759.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kirschner P, Meier PA, Bottger EC. Genotypic identification and detection of mycobacteria. In: Persing DH, Smith TF, Tenover FC, White TC, editors. Diagnostic Molecular Microbiology. Washington, DC, USA: American Society for Microbiology; 1993. pp. 173–190. [Google Scholar]
- 70.Telenti A, Marchesi F, Balz M, Bally F, Bottger EC, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. Journal of Clinical Microbiology. 1993;31(2):175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trueba F, Fabre M, Saint-Blancard P. Rapid identification of Mycobacterium genavense with a new commercially available molecular test, INNO-LiPA MYCOBACTERIA v2. Journal of Clinical Microbiology. 2004;42(9):4403–4404. doi: 10.1128/JCM.42.9.4403-4404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turenne CY, Semret M, Cousins DV, Collins DM, Behr MA. Sequencing of hsp65 distinguishes among subsets of the Mycobacterium avium complex. Journal of Clinical Microbiology. 2006;44(2):433–440. doi: 10.1128/JCM.44.2.433-440.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fatima N. Newer diagnostic techniques for tuberculosis. Respiratory Medicine. 2009;2(4):151–154. [Google Scholar]
- 74.Neonakis IK, Gitti Z, Krambovitis E, Spandidos DA. Molecular diagnostic tools in mycobacteriology. Journal of Microbiological Methods. 2008;75(1):1–11. doi: 10.1016/j.mimet.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 75.Inagaki T, Nishimori K, Yagi T, et al. Comparison of a variable-number tandem-repeat (VNTR) method for typing Mycobacterium avium with mycobacterial interspersed repetitive-unit-VNTR and IS1245 restriction fragment length polymorphism typing. Journal of Clinical Microbiology. 2009;47(7):2156–2164. doi: 10.1128/JCM.02373-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davis DE, Carpenter JL, Trevino S, Koch J, Ognibene AJ. In vitro susceptibility of Mycobacterium avium complex to antibacterial agents. Diagnostic Microbiology and Infectious Diseases. 1987;8(3):149–155. doi: 10.1016/0732-8893(87)90165-9. [DOI] [PubMed] [Google Scholar]
- 77.Rastogi N, Goh KS, Clavel-Seres S. Stazyme, a mycobacteriolytic preparation from a Staphylococcus strain, is able to break the permeability barrier in multiple drug resistant Mycobacterium avium. FEMS Immunology and Medical Microbiology. 1997;19(4):297–305. doi: 10.1111/j.1574-695X.1997.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 78.Gill I, Blandy ML. Control of avian tuberculosis in a commercial poultry flock. Australian Veterinary Journal. 1986;63(12):422–423. doi: 10.1111/j.1751-0813.1986.tb15923.x. [DOI] [PubMed] [Google Scholar]
- 79.Dhama K, Kataria JM, Dash BB, Mahendran M, Tomar S. Biosecurity-the first line of defense in poultry disease control. In: Proceedings of the Souvenir of National Symposium on Emerging and Exotic Diseases of Poultry; 2005; Uttarpradesh, India. IVRI; pp. 73–77. [Google Scholar]
- 80.Dhama K, Mahendran M. Technologies and advances in diagnosis and control of poultry diseases: safeguarding poultry health and productivity. Poultry Technology. 2008;2(12):13–16. [Google Scholar]
- 81.Dhama K, Mahendran M, Gupta PK, Rai A. DNA vaccines and their applications in veterinary practice: current perspectives. Veterinary Research Communications. 2008;32(5):341–356. doi: 10.1007/s11259-008-9040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Falkinham JO, III, Gross WB, Pierson FW. Effect of different cell fractions of Mycobacterium avium and vaccination regimens on Mycobacterium avium infection. Scandinavian Journal of Immunology. 2004;59(5):478–484. doi: 10.1111/j.0300-9475.2004.01419.x. [DOI] [PubMed] [Google Scholar]
- 83.Kríz P, Slaný M, Shitaye JEJE, Pavlík I. Avian mycobacteriosis in humans remains a threat in the Czech Republic. Klin Mikrobiol Infekc Lek. 2010;16(1):10–17. [PubMed] [Google Scholar]
- 84.Martin G, Schimmel D. Mycobacterium avium infections in poultry—a risk for human health or not? Dutch Tierarztl Wochenschr. 2000;107:53–58. [PubMed] [Google Scholar]
- 85.Une Y, Mori T. Tuberculosis as a zoonosis from a veterinary perspective. Comparative Immunology, Microbiology and Infectious Diseases. 2007;30(5-6):415–425. doi: 10.1016/j.cimid.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 86.Bradbury FCS, Belf DPH. Human plumonary tuberculosis due to an avian tubercle bacilli: report of a case. The Lancet. 1946;247(6386):89–91. doi: 10.1016/s0140-6736(46)91227-5. [DOI] [PubMed] [Google Scholar]
- 87.Crompton GK, Schonell ME, Wallace A. Disseminated infection with Mycobacterium avium: part III-sensitivity to avian tuberculin among contacts. Tubercle. 1968;49(1):38–41. doi: 10.1016/s0041-3879(68)80005-4. [DOI] [PubMed] [Google Scholar]
- 88.Flynn MP. Comparative testing with human tuberculin and avian tuberculin in county Westmeath. Tubercle. 1962;43(1):64–75. doi: 10.1016/s0041-3879(62)80050-6. [DOI] [PubMed] [Google Scholar]
- 89.Biet F, Boschiroli ML, Thorel MF, Guilloteau LA. Zoonotic aspects of Mycobacterium bovis and Mycobacterium avium-intracellulare complex (MAC) Veterinary Research. 2005;36(3):411–436. doi: 10.1051/vetres:2005001. [DOI] [PubMed] [Google Scholar]
- 90.Dhople AM, Dhople AA, Ibanez MA. In vitro activities of 2,2′-bipyridyl analogues against Mycobacterium avium and M. tuberculosis. Tubercle and Lung Disease. 1995;76(2):136–140. doi: 10.1016/0962-8479(95)90556-1. [DOI] [PubMed] [Google Scholar]
- 91.Falk GA, Hadley SJ, Sharkey FE, Liss M, Muschenheim C. Mycobacterium avium infections in man. The American Journal of Medicine. 1973;54(6):801–810. doi: 10.1016/0002-9343(73)90069-7. [DOI] [PubMed] [Google Scholar]
- 92.Bermudez LE, Wu M, Miltner E, Inderlied CB. Isolation of two subpopulations of Mycobacterium avium within human macrophages. FEMS Microbiology Letters. 1999;178(1):19–26. doi: 10.1111/j.1574-6968.1999.tb13754.x. [DOI] [PubMed] [Google Scholar]
- 93.Eccles RNE, Ptak RNJ. Mycobacterium avium complex infection in AIDS: clinical features, treatment, and prevention. Journal of the Association of Nurses in AIDS Care. 1995;6(5):37–47. doi: 10.1016/S1055-3290(05)80021-4. [DOI] [PubMed] [Google Scholar]
- 94.Bodmer T, Miltner E, Bermudez LE. Mycobacterium avium resists exposure to the acidic conditions of the stomach. FEMS Microbiology Letters. 2000;182(1):45–49. doi: 10.1111/j.1574-6968.2000.tb08871.x. [DOI] [PubMed] [Google Scholar]
- 95.Fukuoka K, Nakano Y, Nakajima A, Hontsu S, Kimura H. Endobronchial lesions involved in Mycobacterium avium infection. Respiratory Medicine. 2003;97(12):1261–1264. doi: 10.1016/s0954-6111(03)00256-7. [DOI] [PubMed] [Google Scholar]
- 96.Gangadharam PRJ, Perumal VK, Jairam BT, Podapati NR, Taylor RB, LaBrecque JF. Virulence of Mycobacterium avium complex strains from acquired immune deficiency syndrome patients: relationship with characteristics of the parasite and host. Microbial Pathogenesis. 1989;7(4):263–278. doi: 10.1016/0882-4010(89)90045-4. [DOI] [PubMed] [Google Scholar]
- 97.Thoen CO. Mycobacterium avium infections in animals. Research in Microbiology. 1994;145(3):173–177. doi: 10.1016/0923-2508(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 98.Thoresen OF, Saxegaard F. Comparative use of DNA probes for Mycobacterium avium and Mycobacterium intracellulare and serotyping for identification and characterization of animal isolates of the M. avium complex. Veterinary Microbiology. 1993;34(1):83–88. doi: 10.1016/0378-1135(93)90009-v. [DOI] [PubMed] [Google Scholar]
- 99.Limia A, Sangari FJ, Wagner D, Bermudez LE. Characterization and expression of secA in Mycobacterium avium. FEMS Microbiology Letters. 2001;197(2):151–157. doi: 10.1111/j.1574-6968.2001.tb10597.x. [DOI] [PubMed] [Google Scholar]
- 100.Lesslie IW, Birn KJ. Mycobacterium avium infections in cattle and pigs in Great Britain. Tubercle. 1970;51(4):446–451. doi: 10.1016/0041-3879(70)90011-5. [DOI] [PubMed] [Google Scholar]
- 101.Van Ingen J, Wisselink HJ, van Solt-Smits CB, Boeree MJ, van Soolingen D. Isolation of mycobacteria other than Mycobacterium avium from porcine lymph nodes. Veterinary Microbiology. 2010;144(1-2):250–253. doi: 10.1016/j.vetmic.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 102.Tirkkonen T, Pakarinen J, Moisander AM, Makinen J, Soini H, Ali-Vehmas T. High genetic relatedness among Mycobacterium avium strains isolated from pigs and humans revealed by comparative IS1245 RFLP analysis. Veterinary Microbiology. 2007;125(1-2):175–181. doi: 10.1016/j.vetmic.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 103.Suzuki AE, Inamine JM. Genetic aspects of drug resistance in Mycobacterium avium. Research in Microbiology. 1994;145(3):210–213. doi: 10.1016/0923-2508(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 104.Tsukamura M. In vitro bacteriostatic and bactericidal activity of isoniazid on the Mycobacterium avium-Mycobacterium intracellulare complex. Tubercle. 1990;71(3):199–204. doi: 10.1016/0041-3879(90)90076-k. [DOI] [PubMed] [Google Scholar]
- 105.Shafran SD. Prevention and treatment of disseminated Mycobacterium avium complex infection in human immunodeficiency virus-infected individuals. International Journal of Infectious Diseases. 1998;3(1):39–47. doi: 10.1016/s1201-9712(98)90094-7. [DOI] [PubMed] [Google Scholar]
- 106.Raszka WV, Jr., Skillman LP, McEvoy PL. In vitro susceptibility of clinical isolates of Mycobacterium avium and M. intracellulare to folate antagonists. Diagnostic Microbiology and Infectious Disease. 1994;18(3):201–204. doi: 10.1016/0732-8893(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 107.Dunne MW, Foulds G, Retsema JA. Rationale for the use of azithromycin as Mycobacterium avium chemoprophylaxis. American Journal of Medicine. 1997;102(5):37–49. [Google Scholar]
- 108.Horgen L, Legrand E, Rastogi N. Postantibiotic effects of rifampin, amikacin, clarithromycin and ethambutol used alone or in various two-, three- and four-drug combinations against Mycobacterium avium. FEMS Immunology and Medical Microbiology. 1999;23(1):37–44. doi: 10.1111/j.1574-695X.1999.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 109.Saito H, Tomioka H, Sato K, Kawshara S, Hidaka T, Dekio S. Therapeutic effect of KRM-1648 with various antimicrobials against Mycobacterium avium complex infection in mice. Tubercle and Lung Disease. 1995;76(1):51–58. doi: 10.1016/0962-8479(95)90580-4. [DOI] [PubMed] [Google Scholar]
- 110.Lin YE, Vidic RD, Stout JE, McCartney CA, Yu VL. Inactivation of Mycobacterium avium by copper and silver ions. Water Research. 1998;32(7):1997–2000. [Google Scholar]
- 111.David S, Barros V, Guerra KP, Delgado R. Exploring Mycobacterium avium inhibition by macrocyclic compounds. Research in Microbiology. 2005;156(9):904–910. doi: 10.1016/j.resmic.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 112.Bermudez LE, Young LS. Killing of Mycobacterium avium: insights provided by the use of recombinant cytokines. Research in Microbiology. 1990;141(2):241–243. doi: 10.1016/0923-2508(90)90037-q. [DOI] [PubMed] [Google Scholar]
- 113.Chinen L, Cipriano IM, de Oliveira RS, Leao SC, Mariano M, Carneiro CW. Recombinant interleukin-4-treated macrophages, epithelioid cell surrogates, harbor and arrest Mycobacterium avium multiplication in vitro. Microbes and Infection. 2006;8(4):965–973. doi: 10.1016/j.micinf.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 114.Dhama K, Mahendran M, Chauhan RS, Tomar S. Cytokines: their functional roles and prospective in veterinary practice-A review. Journal of Immunology and Immunopathology. 2008;10(2):79–89. [Google Scholar]
- 115.Silvaa TI, Copea A, Goepelb J, Greiga JM. The use of adjuvant granulocyte-macrophage colony-stimulating factor in HIV-related disseminated atypical mycobacterial infection. Journal of Infection. 2007;54(4):207–210. doi: 10.1016/j.jinf.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 116.Salem II, Flasher DL, Duzgunes N. Liposome-encapsulated antibiotics. Methods in Enzymology. 2005;391:261–291. doi: 10.1016/S0076-6879(05)91015-X. [DOI] [PubMed] [Google Scholar]
- 117.Fernandes SS, Nunes AA, Gomes AR, et al. Identification of a new hexadentate iron chelator capable of restricting the intramacrophagic growth of Mycobacterium avium. Microbes and Infection. 2010;12(4):287–294. doi: 10.1016/j.micinf.2010.01.003. [DOI] [PubMed] [Google Scholar]


