Abstract
Lipid rafts, the sphingolipid and cholesterol-enriched membrane microdomains, are able to form different membrane macrodomains or platforms upon stimulations, including redox signaling platforms, which serve as a critical signaling mechanism to mediate or regulate cellular activities or functions. In particular, this raft platform formation provides an important driving force for the assembling of NADPH oxidase subunits and the recruitment of other related receptors, effectors, and regulatory components, resulting, in turn, in the activation of NADPH oxidase and downstream redox regulation of cell functions. This comprehensive review attempts to summarize all basic and advanced information about the formation, regulation, and functions of lipid raft redox signaling platforms as well as their physiological and pathophysiological relevance. Several molecular mechanisms involving the formation of lipid raft redox signaling platforms and the related therapeutic strategies targeting them are discussed. It is hoped that all information and thoughts included in this review could provide more comprehensive insights into the understanding of lipid raft redox signaling, in particular, of their molecular mechanisms, spatial-temporal regulations, and physiological, pathophysiological relevances to human health and diseases. Antioxid. Redox Signal. 15, 1043–1083.
-
IV. Redox Molecules Associated with LRs
-
V. Frequently Used Methods for Identifying LR Redox Signaling Platforms
-
VII. Mechanisms Mediating the Formation of LR Redox Signaling Platforms
-
VIII. Physiology and Pathophysiology of LR Redox Signaling Platforms
-
IX. Possible Therapeutic Strategies Targeting LR Redox Signaling Platforms
I. Introduction
Redox signaling is increasingly regarded as an important cellular process in a variety of cellular activities, including cell proliferation (50, 52, 275), differentiation (72, 153, 219, 337, 338), and apoptosis (162, 242, 254, 304, 413). Redox injury, as a pathological mechanism, is also involved in a wide range of pathophysiological processes such as senescence (65), inflammation (17, 264, 421), hypoxia (32, 148, 200, 245), and ischemia/reperfusion (126, 379, 384), which contribute to the progression of almost all diseases, from cardiovascular ones such as shock (94, 116, 117), hypertension (73, 167, 288, 294, 316, 440), atherosclerosis (208, 297), to metabolic ones such as diabetes mellitus (20, 217), neurodegenerative ones such as Alzheimer's disease (AD) (55, 305), infectious diseases (184, 252, 285, 375), and cancer (16, 292, 409).
Despite extensive research, the exact mechanism by which redox enzymes are promptly activated by different stimuli still remains poorly understood, perhaps because enzymes such as NADPH oxidase, unlike G-protein-coupled enzymes, are not linked directly with any specific receptors. Recently collected evidence suggests that membrane lipid rafts (LRs) and their platforms may represent an important mechanism by which redox signals are produced and transmitted in response to various agonists or stimuli (234, 283, 423, 446). Many studies have shown that LRs or their platforms can participate in the signaling of cell apoptosis or dysfunction associated with oxidative stress during activation of various death receptors (385). Major advances in LR redox signaling in specific cell types have been reported and reviewed by a series of excellent papers that have added much to the literature (192, 235, 283, 446). This review will seek to further extend such LR redox signaling concept to different areas as a common signaling mechanism and thoroughly introduce the latest advances in its molecular mechanisms and the corresponding physiological and pathological relevance. Some special emphasis will be put on the different patterns of LR redox signaling platforms, the different regulation of such redox signaling platforms, and their translational significance in health and diseases.
II. Redox Signaling and Redox Injury
A. Redox signaling
In biological systems, electron-transfer processes play a key messenger role in redox signaling and it is primarily represented by reactive oxygen species (ROS) as a messenger that mediates or regulates cell–cell communication and intracellular signal transduction (28, 352, 402). ROS is a collective term that often includes not only the oxygen radicals such as superoxide (O2•−), hydroxyl (OH−), peroxyl (RO2), alkoxyl (RO•), hydroperoxyl (HO2•) but also such nonradicals as hydrogen peroxide (H2O2), hypochlorous acid (HOCl), ozone (O3), singlet oxygen (ΔgO2), and peroxynitrite (ONOO−). Since these oxygen derivatives, whether they are radicals or nonradicals, are very reactive, they can oxidize or reduce other molecules in living cells or tissues. Therefore, in general, redox signaling is often referred to as the signaling induced by ROS. However, these ROS are often called oxidants, since they can act as both oxidizing and reducing agents. In the literature, ROS, oxygen-derived species, and oxidants are used interchangeably to refer to the same substances active in a biological system (149, 369, 381).
Under physiological or pathological conditions, ROS can be produced as a basic signaling messenger to maintain cell or organ functions, or increasingly generated or released in response to various stimuli. Meanwhile, these active molecules are constantly scavenged by the endogenous antioxidant systems, mainly composed of the enzyme-mediated pathways as superoxide dismutase (SOD), catalase, glutathione peroxidase, glutathione-S-transferase, thioredoxin/thioredoxin reductase, and other peroxidases. In addition, direct reactions between the ROS and different molecules may also result in antioxidant actions such as the interactions between ROS and NO, -SH, vitamin E, β-carotene, ceruloplasmin, ferritin, transferin, hemoglobin, and ascorbates (28, 352, 402). Being tightly regulated under normal conditions, intracellular and extracellular ROS are maintained at very low levels (less than 1% of produced ROS) (102, 199, 250, 307, 404). If the generation of ROS exceeds its removal by scavengers, the intracellular and extracellular levels of ROS will increase, leading to oxidative stress and a progression of various pathophysiological processes and respective diseases (102, 199). If the level of ROS increases to even higher levels, its damaging effects, to DNAs, proteins, lipids, and glycols, become inevitable (28, 102, 199). These damaging effects of ROS are often tightly correlated together and share a common redox system responsible for the generation and scavenging of ROS molecules (102, 199).
B. Redox signaling versus injury
Among ROS, H2O2 was first found to mimic the action of insulin and insulin could activate NADPH oxidase to generate endogenous H2O2. These results demonstrated a concept of redox signaling (263). Thereafter in 1978 both insulin and nerve growth factor were further demonstrated to stimulate H2O2 production (262) and therefore ROS and, in particular, H2O2 were confirmed to have signaling actions. However, because ROS have numerous pathological roles in various diseases and participate in bacteria killing and there is overwhelming evidence that antioxidants can prevent oxidative damage and thus protect against the adverse effects of oxidants, the pathological actions of ROS were largely focused in many studies over decades, which overshadowed the important signaling action of ROS under physiological conditions. During the last decade, the research of ROS as signaling molecules has taken a new turn. It is now clear that in the biological systems ROS may act as autocrine, paracrine, or intracellular second messengers, involved in various signaling processes. Today it is understood that the signaling or damaging actions of ROS in or on cells are very much dependent on the level of oxidants in the cells or tissues (96). There is agreement now that the biological responses to cellular or tissue ROS levels are very different and vary from physiological to pathological reactions. When a small amount of ROS is produced, they may mediate physiological redox signaling. When ROS production increases to certain levels, cell/tissue repair or adaptive responses may be activated. When ROS production is further increased to high levels, cell/tissue damage can occur, resulting in apoptosis and necrosis (96).
C. Common ROS as messengers
It is now widely accepted that ROS and, in particular, H2O2 are involved in all types of signaling, including synaptic signaling (364), paracrine signaling (319, 448), autocrine signaling (43), and intracellular signaling (176), as a mediator or modulator of signal transduction. So far, there are four common ROS, which are reportedly able to serve as secondary messengers. As shown in Table 1, they are O2•−, H2O2, HO−, and ONOO−. These ROS are centered on the O2•− as shown by their chemical reactions. O2•− can be converted into H2O2, HO−, and ONOO−, either enzymatically or nonenzymatically. Although there is evidence that these downstream ROS may be converted back to O2•−, the reaction of O2•− to form these downstream products are dominant in mammalian cell systems. Therefore, in general, O2•− may produce its action primarily through their downstream products, notwithstanding its ability to directly act as a signaling molecule. Given the central role of O2•− in the conversion into other common ROS, the production of O2•− and related regulation in biological systems has been intensively studied. It is, for example, well recognized that for signaling functions, O2•− is primarily produced via several endogenous pathways, including different enzyme systems such as mitochondrial flavin enzymes, NADPH oxidase, xanthine oxidase, cytochrome P450, lipoxygenase, cyclooxygenase, uncoupled nitric oxide synthase (NOS), and peroxisomes. Some nonenzymatic derivatives of O2•− may be formed via photolysis, Fe(III) heme protein, and auto-oxidation reactions. These enzymatic and nonenzymatic pathways responsible for O2•− production in the biological systems are summarized in Table 2 (28, 102, 199). Among these pathways, NADPH oxidase has been reported to be a major source of O2•−, in redox regulation in some cells such as vascular endothelial and smooth muscle cells (51, 138). It is estimated that this nonmitochondrial NADPH oxidase-derived O2•− constitutes more than 95% of the production of O2•− in these cells, especially when stimulated (259, 319). The role of NADPH oxidase in the normal regulation of cell functions has been well documented and is considered as one of the most important redox signaling pathways (82, 91).
Table 1.
Common Signaling Reactive Oxygen Species and Their Chemical Reactions
| Common signaling reactive oxygen species | Chemical reactions |
|---|---|
| Superoxide | (O2•−) O2+e−→O2•−+NO→ONOO− |
| e−↓↑ | |
| Hydrogen Peroxide (H2O2) | H2O2 |
| e−↓↑ | |
| Hydroxyl Radical (HO−) | HO− |
| Peroxynitrite (ONOO−) |
Table 2.
Endogenous Production of O2•−
| Enzymatic | Nonenzymatic |
|---|---|
| Mitochondrial Flavin Enzymes | Photolysis |
| NADPH Oxidase | Heme protein + Fe |
| Xanthine oxidase | Auto-oxidation reactions |
| Cytochrome P450 | |
| Lipoxygenase | |
| Cyclooxygenase | |
| Nitric Oxide Synthase | |
| Peroxisomes |
III. Concepts of LRs and Their Clustering
A. Concepts of LRs and existing debates
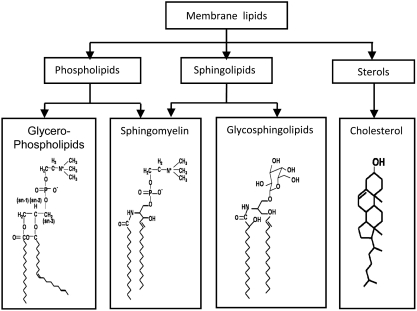
From the point of view of evolution, the formation of cell membranes has led to a separation of the protoplasm from the environment, enduing a cell with more independence and more capability of efficiently maintaining its integrity (139, 253). Cells selectively uptake molecules through the plasma membrane, or secrete molecules into the external cellular environment, keeping an efficient homeostatic balance in substances exchanged. Such membrane-mediated exchanges and regulatory activities facilitate the life of organisms and empower them to evolve to more advanced levels (212, 324, 346). It is well known that the cell membranes are mainly composed of lipids, proteins, and glycols, in variable ratios, in different cell types (139, 253). Membrane lipids comprise more than 50% of the cell membrane and constitute the backbone of the cell wall. These membrane lipids mainly include phospholipids, sphingolipids, glycolipids, and cholesterol, and their chemical structures are shown in Figure 1. For many years, the role of these membrane lipids in the constitution of cells or various organelle membranes has been intensively studied, and several different membrane models developed to explain the structure of various biological membranes and their interaction with other components (212, 324, 346).
FIG. 1.
Composition of membrane lipids and their chemical structures. Lipid rafts (LRs) may consist of dynamic assemblies of cholesterol and lipids with saturated fatty acid chains such as sphingolipids and glycosphingolipids in the exoplasmic leaflet of the membrane bilayer. In addition, phospholipids with saturated fatty acids and cholesterol in the inner leaflet. Here depicted are structures of two sphingolipids including sphingomyelin and glycosphingolipids (GSL), cholesterol, and phospholipid-phosphatidylcholine.
In 1972, Singer and Nicolson first proposed a “fluid mosaic model” of cell membrane structures (360, 361). Since then, numerous studies have advanced our understanding of membrane biology. In 1977, based on experimental observations, Jain and White suggested a “microdomain or lipid domain mode” of membrane structures, which hypothesized that the cell membrane is made of dynamic membrane microdomains (180, 181). This model emphasized the fluid characteristics of mosaic blocks in the cell membrane (180, 181). Further studies, since, have demonstrated that sphingolipids and cholesterol-rich microdomains in the cell membrane have unique physical and chemical properties, which are able to form liquid ordered structures that float in the ocean of fluid glycerophospholipids. Such sphingolipids and cholesterol-rich microdomains have been found to play important roles in biological and physiological processes (256, 327, 328). Until 1997, Simons and Ikonen proposed, based on many studies of lipid patches or membrane microdomains in molecular trafficking in their own labs and others, the so-called LR model for cell membrane structures, based on the organization of sphingolipids and cholesterol mircodomains that can be selectively included or excluded (47, 356, 359, 394). They concluded that the function of such lipid microdomains is to serve as rafts for the transport of selected membranes or as relay stations in intracellular signaling (356–358).
LRs were assumed to consist of dynamic assemblies of cholesterol and lipids with saturated acyl chains such as sphingolipids and glycosphingolipids in the exoplasmic leaflets of the membrane bilayer and phospholipids with saturated fatty acids and cholesterol in the inner leaflets (283). Because long fatty acid of sphingolipids in the outer leaflets couples the exoplasmic and cytoplasmic leaflets by interdigitation and transmembrane proteins stabilize this coupling, LRs are very stable and detergent resistant (247, 277). The sizes of individual LRs are thought to vary in different cell types from 50 to 200 nm in diameter. Given its small size, a raft may contain only a subset of all available raft proteins. It has been estimated that the number of proteins in each raft depends on its packing density, but it probably carries no more than 10–30 proteins (314). This, in turn, suggests that raft clustering is important for transmembrane signaling amplification. By comparing the ratio of the main raft and nonraft exoplasmic leaflet lipids, it was found that about 45% of the cell surface in fibroblasts and about 30% in lymphocytes are made up by sphingolipids (143, 314).
Notwithstanding the extensive research into them, even the existence of LRs is still not beyond doubt and some debates remain due to the lack of direct observations of such LR structures in living cells (266). With the development of advanced technologies in microscopy and spectroscopy, such as scanning probe microscopy (SPM), atomic force microscopy, single-particle tracking (SPT), fluorescence correlation spectroscopy (FCS), fluorescence resonance energy transfer (FRET), and fluorescence photoactivation localization microscopy (FPALM), more and more direct evidence gathered in living cells has shown that the nano-scale dynamic microdomains are rich in sphingolipid, cholesterol, and specific proteins (241). Hancock (151) suggests that rafts at the plasma membrane are present in nanoscale complexes, which are well below the optical resolution limits set by the diffraction of light. This nanometer-size scale was supported by electron microscopic observations of immunogold-labeled raft markers (101). More recently, by using near-field scanning optical microscopic techniques with localization accuracies of approximately 3 nm, a nanodomain of GPI-anchored proteins was observed concentrated in a region smaller than 250 nm in fixed cells (396). In living cells, however, single-particle tracking of colloidal gold-labeled glycosylphosphatidylinositol (GPI)-anchored receptors, CD59, and others has revealed CD59 clusters containing several CD59 molecules, and single molecules of Gαi2 or Lyn that were frequently if only transiently (133 and 200 ms, respectively) recruited to CD59 clusters right after the recruitment of Gαi2 (376). Other evidence obtained through variable waist fluorescence correlation spectroscopy indicates how GPI-anchored proteins, in the form of assemblages of less than 120 nm in diameter, fluctuate on a subsecond time scale (229). In addition, high spatial and temporal resolution fluorescence resonance energy transfers reveal a size estimate of approximately 10 nm in GPI-anchored receptors residing in temporally stable clusters (125). Fluorescence photoactivation localization microscopy has shown a dynamically clustered nanoscale distribution of hemagglutinin (161), a transmembrane protein thought to be raft associated (314). By analysis of the association between cholesterol and sphingolipids, in the assembly formation of membranes, using stimulated emission depletion microscopy, a study has revealed that, unlike glycerophospholipids, plasma-membrane sphingolipids display transient cholesterol-dependent confinement in areas of less than 20 nm, which is a typical LR structure (83). All these lines of evidence obtained by using the most advanced techniques strongly support the idea that membrane molecular constituents form microdomains or LRs in the cell membrane of diverse cell types, suggesting, in turn, the presence of small, dynamic, and selective cholesterol-related microdomain heterogeneity or LRs in the plasma membranes of living cells. It would appear that functioning LRs are not only present in cell membranes, but are responsible for molecular trafficking, transport, and signaling (152, 241).
Yet, many scientists who have failed to identify LRs in their work on living cells are not completely convinced that there are such things as LRs present in living cell membranes. Due, no doubt, greatly to this reason, a recent “Key Stone Symposium on LRs and Cell Functions,” which brought together leading scientists in the raft field, replaced the term “lipid rafts” with “membrane rafts (MR).” Since this conference it is MRs that are referred to in the literature, irrespective of whether the rafts are thought to be driven by lipids (classical LRs proposed by Simons and Ikonen and the classification adopted throughout this article), or thought to be driven by protein interactions, where lipids are merely accompanying components (157).
B. Molecular models of LRs
Two major molecular models are often utilized to describe and explain the nature and behavior of LRs. In the first model, LRs are considered relatively small structures enriched in cholesterol and sphingolipids within which associated proteins are likely to be concentrated (356). In this sphingolipid-enriched model of LRs, the most prevalent component of the sphingolipid fraction in the cell membrane is sphingomyelin (SM), which is composed of a highly hydrophobic ceramide moiety and a hydrophilic phosphorylcholine headgroup. The tight interaction between the cholesterol-sterol-ring system and the ceramide moiety of the SM promotes a lateral association between the sphingolipids and the cholesterol, forming distinct microdomains. In these microdomains, cholesterol exerts a stabilizing role by filling the voids between the large and bulky sphingolipids. The cholesterol-SM interaction determines the transition of these microdomains into a liquid-ordered or gel-like phase that is the unique characteristic of LRs. Other domains in cell membranes primarily exist in a more disordered fluid or liquid phase, precisely because of the absence of this cholesterol-SM interaction (146).
The second model of LRs, known as the shell hypothesis, views the generation of LRs as being based on protein–lipid or protein–protein interactions. According to this model, rafts are constructed of lipid shells, which, as small dynamic membrane assemblies, are formed by proteins preferentially associated with certain types of lipids. Protein–protein interactions create larger functional units corresponding to LRs (13). Other nonshell proteins associate with LRs by additional and new protein–protein interactions. In addition, an oligomerization of these proteins may create and stabilize large raft domains, forming LR platforms, making the formation and clustering of LRs dependent on both protein–lipid interactions and protein–protein interactions (160).
In many studies of the molecular models of LRs or the mechanisms forming LRs in cell membranes, two common questions have often been asked: (i) Why can sphingolipid- and cholesterol-enriched microdomains be separated from glycerophospholipid membrane bilayers and act as rafts floating in the membrane? (ii) What kind of proteins associates with LRs? In trying to answer the first question, evidence is proffered showing that there are three main factors accounting for the formation of LRs and leading to their flotation in the cell membrane. First, compared to glycerophospholipid, the two hydrophobic SM chains are longer and more highly saturated, making them fully extended and tightly packed close to each other, which represents an important feature of LR assemblies (241, 372, 450). The different arrangements between sphingolipids and phospholipids may be the key factor causing the phase separation in their combination (241, 372, 450). More studies have shown that a different phase separation behavior can occur in the mixed system of cholesterol, leading to coexistence of classic mesophase and a new liquid ordered phase. In such a new liquid ordered phase, lipid fatty acid chains are fully stretched and closely arranged into a gel like phase that exhibits a high degree of lateral mobility (241, 372, 450). Second, unlike glycerophospholipids, SMs contain at least one hydroxyl group as shown in Figure 1, which makes hydrogen bonds easy to form not only between SM molecules, but also between SM and cholesterol (39). The formation of intermolecular hydrogen bonding significantly increases the intermolecular forces among these molecules, increasing the melting temperature of the lipid assembly and resulting in a transition of the assembly from a liquid disordered phase (liquid phase), with lower melting temperatures into a liquid ordered phase (gel phase) with higher melting temperatures. Conversion of SM into such liquid ordered phases separates it from the surrounding liquid disordered phase (glycerophospholipids) (39), not unlike sphingolipid rafts floating in a sea of glycerophospholipids, a structural arrangement figuratively referred to as LRs. Finally, cholesterol can promote phase separation behavior. By filling the void space in the bulky sphingolipid molecules and forming hydrogen bonds with sphingolipids, cholesterol serves as a glue that packs the sphingolipid molecules into a more tightly organized assembly (39). Because the sphingolipids required to combine with the cholesterol for the formation of the liquid ordered phase are much less than those without cholesterol, LRs in cells are formed with relatively much less membrane sphingolipids (39). Cholesterol depletion by M-β-CD or cholesterol binding to fillipin leads to the breakdown of LRs because these compounds suppress the glue effect of cholesterol on sphingolipids. This is why both compounds are used as classical tool drugs in the area of LR research (39).
With respect to what types of proteins associate with LRs, there is considerable evidence that only those proteins with specific posttranslational modifications, such as the glycolphosphotidylinosital (GPI)-anchoring proteins, Src family tyrosine kinase, and the marker protein of LR, caveolin, can fuse in or dissociate from LRs (240, 241, 356). Based on their location in the cell membrane, membrane proteins can be divided into three categories: (i) proteins present within LRs, including glyco-phosphatidylinositol-anchored proteins (GPI anchored proteins), some transmembrane proteins, Hedgehog proteins, and doubly acylated proteins such as nonreceptor tyrosine kinase Src, G protein Gα subunits, and vascular endothelial cell NOS; (ii) proteins present outside LRs (the liquid disordered phospholipids); and (iii) proteins present between or around LRs, such as certain proteins in low affinity with LRs; under resting status, they may form oligomerized bodies that are transferred into LRs upon stimulation.
Recent proteomic analysis has demonstrated that there are around 241 authentic proteins detectable in LRs (98). It was found that these proteins underwent several types of posttranslational modifications, thereby increasing their binding capacity to sphingolipids (265, 299). These posttranslational modifications include GPI-anchoring, palmitoylation, and myristoylation. Among these modifications, palmitoylation is attracting particular interest among investigators (265, 299). Although most of these lipid modifications are irreversible, protein S-palmitoylation, also called as thioacylation or S-acylation, is able to reversibly attach, via thioester linkages, to 16-carbon saturated fatty acids that have specific cysteine residues in their protein substrates (239, 399). Such palmitoylation enhances surface hydrophobicity and the membrane affinity of protein substrates and thereby plays important roles in modulating protein trafficking (79, 239), stability (239), sorting (135), etc. It is now widely accepted that the proteins that undergo palmitoylation have a high propensity to be targeted into LRs.
C. LRs on cell membranes
1. Caveolar LRs
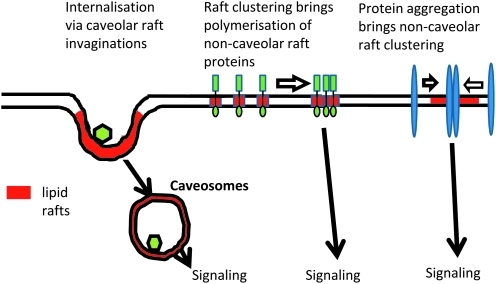
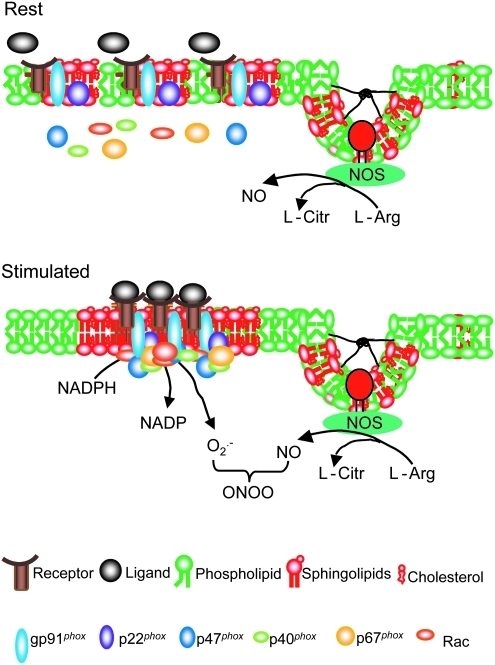
The concept of two types of LRs, namely, caveolar and noncaveolar rafts, in cell membranes, based on their structure and components, are well established. Caveolar rafts are formed in cell types that express caveolin proteins that bend to form scaffoldings that give shape and form caveolae. Although there have been numerous studies about caveolae functions, even before the establishment of a general LR concept (99, 344, 370), the most well-studied function of caveolae has been its role as an important platform for the action of endothelial NOS (eNOS) and the synthesis of NO as a regulator of vascular dilation and constriction (119). There is wide agreement that the binding of eNOS to the caveolin scaffolding can inhibit eNOS activity (112), whereas the absence of any caveolin expression can increase eNOS activity (322). General consensus is also shared in the important role played by endothelium-specific expressions of eNOS and, in turn, the colocalization of eNOS with caveolins in ECs, in NO-mediated vasodilation and, thereby, blood pressure homeostasis (267). The caveolin-1-mediated formation of caveolae in ECs represents a form of LR clustering, which is present even under resting conditions. In general, NOS in caveolae are constitutive and most activators of this enzyme do not alter the location of the NOS in caveolae. This is different from noncaveolar LRs, which largely depend on clustering or de-clustering in response to various stimuli. As shown in Figure 2, caveolar and noncaveolar LRs mediate different signaling pathways, thereby participating in the regulation of different cell functions or cell responses to agonists or other stimuli (119, 131).
FIG. 2.
Demonstration of caveolar and noncaveolar lipid rafts and their function. Caveolar and noncaveolar LRs may mediate different signaling pathways in different cells or even in the same cell in response to different agonists or stimuli. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
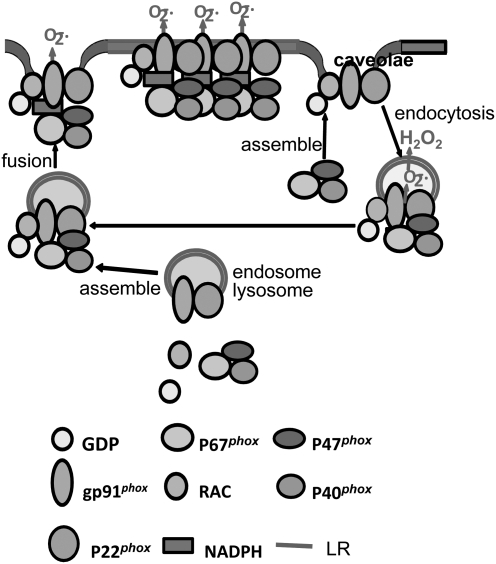
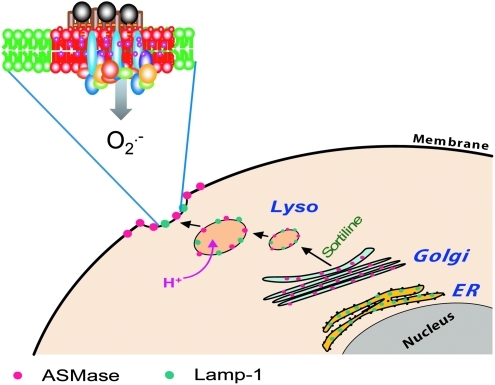
In addition to NOS regulation of caveolae, caveolae is also understood to play an important role in endocytotic or exocytotic transmembrane transport (154). They can bud from the plasma membrane and fuse with intracellular organelles, including caveosomes (276, 303), or bud outward from the cell surface in exocytosis (301). Caveolar endocytosis may well be a mechanism in the regulation of the lipid composition of the plasma membrane (60, 348). More important to redox signaling, recent studies have linked such caveolar raft-associated endocytosis with the formation of redoxosomes. It has been suggested that receptor stimulation may lead to the formation of redoxosomes by caveolin-1-dependent LR-mediated endocytosis of receptors such as IL-1R1, as NADPH oxidase subunit-gp91phox (NOX2) and IL-1R1 enter redoxosomes together from the cell-surface caveolae. Therefore, LR- or caveolae-mediated endocytosis would be critical for the formation of redoxosomes (283, 284). Such caveolae-mediated endocytotic processes have been shown to participate in the regulation of cell functions such as ion channel activities, cell polarization, molecular metabolism, recycling, and membrane repair (60, 204–206, 269, 296, 300, 302, 377).
2. Noncaveolar LRs
According to current understanding, caveolae and noncaveolar LRs may mediate different signaling pathways, participating in the temporal-spatial regulation of the consequent cell responses even in the same type of cells. Despite different signaling functions, the lipid components in caveolar or noncaveolar rafts are difficult to differentiate using common LR research techniques. Yet, there is considerable evidence that while some cell types have only caveolar or noncaveolar membrane rafts, some cell types may have both in their plasma membranes (406). Numerous studies have been done to clarify the association of NOS with caveolae and noncaveolar rafts. They, in turn, have shed vital light on the complex features of such membrane structures as functional units. As mentioned above, the formation of caveolae may be associated with NO production and endocytosis in ECs (119, 370). However, eNOS is also found in noncaveolar LRs and the formation of caveolae promotes interfacing or juxtaposing of NOS with other signaling partners such as caveolin-1, dynamin-2, calmodulin, heat shock protein 90, and akt (315). There is evidence that although caveolin-1 is important to the formation of caveolae, this protein exerts an inhibitory action on NOS activity. In fact, the formation of caveolae appears to play a critical role in clustering or juxtaposing various signaling components for NOS production. From this perspective, caveolin-1-mediated formation of caveolae clearly represents a special form of LR clustering, which is constitutive and present even under resting conditions. Noncaveolar LRs are clustered in response to agonists or stimuli. Therefore, it is not surprising that NOS can be detected in caveolar and noncaveolar LRs. With respect to NADPH oxidase, its subunits have also been identified in caveolar and noncaveolar LRs of certain cell types studied (423, 453). Like NOS, the distribution of NOX in both LRs and caveolae may also mediate different signaling pathways, participating in the temporal–spatial redox regulation of cell functions in different or even same type of cells, although in response to agonists or stimuli. For example, in vascular smooth muscle (VSM) cells there is strong evidence that NADPH oxidase subunits are colocalized with caveolin-1, indicating an association of this enzyme with caveolae (163, 390). Angiotensin II stimulates this caveolae-associated NADPH oxidase to produce O2•−, an integral part of the redox signaling mechanism mediating the action of angiotensin II in the regulation of VSM cellular activities such as protein synthesis, hypertrophy, and proliferation (453). In endothelial cells, however, the action of inflammatory factors, such as the TNF-α or Fas ligand (FasL), to alter endothelial functions, are dependent on both caveolae and noncaveolae-related mechanisms (423, 444). The formation of noncaveolar LR signaling platforms may contribute to aggregation or recruitment of NADPH oxidase components in ECs. Different from caveolae, LRs clustering of these noncaveolar LRs are not constitutively present, but occur only upon stimulations (444).
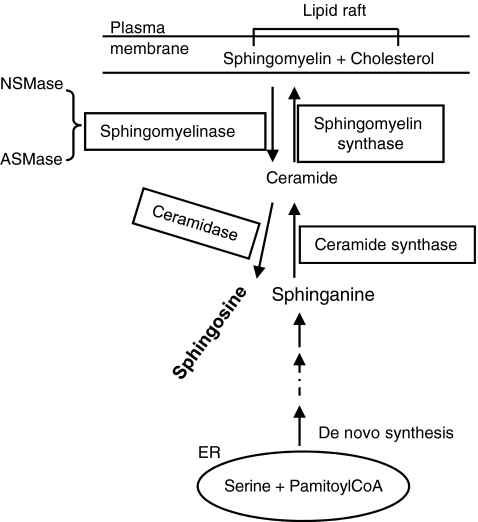
3. Ceramide-enriched micro- and macrodomains
In spite of the difficulty in pinpointing classical LRs with SM in living cells, ceramide-enriched membrane domains are well documented. The biophysical properties of ceramide molecules predict a tight interaction of ceramide molecules with each other, resulting in the formation of stable and tightly packed ceramide-enriched membrane microdomains that spontaneously fuse to form large ceramide-enriched membrane macrodomains or platforms. Although in a broad sense, the ceramide microdomains are also called LRs, it should be noted that ceramide-enriched membrane platforms or macrodomain can be formed without the presence of classically defined rafts, namely, the small structures enriched in cholesterol, sphingolipids, and associated proteins. Ceramide-enriched membrane platforms are often conveniently used to describe the signaling mechanism related to these special membrane lipid platforms. Ceramide is generated in the biological membranes either by hydrolysis of SM, catalyzed, in turn, by various sphingomyelinases (SMase) or by a de novo ceramide synthase pathway. Both SMase and de novo synthesis–derived ceramides have been shown to be involved in cell signaling. Among SMases, acid SMase (ASMase) has been considered as the major enzyme responsible for the formation of ceramide-enriched membrane platforms. The acid SMase is present locally within secretory vesicles, which are mobilized, on stimulation, to fuse with the cell membrane (81, 146). There is evidence that ASMase may also be found locally in lysosomal vesicles and that their activation and fusion with the cell membrane are associated with the functional integrity of lysosomes. Disturbance of lysosomal functions abolish the formation of ceramide-enriched membrane platforms associated with ASMase activation (189, 190). The structure of these ceramide rafts or platforms is similar to classical SM rafts with cholesterol serving, on the one hand, as a spacer between the hydrocarbon chains of ceramide and, on the other, as dynamic glue that keeps the raft assembly together. Cholesterol also provides partitions between the raft and the nonraft phase, having a higher affinity to raft sphingolipids (ceramide here) than to unsaturated phospholipids. This would appear to be confirmed by the fact that removal of raft cholesterol leads to dissociation of most proteins from the rafts, rendering them nonfunctional. During ceramide formation, ASMase hydrolyzes SM to release choline without affecting the hydrocarbon chains that remains in the ceramide, suggesting, in turn, that cholesterol is an important component in ceramide rafts or platforms (46, 354).
D. Intracellular LRs
Although the constituents and the exact function of LRs inside the cell remain poorly understood, there is considerable evidence that LRs may also be present in intracellular membranes including endoplasmic reticulum membranes (21, 47, 160, 356, 454), Golgi apparatus (359), endosomes (270, 355, 397), lysosomes (270, 355, 397) and mitochondria (63, 366). Studies have shown that the concentration of sphingolipids and sterols increase along the biosynthetic pathway from the endoplasmic reticulum (ER) to the trans-Golgi network (TGN). Such occurrence of sphingolipids and sterols may lead to functional raft clustering in these organelles, probably determining the nature of the molecular sorting, trafficking and recycling within the cells (160).
The Golgi apparatus was the first organelle demonstrated to have functional rafts that play a vital role in sorting molecules (359). In this respect, apical sorting of GPI-anchored proteins in polarized epithelial cells has been the subject of intense research (47, 160, 356, 454), which, in turn, have shown that GPI-anchored proteins associate with detergent resistant membranes (DRMs) during their passage through the Golgi apparatus and perturbation of this association by cholesterol or sphingolipid depletion results in impaired transport or altered polarity of the GPI-anchored proteins (47, 160, 356, 454). In addition, Golgi LRs have been reported to participate in the maintenance of Golgi structures and functions. If the cholesterol balance of cells is changed, Golgi morphology and intra-Golgi protein transport may be dramatically altered (155, 373, 435).
GPI-anchored proteins were also found to associate with LRs in the ER in yeast and mammalian cells. These proteins are sorted and processed by the LRs and are then transported from the ER to the Golgi compartments (21, 47, 160, 356, 454). It is assumed that the role of rafts in ER sorting has to do with its stabilizing role in the association of GPI proteins with the ER membrane. In studies of the prion protein PrPC, also a GPI-anchored protein, it was demonstrated that perturbation of ER microdomains affects the folding of the immature protein and increases misfolding of some ER-localized mutants. Therefore, LRs on the ER may well contribute to the regulation or conformation of the PrPC and its dysfunction may be a key mechanism of neurodegenerative diseases known as Prion diseases (160).
LRs have been identified in endosomes and lysosomes (270, 355, 397). The important roles LRs play in the endosomal recycling pathways are well known. Raft-dependent internalization is one of the important mechanisms for the formation of endosomes, where membrane molecules and proteins are processed, transported, or metabolized. Increasing evidence has been found that LRs are present in the membrane of lysosomes. However, the mechanisms mediating the formation of LRs in lysosomal membranes and the functional relevance of such lysosomal LRs are still poorly understood. Pathologically, however, LRs are known to accumulate in late endosomes or lysosomes in patients with lysosomal storage diseases (160, 355). How such pathological changes in lysosomal LRs occur remains unknown.
With respect to LRs in mitochondria, some studies have reported that mitochondria do not contain LRs and that LRs do not contain mitochondrial proteins (451). These studies have used quantitative proteomics and multiple subcellular fractionation procedures to examine, from several angles in different cell types, whether mitochondrial proteins are in LRs. Some studies found no rafts in mitochondria and no mitochondrial proteins in cell surface rafts (451). However, other studies have demonstrated that LR structures are detectable in mitochondria. In particular, there is considerable evidence showing that the activation of death receptors (CD95/Fas or TNF-α receptor) may induce an intracellular movement of LRs components, such as GD3 ganglioside, toward the mitochondria, which may be responsible for the mitochondrial mechanism of cell death. In isolated mitochondria, LR constituents, GD3 and GM3 gangliosides, can be detected when cells are challenged with anti-CD95/Fas. In such LR or LR-like domains, multiple proteins, such as GD3, the voltage-dependent anion channel-1, and the fission protein hFis, are enriched. Functionally, it is presumed that LRs in the mitochondrial complex drive mitochondrial fission, where catalytic domains are provided to associate or cleave related molecules. Disturbance of the framework of such a mitochondrial complex may impair fission and apoptosis. It has been suggested that mitochondrial LRs may represent essential activating platforms where mitochondria-mediated events determine cell survival or death (63, 366).
E. LR clusters or signaling platforms
It is widely accepted that the function of LRs are dependent on the formation of macrodomains or platforms, irrespective of whether they are formed or driven by SM-cholesterol and ceramide-ceramide interactions, as postulated by the sphingolipid model or alternatively by the protein–protein interactions in the shell protein model (241). The fact that LRs, in both surface and intracellular membranes, are able to form membrane lipid platforms, begs the question whether the clustering of membrane LRs may actually produce important signaling platforms instead of being mere silent building blocks (9, 368). These membrane-signaling platforms play important roles in the transmembrane signaling in a variety of mammalian cells. Here, initiation of intracellular signaling cascades is associated with aggregation or reduction of cell surface receptors through LR clustering in the plasma membrane (132, 144). These receptors in LR clusters are, not unexpectedly, many in number, including among them T-cell receptor/CD3 complexes, B-cell receptors, CD2, CD40, CD44, L-selectin, insulin receptors, or integrins, which help conduct signals to transmembrane signaling proteins or proteins in the inner leaflets of the cell membrane, when they aggregate within LR clusters. This completes the transmembrane signaling process (9, 40, 131, 357). Recent studies have indicated that several death receptors, including tumor necrosis factor receptors (TNFR), Fas, and death receptor (DR) 4 and 5, produce their apoptotic effects through this mechanism (243, 358). During LR clustering, aggregated receptors or other signaling molecules are either constitutively located in the LRs or translocated by transporters or recruiters upon stimulations (45, 59). This dynamic clustering of lipid microdomains may represent a critical common mechanism in transmembrane signal transduction.
LRs platforms usually contain different proteins, including different signaling molecules and crosslinkers or enzymes (356, 358). The formation of LR platforms activates, facilitates, and/or amplifies signal transductions. There is considerable evidence that LR clustering is formed as a ceramide-enriched membrane platform, where the ceramide production or enrichment is from SMase catalyzed cleavage of SM cholines in individual LRs (145, 168). However, ceramide-enriched membrane platforms might also be formed without the presence of classically defined LRs simply through a fusion of several ceramide molecules. These ceramide molecules can come from LRs or other membrane fractions. LR clustering or platform formations, especially ceramide-enriched ones are responsible for the regulation of a number of widely varied biological processes in different cells, including cell growth, differentiation and apoptosis, T-cell activation, tumor metastasis, and neutrophil and monocyte infiltration (145). The clustering of receptor molecules within ceramide-enriched membrane platforms might well have several important functions such as the aggregation in close proximity of many receptor molecules (144), facilitation of the transactivation of signaling molecules associating or interacting with a receptor, and the amplification of the specific signal from activated receptors. On the other hand, the formation of ceramide or ceramide platforms at the erythrocyte surface may partially contribute to the scrambling of the cell membrane but not assembling, leading to eryptosis after a second different stimulus such as osmotic shock. Such eryptosis may be linked to apoptotic pathways via ceramide, which, in turn, may be causally linked to local oxidative stress. This may represent another type of LR redox signaling in erythrocytes (221, 222).
There are many different LR signaling platforms that are formed or present in mammalian cells. As summarized in Table 3, these LR signaling platforms include phosphorylation or transphosphorylation signaling platforms (1, 49, 74, 97, 105, 279, 287, 317, 329, 405, 436, 439), GPCR raft signaling platforms (61, 89, 159, 179, 280, 290), TCR signaling platforms (2, 193, 216, 232, 291, 311, 363, 393), Ca2+ channel signaling platforms (10, 108, 111, 334, 403, 407), PI(4,5)P(2) rafts (54, 224), STIM1 raft clustering at ER-plasma membrane junctions (7, 108, 187, 293), cadiolipin platforms on mitochondria (366), raft-cytoskeleton nanodomains and macrodomains (218, 332), and LR redox signaling platforms (191, 192, 234, 235, 283, 306, 320, 335, 343, 401, 444). These LR signaling platforms may work on different type of cells, mediating or regulating cellular activities and cell functions. Given the stated focus of this review to be on LR redox signaling platforms, the following sections will discuss the formation and regulation of this LR signaling platform and explore related physiological and pathological relevances.
Table 3.
Different Lipid Raft Signaling Platforms
| LR platforms | Functions |
|---|---|
| Phosphoryl./Transphosphoryl. raft platforms | Phosphorylation, cell signaling |
| GPCR raft platforms | GPCR cellular signaling |
| TCR raft platforms | T-cell activation |
| Ca2+ channel raft platforms | Ion channel activity |
| PI(4,5)P(2) raft platforms | Vesicle trafficking |
| STIM1 raft clustering at ER-PM junctions | ER-PM Ca2+ signaling complexes |
| Cadiolipin platforms on mitochondria | Apoptotic signals |
| Raft-cytoskeleton nanodomains | Fas signaling, cell death |
| LR redox signaling platforms | Redox signaling and regulation |
LR, lipid raft.
IV. Redox Molecules Associated with LRs
A. The NADPH oxidase family
As mentioned above, NADPH oxidase is now considered as a main resource of signaling ROS under physiological conditions (31). General consensus, further, appears to exist that LR provides the essential physical platform to aggregate and assemble the needed subunits into an active enzyme complex that produces O2•−, other ROS, and conducts redox signaling (227). Detailed information about the structural and functional nature of this family of enzymes will help understand how LR redox signaling is associated with this enzymatic system under both physiological and pathological conditions.
1. Structure of the NADHP oxidase family and their tissue distribution
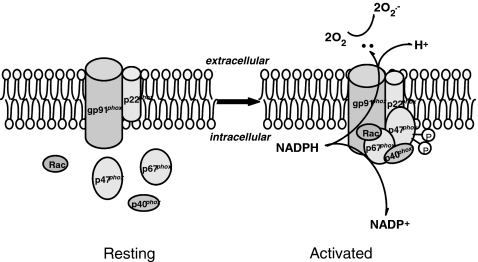
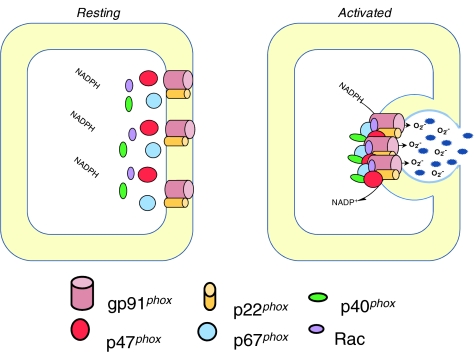
NADPH oxidase is a six-subunit multiprotein complex, first found abundantly expressed in phagocytic cells. Both the structure and function of phagocytic NADPH oxidase have been thoroughly studied and are well understood. For example, it is now well known that the catalytic subunit gp91phox (also known as NOX2) and regulatory subunit p22phox, located in the cell membrane, form heterodimers (also known as flavin cytochrome b558), whilst other regulatory subunits, including p47phox, p40phox, p67phox, and the small G protein Rac (small GTPase Rac), are located in the cytoplasm (5, 18, 31). In the classic model of phagocytic type NADPH oxidase, activation involves translocation of the four cytosolic proteins to the cell membrane and interactions with the membrane spanning subunits p22phox and NOX2, resulting in the transfer of the NADPH electron to oxygen molecules and the generation of O2•− (18, 70).
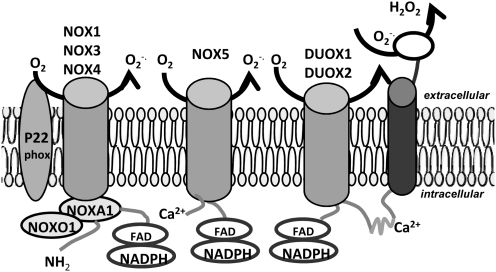
In addition to the above, recent discoveries of other different types of the nonphagocytic homolog NADPH oxidase catalytic subunit, gp91phox (NOX2), have been found in a variety of cells and/or organs and have been classified collectively as the NOX protein family. These nonphagocytic catalytic subunits include NOX1, NOX3, NOX4, NOX5, DUOX1 (dual oxidases1), and DUOX2 (44), which determine ROS production in nonphagocytes (57). It should be noted that NOX is usually named for gp91phox homologs, rather than the entire NADPH oxidase. Some in the literature use Nox as an abbreviation for NADPH oxidase, which can be easily confused with the gp91phox homologs, NOX. Although NOX2 is a phagocytic isoform of NOX, there is increasing evidence suggesting that NOX2 is also expressed in the nonphagocytes, including neurons, cardiac cells, skeletal muscle cells, liver cells, endothelial cells, B lymphocytes, epithelial cells, and hematopoietic cells (308, 374). The structure and function of nonphagocytic NOX are very similar to NOX2. They can also catalyze a single-electron reduction of molecular oxygen, generating O2•− and other ROS (410). Under physiological circumstances, the nonphagocytic NOX expression is merely very low and its activity is maintained at a very low level. Unlike the ROS produced in phagocytes that are mainly involved in host defense, the ROS produced in nonphagocytes primarily serve as a signaling messenger, which directly or indirectly act on the downstream intermittent or effector proteins, such as protein kinase, protein phosphatase, and various transcription factors. In this way, ROS participate in many cellular activities and cell functions, including cell proliferation and differentiation (142). However, upon stimulation of specific agonists, such as angiotensin II (Ang II), the platelet-derived growth factor (PDGF), an expression of nonphagocytic NOXs, appears to be highly upregulated, although through several intracellular redox-related signaling pathways as mitogen activated protein kinases (P38MAPKs), adenylate kinase (AKT), and others (29, 31, 400).
In terms of molecular structure, NOX proteins can be divided into two major domains: (i) the N terminal hydrophobic transmembrane domain and (ii) the C terminal flavin-binding domain. The flavin-binding domain also has some homology with a number of flavin adenine dinucleotide (FAD)-binding proteins, including cytochrome P450 reductase and ferredoxin-NADP oxidoreductase (31). NOX family proteins have a molecular weight between 56,400 and 73,700 Da, all possessing six transmembrane domains. It is these conservative domains that may be responsible for NADPH and FAD bindings (31).
The NOX1 gene is located on X chromosomes and expressed mainly in the colon (5, 378), VSM, the uterus, prostate, osteoblasts, and cells in the outer retina (5, 378). As mentioned above, NOX2 was first found in neutrophils and macrophages and is often called phagocyte NADPH oxidase. It, however, has also been detected in many other cells. NOX3 possesses 56% of homology in amino acid sequences, with NOX2. The human NOX3 gene is located on chromosome 6. Sequence comparison and hydrophilic diagrammatic analysis have shown that the overall structure of NOX3 has a high degree of similarity to both NOX1 and NOX2, whereas other research has conclusively proven that NOX3 is located in the inner ear as an NADPH oxidase (24, 261, 387). NOX3 also has a low expression level in some other tissues, such as the fetal spleen, kidney, skull, and brain (31).
As an NADPH oxidase, NOX4 was originally found in the adult and fetal kidneys. NOX4 and NOX2 have a 39% homology in amino acids sequences. NOX4, expressed primarily in adult kidneys, is possibly one of the renal oxygen-sensitive sensors (36, 123). In addition, NOX4 mRNA have also detected in other cells such as endothelial cells, smooth muscle cells, and fibroblasts, but only at a low expression level in monocytes. In vessels, endothelial cells mainly express NOX2 and NOX4, and VSM cells mainly express NOX4 and NOX1 (31).
NOX5 was found in all embryonic tissues, although with a very low expression level in the ovaries, placenta, and the pancreas (107). In addition to the basic catalytic domains of NOX1∼4, NOX5 is also known to encode amino-terminal domains that contain four helix-loop-helix (EF-hand) calcium-binding sites. In cells transfected with NOX5, it was found that ROS was generated by NOX5 through a calcium-dependent mechanism. Calcium binding to the above EF hand structures can change conformation to facilitate binding with the catalytic domain, thereby transferring electrons from NADPH to oxygen to generate O2•− (25, 107).
Dual oxidase 1 (DUOX1) and dual oxidase 2 (DUOX2) genes are located in the long arm of human chromosome 15. Human DUOX1 and DUOX2 proteins have 83% similarity in sequence (92, 128, 220). DUOX1 and DUOX2 were first found expressed primarily in the thyroid gland. However, low levels of expression were also shown recently in other tissues, such as the salivary glands, bronchus, lung, and prostate. DUOX2 were found to be mainly expressed throughout the digestive tract (11, 92, 128, 220).
Among all the O2•-producing NOXs, NOX1, NOX2, and NOX4 have been the most extensively studied ones (31). Interestingly for the discussion here, almost all NOXs were demonstrated to have some structural or functional link to, or relationship with, LRs. Given that NOX activation requires many cofactors to work together, LRs provide a wonderful platform, for NOX and the other NADPH oxidase subunits and cofactors, to assemble and then work as an active enzymatic complex. Indeed, many studies, in house and outside, have demonstrated that LRs even provide the driving force that promotes the assembling of NOX with other NADPH oxidase subunits or cofactors (26, 27, 190, 235, 388, 389, 391, 443, 444, 446, 452, 453).
2. Assembly and activation of NOX
Among all NOXs, the activation and functions of the phagocytic NOX or NOX2-associated NADPH oxidase have been described in the most detail. As shown in Figure 3, the assembly of the active NADPH oxidase (phagocytic) requires translocation of cytosolic subunits p47phox and p67phox, as well as Rac to the plasma membrane, where these subunits interact with gp91phox and p22phox, associating with other cofactors in the membrane to form a functional enzyme complex. Here again, electron transfer involves cytosolic NADPH binding to gp91phox and releasing two electrons. These electrons, in turn, are then transferred to two molecules of oxygen on the extracellular side of the membrane via FAD and heme, resulting in production of two molecules of O2•− (140, 331). In the assembly and activation process of NADPH oxidase, the p47phox translocation is a key step, and to some extent the marker for the event, since it is the first subunit translocated during the assembly process of these enzyme subunits. p47phox translocation is initiated by the phosphorylation of this subunit by protein kinase C (PKC), protein kinase A (PKA), or mitogen-activated protein kinase (MAPK) at various phosphorylation sites (140, 362). Studies using either tissues from p47phox knockout mice or specific inhibitors have shown a crucial role for p47phox in NADPH oxidase activation by several agonists such as angiotensin II, TNF-α, vascular endothelial growth factor (VEGF), and chronic oscillatory shear (100, 390). However, for a long time it was unknown how p47phox translocation and subsequent assembly of other NADPH oxidase subunits occurred in the cell membrane. Even today, the driving force or physical platform upon which NADPH oxidase functions as an active enzyme complex is still unknown. As noted above, the LR clustering or formation of LR macrodomains or platforms may represent an important mechanism mediating this assembly or activation process of NADPH oxidase.
FIG. 3.
Assembling and activation of NADPH oxidase. Upon stimulation, p47phox is phosphorylated and translocated to the membrane. NADPH oxidase subunits are aggregated in the membrane to form a functional enzyme. The gp91phox with help of other subunits or factors uses NADPH as substrate to transfer two electrons to molecular oxygen on the opposite side of the membrane to produce O2•−.
With respect to the assembly and activation of other NOXs, there is no consensus whether they all, like phagocytic NOX, need subunits or cofactors. Some reports have indicated that NOX1 and 4 also require all subunits and cofactors to assemble into an active enzyme complex (48, 64, 136, 235, 336, 414). However, many other studies have reported that nonphagocytic NOXs may function without a similar assemblage as phagocytic NOX (115, 251, 350). Figure 4 summarizes different types of NOX and their working models, where some differences among these NOXs can be seen (31).
FIG. 4.
Major Nox isoforms and their proposed model of activation. In comparison, different NOXs may work in the same way as phagocytic NOX, which need the assembly of all subunits and cofactors, or in different way as phagocytic NOX, which nonphagocytic NOXs may be functioning without assembling other subunits or cofactors.
In some cells such as VSM cells, O2•− has been shown to accumulate within cells when NADPH oxidase is activated by different agonists such as angiotensin II (137). This understanding about intracellular accumulation of O2•−, in turn, has led to an assumption which is different from the orientation of phagocytic NADPH oxidase, that a plasma membrane-bound NADPH oxidase may produce and release O2•− into cells (138). This despite the proposed topology of NADPH oxidase subunits, which indicates that membrane-associated NADPH oxidase should not release O2•− into the cytosol (44, 223). Studies on subcellular localization of vascular NADPH oxidase subunits also suggest that O2•− within VSM cells may not be derived from plasma membrane NADPH oxidase but rather from intracellular compartmental NADPH oxidase (147, 416, 433). More recently, using patch-clamp techniques, an inhouse research team recorded an inward current associated with NADPH oxidase in coronary arterial myocytes that was similar to that recorded in phagocytes, indicating that an outward electron flow and O2•− production occurred in these cells. It seems therefore reasonable to suggest that membrane-bound NADPH oxidase generate O2•− toward the outside of VSM cells, and in this way O2•− may exert regulatory roles as an autocrine or paracrine. Indeed, such paracrine and autocrine release of O2•− were identified in coronary arterial myocytes by using some sophisticated techniques such as simultaneous recording of extracellular and intracellular O2•− and confocal microscopy (448). It seems therefore reasonable to suggest that the compartmentalization of O2•− production is of the utmost importance in activating or regulating different redox signaling pathways (147, 388, 389, 415–417).
3. Regulation of NOX activity
It has been reported that NADPH oxidase exists in four different states: resting, primed, activation, and inactivation states (85). Being stimulated by different factors and linking to different signaling pathways, the phosphorylation and subsequent translocation of cytoplasmic subunits result in the production of a small amount of O2•−. Needless to say, this mechanism by which NADPH oxidase results in O2•− and its different states are finely regulated by multiple factors (85).
Reference has already been made above to the NADPH oxidase activity that is regulated by its subunit phosphorylation. There is evidence that factors that stimulate the neutrophil NADPH oxidase subunit phosphorylation can be divided into two categories: (i) those stimulations or agonists that produce rapid effects (these factors may stimulate cells to activate NADPH oxidase in 3∼5 minutes) to activate NADPH oxidase, including the complement fragments (C5a), leukotriene B4 (LTB4), platelet-activating factors (PAF), lysophosphatidic choline (LPC), and (ii) those known as delayed-onset types, including tumor necrosis factor-α (TNF-α), lipopolysaccharide (LPS), and granulocyte-macrophage colony-stimulating factor (GM-CSF), which will normally take 15∼60 min to trigger any detectable effects (349). Most of these triggering factors act through the cell surface receptors to interact with the oxidase due to protein kinase C (PKC)-dependent phosphorylation of p47phox (140). The conformational rearrangement of p47phox drives the cytosolic subunit to translocate to the plasma membrane (140). In most cases, interaction between p47phox and p22phox promotes p67phox and p40phox integration with Cytob558. As a delayed-onset triggering factor, TNF-α causes only the partial phosphorylation of p47phox, translocation does not occur in neutrophils (349), but stimulates both phosphorylation and translocation of p47phox in pulmonary artery endothelial cells (100). As a rapid onset triggering factor, however, PAF causes phosphorylation of p67phox, p40phox, and Rac2, but not phosphorylation of p47phox. Phosphorylation of p67phox is, however, necessary not only for its own translocation, but also for the translocation of p40phox and Rac2 to the plasma membrane (349). After LPS incubation with neutrophils, Cyto b558 is translocated to the plasma membrane, and p47phox phosphorylation and translocation are increased, respectively. In addition, homocysteine (Hcys), angiotensin II (Ang-II), the opsonized yeast polysaccharides (OpZ), and β22 Integrins lead to the phosphorylation of p47phox and p67phox (362).
In addition to their effects on NADPH oxidase activity, many factors can regulate the expression of NOXs and their subunits. The protein expression of NADPH oxidase subunits, for example, will increase in activity. In this regard, angiotensin II has been reported to induce the expression of p47phox, p67phox, gp91phox, and p22phox in skeletal muscle cells or other cells that increases the NADPH oxidase activity (411). In FcγR of immune globulin, GIIA-induced receptor-mediated phagocytic processes, the overexpression of the phosphoinositide binding protein, p40phox,results in the activation of NADPH oxidase, which works through phosphatidyl inositol 3 (PI3P) to stimulate O2•− generation in phagosomes (383). By increasing p47phox, p67phox, and gp91phox mRNA levels and protein expression through NF-κB pathway, TNF-α is also able to enhance the NADPH oxidase activity (114). Numerous studies have demonstrated that various subunits or cofactors can be upregulated or downregulated by different stimuli such as cytokines, inflammatory factors, hormones, autocrines, paracrines, physical stress, and some drugs, which may be involved in transcriptional or posttranscriptional regulation of gene expression and translational or posttranslational regulation of proteins (12, 408).
B. Superoxide dismutase
Recently, proteomic analysis demonstrated that membrane SOD (SOD1) is present in LR fractions (441), a fact consistent with previous reports that SOD1 is detectable in LRs (351). Reported SOD1 levels, for example, in LRs fractions were much higher than that in other areas of the plasma membrane. These results support the view that in aggregation the LRs may play an important role for the SOD1 actions (6, 201). It is assumed that localization and subsequent aggregation of SOD1 in LRs could affect cellular functions as well as the interplay between different cell types, as LRs are rich in receptors and the signaling molecules necessary for cell–cell communications (441). Indeed, a more recent study has reported that H2O2, generated extracellularly by extracellular SOD, anchored to ECs surface via the heparin-binding domain (HBD), enhances VEGF-induced VEGF receptor 2 (VEGFR2) autophosphorylation in caveolin-enriched LRs, but not in noncaveolar LRs. The HBD of endothelial SOD is required for its localization in plasma membrane LRs, suggesting that localization of endothelial SOD in caveolae/LRs via HBD can serve as an important mechanism by which SOD-derived extracellular H2O2 efficiently promotes VEGFR2 signaling in ECs and postnatal angiogenesis (289).
C. Catalase
In neutrophils, proteomic analysis (90) has found catalase in LR fractions that play critical roles in redox signaling by cleavage of H2O2. Although some studies have demonstrated that LR-associated catalase may be related to peroxisome biogenesis, the function of this catalase association with LRs remains largely unknown. It is possible that LRs in hepatic peroxisomal membrane cells are able to help catalase sorting and distribution to different compartments of these cells, assigning them an important role in hepatocyte proliferation and lipid metabolism. Given that hepatic caveolin-1 plays an important role in liver regeneration and lipid metabolism, caveolae with catalase may be critically involved in this liver regeneration and lipid metabolism. However, recent studies found that the absence of caveolin-1 did not affect the peroxisomal location of catalase in mouse liver. It seems caveolin-1 is not required for peroxisome biogenesis, whereas other types of peroxisomal LRs are required (418). Obviously more research and thinking needs to be invested into the formation and function of LR-associated catalase complexes.
D. Thioredoxin
Although it is not yet extensively studied, thioredoxin has also been reported as a LR-associated protein. In some reports, LRs have been shown to mediate the effects of thioredoxin (TRX). There is convincing evidence that LRs may mediate the actions of TRX on leukocyte–endothelial cell interaction related to redox regulation during inflammation. TRX is a ubiquitous protein with a redox-active disulfide that functions in concert with NADPH and TRX reductase to control the redox state of cysteine residues of different oxidant-targeted proteins. Given the antioxidant role of TRX, the LR–mediated role of TRX in the interaction between leukocytes and endothelial cells may importantly regulate inflammatory responses through counteracting oxidative stress and ROS (146). In addition, TRX can be internalized into the cells through LR–mediated endocytosis. In particular, a TRX mutant, TRX-C35S (with replacement of cysteine 35 by serine), was found to bind rapidly to the cell surface and be internalized into the cells through LRs in the plasma membrane. This indicates that the cysteine at the active site of TRX is important for the internalization and signal transduction of extracellular TRX through LRs (156, 210).
E. Transient receptor protein C3 and C4: redox sensors
In addition to the association of LRs with ROS-producing or scavenging enzymes, another noteworthy point in LR-associated signaling molecules is the help LRs give to molecules aggregation, gating, or activation and their downstream impact on redox-sensing or enhancement of effector responses to redox signaling. Among these molecules, a currently identified redox-sensitive protein-transient receptor protein (TRP) is particularly noteworthy. TRPs are a family of voltage-independent nonspecific cation-permeable channels. Evidence exists that transient receptor protein C3 (TRPC3) and TRPC4 localize or relocalize in LRs, and can form a TRPC3–TRPC4 complex with different properties from their respective homomeric channels, which are redox sensitive (313). Perhaps these TRP channels are directly gated or influenced by the formation of LR platforms and therefore their redox-sensing function are altered. Indeed, the TRPC3 channel activity is increased by cholesterol loading of the cell membrane when TRPC3 is overexpressed. This increased channel activity may lead to enhanced redox sensitivity of the channels, exerting an important redox regulation or resulting in pathologic consequences in different cells (313).
F. Effects of redox molecules on LRs
The preceding pages have provided some insights into the role of LRs in mediating or modulating redox signaling. On the other hand, there is increasing evidence indicating that the formation of LR–derived signaling platforms can also be altered or regulated by redox molecules. For example, the formation of ceramide-enriched membrane platforms in the membrane of coronary arterial ECs can be reduced by SOD, but increased by O2•− donor or generating systems (235). H2O2 was also found to activate pro-survival signaling pathways, including activation of PI3 kinase/Akt and Extracellular signal-regulated kinases (ERK)1/2 by changes in LRs behaviors (422). In addition, various ROS species were found to influence LR signaling or function through their actions on many LR constituents such as ceramide production, cholesterol, and related raft proteins (81, 260). ASMase, which play a key role in the formation of ceramide-enriched membrane platforms have been extensively studied. ROS generation, for example, is known to be intimately involved in the activation of the enzyme in response to various stimuli. Pretreatment of neutrophils with the antioxidants N-acetylcysteine (NAC) and desferrioxamine significantly inhibited the downstream ASMase activities, such as ceramide generation and CD95 clustering. The results suggest that ROS release is an essential prerequisite for ASMase activation (340).
A new model proposed by Gulbins et al. has summarized the mechanisms by which ASMase is activated by ROS. Based on this model, the free C-terminal cysteine of ASMase can be modified and lost by the actions of ROS, wherein a zinc coordination in this enzyme is altered, leading to the activation or inhibition of the enzyme. This model is basically similar to the “cysteine switch” activation mechanism described elsewhere for the matrix metalloproteinase family (395). Confirmation of the links between redox regulation of ceramide-enriched membrane platforms and glioma chemotherapy illustrated this. By transfection of human or murine glioma cells with ASMase, marked sensitization of the glioma cells to gemcitabine and doxorubicin occurred, accompanied by increased activation of ASMase, elevated ceramide levels and enhanced formation of ceramide-enriched membrane platforms. Scavenging of ROS prevented these events, suggesting that the activation of ASMase by these therapeutic agents is associated with the actions of ROS (127). Taken together, ROS also regulates the formation of LR signaling platforms and therefore LRs and ROS may constitute an amplification of signals in different biological membranes, insuring the efficiency of signal transduction. Such feedforwarding regulation will be further discussed below in the regulation of LR redox signaling platforms.
V. Frequently Used Methods for Identifying LR Redox Signaling Platforms
Frequently used methods for identifying LR redox signaling platforms include: fluorescent staining and confocal imaging of the LR redox signaling platforms; fluorescent resonance energy transfer (FRET) analysis between tightly associated molecules; immunoblot analysis of detergent resistant membrane fractions (LR fractions) isolated by gradient ultracentrifugation; measurement of O2•− produced in LR redox signaling platforms by electron spin resonance (ESR) spectroscopy (429) and several others.
A. Fluorescent confocal microscopic imaging
The most important factors in the detection of LR redox signaling platforms are the colocalization of lipid components and aggregated or recruited NADPH oxidase subunits or other molecules. Individual LRs on the cell membrane are too small (suggested to be around 50 nm in diameter) to be resolved by standard light microscopy, but once several separate small LRs were clustered upon stimulation, these LR clusters could be observed as patches or spots under microscope (444). Therefore, fluorescent staining and confocal microscopic imaging of LR patches or spots on the cell membrane is the most frequently used method to identify the formation of LR signaling platforms including LR redox signaling platforms. The fluorescence labeling of the B subunit of cholera toxin (CTXB) is widely used as a common LR marker to perform colocalization with some LR-associated redox molecules such as NOXs and other subunits including p47phox, p21phox, p67phox and others. The use of CTXB is because the sphingolipids normally contain the prevalent type of glycosphingolipid, GM1 ganglioside, which is known to have a high affinity with CTXB. In addition, given that ceramide-enriched signaling platforms are considered as another type of LRs, anticeramide antibodies can also be used as a marker of LRs or sphingolipids to detect LR-associated redox enzymes or related molecules (429).
B. Fluorescence resonance energy transfer
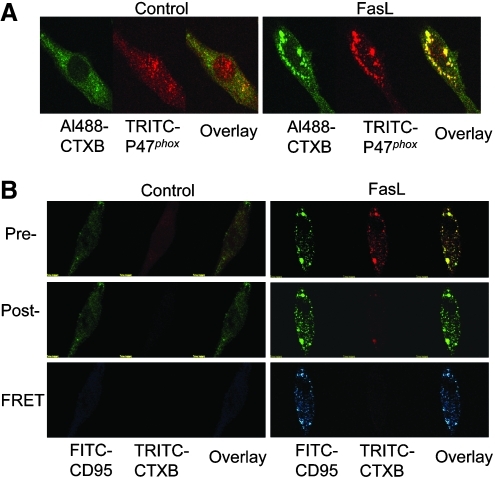
Fluorescence resonance energy transfer (FRET) is a phenomenon that occurs between a fluorophore pair, donor and acceptor (e.g., FITC and TRITC). The fluorophore pair both share the same characteristics in the transfer of energy from the donor to the acceptor, namely the overlap of the emission wavelength of the donor with the excitation of the acceptor's wavelength (198). The two key factors determining the occurrence of FRET are molecular orientation and distance between the molecules. It is proposed that FRET can only take place between two molecules within 7–10 nm range. Detected FRET generally indicates that two molecules are closely located, allowing them to generate an energy transfer from one to the other that leads to molecular reactions. FRET analysis, with resolutions believed to be at lower than 10 nm of separations between the two molecules, may significantly enhance [colocalization using regular confocal microcopy requires a separation of greater than 400 nm (198)] the resolution of common confocal microscopic observations. For example, in FRET between FITC and TRITC, cells can be stained with TRITC-labeled CTXB and FITC-labeled ASMase, gp91phox or redox enzymes or proteins constituents (190) and then observed under a confocal microscope. Both donor and acceptor bleaching protocols can be employed to measure the FRET efficiency. As described elsewhere (190, 198, 203, 353), acceptor bleaching protocols first prepared prebleaching acceptor images followed by increases of the excitation wavelength of the acceptor (TRITC) laser intensity (from 50 to 98) for 2 min bleaching the acceptor fluorescence. After the intensity of the excitation laser of the acceptor was adjusted back to 50, the postbleaching image was then taken. The FRET image was obtained by subtracting the prebleaching image from the postbleaching image (in blue). After measuring the FITC fluorescence intensity in the pre-, post-, and FRET image, the FRET efficiency was calculated using the following formula: E=(FITCpost-FITCpre)/FITCpost*100% (190, 278). Some examples of such confocal microscopic colocalization and FRET detections in endothelial cells are presented in Figure 5. Panel A shows colocalization of CTXB and gp91phox as indicated by yellow spots or dots in overlaid images. Panel B depicts the FRET as indicated by FITC-CD95 image and overlay image in blue. CD95 is Fas, which is a typical LR clustered receptor that activates LR clustering and redox signaling in ECs.
FIG. 5.
Confocal microscopic colocalization and FRET detection. (A) Colocalization of CTXB and p47phox as indicated by yellow spots or dots in overlay image, suggesting LR platforms or clusters. (B) The FRET as indicated by FITC-CD95 image and overlay image in blue. CD95 (Fas) is a typical LR-clustered receptor that activates LR clustering and redox signaling in ECs. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
C. Membrane fraction flotation
Biochemically, the method most often used for detection of LRs is the flotation of DRMs in combination with Western blots to identify associated proteins or receptors in LR fractions (429). During sucrose gradient centrifugation, DRMs complexes or detergent insoluble glycolipid-enriched domains (DIG) can float to low-density fractions and reinforce the integrity of LRs structure. These LR fractions contain abundant raft proteins and therefore analyzing the raft proteins in DRMs by immunoblotting provides a reliable and simple means for identifying possible LR components, especially LR-associated proteins such NOXs or related subunits or cofactors (429). Further, if large scale proteomic analyses could reach sufficient resolutions and sensitivities, in combination with proteomic techniques developed recently, this membrane flotation technique could help identify many as yet unobserved molecules including receptors, enzymes, regulators and adaptors (271).
Recently, there have been some challenges to the use of DRMs (4, 339) and their possible artifacts, such as LR fractions. The procedure for the isolation of nondetergent MR fractions has been developed and used (365), significantly increasing the sensitivity and specificity of isolated LR proteins or components. In addition, using 3-layer gradient centrifugation for isolation of LR fractions, researchers have succeeded in separating noncaveolar and caveolar fractions in classical DRMs flotations (425, 438). A modified nondetergent 4-layer gradient centrifugation is now used to isolate LR fractions. This method separates, respectively, light low density fractions, heavy low density fractions and other high density fractions, which represent noncaveolar, caveolar and other fractions of membrane proteins, making it now possible to identify and separate signaling molecules or enzymes in LR clusters in both caveolar and noncaveolar compartments. Such membrane flotation will provide more and increasingly accurate information about the location of LR redox signaling platforms by detecting their distribution in different fractions. A typical gel document using nondetergent and modified 4-layer gradient flotation and then Western blot analysis of gp91phox is presented in Figure 6. Among 24 fractions, 3–6 and 10–14 represent light and heavy, low-density fractions, respectively, which correspond to noncaveolar and caveolar LRs. Under controlled conditions, interestingly, gp91phox is present in caveolar fractions, but not in noncaveolar fractions. When the cells were treated with Fas ligand, the fractions were shifted to noncaveolar fractions. In addition, consistent with other reports, caveolin-1 and flotilin-1 were present in both light and heavy low-density fractions, suggesting that they may not be good markers to separate noncaveolar and caveolar LRs.
FIG. 6.
Flotation of membrane MR fractions by nondetergent 4- layer gradient flotation. A typical gel document shows that among 24 fractions, 3–6 and 10–14 are light and heavy low-density fractions, respectively. They represent noncaveolar and caveolar LRs. Under control condition, gp91phox was seen in caveolar fractions, but not in noncaveolar fractions. When the cells were treated with Fas ligand, the fractions were shifted to noncaveolar fractions.
D. Superoxide production in LR platforms
Methods for analyzing the activity and modulation of O2•− producing or redox-related enzymes, such as NADPH oxidase, include lucigenin-enhanced chemiluminescence, dihydroethidium (DHE) fluorescent spectrometric assay, HPLC analysis, fluorescent dye intracellular trapping detection and electron spin resonance (ESR). Among these methods, the most direct and definitive method is ESR spectrometric analysis (174, 429). ESR, also called electron paramagnetic resonance (EPR) spectroscopy, is a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion (429). Several ROS are free radicals with unpaired electrons, which are very short-lived. Such ESR assays have made measurements of ROS, in particular, O2•−,as highly specific, quantitative and reproducible. Today ESR is commonly used for measurements of NO, O2•− and other ROS from live cells, organelles and tissues (174, 429).
E. Others
Besides these frequently used methods, other general observation techniques for LRs are also used for further studies of LR redox signaling platforms. For example, total internal reflection (TIRF) microscopy provides information on the diffusivity of particles in the membrane as well as revealing membrane corrals, barriers and sites of confinement (14, 93, 170). Fluorescence correlation and cross-correlation spectroscopy (FCS/FCCS) are used to gain information of fluorophore mobility in the membrane (19, 175, 258). In addition, atomic force microscopy (86, 238), scanning ion conductance microscopy (SICM) (37), nuclear magnetic resonance (NMR) (120, 367) and super-resolution microscopy such as stimulated emission depletion (STED) (22) may also be used if related equipment or instruments are available. Table 4 summarizes all possible methods for studies of LRs or LR redox signaling, which can serve as a reference for selection in different experiments.
Table 4.
Frequently Used Method for Identifying Lipid Raft Redox Signaling Platforms
| Morphological observation of LR redox signaling platforms |
| Fluorescence confocal microscopy |
| Fluorescent resonance energy transfer |
| Flow cytometry or fluorescence activated cell sorter |
| Total internal reflection fluorescence microscopy |
| Fluorescence correlation and cross-correlation spectroscopy |
| Atomic force microscopy |
| Scanning ion conductance microscopy |
| Stimulated emission depletion |
| Biochemical characterization of LR redox signaling platforms |
| Floatation of detergent resistant membranes |
| Immunoblot analysis |
| Immunoprecipitation |
| Reactive oxygen species measurements |
| Electron spin resonance |
| Lucigenin-enhanced chemiluminescence |
| Dihydroethidium fluorescent spectrometric assay |
| Fluorescent dye intracellular trapping detection |
| HPLC analysis |
| Fluorometric or colorimetric assay of H2O2 |
VI. Downstream Targets of LR Redox Signaling
A. Signaling in phagocytic process
Redox signaling was first demonstrated to have an association with LRs in neutrophils in 2003 (347). When neutrophils deal with the pathogen to produce respiratory bursts, Fcγ receptors on the cell membrane are the major receptors responsible for the phagocytic uptake of IgG-opsonised pathogenic particles and have been shown to be coupled to the activation of NADPH oxidase (78). IgG-opsonised pathogen-induced activation of NADPH oxidase in neutrophils is very rapid, indicating a possibly very high efficiency in coupling of the receptors to the oxidase activation. Currently, two types of Fcγ receptors, FcγRIIA and FcγRIIIB, are cloned on neutrophils (71, 321). FcγRIIIB is a glycosylated molecule of 50–70 kDa, linked with the outer leaflet of the plasma membrane by a GPI anchor and its expression on the plasma membrane is 10–15-folds greater than that of FcγRIIA, suggesting it might be the major receptor for IgG-opsonised particles on these cells (71). As we have discussed above, an important feature of GPI-anchored proteins is their association with LRs (45, 46). Meanwhile, numerous reports have shown the presence of LRs in neutrophils and identified different signaling components in these rafts, including cytoskeletal proteins and several membrane proteins including Fc receptor proteins. This constitutes the basis for the notion that the lipid compartments function as physical platforms for signal integration at the plasma membrane of phagocytes (169, 211, 271). Under this condition, NADPH oxidase assembled and activated in LR of neutrophils produces O2•−, causing respiratory bursts and killing bacteria (347).
By isolation of LRs through sucrose gradient ultracentrifugation to obtain the low density Triton X-100-insoluble fractions, the following immunoblottings were performed and the basic expression of NADPH oxidase components was analyzed. In resting neutrophils, only a small amount of total gp91phox and p22phox were present in raft fractions. Preincubation of the cells with M-β-CD resulted in a loss of association of gp91phox with the LR fractions, confirming that gp91phox is localized to membrane LRs. It has been suggested that in resting neutrophils, the core flavocytochrome of the NADPH oxidase is present in the raft compartment of the plasma membrane and that the distribution of cytosolic components of the NADPH oxidase, p40phox and p67phox are very low in cell membrane rafts. p47phox has never been detected in such membrane raft fractions when these cells were at a resting status. Upon activation by IgG-opsonised S. Aureus particles, the levels of NADPH oxidase components in LR fractions greatly increased. In particular, p47phox appeared to increase most significantly, as it has reached an increase of 40-folds. It is now well established that the translocation and late association of p47phox with the membrane-bound phox proteins is a rate-limiting step, tightly correlated with NADPH oxidase activation (140). Association of p47phox with the flavocytochrome may stabilize the entire complex, thus explaining the increased levels of gp91phox in the raft fractions.
Analysis of the kinetics of NADPH oxidase activation upon Fcγ receptor stimulation at different stimulation intensities, under normal conditions and under raft depletion, has found that M-β-CD itself does not affect the rate of NADPH oxidase activity. However, M-β-CD can significantly delay activation of the NADPH oxidase. This delaying effect of M-β-CD was observed when lower intensive stimuli were employed. It never occurred at high stimulation intensity. Thus, activation of the NADPH oxidase effector system at the raft is specifically associated with the efficient initiation of the response at low stimulus intensity. Additionally, a second structurally different raft-disrupting agent, filipin, was also employed to confirm the importance of LRs on activation of NADPH oxidase in response to application of IgG-opsonised particles. Unlike M-β-CD, filipin is able to inhibit the function of LRs by tightly binding to the cholesterol in LRs instead of depleting. Very similarly, it was found that pretreatment of neutrophils with filipin did not affect the maximal rate of NADPH oxidase activation, but reduced the onset of the NADPH oxidase response. The time interval required after the application of the stimulus to reach maximal rates was also decreased. These observations indicate that LRs are involved in the activation of NADPH oxidase by Fcγ receptors. Under conditions of low receptor occupancy, the coupling process is improved by the physical association of the receptors and the effectors in LRs, whereas under conditions of high receptor occupancy, no such mechanisms are required to allow efficient receptor-effector interactions (347).
Almost at the same time, Vilhardt et al. also reported that in the murine microglia cell line, Ra2, and the human promyelocytic leukemia cell line, HL60, LRs act to recruit and/or organize the cytosolic NADPH oxidase factors in the assembly of active NADPH oxidases in cell membranes (398). The basic concept is very similar to the discussion above pertaining to neutrophils. Cholesterol depletion by M-β-CD, however, was reported as having significantly reduced O2•− production, but only caused a delay of the NADPH oxidase activation in both intact cells and a cell-free reconstituted systems. This M-β-CD effect was accompanied by a parallel reduction of the translocation of cytosolic phox subunits to the membrane. The difference was explained by the rapid replenishment of plasma membrane cholesterol from intracellular stores that had not been treated with lovastatin to reduce intracellular cholesterol (398). Later, Fuhler et al. further demonstrated that treatment of neutrophils with the LR-disrupting agent, M-β-CD, abrogated fMLP-induced ROS production and activation of ERK1/2 and protein kinase B/Akt in both unprimed and GM-CSF-primed neutrophils, further supporting the view that LR-associated NADPH oxidase produces ROS and contributes importantly to the onset of phagocytic respiratory bursts (103). This function of LR-associated NADPH oxidase is summarized in Figure 7.
FIG. 7.
LR-associated NADPH oxidase in respiratory burst. During phagocytic uptake of pathogens, LRs cluster, assemble, and activate NADPH oxidase in neutrophils to produce O2•−, causing respiratory burst. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
B. Transmembrane signaling via receptors in nonphagocytic cells
In nonphagocytic cells, endothelial cells (ECs) were first reported to have functional LR redox signaling platforms on their membranes (444). Similar to neutrophils, NADPH oxidase, expressed in vascular ECs, also have several subunits, including gp91phox, p22phox, p47phox, p40phox and p67phox. Further, the cytosolic GTPase activates a small G-protein known as Rac that, participates in the activation of NADPH oxidase by assembling NADPH oxidase complexes on the cell membrane. When ECs are stimulated by death receptor ligands, an increase in the ROS production is always observed, suggesting the latter has a physiological mediating role or a proapoptotic one on the cells. Among all isoforms of NOXs, gp91phox has been considered as the major isoform of NOX proteins in vascular ECs. This was supported by the findings that phorbol ester-induced O2•− production decreased and endothelium-dependent relaxation was improved in gp91phox−/− mice (195). This NOX-derived O2•− may constitute more than 95% of the production of O2•− in ECs, especially when cells are stimulated by cellular death factors. All these data highlight the specific role of NADPH oxidase in augmentation of local tissue oxidative stress (124, 195).
For a long period of time, how the activation of NADPH oxidase coupled to activation of the death receptor in ECs remained illusive. Over the last 5 years, a growing body of evidence has indicated that the clustering of LRs may serve as a main mechanism mediating the coupling of death receptors to NADPH oxidase. Upon agonist stimulation in coronary arterial EC, the formation of large membrane LR patches or macrodomains is often observed. The agonists or stimulations tested and found to stimulate LR redox signaing platforms include Fas ligand, anti-Fas agonistic antibody, TNF-α, endostatin, H2O2, homocysteine, 7-dehydrocholesterol, platelet aggregation factor, acetylcholine and prostaglandins (235, 446). Among these agonists or death factors, Fas ligands were first reported to stimulate LR clustering with aggregation of gp91phox and some other cytosolic subunits, which lead to the activation of NADPH oxidase in ECs (191, 444). Such LR NADPH oxidase clusters or complexes produce ROS and thereby mediate transmembrane signaling from death receptors to intracellular effectors or targets such as NO, ryanodine receptors, vav and other molecules (188, 428, 431, 444).
In addition to Fas ligand, endostatin was also found to have similar effects of inducing LR clustering and thereby forming redox signaling platforms (191). Endostatin (EST) is the ∼20 kDa C-terminal fragment of collagen XVIII located in the basement membrane zones around blood vessels, which is a naturally occurring peptide in the body (282, 323). In vivo, endostatin is one of the most potent EC-specific inhibitors of angiogenesis and tumor growth. In vitro, extensive studies have also demonstrated that endostatin specifically inhibits many cell processes, such as EC proliferation, migration, apoptosis, etc. (95). Besides these generally acknowledged actions, endostatin was learned to reduce NO bioavailability through enhanced O2−·production in the intact coronary endothelium, suggesting a potential role for it in the impairment of endothelium-dependent vasodilation responses, which ultimately contribute to endothelial dysfunction (442). Such actions of endostatin are associated with the formation of LR redox signaling platforms (444).
As demonstrated in Figure 8, under resting conditions, individual LRs, with or without NOX, are present in the membrane of ECs. When receptors bind to a ligand, LRs cluster to form LR macrodomains or platforms, with aggregation and assembling of NADPH oxidase subunits and other proteins such as Rac GTPase. This leads to the activation of NADPH oxidase and production of O2•−, resulting in prominent amplification of the transmembrane signals. Besides lipid components such as SM, ceramide and cholesterol, many other molecules, such as NADPH oxidase subunits, Rac, and other regulatory components are clustered to form complex signalosomes (27, 190, 335, 446). O2•− in ECs acts with NO to increase ONOO− levels and thereby reduces the bioavailability of NO, leading to endothelial dysfunction. Further, O2•− may be converted into H2O2 by SOD, sending out a wide range of signals and by doing so influences vascular functions (76, 106, 121, 333).
FIG. 8.
LR redox signaling platforms associated with NADPH oxidase in transmembrane signaling. Under resting condition without ligand binding, individual LR with or without NOX are present in the membrane of ECs. When receptor ligand binding occurs, LRs are clustered to form LR platforms, with aggregation and assembling of NADPH oxidase subunits and other proteins such as Rac GTPase. Then, NADPH oxidase is activated to produce O2•−, which reacts with NO to produce ONOO−, resulting in endothelial dysfunction. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Evidence has been proffered at LRs keep NADPH oxidase in the inactive state in human renal proximal tubule (RPT) cells. Disruption of such inactive LRs may result in their activation (150). Different cells use LRs to conduct redox signaling in different ways. As Li et al., have reported, NADPH oxidase-dependent ROS production is differentially regulated in LRs and non-LR compartments of RPT epithelial cells (233). This differential regulation or LR-associated inactive NADPH oxidase is mainly attributed to the action of the neurotransmitter dopamine. Dopamine is an essential neurotransmitter involved, mainly via its peripheral receptors, in the control of blood pressure, sodium balance, various renal and adrenal functions (194). As G protein–coupled receptors, dopamine receptors, are associated with both caveolar and noncaveolar LRs (8, 240, 356, 437). It has been shown that D1-like receptors can exert an inhibitory action on ROS production in VSM and RPT cells (412, 424, 427). However, the molecular mechanisms involved still remain unknown. By sucrose density gradient ultracentrifugation and analysis of NADPH oxidase isoforms and subunits in LRs, it was found that the majority of membrane proteins was in non-LR fractions; only a small portion of proteins were in LR fractions. The D1-like receptor agonist, fenoldopam decreases NOX2 and Rac1 proteins in LRs, although to a greater extent in hypertensive than normotensive rats. Fenoldopam decreased the amount of Nox2 that coimmunoprecipitated with p67phox in cells from normotensive rats. These observations suggest that fenoldopam causes a redistribution of NOX2, NOX4, and Rac1 from LRs and to non-LR fractions. Further studies have shown that disruption of LRs results in the reactivation of NADPH oxidase that was destroyed by antioxidants and the silencing of NOX2 or NOX4. Perhaps this explains why in human RPT cells, LRs maintain NADPH oxidase in an inactive state (150).
C. LR redox signaling not via receptors
In addition to membrane receptor-mediated cell responses, LR redox signaling has also been observed in nonreceptor-mediated cell responses, such as H2O2, hypoxia/reoxygenation, ultraviolet (UV) irradiation and chemicals as polychlorinated biphenyls and ASMase activators (27, 56, 88, 295, 422). Further, M-β-CD or filipin pretreatment of aortic ECs was found to significantly reduce the H2O2-induced phosphorylation of Akt and ERK 1/2, but reconstitution of LR domains by exogenous cholesterol restored H2O2-induced Akt and ERK1/2 phosphorylation. This indicates that LRs participate in signaling pathways activated by H2O2 (422). This H2O2-induced prosurvival signaling pathway was also dependent on the oxidation and subsequent inhibition of the tumor suppressor PTEN (phosphatase and tensin homolog deleted from chromosome 10) (66, 246). Similarly, in rat primary astrocytes, exogenous treatment with H2O2 or application of hypoxia/reoxygenation triggered SHP-2 phosphorylation in a time- and dose-dependent manner and led to its translocation into LRs, forming a complex with STAT-3 that activated downstream signaling molecules. It is quite clear that the SHP-2 here is activated by ROS in astrocytes and then translocated into LR clusters to produce dephosphorylation and inactivation of other phosphotyrosine-containing proteins such as STAT-3 (295). In addition, ultraviolet irradiation was found to induce ROS production via clustering of membrane rafts and ceramide (56) in different cell lines such as 293 cells (kidney), Jurkat (lymphocytes) and MCF-7 cells. Polychlorinated biphenyls, such as 153 (PCB153) are also reported as stimulating the formation of LR redox signaling platforms on brain ECs to mediate the expression of cell adhesion molecules (88). These nonreceptor-associated LR redox signaling pathways may be play an important role in the pathogenesis of different diseases related to physical or chemical stimuli and in the therapeutically intended different treatments such as those that target tumor cells with irradiation and chemicals (145). More recently, uric acid (MSU) crystals were found to be internalized via a lipid sorting mechanism (273). LRs have a natural affinity to MSU crystals, possibly due to hydrogen bonding interactions, and the exposure of MSU crystals to LR microdomains may result in aggregation of cholesterol-rich regions to MSU crystals, thereby activating Syk signaling in an extracellular protein receptor-independent manner. Syk signaling, in turn, results in PI3K activation, cytoskeletal rearrangement, and phagocytosis of the crystal, leading to the activation of NALP3 inflammasomes, which are associated with K+ efflux and ROS production (141, 272). It is possible that LR redox signaling is also critical for activation of inflammasomes, an intracellular inflammatory machinery.
D. Interactions of intracellular vesicles or organelles through LR redox signaling
IL-1β is an essential cytokine responsible for immune and inflammatory responses, but also a well known activator of the ubiquitous transcription factor NF-kappa B, participating in the pathogenesis of a variety of diseases, such as sepsis, autoimmune, diabetes, atherosclerosis, asthma, cancer and other pathological processes. It has long been noted that the downstream effects of IL-1β are mediated by their ability to produce intracellular ROS. NOX1 and NOX2 have been detected in LRs (228, 309, 347, 398) giving rise to the possibility that LRs may act as a critical mediator of redox signaling through IL-1β. However, it was not until systematic studies done by Oakley et al., that the exact methods employed by the induction of ROS following ligand binding to receptors was understood (284).
Oakley et al. found that IL-1R1 was constitutively present in LR factions, regardless of IL-1β stimulation, in MCF-7 mammary cancer cell lines. NOX2 was also found in caveolae, which are also constitutively present in LR fractions. Upon IL-1β stimulation, the phosphorylated active form of caveolin-1 was found to be richer in LR fractions, which could be blocked by pretreatment of cells with nystatin and filipin. It has been suggested that the IL-1 signaling pathway is dependent on LRs-associated redox signaling (284).
In further studies, significant increases in the percentage of LR-associated IL-1R1, colocalized with the early endosomal marker, early endosome antigen 1, were found after cell stimulation with IL-1β. However, in unstimulated cells, the majority of IL-1R1 resided in the plasma membrane. NOX2 in the plasma membrane was demonstrated to colocalize with IL-1R1. After IL-1β stimulation, the colocalization of NOX2 and IL-1R1 were shown to have moved to intracellular endosomal compartments (284), as evidenced by increased CTX/IL-1R1- and NOX2/CTX on the endosomes after IL-1β stimulation. These findings support the view that intracellular LR platforms may be formed via the ligand-stimulated internalization of NOX2 and IL-1R1 into endosomes, which form a redoxosome that conducts intracellular redox signaling through some LR complex located in the endosomes (270, 355, 397, 444). This redoxosome redox signaling was recently discussed in detail in another review of this journal (283).
Bedard et al. have suggested another LR-associated redox signaling involving exocytosis, with links to intracellular vesicles or organelles. They reported that NOX2 which was under resting conditions, initially, located in the intracellular vesicle membranes moved and fused, after activation, with the plasma membrane,,thereby generating and releasing O2•− outside the cell (31, 41, 113). Other studies have corroborated this NOX movement (286). Alas, these studies do not clarify how LRs are involved in this process, an issue needing clarification. Given the presence of LRs in lysosomes and other vesicles (270, 355, 397), it is possible that the movement to cell membranes and the fusion of lysosomes with LR-associated NOXs may either assist in the formation of plasma membrane LR platforms, in combination of course with other membrane components, or recruit and aggregate various subunits or cofactors during their movement, directly releasing O2•− and conducting redox signaling.
E. Hypothetic models of LR redox signaling platforms
Based on the foregoing discussions,, it is possible to postulate three LR redox signaling platforms in different mammalian cells that efficiently and robustly conduct redox signaling. As shown in Figure 9, the first type of LR redox signaling platforms is represented by clustering of LRs in plasma membranes in response to a variety of stimulations such as death receptor ligands, cytokines and ASMase activators, wherein NOX with related subunits and cofactors are aggregated and recruited to form LR clusters or platforms. These platforms are responsible for the extracellular or intracellular O2•− production depending upon cell types. This type of LR redox signaling platforms is shown in the middle among three platforms in Figure 9.
FIG. 9.
Three models of LR redox signaling platforms. (i) LR redox signaling platforms is formed by clustering of LRs in plasma membrane upon stimulations of receptors to produce O2•− extracellularly or intracellularly. (ii) Upon stimulations, endocytosis occurs via caveolae to form intracellular LR-containing redoxosomes, producing O2•− and other reactive oxygen species to conduct redox signaling. (iii) During clustering of LRs, NOX and related subunits or cofactors are aggregated and then traffic to the plasma membrane to produce O2•− and conduct redox signaling.
It should be noted that the membrane raft redox signaling could also occur in the caveolae, where NADPH oxidase subunits are preassembled under resting condition and the enzyme functional in membrane rafts, specifically in caveolae. Stimulation with some agonists such as TNF-alpha induces additional recruitment of p47 to raft-localized NADPH oxidase complex and thereby enhances ROS production within raft domains. ROS may interact with NO to regulate the nitration of tyrosine-containing proteins. These tyrosine-containing proteins are targets of caveolar raft redox signaling to regulate cell function (423).
Another type of LR redox signaling platform is involved with caveolae-associated endocytosis. Under resting conditions, NOX and its subunits or cofactors are located in caveolar LRs. Upon stimulations these redox molecules aggregate and assemble into an enzyme complex through LRs clustering. At the same time, endocytosis occurs to form intracellular LR-containing redoxosomes, which produce O2•− and other ROS to conduct redox signaling. Such redoxosome-related redox signaling is presented on the right of Figure 9.
The third type of LR redox signaling platforms depends upon exocytosis of endosomes, lysosomes or related vesicles, where LRs may first be clustered in response to some stimuli. During clustering of LRs, NOX and related subunits or cofactors aggregate and simultaneously move to the plasma membrane and fuse there. The now assembled NADPH oxidase produces O2•− and conducts redox signaling. Again, depending upon the cell type, O2•− is produced inside or outside cells. This type of LR redox signaling platform is depicted on the left of Figure 9.
Different cells may form different types of LR redox signaling platforms. In some cells, there may be one type, whilst in others two or three LR redox signaling platforms might be formed in response to stimulations. Given that LRs are very dynamic microdomains, the possibility that the coexisting three different LR redox signaling platforms in one cell can not be ruled out and these coexisting different LR redox signaling platforms might have the internal tendency to transform to each other, thus forming a dynamic cycle between these platforms. This, however, is in need of evidence that confirms such interactions of different LR signaling platforms.
VII. Mechanisms Mediating the Formation of LR Redox Signaling Platforms
A. Ceramide metabolizing pathways
In the literature of LRs, the formation and function of ceramide-enriched platforms have been well documented. Therefore, understanding the mechanisms mediating the formation of ceramide-enriched platforms may provide vistas to explore the mechanism by which other different LR platforms are formed and regulated. Ceramide belongs to a highly hydrophobic lipid family, which consists of fatty acids with carbon chains in variable lengths (2–28 carbons) and sphingosine. It plays an important physiological role in cell homeostasis (255, 341). Ceramide as a central molecule in the SM signaling pathway is involved in the activation of a variety of protein kinases and protein phosphatases, such as the stress-activated protein kinase (SAPK), kinase inhibitor of Ras (KSR), c-Jun N-terminal kinase (JNKs), protein kinase C (PKC), protein phosphatase l (PP1), and protein phosphatase 2A (PP2A). Ceramide is known to mediate or modulate many cell activities or function such as cell apoptosis, proliferation, differentiation and growth arrest (341). A better understanding of ceramide-mediated signaling pathway at the molecular level will further help understand the pathogenesis of different disorders and diseases such as infections, cancers, cardiovascular diseases, neurological degenerations, and autoimmune diseases (3, 34, 129, 213, 237, 281, 341).
As shown in Figure 10, the metabolism of LRs-related ceramide relates to several synthase and hydrolases. To date, several of these enzymes have been cloned, and recently more and more evidence suggests that multiple pathways are responsible for ceramide generation and clearance, perhaps, in response to different agonists or stimuli. Ceramide is mainly produced via two enzymatic pathways, including the (i) de novo synthesis pathway and the (ii) SMase pathway. The de novo synthesis of ceramide is conducted within the endoplasmic reticulum, beginning with the conversion of dihydro-sphingosine into dihydro-ceramide by the ceramide synthase and on the catalytic action of dihydro-sphingosine reductase. Then, dihydro-ceramide is further converted into ceramide (231). With respect to SMases, acid or neutral SMase is the primary source for ceramide with second messenger characteristics in cells. SMase catalyzes the hydrolysis of phosphodiester bond in SM and produces ceramide and choline phosphate (207).
FIG. 10.
Ceramide-metabolizing pathways. The de novo synthesis of ceramide begins with the conversion of dihydro-sphingosine into dihydro-ceramide by ceramide synthase and then further converted into ceramide. ASMase and NSMase catalyze the hydrolysis of phosphodiester bond in SM and produces ceramide and choline phosphate. Ceramide is converted back into SM by SM synthase and degraded by ceramidase.
SMases belong to the phospholipase C (PLC) superfamily and are able to act on SM as a hydrolase (68). SMase is released to the cell surface or extracellular space in an autocrine or paracrine manner that hydrolyzes cell surface SM, inducing cell-cell communications. More importantly, SMase may act on SM incorporated in the LR area of cell membranes and thereby produce ceramide in the cell membranes, resulting in the formation of ceramide-enriched platforms that constitute a solid basis for the formation of redox signaling platforms (68).
Based on its working pH, cellular localization and ion-dependence, SMases can be divided into six subtypes, including ASMase, secretory SMase, neutral SMase (NSMase) including Mg2+-dependent and Mg2+-independent NSMase, and alkaline SMase (122). The subcellular distributions and the particular characteristics of these types of SMases vary in different types of cells. It is now well accepted that only ASMase and NSMase may be involved in mammalian cell signaling. tASMase activity accounts for 90% of total SMase activity in many cell types (122). SMase is a soluble glycoprotein with a molecular weight of 64,000 Daltons, and its coding gene is located in chromosome location 11p15.1∼15.4, which has approximately 5 kb in length and possesses six exons, encoding 627 amino acids. ASMase is located mainly in lysosomes and participates in the flip-flop of cell membranes (268). In an environment with pH<5 such as that in lysosomes, ASMase exhibits the highest biological activity. Upon certain exogenous stimulations, ASMase can be rapidly activated and released to the cell surface to hydrolyze cell membrane SM, leading to rapid increases in ceramide levels within seconds to minutes. Macrophages, fibroblasts and ECs can secrete ASMase or are able to produce lysosome fusions and release ASMase into the cell membrane, in particular, in LRs-enriched areas. Some reports have indicated that ECs may possess almost 20-folds higher ASMase activity than other cell types (268).
Ceramide is metabolized via two enzymatic pathways: (i) ceramide as substrate that synthesizes SM, where SM synthase catalyzes the transfer of choline phosphate groups from phosphatidylcholine to ceramide, generating both SM and diacylglcyerol (DAG) (69); and (ii) degradation by ceramidase. After phosphorylated by certain kinases, ceramide is converted into ceramide-1 phosphate and then into sphingosine by the action of ceramidase. Following upon the action of sphingosine kinase, sphingosine is converted into sphingosine-1 phosphate (S1P) (382). According to its pH dependency, ceramidase can also be divided into three types: acidic, neutral and alkaline ceramidase. Three ceramidases will work in different cells individually or together under different pH to metabolize ceramide after its action as a signaling molecule (382).
B. Association of ceramide metabolism and its signaling pathway
Ceramide is limited in the membrane because of its hydrophobicity. It is likely that ceramide only functions in its generating site. The subcellular localization of ceramide-metabolizing enzymes may in part determine which signaling pathways are initiated by its activation (392). For example, ceramide in the mitochondrial membrane by de novo synthesis mediates mitochondria-based chemotherapeutical drug-induced apoptosis (392). Upon stimulation of a same agonist, the activation of different SMase showed different results. Ceramide generated by activation of TNF-α 55 kDa receptor (p55) may mediate either inflammation or apoptosis signaling pathways. For example, NSMase activation through the p55-protein-coupled FAN causes inflammation, whereas ASMase promotes apoptosis through the p55 death domain (392).
C. Role of ceramide-enriched microdomains in LRs clustering
Because ceramide is a much less polaric molecule than other sphingolipids, it is very easily and spontaneously aggregated, leading some reports to call it a membrane fusigen that promotes fusion of cell membranes or intracellular vesicles (192, 392). As noted earlier in the section of ceramide-enriched platforms or macrodomains, the formation of ceramide-enriched platforms can be based on SM-LRs in the cell membrane, the classical mechanism, or without them. When ASMase is activated, it can hydrolyze SM anywhere in the membrane as long as SM can be reached. In such ceramide enriched platforms, NOX and related subunits or cofactors aggregate and activate NADPH oxidase, producing O2•− to conduct signaling (30, 39, 236, 371, 449).
Recent studies have demonstrated that this ceramide-enriched redox signaling platform can be formed in response to different death receptor ligands such as Fas ligand, TNF-α, or endostatin (190, 191). Further, ultraviolet irradiation- induced ROS production was also reported to be associated with the formation of ceramide-enriched platforms that mediate ROS production (56). Given that ASMase is mainly present in lysosomes, the importance of this enzyme as a resource for ceramide production in cell membranes was not clear until we demonstrated that a rapid movement and consequent fusion of lysosomes to supply ASMase into the LR area of cell membranes occur in response to various stimuli. This lysosome fusion is critical for the formation of ceramide-enriched platforms and therefore determines LR redox signaling in different cells, in particular, in ECs (190, 191).
D. Lysosome fusion and targeting of ASMase in LRs clustering
Lysosomes are membrane-bound organelles, which originate from the Golgi apparatus and exist in the cytoplasm of all eukaryotic cells. These cytoplasmic organelles contain several dozen acid hydrolases that are primarily responsible for intracellular digestion (23, 42). Based on their differing functions, lysosomes are divided into two types, conventional lysosomes and secretory lysosomes. Conventional lysosomes are the common lysosomes found in cell biology textbooks and the literature. These lysosomes are the digestive organelles of the cell. Another type of lysosome, the secretory lysosome, however, is able to fuse with the plasma membrane and secrete its content outside the cell. Many cells, including ECs, are found to have secretory lysosomes, which secrete different substances by exocytosis. Recently, a third type of lysosome has been reported, which has conventional lysosome features, but works like secretory lysosomes by fusing to the plasma membranes and repairing damaged membrane areas (173, 257).
Besides their intracellular digestion function, recent studies have extended lysosomal functions to cellular signaling in different cells (202, 447). Lysosomal vesicles have been reported to contribute to exocytosis in nonsecretory cells, where these vesicles fuse with the plasma membrane to excrete the contents of vesicles and incorporate the vesicle membrane components into the cell membrane (182). In addition, some studies have demonstrated that lysosomes, as Ca2+ store houses, are an important regulator on of cell functions in a variety of tissues or cells, where lysosome Ca2+ stores can be mobilized to mediate NAADP-induced Ca2+ release (62, 226).
In house and other researchers have recently explained how lysosomes can rapidly fuse with cell membranes, leading to ASMase translocation to the surface of ECs (182, 189, 190). In house research used coronary arterial ECs, LysoTrackers and Alexa488-labeled CTXB to detect lysosome movements into the cell membrane by detection of lysosome distance to LRs on the cell membrane. Under a confocal microscope, Fas ligands as an agonist were found to induce the formation of LR clusters. Accompanied by the aggregation of NOX2 and related subunits, in the plasma membrane of ECs the LR clusters produced O2•−. When these cells were pretreated with two structurally different lysosomal vesicle function inhibitors, bafilomycin A1 and glycyl-L-phenylalanine-beta- naphthylamide (GPN), Fas ligand-induced LRs clustering was substantially blocked and corresponding ROS production significantly decreased. Using LysoTracker staining, it was found that colocalization of LRs and lysosomal vesicles located around the cell membrane, was abolished by bafilomycin A1 or GPN. This has, in turn, led to the conclusion that lysosomal vesicles are vital contributors to the formation of LR-redox signaling platforms associated with NADPH oxidase. In addition, lysosome FM1–43 quenching or dequenching and FM1–20 destaining experiments have confirmed that activation of Fas ligands and some ASMase activators such as phatidylinositol (PI) and bis(monoacylglycero)phosphate lead to lysosomal fusion to the cell membrane, triggering LRs clustering and the formation of signaling platforms or macrodomains (27). However, silencing the ASMase gene or pretreatment of the cells with vacuolin-1, a lysosome fusion inhibitor, was found to block LR clustering, activation of ASMase and membrane proximal lysosome fusion (27). It is now confident that a rapid lysosome fusion into cell membrane is a critical step to form LR redox signaling platforms, leading to the signaling in O2•− production.
Further studies have revealed that sortilin, a glycoprotein responsible for transferring ASMase from the Golgi apparatus to lysosomes is also important in initiating the movement of lysosomes and promoting their fusion to the cell membrane in ECs (26, 27). Sortilin is a 95-kDa glycoprotein, which has been reported to play an important role in targeting or transferring proteins to lysosomes (274). Its Vps10p domain in the luminal region may be the binding site for the saposin-like motif of ASMase, whereas its cytoplasmic tail containing an acidic cluster-dileucine motif binds the monomeric adaptor protein GGA and is structurally similar to the cytoplasmic domain of M6P. All of these structural features suggest sortilin is an intracellular protein transporter responsible for the sorting of soluble hydrolases such as ASMase to lysosomes. The results about colocalization of sortilin with lysosome proteins during death receptor activation further suggests that sortilin not only simply mediates the targeting of ASMase to lysosomes, but also functionally interacts with ASMase (26). The coupled sortilin-1 and ASMase work together to promote the movement of lysosomes toward the cell membrane, which, in turn, leads to LRs clustering and NOX activation in ECs. This ASMase-dependent clustering of receptors was also observed for other receptors such as CD20, CD40, TNFR and epidermal growth factor receptors (EGFR) (330).
More recently, we addressed how lysosomes fuse to cell membrane and transport ASMase into LR platforms. It was found that SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor)-centered exocytic machinery is involved in LR clustering to form redox signaling platforms. SNAREs comprise a superfamily of small, mostly membrane-anchored proteins, which mediate membrane fusion between organelles or from organelles to cell plasma membranes (118). In particular, SNARE-mediated membrane fusions plays an essential role in the secretory pathway of various eukaryotic cells, which is named as the SNARE or SNARE-centered exocytic machinery (35). In our experiments, pretreatment of coronary ECs with a specific inhibitor of vesicle-associated membrane protein 2 (VAMP2, a v-SNARE protein), almost completely blocked the formation of LR clusters. Using FITC-labeled anti-v-SNARE antibodies and TRITC-labeled CTXB (labeling raft marker GM1), the aggregated v-SNARE was found to be colocalized with CTXB on the cell membrane and that both the colocalized molecules produced FRET. Fas ligand stimulation significantly increased the FRET efficiency between v-SNARE and GM1 when they were aggregated on the cell membrane (446). It seems that SNARE as a membrane fusion facilitator is also present in LR redox signaling platforms although its major function is to help lysosome fusion (446).
These findings provide a comprehensive working model for the mediation of LR clustering and the formation of LR signaling platforms in arterial ECs. As shown in Figure 11, this model emphasizes the derivation of membrane ASMase as being from lysosomes, which target ASMase when it is synthesized from ER and transported through Golgi apparatus. Many mature lysosomes with ASMase are proximal to the cell membrane. When a receptor such as death receptor is activated by a ligand binding to it or by other stimulations, these lysosomes proximal to the cell membrane become mobilized to move and fuse with the cell membrane, activating ASMase and synthesizing ceramide, thereby resulting in LRs clustering and the formation of ceramide-enriched platforms. These LR platforms, in turn, recruit, translocate and aggregate NOX and its subunits or cofactors and assemble them into an active enzyme complex, which produces O2•−, promoting transmembrane signaling. An insert in the figure presents a LR redox signaling platform or redox signalosome on cell membrane after lysosome fusion and activation of NADPH oxidase.
FIG. 11.
Lysosome biogenesis and fusion to cell membrane to form LR platforms. ASMase is synthesized from the ER and transported through Golgi apparatus to lysosomes. These lysosomes can be mobilized to traffic and fuse into cell membrane, where ASMase is activated and ceramide produced, resulting in LRs clustering and formation of ceramide-enriched platforms. The insert presents an LR redox signaling platform or redox signalosome on cell membrane after lysosome fusion and activation of NADPH oxidase. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
E. Cytoskeletal components and LR clustering
Some in the literature have proposed that LRs be defined as a membrane structure enriched with cholesterol and associated with the cytoskeleton. The relationship between cytoskeletal elements and LRs is becoming clearer. It has been shown, for example, that tubulin is present in LRs and can be coimmunoprecipitated with caveolin-1 in rat forebrain extracts (80). One possible mechanism for the contribution of LRs to alterations in microtubules is indicated by experiments in smooth muscle cells (197). In these cells, caveolins stabilize the microtubules by interfering with the interaction between the microtubule-destabilizing protein stathmin and tubulin. Reports in the literature also indicate that treatment of glias or cardiac myocytes with microtubule-disrupting agents, such as colchicines, results in the loss of many signaling molecules from LRs, in particular, those involved in adrenergic receptor signaling (77, 159).
The actin cytoskeleton is found to have a bidirectional relationship with LRs. As an actin binding lipid, phosphoinositide lipids such as PtdIns(4,5)P2 and PtdIns(3,4)P2, known to direct actin assembly into filaments, can accumulate in the LRs and these lipids are also (54, 312). In addition to binding to these lipids, actin also helps cluster signaling molecules in LRs. For example, small G protein clustering in LRs is dependent on the actin cytoskeleton (310) and these GTPases may change their raft localization in response to the external signals that modify the actin cytoskeleton (183). Therefore, agents that modify the raft association of actin can utilize small G proteins and other signaling molecules to form signaling platforms in cell membrane. However, precious little is known about whether the cytoskeleton is involved in the formation of LR redox signaling platforms, an interesting topic for further studies.
F. Feedforward amplifying mechanism
It is well accepted that the major function of LR redox signaling platforms is to transmit and amplify signals received by corresponding receptors due to aggregation of signaling molecules to work in a platform manner. However, there is also evidence that a feedforward amplifying mechanism exists in the formation of such LR redox signaling platforms. It has been proposed that the production of ROS within LR redox signaling platforms can further enhance the formation of larger platforms, thus amplifying the whole process. In this enhancement of platform formation, increased activation of ASMase by ROS is found to be a contributor. As discussed earlier, the formation of ASMase dimers by modification of the free C-terminal cysteine plays a vital role in the enhancement of ASMase activity (318). Exogenous administration of xanthine/xanthine oxidase, a O2•− generating system, has also demonstrated a dramatic increase in LRs clustering in the membrane of ECs (318, 443), providing an analogous model of redox enhancement of LRs platform formation.
Further, ROS in T lymphocytes were also shown to enhance LR signaling. Blockage of ROS production by SOD-mimic MnTBAP reduced the localization of several signaling molecules such as LAT, phospho-LAT, and PLC-gamma in LRs fractions. Treatment of T cells with the ROS-synthesizer, tert-butyl hydrogen peroxide (TBHP), greatly enhanced membrane raft formation and the distribution of phospho-LAT into LRs. Moreover, lipid peroxides were found to promote the formation of larger rafts or platforms on the membrane, and photooxidation, at the lipid double bonds, caused raft enlargement (15). These observations corroborate and reinforce the conclusion that ROS are able to enhance LR clustering or formation of macrodomains and must contribute to the formation of membrane raft platforms (244). Within LR redox signaling platforms, the formation of a feedforward amplifying loop for LR redox signaling will amplify and enhance cellular signaling and, if excessively enhanced, this feedforward signaling amplification may result in the progress and development of multiple oxidative stress related diseases.
VIII. Physiology and Pathophysiology of LR Redox Signaling Platforms
As discussed at beginning of this review, the biological responses to cellular or tissue ROS levels are very different and vary from physiological to pathological reactions. When small amounts of ROS are produced, they may mediate physiological redox signaling, but when large amounts of ROS are produced, cell/tissue damage may occur, resulting in cellular apoptosis, necrosis and ultimately causing various systemic or organ based diseases (96, 423, 444, 453). Below are a few typical pathological processes associated with LR redox signaling from the many reported in the literature.
A. Host defense and infection
Neutrophils are essential in the innate immune or host defense response to microbial invasion. By phagocytosis of the pathogen and the release of toxic “free radicals,” neutrophils are able to kill a large number of the invading microorganisms and/or pathogens [1]. Among these toxic “free radicals,” O2•− exerts a primary role and is mainly generated by the NADPH oxidase enzyme complex (331). As discussed earlier, O2•− production in neutrophils are mainly dependent on the formation of LR platforms with NADPH oxidase. If the formation of such LR redox signaling platforms is deficient, the host defense response to microbial invasion will also be deficient or inadequate. For example, patients with myelodysplasia (MDS) always suffer from multiple types of infection. An impaired ROS production in their neutrophils is an important part of the pathogenesis of their illness. In this regard, Fuhler et al. have provided some direct evidence of a decreased presence of Lyn, gp91phox, and p22phox in LR fractions and that plasma membrane expression of LR components were suppressed in neutrophils of MDS patients. This, in turn, begs the critical question, “will enhancing the LR redox signaling help treat MDS by stimulating the formation and the homeostatic restoration of the function of LR redox signaling platforms (103)?”
The need to focus more attention on LRs is made even more critical and essential if as reported LRs are closely involved in pathogen-receptor interactions, clustering and internalization of pathogens and if it is true, as increasing evidence appears to suggest, that many pathogens including bacteria, viruses, parasites and even fungi target and employ LRs for infection of cells. These pathogens include Escherichia coli, Mycobacterium tuberculosis, Campylobacter jejuni, Vibrio cholerae, Clostridium difficile, Clostridium tetani, Salmonella typhi and typhimurium, Shigella flexneri, influenza virus, HIV, measles virus, respiratory syncytial virus, Ebolavirus, Papillomaviridae, EBV, Echovirus, Sindbisvirus, Plasmodium falciparum, Trypanosoma, Leishmania, Prions, and Toxoplasma gondii (249, 354). In particular, ceramide-enriched membrane platforms were found to mediate the infection of mammalian cells with P. aeruginosa (133), Staphylococcus aureus (87), Neisseriae gonorrhoeae (N. gonorrhoeae) (130, 158), Rhinoviruses (134), and Sindbis virus (185). It remains unknown whether such microbial infection mediated by LR platforms are associated with redox regulations, although some bacteria were found to inhibit activation of NADPH oxidase and assembly of this enzyme in their inclusion compartments, to survive in host cells (326). One area, for example, where research and better understanding of LRs have already paid dividends is in the use of the antioxidant N-acetylcysteine that decreases cell levels of ROS and increases the concentration of reduced glutathione thereby preventing the successful invasion of pathogenic Escherichia coli. It appears as if, at least in this case, the fate of this microbial invasion was dependent upon the local redox environment, provided by LR redox signaling platforms during the invasion (110).
B. Vascular inflammation and atherosclerosis
Many in house studies have demonstrated the contributions of LR redox signaling platforms and NADPH oxidase to endothelial dysfunction induced by various stimuli such as death receptor activation, homocysteine, cytokines or adipokines. As a commonly used functional study, endothelium-dependent vasodilation (EDVD) response in isolated perfused arteries was intensively tested. It was found that various stimulations that led to the formation of LR redox signaling platforms such as FasL, endostatin, homocysteine and visfatin all led to impairment of EDVD. This impairment was homeostatically recovered by NADPH oxidase inhibition using apocynin, M-β-CD, filipin or ASMase siRNA, suggesting, in turn, that LR redox signaling platforms with NADPH oxidase participate in the impairment of endothelial function (189, 191, 443, 444). This LR redox enhancement in endothelial injury and dysfunction may be intimately involved in the pathophysiology of diverse cardiovascular diseases such as atherosclerosis, hypertension, shock and ischemia/reperfusion injuries.
More perhaps directly to the point, there are some reports that the formation or enhancement of LR redox signaling platforms may contribute to macrophage reprogramming, foam cell formation, and cell deformability (234). Induction of lipid oxidation through ROS was found to amplify foam cell formation through oxidized low-density lipoprotein (Ox-LDL) uptake and a subsequent clustering of ceramide-enriched lipid domains. In addition, Ox-LDL was found to affect cell-surface turnover of ceramide-backbone sphingolipids and apoE-mediated uptake, by low-density lipoprotein receptor related protein (LRP) family members, leading, in turn, to cell-surface expansion of ceramide-enriched domains and activation of apoE/LRP1/CD1-mediated antigen presentation. On the other hand, high-density lipoprotein (HDL)-mediated lipid efflux can disrupt LRs and prevent foam cell formation. It has been suggested that LR redox signaling or regulation plays an important role in the formation of foam cells and thus in the progression of atherosclerosis (345).
In addition to the role in alterations of macrophage behavior, LR redox signaling may also play an important role in cell deformability, thereby initiating or promoting atherogenesis. Studies have demonstrated that disruption of LRs by oxidants such as Ox-LDL altered the cytoskeletal structure, including the extent of polymerization, stabilization, crosslinking, and membrane association. These molecular alterations may increase the force generated by the cytoskeleton, resulting in a stiffening of the cytoskeleton and hence stiffening of the cell and plasma membrane. Increased force in the cytoskeleton and its downstream increased stiffness may also elevate membrane tension and thereby influence the activity of various mechanosensitive ion channels. Direct evidence suggests that oxLDL can disrupt LRs, resulting in a series of pathological changes in the biomechanical properties of vascular ECs and ultimately induce endothelial dysfunction and atherogenesis (230).
C. AD and neurological disease
AD is the most common neurodegenerative disease, and a leading cause of progressive dementia. The principal neuropathological features of AD are the two lesions first described by Alzheimer, namely, neurofibrillary tangles and senile plaques (58). Recent studies have demonstrated that the pathogenesis of AD may be mainly due to extracellular deposits of 39–42 amino-acid-long amyloid-β (Aβ) peptides, which are generated by sequential proteolytic processing of large type I transmembrane protein, known as amyloid precursor protein (APP). There is increasing evidence suggesting that amyloidogenic processing of APP is closely associated with LRs. The literature has suggested that targeting APP processing in LRs may selectively reduce Aβ burden without the adverse effects inherent in the alternative standard strategy of inhibiting BACE1 and γ-secretase by blocking their active sites. Using a variety of techniques, amyloidogenic APP processing has been found to preferentially occur in the cholesterol-rich regions of membranes, namely, LRs. These LRs may be involved not only in the aggregation of Aβ, but also in its clearance by amyloid-degrading enzymes such as plasmin or possibly neprilysin (67).
Although there is no study that has directly addressed the role of LR redox signaling platforms in the development of AD, some recent studies have shown that the concomitant enrichment of Aβ and copper within LRs promotes the formation of redox-active Aβ·Cu2+ complexes, fostering the catalytic oxidation of cholesterol, lipids, and the generation of neurotoxic H2O2. This LR redox Aβ·Cu2+ complex further creates a vulnerable environment for Aβ to cross-link, forming SDS-resistant oligomers, which are characteristic of the Aβ samples extracted from diseased brain cells (172, 342). It is possible that the LR redox-active Aβ·Cu2+ complex represents another type of LR redox signaling platform that plays a critical role in the development of AD.
NADPH oxidase was also found to be activated by Aβ and thereby to cause oxidative stress in astrocytes, influencing, in turn, neuronal viability. Interestingly, there is evidence that activation of NADPH oxidase may increase the activity of SMases and the apoptosis induced by Aβ in neurons. Activation of NADPH oxidase may play a major role in the Aβ-induced neurodegeneration in AD. It is imperative to determine by further research whether such action of NADPH oxidase activation by Aβ is also associated with LR clustering and the formation of redox signaling platforms (53, 186).
D. Kidney diseases
There is considerable evidence that ceramide is implicated in the regulation of renal function and may be involved in renal glomerular and tubular pathologies (196, 386, 432, 434). Recent in house research has demonstrated that ceramide plays an important role in the development of chronic glomerular injuries associated with hyperhomocysteinemia and therefore ceramide contributes to the pathogenesis of end-stage renal diseases (432). Hyperhomocysteinemia was found to significantly increase ceramide levels in the renal cortex of rats, and L-homocysteine stimulated ceramide production in different glomerular cells such as glomerular capillary endothelial cells, podocytes and mesangial cells. Ceramides appear to be an important regulator of the function of glomerular filtration membrane. Indeed, there are some reports indicating that ceramide may be involved in the regulation of normal renal function (196, 386, 434, 442).
In studies of the mechanisms by which ceramide acts to regulate renal glomerular function or to cause pathological changes, it was found that blockage of ceramide de novo synthesis in hyperhomocysteinimic rats substantially inhibited the enhancement of NADPH oxidase activity and production of O2•− in the kidney (432). Although translocation of p47phox, seen in ECs, was not shown to occur in L-homocysteine- or ceramide-induced activation of NADPH oxidase in rat mesangial cells (432), recent in house studies have demonstrated that in podocytes and glomerular capillary endothelial cells, homocysteine did induce the formation of LR redox signaling platforms associated with NADPH oxidase (430, 445). Perhaps the transformation of small LRs to ceramide-enriched membrane platforms results in a clustering of NAD(P)H oxidase molecules, producing redox signaling or injury in glomerular cells, in particular, in podocytes and glomerular capillary ECs. In previous studies, oxidative stress mediated by NADPH oxidase was found to play an important role in progressive glomerular injuries or glomerulosclerosis associated with hHcys and other diseases such as diabetes and hypertension (84, 104, 432). In further studies of how the formation of LR redox platforms produces glomerular injuries and consequent sclerosis, in house studies have found that Hcys-induced enhancement of glomerular permeability is associated with the regulation of microtubule stability through LR-redox platforms. It seems that the early injurious effects of Hcys and other pathogenic factors acting on NADPH oxidase are associated with the formation of redox signaling platforms via LR clustering, leading, in turn, to increases in glomerular permeability by disruption of microtubule networks in the glomerular filtration membrane (428, 445).
E. Obesity
LRs have been implicated in the development of insulin resistance and the obesity associated with metabolic syndrome and type 2 diabetes. Accumulating evidence has demonstrated that LRs or caveolae are present in various target tissues of insulin resistance such as striated muscles, adipose tissues, the liver, and pancreatic β cells that secret insulin (177). Although the role of LRs in mediating insulin signaling is controversial, it is well accepted that LR-dependent interactions may help segregate signaling components because raft perturbation changes the sensitivity of two key insulin receptor-mediated signaling pathways, activating the small guanosine triphosphatase TC10 and phosphoinositide 3-kinase (PI3K). LRs are clearly important to insulin signaling and may thereby determine the insulin resistance during obesity or diabetes. Another line of evidence corroborating the involvement of LRs in obesity is related to observations in Obese Zucker fa/fa rats and ob/ob mice with increased levels of GM3 synthase mRNA in their adipose tissues. Addition of GM3 to 3T3-LI adipocytes suppresses insulin-stimulated phosphorylation of the insulin receptor, suggesting that LRs containing GM3 are involved in the signaling process of the insulin receptors. Indeed, other studies have shown that insulin signaling is initiated in glycosphingolipid-enriched rafts and caveolae (178, 426).
With respect to the role of LR redox signaling platforms in the development of obesity, some preliminary experiments were recently performed to test whether excessive accumulation of sphingolipids, ceramide, and their metabolites, or a combination of them contributes to the development of obesity and associated organ damages. In these experiments, high-fat diet (HFD) significantly increased plasma total ceramide levels compared with animals fed a low-fat diet (LFD). Treatment of mice with the ASMase inhibitor amitriptyline significantly attenuated the HFD-induced plasma ceramide levels. Correspondingly, HFD-induced increases in the body weight gain, plasma leptin concentration, urinary total protein and albumin excretion, glomerular damage indexes, and adipose tissue ASMase activities were almost completely suppressed. HFD-induced reduction of insulin receptor sensitivities were also blocked by ASMase inhibition. These results provided evidence that ceramide biosynthesis may play a pivotal role in the development of obesity, metabolic syndrome, and associated kidney damages (38).
Although it is admittedly difficult to detect LR redox signaling platforms in vivo experiments, some in vivo detection of such platforms associated with obesity was currently performed in our laboratory. Using glomerular capillary endothelial cells (GECs), visfatin, an adipokine was found to stimulate ASMase activity and led to aggregation of ceramide with NADPH oxidase subunits, gp91phox and p47phox, a typical LR redox signaling platform, where O2•− production is increased. The ASMase inhibitor, amitriptyline, or ASMase siRNA blocked this visfatin-induced formation of LR redox signaling platforms associated with NADPH oxidase and O2•− production. The results suggest that the injurious effect of the adpokine, visfatin, is associated with the formation of LR redox signaling platforms via LR clustering, where O2•− production increases the glomerular permeability by disruption of microtubule networks in GECs leading to glomerular injuries (38).
F. Tumors
LRs have also been implicated in tumor growth and aggressiveness. In some tumors such as prostate cancer, substantial levels of cholesterol and other fatty substances may promote their progression, and prolonged inhibition of the cholesterol synthesis pathway by pharmacologic interventions may well reduce the risk of advanced prostate cancer. It has been reported in the literature that membrane cholesterol promotes prostate cancer progression by a mechanism that involves dysregulation of LR signaling complexes (75).
In other studies, proteomic analysis of LRs/caveolae demonstrated the enigmatic role of various signaling proteins associated with LRs in cancer development. There are two subsets of raft assemblage in cell membranes, cholesterol-SM-ganglioside-cav-1/Src/EGFR and ceramide-SM-ganglioside-FAS/Ezrin. The raft with cholesterol-SM-ganglioside-cav-1/Src/EGFR is involved in normal cell signaling, and its dysregulation will promote cell transformation and tumor progression. However, the second type of raft complex with ceramide-SM-ganglioside-FAS/Ezrin can promote apoptosis. When such raft complexes dysfunction,, apoptosis is disturbed and tumor progression occurs unrestrained (298). Bionda et al. have reported that gamma-irradiation treatment on a radiosensitive human head and neck squamous carcinoma cell line (SCC61) can trigger raft coalescence to larger membrane platforms associated with the externalization of ASMase, leading to ceramide release in rafts, increasing ROS production and ultimately inducing cell death. However, this structural rearrangement is defective in radioresistant (SQ20B) cells and associated with the lack of ASMase activation and translocation. Blockade of endogenous antioxidant defenses of SQ20B cells triggered ASMase activation and translocation, raft coalescence, and apoptosis. Based on these results, manipulation of LRs through redox equilibrium may provide opportunities for radiosensitization of tumor cells (33, 109).
Corroboration for this hypothesis has come from recent studies in the chemotherapy of glioma. Transfection of human or murine glioma cells with ASMase resulted in a marked sensitization of glioma cells to gemcitabine and doxorubicin, respectively. These results, in turn, were accompanied by an increased activation of ASMase, elevated ceramide levels and enhanced formation of ceramide-enriched membrane platforms. Scavenging of ROS prevented these events, suggesting that the activation of ASMase by these therapeutic agents is associated with the actions of ROS (127).
IX. Possible Therapeutic Strategies Targeting LR Redox Signaling Platforms
Given that LR redox signaling platforms are importantly involved in a variety of diseases, targeting LRs and ceramide-mediated pathways may be useful strategies in the development of new therapies to prevent or treat these diseases. Most tool compounds used to alter LRs, raft platforms and ASMase activity or the action of ceramide could be possible drug candidates.
A. Targeting cholesterol
Although sphingolipids are the major constituents in LRs or their platforms, so far there are no effective ways to decrease such important lipids in cell membrane without severe disruption of the cell structure or function. Therefore, targeting membrane cholesterol becomes one of the most popular methods in manipulation of LRs structures and functions. Due to the inclusion capability of cholesterol, M-β-CD is widely used as a tool drug to disrupt LRs by depletion of cholesterol from cells in LR-related research. This is based on an assumption that M-β-CD can preferentially deplete cholesterol in LRs and that the sensitivity to M-β-CD is proof of LR involvement in cellular processes. However, a recent study demonstrated that at 37 degrees Centigrade or body temperature, M-β-CD extracts similar proportions of cholesterol from Triton X-100 resistant membrane fractions (LRs enriched) as it does from other cellular fractions. Moreover, cells restore the cholesterol level in the plasma membrane by mobilizing cholesterol from intracellular cholesterol stores. However, incubation at 0OC caused a loss of plasma membrane cholesterol with a concomitant increase in cholesterol esters and adiposomes. This study showed that M-β-CD does not specifically extract cholesterol from any cellular fraction and that intracellular cholesterol can replenish plasma membrane cholesterol (248). Therefore, M-β-CD may disrupt not only LRs, but also deplete intracellular cholesterol. However, extending the LR concept to intracellular raft platforms, as discussed in section III.D above, M-β-CD may still be considered a good disruptor of LRs in both plasma membranes and intracellular organelles. However, the specificity of M-β-CD in specific desirable actions that interfere with LRs structure or functions has often been challenged. It should be aware that this type of compounds has also been reported to have effects on membrane depolarization, calcium influx and alteration of the cytoskeleton (380, 420, 430).
Filipin is also a frequently used tool drug in disrupting LRs. Filipin was isolated from the mycelium and culture filtrates of Actinomycete, Streptomyces and Filipinensis. It was discovered in a soil sample collected in the Philippine Islands. The isolate possessed potent antifungal activity. Filipin was identified as a polyene macrolide based on its characteristic UV-Vis and IR spectra. Unlike M-β-CD, filipin interferes with LRs by binding to cholesterol in LRs or their platforms, thus decreasing the fluidibility of LRs and serving as functional inhibitor of LRs. Although filipin exhibits potent antifungal activity, it is too toxic for therapeutic applications. Further modification of this compound may be needed to use it for targeting LR redox signaling platforms as a therapeutic drug.
The statins are a class of drugs used to lower plasma cholesterol levels by inhibition of the enzyme HMG-CoA reductase, which is a rate-limiting enzyme of the mevalonate pathway of cholesterol synthesis. Inhibition of this enzyme, in the liver, results in decreased cholesterol synthesis as well as increased synthesis of LDL receptors, which, in turn, leads to an increased clearance of LDL from the blood stream. The pleiotropic effects of statins may also reflect changes in membrane cholesterol and, specifically, the density of membrane rafts. Moreover, there is likely to be a relationship between membrane cholesterol, membrane rafts and cell functions that are involved in the pathogenesis of cardiovascular and metabolic diseases (165, 166, 419). Therefore, in addition to lipid lowering effects which bring beneficial effects to the cardiovascular system, statins may also have an impact on the LR redox signaling platforms. Indeed, we have recently found that in human coronary arterial ECs (HCAECs), Fas ligand-induced LR clustering with aggregation of NADPH oxidase subunits was almost completely blocked by statins (lovastatin, provastatin and simvastatin). The Fas ligand-induced fivefold increase in O2•− production in the LR fractions was also substantially blocked by pretreatment of HCAECs with statins. This leads to the conclusion that blockage of LR redox signaling platform formation in EC membranes is another important therapeutic mechanism of statins in preventing endothelial injury and atherosclerosis (171). In addition, statins have also been linked to a reduced risk of developing Alzheimer's dementia. Long-term statin therapy was reported to protect individuals from AD through its action on LR clustering or related functions (164).
B. Targeting ASMase activity
By using structure-property-activity (SPAR) models, chemists have characterized some organic weak bases as ASMase inhibitors that function by inducing a detachment of ASMase from inner lysosomal membranes and subsequent inactivation of the enzyme (215). Moreover, cationic amphiphilic substances can induce the detachment of ASMase proteins from inner lysosomal membranes, thereby inactivating them. These can be utilized as functional inhibitors of ASMase and are minimally toxic, which may be applied for disease states associated with increased activity of ASMase and ceramide-enriched platforms (214).
Recently, a potent and selective novel inhibitor of ASMase, L-alpha-phosphatidyl-D-myo-inositol-3,5-bisphosphate (PtdIns3,5P2), was reported. As a naturally occurring substance detected in mammalian, plant and yeast cells it may also be used as starting point for the development of new potent ASMase inhibitors optimized for diverse applications (209). Based on many experimental results, inhibition of ASMase or gene silencing of ASMase genes can be an appropriate strategy for prevention or treatment of diseases related to LR redox signaling platforms. There is no compound known to us that is effective in inhibiting AMSase and available for clinical use. However, a group of German scientists led by Dr. Eric Gulbins introduced a large group of compounds with a broad range of new clinical indications, they named “FIASMA” (Functional Inhibitor of ASMase). FIASMAs differ markedly with respect to molecular structure and current clinical indications, and most of the available compounds of this group of ASMase inhibitors are licensed for medical use in humans, which are minimally toxic and may therefore be applied for disease states associated with increased activity of ASMase or LR redox signaling (214).
C. Targeting protein palmitoylation
Covalent attachment of palmitates to proteins is a post-translational modification that exerts diverse effects on protein localization and functions. Techniques to inhibit protein palmitoylation include site-specific mutagenesis and treatment of cells with inhibitors of protein palmitoylation, including 2-bromopalmitate, cerulenin, and tunicamycin. Moreover, general approaches to determining the effect of altered palmitoylation status on YFPP association with membranes and LRs, as well as signal transduction, are described in detail elsewhere in the literature (325). The natural product, cerulenin ([2R, 3S]-2,3-epoxy-4-oxo-7,10-trans, trans-dodecadienamide) inhibits the palmitoy- lation of H-ras- and N-ras-encoded p21s, in parallel to inhibition of cell proliferation. More than 30 analogs of cerulenin, both aromatic and aliphatic, with various chain lengths and amide substitutions, have been synthesized for use. Regression analysis has indicated that inhibition of palmitoylation is more closely related to inhibition of proliferation than inhibition of fatty acid synthases. Further characterization of the molecular pharmacology of these compounds and analogs may define a new class of drugs with antitumor activities at least partially through its action on LRs structures or functions, and in particular, on LR redox signaling platforms (225).
X. Conclusions and Perspectives
To date, there is no doubt that redox signaling through NADPH oxidase is tightly correlated with the unique membrane structures known as LRs. LRs serve as platforms to aggregate the membrane spanning or cytosolic subunits, which allow the assembly of NADPH oxidase subunits and cofactors into an active enzyme complex. They also provide the driving force for clustering of small individual rafts, where ceramide derived from the hydrolysis of SM is involved. Such LR redox platforms produce O2•−, and thereby conduct redox signaling with compartmentalization and amplifications in response to different receptor bindings or other stimuli. Three types of LR redox signaling platforms are proposed currently. First are LR clusterings with NADPH oxidase subunits in the cell membrane where O2•− is produced, outside or inside the cell, leading to cellular signaling. Second are the redoxosomes due to endocytosis of LR clusters with NADPH oxidase subunits that produce O2•− intracellularly along with transportation of redoxosomes. Third are the exocytosic fusion of LR redox platforms that are formed in endosomes or lysosomes and then move and fuse with cell membranes to produce O2•− for signaling. These different types of LR redox signaling platforms each have different functions. For example, the major function of LR redox signaling platforms on the cell membrane is to produce ROS to kill pathogens or mediate transmembrane signaling. However, the endocytosed or preassembled intracellular redoxsomes are mainly to produce ROS as signaling molecules to evoke downstream responses inside cells. Such functional differences may be due to the differences in cell types, cell response to agonists or other stimuli or cell reactivity under different states. The formation of LR redox signaling platforms is associated with activation of ASMase, production of ceramide, ROS feedforward amplification, and cytoskeleton-mediated regulation. Increasing evidence has been reported in the literature that LR redox signaling may contribute to infection and host defenses and to the development of different diseases such as atherosclerosis, glomerular sclerosis, obesity or metabolic syndrome and tumor progression. It is imperative to develop therapeutic strategies to target such LR redox signaling platforms for possible treatment or prevention of these diseases that LR redox signaling serves as pathogenic mechanisms. The further study and research into the molecular mechanisms mediating the formation of this membrane signaling complex and how signaling through these platforms can be specific to agonists and downstream effectors are imperative and essential. Although considerable evidence suggests that this LR redox signaling is linked to a number of cell responses to pathological stimuli and can be a target of therapeutic interventions, the physiological relevance of this signaling still remains to be adequately addressed. In addition, most of results or conclusions about LR redox signaling or signaling platforms were obtained from isolated cell preparations in previous studies. More attention is needed to develop new in vivo research strategies that are able to address the contribution of LR redox signaling or signaling platforms to cell activities or organ functions and related regulatory mechanisms. Like other research areas, more studies are essential to translate experimental results related to LR redox signaling to clinical use.
Abbreviations Used
- AD
Alzheimer's disease
- AKT
adenylate kinase
- Ang-II
angiotensin II
- APP
amyloid precursor protein
- ASMase
acid sphingomyelinase
- CD
cyclodextrin
- CTXB
B subunit of cholera toxin
- DAG
diacylglcyerol
- DES
desipramine
- DHE
dihydroethidium
- DR
death receptor
- DRMs
detergent-resistant membranes
- DUOX
dual oxidases
- ECs
endothelial cells
- EDVD
endothelium-dependent vasodilation
- eNOS
endothelial nitric oxide synthase
- EPR
electron paramagnetic resonance
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinases
- ESR
electron spin resonance
- EST
endostatin
- FAD
flavin adenine dinucleotide
- FasL
Fas ligand
- FCCS
fluorescence cross-correlation spectroscopy
- FCS
fluorescence correlation spectroscopy
- FIASMA
functional inhibitor of sphingomyelinase
- FITC
fluorescein isothiocyanate
- fMLP
N-Formyl-Met-Leu-Phe
- FPALM
fluorescence photoactivation localization microscopy
- FRET
fluorescence resonance energy transfer
- GEC
glomerular endothelial cell
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GPCR
G-protein coupled receptor
- GPI
glycosylphosphatidylinositol
- GPN
glycyl-L-phenylalanine-beta- naphthylamide
- HBD
heparin-binding domain
- HCAEC
human coronary arterial endothelial cell
- Hcys
homocysteine
- HDL
high-density lipoprotein
- HFD
high-fat diet
- HMG-CoA
3-hydroxy-3-methyl-glutaryl-CoA
- IL-1β
interleukin-1β
- JNK
c-Jun N-terminal kinase
- KSR
kinase suppressor of Ras
- LFD
low-fat diet
- LPC
lysophosphatidic choline
- LPS
lipopolysaccharide
- LR
lipid raft
- LRP
low-density lipoprotein receptor related protein
- LTB4
leukotriene B4
- M-6-P
mannose-6-phosphate
- MAPK
mitogen activated protein kinases
- MCF
mammary cancer cell
- MDS
myelodysplasia
- MR
membrane raft
- MSU
uric acid
- M-β-CD
methyl-β-cyclodextrin
- NAADP
reduced form of nicotinic acid adenine dinucleotide phosphate
- NAC
N-acetylcysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NMR
nuclear magnetic resonance
- NO
nitric oxide
- NOS
nitric oxide synthase
- NOX
NAD(P)H oxidase gp91 analogs
- NSMase
neutral sphingomyelinase
- O2•−
superoxide
- OpZ
opsonized yeast polysaccharides
- Ox-LDL
oxidized low density lipoprotein
- PAF
platelet-activating factor
- PDGF
platelet-derived growth factor
- PI
phosphatidyl inositol
- PI(4,5)P(2)
phosphatidylinositol 4,5-bisphosphate
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- PM
plasma membrane
- PP
protein phosphatase
- ROS
reactive oxygen species
- RPT
renal proximal tubule
- S1P
sphingosine-1 phosphate
- SAPK
stress-activated protein kinase
- SCC
squamous carcinoma cell
- SICM
scanning ion conductance microscopy
- siRNA
small interfering RNA
- SM
sphingomyelin
- SMase
sphingomyelinase
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- SOD
superoxide dismutase
- SPAR
structure-property-activity relation
- SPM
scanning probe microscopy
- SPT
single-particle tracking
- STAT
signal transducer and activator of transcription
- STED
stimulated emission depletion
- STIM1
stromal interaction molecule 1
- TBHP
tert-butyl hydrogen peroxide
- TCR
T cell receptor
- TGN
trans-Golgi network
- TIRFM
total internal reflection microscopy
- TNFR1
tumor necrosis factor receptor 1
- TNF-α
tumor necrosis factor-α
- TRITC
tetramethyl rhodamine iso-thiocyanate
- TRPC3
transient receptor protein C3
- TRX
thioredoxin
- VEGF
vascular endothelial growth factor
- VSM
vascular smooth muscle
Footnotes
Reviewing Editors: Cristina Cecchi, Hua Cheng, Florian Lang, Irena Levitan, Zheng-Gang Liu, Victor Rizzo, Yi Wu, Fan Yi, Junji Yodoi, and Vanina Zaremberg
Acknowledgments
The authors are funded by grants from the National Natural Science Foundation of China (30870997, 81072634) and a Program for New Century Excellent Talents from the Ministry of Education of China, and most of the works cited in this review from authors' laboratories were supported by the National Institute of Health grants (HL075316 and HL057244).
References
- 1.Abdel Shakor AB. Kwiatkowska K. Sobota A. Cell surface ceramide generation precedes and controls FcgammaRII clustering and phosphorylation in rafts. J Biol Chem. 2004;279:36778–36787. doi: 10.1074/jbc.M402170200. [DOI] [PubMed] [Google Scholar]
- 2.Abrahamsen H. Baillie G. Ngai J. Vang T. Nika K. Ruppelt A. Mustelin T. Zaccolo M. Houslay M. Tasken K. TCR- and CD28-mediated recruitment of phosphodiesterase 4 to lipid rafts potentiates TCR signaling. J Immunol. 2004;173:4847–4858. doi: 10.4049/jimmunol.173.8.4847. [DOI] [PubMed] [Google Scholar]
- 3.Adam D. Heinrich M. Kabelitz D. Schutze S. Ceramide: does it matter for T cells? Trends Immunol. 2002;23:1–4. doi: 10.1016/s1471-4906(01)02091-9. [DOI] [PubMed] [Google Scholar]
- 4.Adam RM. Yang W. Di Vizio D. Mukhopadhyay NK. Steen H. Rapid preparation of nuclei-depleted detergent-resistant membrane fractions suitable for proteomics analysis. BMC Cell Biol. 2008;9:30. doi: 10.1186/1471-2121-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ago T. Kitazono T. Kuroda J. Kumai Y. Kamouchi M. Ooboshi H. Wakisaka M. Kawahara T. Rokutan K. Ibayashi S. Iida M. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke. 2005;36:1040–1046. doi: 10.1161/01.STR.0000163111.05825.0b. [DOI] [PubMed] [Google Scholar]
- 6.Aisenbrey C. Borowik T. Bystrom R. Bokvist M. Lindstrom F. Misiak H. Sani MA. Grobner G. How is protein aggregation in amyloidogenic diseases modulated by biological membranes? Eur Biophys J. 2008;37:247–255. doi: 10.1007/s00249-007-0237-0. [DOI] [PubMed] [Google Scholar]
- 7.Alicia S. Angelica Z. Carlos S. Alfonso S. Vaca L. STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: moving TRPC1 in and out of lipid rafts. Cell Calcium. 2008;44:479–491. doi: 10.1016/j.ceca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Allen JA. Halverson-Tamboli RA. Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 9.Alonso MA. Millan J. The role of lipid rafts in signalling and membrane trafficking in T lymphocytes. J Cell Sci. 2001;114:3957–3965. doi: 10.1242/jcs.114.22.3957. [DOI] [PubMed] [Google Scholar]
- 10.Aman MJ. Ravichandran KS. A requirement for lipid rafts in B cell receptor induced Ca(2+) flux. Curr Biol. 2000;10:393–396. doi: 10.1016/s0960-9822(00)00415-2. [DOI] [PubMed] [Google Scholar]
- 11.Ameziane-El-Hassani R. Morand S. Boucher JL. Frapart YM. Apostolou D. Agnandji D. Gnidehou S. Ohayon R. Noel-Hudson MS. Francon J. Lalaoui K. Virion A. Dupuy C. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem. 2005;280:30046–30054. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 12.Ammons MC. Siemsen DW. Nelson-Overton LK. Quinn MT. Gauss KA. Binding of pleomorphic adenoma gene-like 2 to the tumor necrosis factor (TNF)-alpha-responsive region of the NCF2 promoter regulates p67(phox) expression and NADPH oxidase activity. J Biol Chem. 2007;282:17941–17952. doi: 10.1074/jbc.M610618200. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RG. Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 14.Axelrod D. Total internal reflection fluorescence microscopy in cell biology. Traffic. 2001;2:764–774. doi: 10.1034/j.1600-0854.2001.21104.x. [DOI] [PubMed] [Google Scholar]
- 15.Ayuyan AG. Cohen FS. Lipid peroxides promote large rafts: effects of excitation of probes in fluorescence microscopy and electrochemical reactions during vesicle formation. Biophys J. 2006;91:2172–2183. doi: 10.1529/biophysj.106.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azad MB. Chen Y. Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–790. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- 17.Azad N. Rojanasakul Y. Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 2008;11:1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
- 18.Babior BM. Lambeth JD. Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 19.Bacia K. Schwille P. A dynamic view of cellular processes by in vivo fluorescence auto- and cross-correlation spectroscopy. Methods. 2003;29:74–85. doi: 10.1016/s1046-2023(02)00291-8. [DOI] [PubMed] [Google Scholar]
- 20.Bagi Z. Feher A. Beleznai T. Preserved coronary arteriolar dilatation in patients with type 2 diabetes mellitus: implications for reactive oxygen species. Pharmacol Rep. 2009;61:99–104. doi: 10.1016/s1734-1140(09)70011-8. [DOI] [PubMed] [Google Scholar]
- 21.Bagnat M. Keranen S. Shevchenko A. Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci U S A. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bain AJ. Marsh RJ. Armoogum DA. Mongin O. Porres L. Blanchard-Desce M. Time-resolved stimulated emission depletion in two-photon excited states. Biochem Soc Trans. 2003;31:1047–1051. doi: 10.1042/bst0311047. [DOI] [PubMed] [Google Scholar]
- 23.Bainton DF. The discovery of lysosomes. J Cell Biol. 1981;91:66s–76s. doi: 10.1083/jcb.91.3.66s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banfi B. Malgrange B. Knisz J. Steger K. Dubois-Dauphin M. Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 25.Banfi B. Tirone F. Durussel I. Knisz J. Moskwa P. Molnar GZ. Krause KH. Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J Biol Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 26.Bao JX. Jin S. Zhang F. Wang ZC. Li N. Li PL. Activation of membrane NADPH oxidase associated with lysosome-targeted acid sphingomyelinase in coronary endothelial cells. Antioxid Redox Signal. 2010;12:703–712. doi: 10.1089/ars.2009.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao JX. Xia M. Poklis JL. Han WQ. Brimson C. Li PL. Triggering role of acid sphingomyelinase in endothelial lysosome-membrane fusion and dysfunction in coronary arteries. Am J Physiol Heart Circ Physiol. 2010;298:H992–H1002. doi: 10.1152/ajpheart.00958.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baran CP. Zeigler MM. Tridandapani S. Marsh CB. The role of ROS and RNS in regulating life and death of blood monocytes. Curr Pharm Des. 2004;10:855–866. doi: 10.2174/1381612043452866. [DOI] [PubMed] [Google Scholar]
- 29.Bataller R. Schwabe RF. Choi YH. Yang L. Paik YH. Lindquist J. Qian T. Schoonhoven R. Hagedorn CH. Lemasters JJ. Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker KA. Gellhaus A. Winterhager E. Gulbins E. Ceramide-enriched membrane domains in infectious biology and development. Subcell Biochem. 2008;49:523–538. doi: 10.1007/978-1-4020-8831-5_20. [DOI] [PubMed] [Google Scholar]
- 31.Bedard K. Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 32.Bell EL. Chandel NS. Mitochondrial oxygen sensing: regulation of hypoxia-inducible factor by mitochondrial generated reactive oxygen species. Essays Biochem. 2007;43:17–27. doi: 10.1042/BSE0430017. [DOI] [PubMed] [Google Scholar]
- 33.Bionda C. Hadchity E. Alphonse G. Chapet O. Rousson R. Rodriguez-Lafrasse C. Ardail D. Radioresistance of human carcinoma cells is correlated to a defect in raft membrane clustering. Free Radic Biol Med. 2007;43:681–694. doi: 10.1016/j.freeradbiomed.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 34.Bismuth J. Lin P. Yao Q. Chen C. Ceramide: a common pathway for atherosclerosis? Atherosclerosis. 2008;196:497–504. doi: 10.1016/j.atherosclerosis.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blank U. Cyprien B. Martin-Verdeaux S. Paumet F. Pombo I. Rivera J. Roa M. Varin-Blank N. SNAREs and associated regulators in the control of exocytosis in the RBL-2H3 mast cell line. Mol Immunol. 2002;38:1341–1345. doi: 10.1016/s0161-5890(02)00085-8. [DOI] [PubMed] [Google Scholar]
- 36.Block K. Gorin Y. Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bocker M. Muschter S. Schmitt EK. Steinem C. Schaffer TE. Imaging and patterning of pore-suspending membranes with scanning ion conductance microscopy. Langmuir. 2009;25:3022–3028. doi: 10.1021/la8034227. [DOI] [PubMed] [Google Scholar]
- 38.Boini KM. Zhang C. Xia M. Poklis JL. Li PL. Role of sphingolipid mediator ceramide in obesity and renal injury in mice fed a high-fat diet. J Pharmacol Exp Ther. 2010;334:839–846. doi: 10.1124/jpet.110.168815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bollinger CR. Teichgraber V. Gulbins E. Ceramide-enriched membrane domains. Biochim Biophys Acta. 2005;1746:284–294. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Boniface JJ. Rabinowitz JD. Wulfing C. Hampl J. Reich Z. Altman JD. Kantor RM. Beeson C. McConnell HM. Davis MM. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands [corrected] Immunity. 1998;9:459–466. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- 41.Borregaard N. Heiple JM. Simons ER. Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowers WE. Christian de Duve and the discovery of lysosomes and peroxisomes. Trends Cell Biol. 1998;8:330–333. doi: 10.1016/s0962-8924(98)01314-2. [DOI] [PubMed] [Google Scholar]
- 43.Brandes RP. Fleming I. Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 44.Brandes RP. Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005;65:16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Brown DA. London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 46.Brown DA. London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 47.Brown DA. Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 48.Brown DI. Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bryant MR. Marta CB. Kim FS. Bansal R. Phosphorylation and lipid raft association of fibroblast growth factor receptor-2 in oligodendrocytes. Glia. 2009;57:935–946. doi: 10.1002/glia.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burdon RH. Control of cell proliferation by reactive oxygen species. Biochem Soc Trans. 1996;24:1028–1032. doi: 10.1042/bst0241028. [DOI] [PubMed] [Google Scholar]
- 51.Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res. 2005;96:818–822. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- 52.Cai H. A new mechanism for flow-mediated vasoprotection? Focus on “lung endothelial cell proliferation with decreased shear stress is mediated by reactive oxygen species.”. Am J Physiol Cell Physiol. 2006;290:C35–C36. doi: 10.1152/ajpcell.00414.2005. [DOI] [PubMed] [Google Scholar]
- 53.Canevari L. Clark JB. Alzheimer's disease and cholesterol: the fat connection. Neurochem Res. 2007;32:739–750. doi: 10.1007/s11064-006-9200-1. [DOI] [PubMed] [Google Scholar]
- 54.Caroni P. New EMBO members' review: actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts. EMBO J. 2001;20:4332–4336. doi: 10.1093/emboj/20.16.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casadesus G. Smith MA. Zhu X. Aliev G. Cash AD. Honda K. Petersen RB. Perry G. Alzheimer disease: evidence for a central pathogenic role of iron-mediated reactive oxygen species. J Alzheimers Dis. 2004;6:165–169. doi: 10.3233/jad-2004-6208. [DOI] [PubMed] [Google Scholar]
- 56.Chatterjee M. Wu S. Cell line dependent involvement of ceramide in ultraviolet light-induced apoptosis. Mol Cell Biochem. 2001;219:21–27. doi: 10.1023/a:1011083818452. [DOI] [PubMed] [Google Scholar]
- 57.Cheng G. Cao Z. Xu X. van Meir EG. Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 58.Cheng H. Vetrivel KS. Gong P. Meckler X. Parent A. Thinakaran G. Mechanisms of disease: new therapeutic strategies for Alzheimer's disease—targeting APP processing in lipid rafts. Nat Clin Pract Neurol. 2007;3:374–382. doi: 10.1038/ncpneuro0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng PC. Dykstra ML. Mitchell RN. Pierce SK. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J Exp Med. 1999;190:1549–1560. doi: 10.1084/jem.190.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng ZJ. Singh RD. Marks DL. Pagano RE. Membrane microdomains, caveolae, and caveolar endocytosis of sphingolipids. Mol Membr Biol. 2006;23:101–110. doi: 10.1080/09687860500460041. [DOI] [PubMed] [Google Scholar]
- 61.Chini B. Parenti M. G-protein-coupled receptors, cholesterol and palmitoylation: facts about fats. J Mol Endocrinol. 2009;42:371–379. doi: 10.1677/JME-08-0114. [DOI] [PubMed] [Google Scholar]
- 62.Chini EN. Beers KW. Dousa TP. Nicotinate adenine dinucleotide phosphate (NAADP) triggers a specific calcium release system in sea urchin eggs. J Biol Chem. 1995;270:3216–3223. doi: 10.1074/jbc.270.7.3216. [DOI] [PubMed] [Google Scholar]
- 63.Ciarlo L. Manganelli V. Garofalo T. Matarrese P. Tinari A. Misasi R. Malorni W. Sorice M. Association of fission proteins with mitochondrial raft-like domains. Cell Death Differ. 2010;17:1047–1058. doi: 10.1038/cdd.2009.208. [DOI] [PubMed] [Google Scholar]
- 64.Clempus RE. Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res. 2006;71:216–225. doi: 10.1016/j.cardiores.2006.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colavitti R. Finkel T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life. 2005;57:277–281. doi: 10.1080/15216540500091890. [DOI] [PubMed] [Google Scholar]
- 66.Connor KM. Subbaram S. Regan KJ. Nelson KK. Mazurkiewicz JE. Bartholomew PJ. Aplin AE. Tai YT. Aguirre-Ghiso J. Flores SC. Melendez JA. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem. 2005;280:16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- 67.Cordy JM. Hooper NM. Turner AJ. The involvement of lipid rafts in Alzheimer's disease. Mol Membr Biol. 2006;23:111–122. doi: 10.1080/09687860500496417. [DOI] [PubMed] [Google Scholar]
- 68.Cremesti AE. Goni FM. Kolesnick R. Role of sphingomyelinase and ceramide in modulating rafts: do biophysical properties determine biologic outcome? FEBS Lett. 2002;531:47–53. doi: 10.1016/s0014-5793(02)03489-0. [DOI] [PubMed] [Google Scholar]
- 69.Cutler RG. Mattson MP. Sphingomyelin and ceramide as regulators of development and lifespan. Mech Ageing Dev. 2001;122:895–908. doi: 10.1016/s0047-6374(01)00246-9. [DOI] [PubMed] [Google Scholar]
- 70.Dang PM. Cross AR. Quinn MT. Babior BM. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67PHOX and cytochrome b558 II. Proc Natl Acad Sci U S A. 2002;99:4262–4265. doi: 10.1073/pnas.072345299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Haas M. Vossebeld PJ. von dem Borne AE. Roos D. Fc gamma receptors of phagocytes. J Lab Clin Med. 1995;126:330–341. [PubMed] [Google Scholar]
- 72.Del Prete A. Zaccagnino P. Di Paola M. Saltarella M. Oliveros Celis C. Nico B. Santoro G. Lorusso M. Role of mitochondria and reactive oxygen species in dendritic cell differentiation and functions. Free Radic Biol Med. 2008;44:1443–1451. doi: 10.1016/j.freeradbiomed.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 73.Delles C. Miller WH. Dominiczak AF. Targeting reactive oxygen species in hypertension. Antioxid Redox Signal. 2008;10:1061–1077. doi: 10.1089/ars.2007.2008. [DOI] [PubMed] [Google Scholar]
- 74.Desplanques AS. Nauwynck HJ. Tilleman K. Deforce D. Favoreel HW. Tyrosine phosphorylation and lipid raft association of pseudorabies virus glycoprotein E during antibody-mediated capping. Virology. 2007;362:60–66. doi: 10.1016/j.virol.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 75.Di Vizio D. Solomon KR. Freeman MR. Cholesterol and cholesterol-rich membranes in prostate cancer: an update. Tumori. 2008;94:633–639. doi: 10.1177/030089160809400501. [DOI] [PubMed] [Google Scholar]
- 76.Dikalova AE. Gongora MC. Harrison DG. Lambeth JD. Dikalov S. Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via enos uncoupling. Am J Physiol Heart Circ Physiol. 2010;299:H673–H679. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Donati RJ. Rasenick MM. Chronic antidepressant treatment prevents accumulation of gsalpha in cholesterol-rich, cytoskeletal-associated, plasma membrane domains (lipid rafts) Neuropsychopharmacology. 2005;30:1238–1245. doi: 10.1038/sj.npp.1300697. [DOI] [PubMed] [Google Scholar]
- 78.Downey GP. Fukushima T. Fialkow L. Signaling mechanisms in human neutrophils. Curr Opin Hematol. 1995;2:76–88. doi: 10.1097/00062752-199502010-00011. [DOI] [PubMed] [Google Scholar]
- 79.Draper JM. Xia Z. Smith CD. Cellular palmitoylation and trafficking of lipidated peptides. J Lipid Res. 2007;48:1873–1884. doi: 10.1194/jlr.M700179-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dremina ES. Sharov VS. Schoneich C. Protein tyrosine nitration in rat brain is associated with raft proteins, flotillin-1 and alpha-tubulin: effect of biological aging. J Neurochem. 2005;93:1262–1271. doi: 10.1111/j.1471-4159.2005.03115.x. [DOI] [PubMed] [Google Scholar]
- 81.Dumitru CA. Zhang Y. Li X. Gulbins E. Ceramide: a novel player in reactive oxygen species-induced signaling? Antioxid Redox Signal. 2007;9:1535–1540. doi: 10.1089/ars.2007.1692. [DOI] [PubMed] [Google Scholar]
- 82.Dworakowski R. Anilkumar N. Zhang M. Shah AM. Redox signalling involving NADPH oxidase-derived reactive oxygen species. Biochem Soc Trans. 2006;34:960–964. doi: 10.1042/BST0340960. [DOI] [PubMed] [Google Scholar]
- 83.Eggeling C. Ringemann C. Medda R. Schwarzmann G. Sandhoff K. Polyakova S. Belov VN. Hein B. von Middendorff C. Schonle A. Hell SW. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature. 2009;457:1159–1162. doi: 10.1038/nature07596. [DOI] [PubMed] [Google Scholar]
- 84.Eid AA. Gorin Y. Fagg BM. Maalouf R. Barnes JL. Block K. Abboud HE. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58:1201–1211. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El-Benna J. Dang PM. Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 86.El Kirat K. Morandat S. Dufrene YF. Nanoscale analysis of supported lipid bilayers using atomic force microscopy. Biochim Biophys Acta. 2010;1798:750–765. doi: 10.1016/j.bbamem.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 87.Esen M. Schreiner B. Jendrossek V. Lang F. Fassbender K. Grassme H. Gulbins E. Mechanisms of Staphylococcus aureus induced apoptosis of human endothelial cells. Apoptosis. 2001;6:431–439. doi: 10.1023/a:1012445925628. [DOI] [PubMed] [Google Scholar]
- 88.Eum SY. Andras I. Hennig B. Toborek M. NADPH oxidase and lipid raft-associated redox signaling are required for PCB153-induced upregulation of cell adhesion molecules in human brain endothelial cells. Toxicol Appl Pharmacol. 2009;240:299–305. doi: 10.1016/j.taap.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fallahi-Sichani M. Linderman JJ. Lipid raft-mediated regulation of G-protein coupled receptor signaling by ligands which influence receptor dimerization: a computational study. PLoS One. 2009;4:e6604. doi: 10.1371/journal.pone.0006604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feuk-Lagerstedt E. Movitz C. Pellme S. Dahlgren C. Karlsson A. Lipid raft proteome of the human neutrophil azurophil granule. Proteomics. 2007;7:194–205. doi: 10.1002/pmic.200600482. [DOI] [PubMed] [Google Scholar]
- 91.Finkel T. Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 92.Fischer H. Mechanisms and function of DUOX in epithelia of the lung. Antioxid Redox Signal. 2009;11:2453–2465. doi: 10.1089/ars.2009.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fish KN. Total internal reflection fluorescence (TIRF) microscopy. Curr Protoc Cytom Chapter. 2009;12 doi: 10.1002/0471142956.cy1218s50. Unit12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flowers F. Zimmerman JJ. Reactive oxygen species in the cellular pathophysiology of shock. New Horiz. 1998;6:169–180. [PubMed] [Google Scholar]
- 95.Folkman J. Antiangiogenesis in cancer therapy—endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 96.Forman HJ. Torres M. Redox signaling in macrophages. Mol Aspects Med. 2001;22:189–216. doi: 10.1016/s0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- 97.Foster JD. Adkins SD. Lever JR. Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Foster LJ. De Hoog CL. Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci U S A. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frank PG. Woodman SE. Park DS. Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23:1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- 100.Frey RS. Rahman A. Kefer JC. Minshall RD. Malik AB. PKCzeta regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ Res. 2002;90:1012–1019. doi: 10.1161/01.res.0000017631.28815.8e. [DOI] [PubMed] [Google Scholar]
- 101.Friedrichson T. Kurzchalia TV. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature. 1998;394:802–805. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- 102.Fubini B. Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol Med. 2003;34:1507–1516. doi: 10.1016/s0891-5849(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 103.Fuhler GM. Blom NR. Coffer PJ. Drayer AL. Vellenga E. The reduced GM-CSF priming of ROS production in granulocytes from patients with myelodysplasia is associated with an impaired lipid raft formation. J Leukoc Biol. 2007;81:449–457. doi: 10.1189/jlb.0506311. [DOI] [PubMed] [Google Scholar]
- 104.Fujimoto S. Satoh M. Horike H. Hatta H. Haruna Y. Kobayashi S. Namikoshi T. Arakawa S. Tomita N. Kashihara N. Olmesartan ameliorates progressive glomerular injury in subtotal nephrectomized rats through suppression of superoxide production. Hypertens Res. 2008;31:305–313. doi: 10.1291/hypres.31.305. [DOI] [PubMed] [Google Scholar]
- 105.Fujimura Y. Tachibana H. Yamada K. Lipid raft-associated catechin suppresses the FcepsilonRI expression by inhibiting phosphorylation of the extracellular signal-regulated kinase1/2. FEBS Lett. 2004;556:204–210. doi: 10.1016/s0014-5793(03)01432-7. [DOI] [PubMed] [Google Scholar]
- 106.Fukai T. Folz RJ. Landmesser U. Harrison DG. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc Res. 2002;55:239–249. doi: 10.1016/s0008-6363(02)00328-0. [DOI] [PubMed] [Google Scholar]
- 107.Fulton DJ. Nox5 and the regulation of cellular function. Antioxid Redox Signal. 2009;11:2443–2452. doi: 10.1089/ars.2009.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galan C. Woodard GE. Dionisio N. Salido GM. Rosado JA. Lipid rafts modulate the activation but not the maintenance of store-operated Ca(2+) entry. Biochim Biophys Acta. 2010;1803:1083–1093. doi: 10.1016/j.bbamcr.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 109.Galli F. Membrane rafts and redox therapies in cancer: a commentary on Radioresistance of human carcinoma cells is correlated to a defect in raft membrane clustering. Free Radic Biol Med. 2007;43:678–680. doi: 10.1016/j.freeradbiomed.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 110.Gamalei IA. Efremova TN. Kirpichnikova KM. Komissarchik YY. Kever LV. Polozov YV. Khaitlina SY. Decreased sensitivity of transformed 3T3-SV40 cells treated with N-acetylcysteine to bacterial invasion. Bull Exp Biol Med. 2006;142:90–93. doi: 10.1007/s10517-006-0300-3. [DOI] [PubMed] [Google Scholar]
- 111.Gambara G. Billington RA. Debidda M. D'Alessio A. Palombi F. Ziparo E. Genazzani AA. Filippini A. NAADP-induced Ca(2+ signaling in response to endothelin is via the receptor subtype B and requires the integrity of lipid rafts/caveolae. J Cell Physiol. 2008;216:396–404. doi: 10.1002/jcp.21407. [DOI] [PubMed] [Google Scholar]
- 112.Garcia-Cardena G. Oh P. Liu J. Schnitzer JE. Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci U S A. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garcia RC. Segal AW. Changes in the subcellular distribution of the cytochrome b-245 on stimulation of human neutrophils. Biochem J. 1984;219:233–242. doi: 10.1042/bj2190233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gauss KA. Nelson-Overton LK. Siemsen DW. Gao Y. DeLeo FR. Quinn MT. Role of NF-kappaB in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-alpha. J Leukoc Biol. 2007;82:729–741. doi: 10.1189/jlb.1206735. [DOI] [PubMed] [Google Scholar]
- 115.Geiszt M. Kopp JB. Varnai P. Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gendzwill A. [Reactive oxygen species and vascular hyporeactivity in septic shock. Part I—reactive oxygen species and vascular hyporeactivity] Pol Merkur Lekarski. 2007;23:280–283. [PubMed] [Google Scholar]
- 117.Gendzwill A. [Reactive oxygen species and vascular hyporeactivity in septic shock. Part II—scavengers and vascular hyporeactivity in septic shock] Pol Merkur Lekarski. 2007;23:284–287. [PubMed] [Google Scholar]
- 118.Gerst JE. SNAREs and SNARE regulators in membrane fusion and exocytosis. Cell Mol Life Sci. 1999;55:707–734. doi: 10.1007/s000180050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goligorsky MS. Li H. Brodsky S. Chen J. Relationships between caveolae and eNOS: everything in proximity and the proximity of everything. Am J Physiol Renal Physiol. 2002;283:F1–F10. doi: 10.1152/ajprenal.00377.2001. [DOI] [PubMed] [Google Scholar]
- 120.Gong XM. Franzin CM. Thai K. Yu J. Marassi FM. Nuclear magnetic resonance structural studies of membrane proteins in micelles and bilayers. Methods Mol Biol. 2007;400:515–529. doi: 10.1007/978-1-59745-519-0_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gongora MC. Lob HE. Landmesser U. Guzik TJ. Martin WD. Ozumi K. Wall SM. Wilson DS. Murthy N. Gravanis M. Fukai T. Harrison DG. Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: a potential mechanism underlying adult respiratory distress syndrome. Am J Pathol. 2008;173:915–926. doi: 10.2353/ajpath.2008.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goni FM. Alonso A. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 2002;531:38–46. doi: 10.1016/s0014-5793(02)03482-8. [DOI] [PubMed] [Google Scholar]
- 123.Gorin Y. Block K. Hernandez J. Bhandari B. Wagner B. Barnes JL. Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 124.Gorlach A. Brandes RP. Nguyen K. Amidi M. Dehghani F. Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 125.Goswami D. Gowrishankar K. Bilgrami S. Ghosh S. Raghupathy R. Chadda R. Vishwakarma R. Rao M. Mayor S. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell. 2008;135:1085–1097. doi: 10.1016/j.cell.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goswami SK. Maulik N. Das DK. Ischemia-reperfusion and cardioprotection: a delicate balance between reactive oxygen species generation and redox homeostasis. Ann Med. 2007;39:275–289. doi: 10.1080/07853890701374677. [DOI] [PubMed] [Google Scholar]
- 127.Grammatikos G. Teichgraber V. Carpinteiro A. Trarbach T. Weller M. Hengge UR. Gulbins E. Overexpression of acid sphingomyelinase sensitizes glioma cells to chemotherapy. Antioxid Redox Signal. 2007;9:1449–1456. doi: 10.1089/ars.2007.1673. [DOI] [PubMed] [Google Scholar]
- 128.Grandvaux N. Soucy-Faulkner A. Fink K. Innate host defense: Nox and Duox on phox's tail. Biochimie. 2007;89:1113–1122. doi: 10.1016/j.biochi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 129.Grassme H. Becker KA. Zhang Y. Gulbins E. Ceramide in bacterial infections and cystic fibrosis. Biol Chem. 2008;389:1371–1379. doi: 10.1515/BC.2008.162. [DOI] [PubMed] [Google Scholar]
- 130.Grassme H. Gulbins E. Brenner B. Ferlinz K. Sandhoff K. Harzer K. Lang F. Meyer TF. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell. 1997;91:605–615. doi: 10.1016/s0092-8674(00)80448-1. [DOI] [PubMed] [Google Scholar]
- 131.Grassme H. Jekle A. Riehle A. Schwarz H. Berger J. Sandhoff K. Kolesnick R. Gulbins E. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 132.Grassme H. Jendrossek V. Bock J. Riehle A. Gulbins E. Ceramide-rich membrane rafts mediate CD40 clustering. J Immunol. 2002;168:298–307. doi: 10.4049/jimmunol.168.1.298. [DOI] [PubMed] [Google Scholar]
- 133.Grassme H. Jendrossek V. Riehle A. von Kurthy G. Berger J. Schwarz H. Weller M. Kolesnick R. Gulbins E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9:322–330. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- 134.Grassme H. Riehle A. Wilker B. Gulbins E. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem. 2005;280:26256–26262. doi: 10.1074/jbc.M500835200. [DOI] [PubMed] [Google Scholar]
- 135.Greaves J. Chamberlain LH. Palmitoylation-dependent protein sorting. J Cell Biol. 2007;176:249–254. doi: 10.1083/jcb.200610151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart. 2004;90:491–493. doi: 10.1136/hrt.2003.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Griendling KK. Minieri CA. Ollerenshaw JD. Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 138.Griendling KK. Sorescu D. Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 139.Griffiths G. Cell evolution and the problem of membrane topology. Nat Rev Mol Cell Biol. 2007;8:1018–1024. doi: 10.1038/nrm2287. [DOI] [PubMed] [Google Scholar]
- 140.Groemping Y. Lapouge K. Smerdon SJ. Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 141.Gross O. Poeck H. Bscheider M. Dostert C. Hannesschlager N. Endres S. Hartmann G. Tardivel A. Schweighoffer E. Tybulewicz V. Mocsai A. Tschopp J. Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 142.Guichard C. Pedruzzi E. Fay M. Ben Mkaddem S. Coant N. Daniel F. Ogier-Denis E. [The Nox/Duox family of ROS-generating NADPH oxidases] Med Sci (Paris) 2006;22:953–959. doi: 10.1051/medsci/20062211953. [DOI] [PubMed] [Google Scholar]
- 143.Gulbins E. Highlight: sphingolipids—signals and disease. Biol Chem. 2008;389:1347–1348. doi: 10.1515/BC.2008.164. [DOI] [PubMed] [Google Scholar]
- 144.Gulbins E. Grassme H. Ceramide and cell death receptor clustering. Biochim Biophys Acta. 2002;1585:139–145. doi: 10.1016/s1388-1981(02)00334-7. [DOI] [PubMed] [Google Scholar]
- 145.Gulbins E. Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 146.Gulbins E. Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol. 2006;290:R11–R26. doi: 10.1152/ajpregu.00416.2005. [DOI] [PubMed] [Google Scholar]
- 147.Gupte SA. Wolin MS. Oxidant and redox signaling in vascular oxygen sensing: implications for systemic and pulmonary hypertension. Antioxid Redox Signal. 2008;10:1137–1152. doi: 10.1089/ars.2007.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guzy RD. Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 149.Halliwell B. Cross CE. Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect. 1994;102(Suppl 10):5–12. doi: 10.1289/ehp.94102s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Han W. Li H. Villar VA. Pascua AM. Dajani MI. Wang X. Natarajan A. Quinn MT. Felder RA. Jose PA. Yu P. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension. 2008;51:481–487. doi: 10.1161/HYPERTENSIONAHA.107.103275. [DOI] [PubMed] [Google Scholar]
- 151.Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hannun YA. Bell RM. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989;243:500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- 153.Hansberg W. de Groot H. Sies H. Reactive oxygen species associated with cell differentiation in Neurospora crassa. Free Radic Biol Med. 1993;14:287–293. doi: 10.1016/0891-5849(93)90025-p. [DOI] [PubMed] [Google Scholar]
- 154.Hansen CG. Nichols BJ. Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol. 2010;20:177–186. doi: 10.1016/j.tcb.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 155.Hansen GH. Niels-Christiansen LL. Thorsen E. Immerdal L. Danielsen EM. Cholesterol depletion of enterocytes. Effect on the Golgi complex and apical membrane trafficking. J Biol Chem. 2000;275:5136–5142. doi: 10.1074/jbc.275.7.5136. [DOI] [PubMed] [Google Scholar]
- 156.Hara T. Kondo N. Nakamura H. Okuyama H. Mitsui A. Hoshino Y. Yodoi J. Cell-surface thioredoxin-1: possible involvement in thiol-mediated leukocyte-endothelial cell interaction through lipid rafts. Antioxid Redox Signal. 2007;9:1427–1437. doi: 10.1089/ars.2007.1661. [DOI] [PubMed] [Google Scholar]
- 157.Harder T. Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 158.Hauck CR. Grassme H. Bock J. Jendrossek V. Ferlinz K. Meyer TF. Gulbins E. Acid sphingomyelinase is involved in CEACAM receptor-mediated phagocytosis of Neisseria gonorrhoeae. FEBS Lett. 2000;478:260–266. doi: 10.1016/s0014-5793(00)01851-2. [DOI] [PubMed] [Google Scholar]
- 159.Head BP. Patel HH. Roth DM. Murray F. Swaney JS. Niesman IR. Farquhar MG. Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem. 2006;281:26391–26399. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- 160.Helms JB. Zurzolo C. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic. 2004;5:247–254. doi: 10.1111/j.1600-0854.2004.0181.x. [DOI] [PubMed] [Google Scholar]
- 161.Hess ST. Gould TJ. Gudheti MV. Maas SA. Mills KD. Zimmerberg J. Dynamic clustered distribution of hemagglutinin resolved at 40 nm in living cell membranes discriminates between raft theories. Proc Natl Acad Sci U S A. 2007;104:17370–17375. doi: 10.1073/pnas.0708066104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hildeman DA. Regulation of T-cell apoptosis by reactive oxygen species. Free Radic Biol Med. 2004;36:1496–1504. doi: 10.1016/j.freeradbiomed.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 163.Hilenski LL. Clempus RE. Quinn MT. Lambeth JD. Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 164.Hill JM. Steiner I. Matthews KE. Trahan SG. Foster TP. Ball MJ. Statins lower the risk of developing Alzheimer's disease by limiting lipid raft endocytosis and decreasing the neuronal spread of Herpes simplex virus type 1. Med Hypotheses. 2005;64:53–58. doi: 10.1016/j.mehy.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 165.Hillyard DZ. Jardine AG. McDonald KJ. Cameron AJ. Fluvastatin inhibits raft dependent Fcgamma receptor signalling in human monocytes. Atherosclerosis. 2004;172:219–228. doi: 10.1016/j.atherosclerosis.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 166.Hillyard DZ. Nutt CD. Thomson J. McDonald KJ. Wan RK. Cameron AJ. Mark PB. Jardine AG. Statins inhibit NK cell cytotoxicity by membrane raft depletion rather than inhibition of isoprenylation. Atherosclerosis. 2007;191:319–325. doi: 10.1016/j.atherosclerosis.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 167.Hirooka Y. Role of reactive oxygen species in brainstem in neural mechanisms of hypertension. Auton Neurosci. 2008;142:20–24. doi: 10.1016/j.autneu.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 168.Hoekstra D. Maier O. van der Wouden JM. Slimane TA. van ISC. Membrane dynamics and cell polarity: the role of sphingolipids. J Lipid Res. 2003;44:869–77. doi: 10.1194/jlr.R300003-JLR200. [DOI] [PubMed] [Google Scholar]
- 169.Holowka D. Sheets ED. Baird B. Interactions between Fc(epsilon)RI and lipid raft components are regulated by the actin cytoskeleton. J Cell Sci. 2000;113(Pt 6):1009–1019. doi: 10.1242/jcs.113.6.1009. [DOI] [PubMed] [Google Scholar]
- 170.Holz RW. Axelrod D. Secretory granule behaviour adjacent to the plasma membrane before and during exocytosis: total internal reflection fluorescence microscopy studies. Acta Physiol (Oxf) 2008;192:303–307. doi: 10.1111/j.1748-1716.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 171.Hu J. Jia S. Li P. Statins Block the Formation of Lipid Raft Redox Signaling Platforms in Coronary Endothelial Cells. FASEB J. 2009:23. [Google Scholar]
- 172.Hung YH. Robb EL. Volitakis I. Ho M. Evin G. Li QX. Culvenor JG. Masters CL. Cherny RA. Bush AI. Paradoxical condensation of copper with elevated beta-amyloid in lipid rafts under cellular copper deficiency conditions: implications for Alzheimer disease. J Biol Chem. 2009;284:21899–21907. doi: 10.1074/jbc.M109.019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Huynh C. Roth D. Ward DM. Kaplan J. Andrews NW. Defective lysosomal exocytosis and plasma membrane repair in Chediak-Higashi/beige cells. Proc Natl Acad Sci U S A. 2004;101:16795–16800. doi: 10.1073/pnas.0405905101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hwang J. Kleinhenz DJ. Lassegue B. Griendling KK. Dikalov S. Hart CM. Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. Am J Physiol Cell Physiol. 2005;288:C899–C905. doi: 10.1152/ajpcell.00474.2004. [DOI] [PubMed] [Google Scholar]
- 175.Hwang LC. Wohland T. Recent advances in fluorescence cross-correlation spectroscopy. Cell Biochem Biophys. 2007;49:1–13. doi: 10.1007/s12013-007-0042-5. [DOI] [PubMed] [Google Scholar]
- 176.Iadecola C. Gorelick PB. Hypertension, angiotensin, and stroke: beyond blood pressure. Stroke. 2004;35:348–350. doi: 10.1161/01.STR.0000115162.16321.AA. [DOI] [PubMed] [Google Scholar]
- 177.Ikonen E. Vainio S. Lipid microdomains and insulin resistance: is there a connection? Sci STKE. 2005;2005:pe3. doi: 10.1126/stke.2682005pe3. [DOI] [PubMed] [Google Scholar]
- 178.Inokuchi J. Insulin resistance as a membrane microdomain disorder. Biol Pharm Bull. 2006;29:1532–1537. doi: 10.1248/bpb.29.1532. [DOI] [PubMed] [Google Scholar]
- 179.Insel PA. Head BP. Ostrom RS. Patel HH. Swaney JS. Tang CM. Roth DM. Caveolae and lipid rafts: G protein-coupled receptor signaling microdomains in cardiac myocytes. Ann N Y Acad Sci. 2005;1047:166–172. doi: 10.1196/annals.1341.015. [DOI] [PubMed] [Google Scholar]
- 180.Israelachvili JN. Refinement of the fluid-mosaic model of membrane structure. Biochim Biophys Acta. 1977;469:221–225. doi: 10.1016/0005-2736(77)90185-7. [DOI] [PubMed] [Google Scholar]
- 181.Jain MK. White HB., 3rd Long-range order in biomembranes. Adv Lipid Res. 1977;15:1–60. doi: 10.1016/b978-0-12-024915-2.50007-4. [DOI] [PubMed] [Google Scholar]
- 182.Jaiswal JK. Andrews NW. Simon SM. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol. 2002;159:625–635. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jaksits S. Bauer W. Kriehuber E. Zeyda M. Stulnig TM. Stingl G. Fiebiger E. Maurer D. Lipid raft-associated GTPase signaling controls morphology and CD8+ T cell stimulatory capacity of human dendritic cells. J Immunol. 2004;173:1628–1639. doi: 10.4049/jimmunol.173.3.1628. [DOI] [PubMed] [Google Scholar]
- 184.Jamaluddin M. Tian B. Boldogh I. Garofalo RP. Brasier AR. Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J Virol. 2009;83:10605–10615. doi: 10.1128/JVI.01090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jan JT. Chatterjee S. Griffin DE. Sindbis virus entry into cells triggers apoptosis by activating sphingomyelinase, leading to the release of ceramide. J Virol. 2000;74:6425–6432. doi: 10.1128/jvi.74.14.6425-6432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jana A. Pahan K. Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase. Implications for Alzheimer's disease. J Biol Chem. 2004;279:51451–51459. doi: 10.1074/jbc.M404635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jardin I. Salido GM. Rosado JA. Role of lipid rafts in the interaction between hTRPC1, Orai1 and STIM1. Channels (Austin) 2008;2:401–403. doi: 10.4161/chan.2.6.7055. [DOI] [PubMed] [Google Scholar]
- 188.Jia SJ. Jin S. Zhang F. Yi F. Dewey WL. Li PL. Formation and function of ceramide-enriched membrane platforms with CD38 during M1-receptor stimulation in bovine coronary arterial myocytes. Am J Physiol Heart Circ Physiol. 2008;295:H1743–H1752. doi: 10.1152/ajpheart.00617.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jin S. Yi F. Li PL. Contribution of lysosomal vesicles to the formation of lipid raft redox signaling platforms in endothelial cells. Antioxid Redox Signal. 2007;9:1417–1426. doi: 10.1089/ars.2007.1660. [DOI] [PubMed] [Google Scholar]
- 190.Jin S. Yi F. Zhang F. Poklis JL. Li PL. Lysosomal targeting and trafficking of acid sphingomyelinase to lipid raft platforms in coronary endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:2056–2062. doi: 10.1161/ATVBAHA.108.172478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jin S. Zhang Y. Yi F. Li PL. Critical role of lipid raft redox signaling platforms in endostatin-induced coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2008;28:485–490. doi: 10.1161/ATVBAHA.107.159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jin S. Zhou F. Lipid raft redox signaling platforms in vascular dysfunction: features and mechanisms. Curr Atheroscler Rep. 2009;11:220–226. doi: 10.1007/s11883-009-0034-6. [DOI] [PubMed] [Google Scholar]
- 193.Jin ZX. Huang CR. Dong L. Goda S. Kawanami T. Sawaki T. Sakai T. Tong XP. Masaki Y. Fukushima T. Tanaka M. Mimori T. Tojo H. Bloom ET. Okazaki T. Umehara H. Impaired TCR signaling through dysfunction of lipid rafts in sphingomyelin synthase 1 (SMS1)-knockdown T cells. Int Immunol. 2008;20:1427–1437. doi: 10.1093/intimm/dxn100. [DOI] [PubMed] [Google Scholar]
- 194.Jose PA. Eisner GM. Felder RA. Role of dopamine receptors in the kidney in the regulation of blood pressure. Curr Opin Nephrol Hypertens. 2002;11:87–92. doi: 10.1097/00041552-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 195.Jung O. Schreiber JG. Geiger H. Pedrazzini T. Busse R. Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109:1795–1801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- 196.Kaushal GP. Singh AB. Shah SV. Identification of gene family of caspases in rat kidney and altered expression in ischemia-reperfusion injury. Am J Physiol. 1998;274:F587–F595. doi: 10.1152/ajprenal.1998.274.3.F587. [DOI] [PubMed] [Google Scholar]
- 197.Kawabe J. Okumura S. Nathanson MA. Hasebe N. Ishikawa Y. Caveolin regulates microtubule polymerization in the vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;342:164–169. doi: 10.1016/j.bbrc.2006.01.125. [DOI] [PubMed] [Google Scholar]
- 198.Kenworthy AK. Petranova N. Edidin M. High-resolution FRET microscopy of cholera toxin B-subunit and GPI-anchored proteins in cell plasma membranes. Mol Biol Cell. 2000;11:1645–1655. doi: 10.1091/mbc.11.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Khan N. Swartz H. Measurements in vivo of parameters pertinent to ROS/RNS using EPR spectroscopy. Mol Cell Biochem. 2002:234–235. 341–357. [PubMed] [Google Scholar]
- 200.Kietzmann T. Gorlach A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin Cell Dev Biol. 2005;16:474–486. doi: 10.1016/j.semcdb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 201.Kim YJ. Nakatomi R. Akagi T. Hashikawa T. Takahashi R. Unsaturated fatty acids induce cytotoxic aggregate formation of amyotrophic lateral sclerosis-linked superoxide dismutase 1 mutants. J Biol Chem. 2005;280:21515–21521. doi: 10.1074/jbc.M502230200. [DOI] [PubMed] [Google Scholar]
- 202.Kinnear NP. Boittin FX. Thomas JM. Galione A. Evans AM. Lysosome-sarcoplasmic reticulum junctions. A trigger zone for calcium signaling by nicotinic acid adenine dinucleotide phosphate and endothelin-1. J Biol Chem. 2004;279:54319–54326. doi: 10.1074/jbc.M406132200. [DOI] [PubMed] [Google Scholar]
- 203.Kinoshita A. Fukumoto H. Shah T. Whelan CM. Irizarry MC. Hyman BT. Demonstration by FRET of BACE interaction with the amyloid precursor protein at the cell surface and in early endosomes. J Cell Sci. 2003;116:3339–3346. doi: 10.1242/jcs.00643. [DOI] [PubMed] [Google Scholar]
- 204.Kirkham M. Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1746:349–363. doi: 10.1016/j.bbamcr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 205.Kirkham M. Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1745:273–286. doi: 10.1016/j.bbamcr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 206.Kiss AL. Botos E. Endocytosis via caveolae: alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J Cell Mol Med. 2009;13:1228–1237. doi: 10.1111/j.1582-4934.2009.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kitatani K. Idkowiak-Baldys J. Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–1018. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kojda G. Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 209.Kolzer M. Arenz C. Ferlinz K. Werth N. Schulze H. Klingenstein R. Sandhoff K. Phosphatidylinositol-3,5-Bisphosphate is a potent and selective inhibitor of acid sphingomyelinase. Biol Chem. 2003;384:1293–1298. doi: 10.1515/BC.2003.144. [DOI] [PubMed] [Google Scholar]
- 210.Kondo N. Ishii Y. Kwon YW. Tanito M. Sakakura-Nishiyama J. Mochizuki M. Maeda M. Suzuki S. Kojima M. Kim YC. Son A. Nakamura H. Yodoi J. Lipid raft-mediated uptake of cysteine-modified thioredoxin-1: apoptosis enhancement by inhibiting the endogenous thioredoxin-1. Antioxid Redox Signal. 2007;9:1439–1448. doi: 10.1089/ars.2007.1665. [DOI] [PubMed] [Google Scholar]
- 211.Kono H. Suzuki T. Yamamoto K. Okada M. Yamamoto T. Honda Z. Spatial raft coalescence represents an initial step in Fc gamma R signaling. J Immunol. 2002;169:193–203. doi: 10.4049/jimmunol.169.1.193. [DOI] [PubMed] [Google Scholar]
- 212.Korn ED. Structure and function of the plasma membrane. A biochemical perspective. J Gen Physiol. 1968;52:257–278. [PMC free article] [PubMed] [Google Scholar]
- 213.Kornhuber J. Reichel M. Tripal P. Groemer TW. Henkel AW. Muhle C. Gulbins E. The role of ceramide in major depressive disorder. Eur Arch Psychiatry Clin Neurosci. 2009;259(Suppl 2):S199–S204. doi: 10.1007/s00406-009-0061-x. [DOI] [PubMed] [Google Scholar]
- 214.Kornhuber J. Tripal P. Reichel M. Muhle C. Rhein C. Muehlbacher M. Groemer TW. Gulbins E. Functional Inhibitors of Acid Sphingomyelinase (FIASMAs): a novel pharmacological group of drugs with broad clinical applications. Cell Physiol Biochem. 2010;26:9–20. doi: 10.1159/000315101. [DOI] [PubMed] [Google Scholar]
- 215.Kornhuber J. Tripal P. Reichel M. Terfloth L. Bleich S. Wiltfang J. Gulbins E. Identification of new functional inhibitors of acid sphingomyelinase using a structure-property-activity relation model. J Med Chem. 2008;51:219–237. doi: 10.1021/jm070524a. [DOI] [PubMed] [Google Scholar]
- 216.Kosugi A. Sakakura J. Yasuda K. Ogata M. Hamaoka T. Involvement of SHP-1 tyrosine phosphatase in TCR-mediated signaling pathways in lipid rafts. Immunity. 2001;14:669–680. doi: 10.1016/s1074-7613(01)00146-7. [DOI] [PubMed] [Google Scholar]
- 217.Ksiazek K. Wisniewska J. [The role of glucose and reactive oxygen species in the development of vascular complications of diabetes mellitus] Przegl Lek. 2001;58:915–918. [PubMed] [Google Scholar]
- 218.Kulma M. Herec M. Grudzinski W. Anderluh G. Gruszecki WI. Kwiatkowska K. Sobota A. Sphingomyelin-rich domains are sites of lysenin oligomerization: implications for raft studies. Biochim Biophys Acta. 2010;1798:471–481. doi: 10.1016/j.bbamem.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 219.Kusmartsev S. Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol. 2003;74:186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 220.Lambeth JD. Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidases. Curr Opin Hematol. 2002;9:11–17. doi: 10.1097/00062752-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 221.Lang F. Gulbins E. Lang PA. Zappulla D. Foller M. Ceramide in suicidal death of erythrocytes. Cell Physiol Biochem. 2010;26:21–28. doi: 10.1159/000315102. [DOI] [PubMed] [Google Scholar]
- 222.Lang F. Lang KS. Lang PA. Huber SM. Wieder T. Mechanisms and significance of eryptosis. Antioxid Redox Signal. 2006;8:1183–1192. doi: 10.1089/ars.2006.8.1183. [DOI] [PubMed] [Google Scholar]
- 223.Lassegue B. Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 224.Laux T. Fukami K. Thelen M. Golub T. Frey D. Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J Cell Biol. 2000;149:1455–1472. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lawrence DS. Zilfou JT. Smith CD. Structure-activity studies of cerulenin analogues as protein palmitoylation inhibitors. J Med Chem. 1999;42:4932–4941. doi: 10.1021/jm980591s. [DOI] [PubMed] [Google Scholar]
- 226.Lee HC. Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling. J Biol Chem. 2005;280:33693–33696. doi: 10.1074/jbc.R500012200. [DOI] [PubMed] [Google Scholar]
- 227.Lefebvre B. Furt F. Hartmann MA. Michaelson LV. Carde JP. Sargueil-Boiron F. Rossignol M. Napier JA. Cullimore J. Bessoule JJ. Mongrand S. Characterization of lipid rafts from Medicago truncatula root plasma membranes: a proteomic study reveals the presence of a raft-associated redox system. Plant Physiol. 2007;144:402–418. doi: 10.1104/pp.106.094102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Legler DF. Micheau O. Doucey MA. Tschopp J. Bron C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity. 2003;18:655–664. doi: 10.1016/s1074-7613(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 229.Lenne PF. Wawrezinieck L. Conchonaud F. Wurtz O. Boned A. Guo XJ. Rigneault H. He HT. Marguet D. Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO J. 2006;25:3245–3256. doi: 10.1038/sj.emboj.7601214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Levitan I. Gooch KJ. Lipid rafts in membrane-cytoskeleton interactions and control of cellular biomechanics: actions of oxLDL. Antioxid Redox Signal. 2007;9:1519–1534. doi: 10.1089/ars.2007.1686. [DOI] [PubMed] [Google Scholar]
- 231.Levy M. Futerman AH. Mammalian ceramide synthases. IUBMB Life. 2010;62:347–356. doi: 10.1002/iub.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Leyton L. Quest AF. Bron C. Thy-1/CD3 coengagement promotes TCR signaling and enhances particularly tyrosine phosphorylation of the raft molecule LAT. Mol Immunol. 1999;36:755–768. doi: 10.1016/s0161-5890(99)00086-3. [DOI] [PubMed] [Google Scholar]
- 233.Li H. Han W. Villar VA. Keever LB. Lu Q. Hopfer U. Quinn MT. Felder RA. Jose PA. Yu P. D1-like receptors regulate NADPH oxidase activity and subunit expression in lipid raft microdomains of renal proximal tubule cells. Hypertension. 2009;53:1054–1061. doi: 10.1161/HYPERTENSIONAHA.108.120642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li PL. Gulbins E. Lipid rafts and redox signaling. Antioxid Redox Signal. 2007;9:1411–1415. doi: 10.1089/ars.2007.1736. [DOI] [PubMed] [Google Scholar]
- 235.Li PL. Zhang Y. Yi F. Lipid raft redox signaling platforms in endothelial dysfunction. Antioxid Redox Signal. 2007;9:1457–1470. doi: 10.1089/ars.2007.1667. [DOI] [PubMed] [Google Scholar]
- 236.Li X. Becker KA. Zhang Y. Ceramide in redox signaling and cardiovascular diseases. Cell Physiol Biochem. 2010;26:41–48. doi: 10.1159/000315104. [DOI] [PubMed] [Google Scholar]
- 237.Lin CF. Chen CL. Lin YS. Ceramide in apoptotic signaling and anticancer therapy. Curr Med Chem. 2006;13:1609–1616. doi: 10.2174/092986706777441986. [DOI] [PubMed] [Google Scholar]
- 238.Lin WC. Blanchette CD. Ratto TV. Longo ML. Lipid domains in supported lipid bilayer for atomic force microscopy. Methods Mol Biol. 2007;400:503–513. doi: 10.1007/978-1-59745-519-0_34. [DOI] [PubMed] [Google Scholar]
- 239.Linder ME. Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 240.Lingwood D. Kaiser HJ. Levental I. Simons K. Lipid rafts as functional heterogeneity in cell membranes. Biochem Soc Trans. 2009;37:955–960. doi: 10.1042/BST0370955. [DOI] [PubMed] [Google Scholar]
- 241.Lingwood D. Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 242.Liu CY. Lee CF. Wei YH. Role of reactive oxygen species-elicited apoptosis in the pathophysiology of mitochondrial and neurodegenerative diseases associated with mitochondrial DNA mutations. J Formos Med Assoc. 2009;108:599–611. doi: 10.1016/s0929-6646(09)60380-6. [DOI] [PubMed] [Google Scholar]
- 243.Lotocki G. Alonso OF. Dietrich WD. Keane RW. Tumor necrosis factor receptor 1 and its signaling intermediates are recruited to lipid rafts in the traumatized brain. J Neurosci. 2004;24:11010–11016. doi: 10.1523/JNEUROSCI.3823-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lu SP. Lin Feng MH. Huang HL. Huang YC. Tsou WI. Lai MZ. Reactive oxygen species promote raft formation in T lymphocytes. Free Radic Biol Med. 2007;42:936–944. doi: 10.1016/j.freeradbiomed.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 245.MacFarlane PM. Wilkerson JE. Lovett-Barr MR. Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol. 2008;164:263–271. doi: 10.1016/j.resp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mackey AM. Sanvicens N. Groeger G. Doonan F. Wallace D. Cotter TG. Redox survival signalling in retina-derived 661W cells. Cell Death Differ. 2008;15:1291–1303. doi: 10.1038/cdd.2008.43. [DOI] [PubMed] [Google Scholar]
- 247.Magee AI. Parmryd I. Detergent-resistant membranes and the protein composition of lipid rafts. Genome Biol. 2003;4:234. doi: 10.1186/gb-2003-4-11-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mahammad S. Parmryd I. Cholesterol homeostasis in T cells. Methyl-beta-cyclodextrin treatment results in equal loss of cholesterol from Triton X-100 soluble and insoluble fractions. Biochim Biophys Acta. 2008;1778:1251–1258. doi: 10.1016/j.bbamem.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 249.Manes S. del Real G. Martinez AC. Pathogens: raft hijackers. Nat Rev Immunol. 2003;3:557–568. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- 250.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616–619. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 251.Martyn KD. Frederick LM. von Loehneysen K. Dinauer MC. Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 252.Mashimo M. Nishikawa M. Higuchi K. Hirose M. Wei Q. Haque A. Sasaki E. Shiba M. Tominaga K. Watanabe T. Fujiwara Y. Arakawa T. Inoue M. Production of reactive oxygen species in peripheral blood is increased in individuals with Helicobacter pylori infection and decreased after its eradication. Helicobacter. 2006;11:266–271. doi: 10.1111/j.1523-5378.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 253.Mas-Oliva J. Delgado-Coello B. Protein stability and the evolution of the cell membrane. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:207–213. doi: 10.1016/j.cbpc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 254.Mates JM. Sanchez-Jimenez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000;32:157–170. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 255.Mathias S. Kolesnick R. Ceramide: a novel second messenger. Adv Lipid Res. 1993;25:65–90. [PubMed] [Google Scholar]
- 256.Mattjus P. Slotte JP. Does cholesterol discriminate between sphingomyelin and phosphatidylcholine in mixed monolayers containing both phospholipids? Chem Phys Lipids. 1996;81:69–80. doi: 10.1016/0009-3084(96)02535-2. [DOI] [PubMed] [Google Scholar]
- 257.McNeil PL. Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- 258.Mikuni S. Kinjo M. [Fluorescence correlation spectroscopy and fluorescence cross correlation spectroscopy for cell biology] Tanpakushitsu Kakusan Koso. 2006;51:1998–2005. [PubMed] [Google Scholar]
- 259.Mohazzab KM. Kaminski PM. Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol. 1994;266:H2568–H2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- 260.Morgan MJ. Kim YS. Liu Z. Lipid rafts and oxidative stress-induced cell death. Antioxid Redox Signal. 2007;9:1471–1483. doi: 10.1089/ars.2007.1658. [DOI] [PubMed] [Google Scholar]
- 261.Mukherjea D. Jajoo S. Kaur T. Sheehan KE. Ramkumar V. Rybak LP. Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid Redox Signal. 2010;13:589–598. doi: 10.1089/ars.2010.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mukherjee SP. Lane RH. Lynn WS. Endogenous hydrogen peroxide and peroxidative metabolism in adipocytes in response to insulin and sulfhydryl reagents. Biochem Pharmacol. 1978;27:2589–2594. doi: 10.1016/0006-2952(78)90332-5. [DOI] [PubMed] [Google Scholar]
- 263.Mukherjee SP. Lynn WS. Reduced nicotinamide adenine dinucleotide phosphate oxidase in adipocyte plasma membrane and its activation by insulin. Possible role in the hormone's effects on adenylate cyclase and the hexose monophosphate shunt. Arch Biochem Biophys. 1977;184:69–76. doi: 10.1016/0003-9861(77)90327-7. [DOI] [PubMed] [Google Scholar]
- 264.Muller-Peddinghaus R. [Reactive oxygen species and inflammation] Dtsch Tierarztl Wochenschr. 1989;96:210–212. [PubMed] [Google Scholar]
- 265.Munday AD. Lopez JA. Posttranslational protein palmitoylation: promoting platelet purpose. Arterioscler Thromb Vasc Biol. 2007;27:1496–1499. doi: 10.1161/ATVBAHA.106.136226. [DOI] [PubMed] [Google Scholar]
- 266.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 267.Murata T. Lin MI. Huang Y. Yu J. Bauer PM. Giordano FJ. Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373–2382. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Murate T. Suzuki M. Hattori M. Takagi A. Kojima T. Tanizawa T. Asano H. Hotta T. Saito H. Yoshida S. Tamiya-Koizumi K. Up-regulation of acid sphingomyelinase during retinoic acid-induced myeloid differentiation of NB4, a human acute promyelocytic leukemia cell line. J Biol Chem. 2002;277:9936–9943. doi: 10.1074/jbc.M111594200. [DOI] [PubMed] [Google Scholar]
- 269.Nabi IR. Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161:673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Nada S. Hondo A. Kasai A. Koike M. Saito K. Uchiyama Y. Okada M. The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. EMBO J. 2009;28:477–489. doi: 10.1038/emboj.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nebl T. Pestonjamasp KN. Leszyk JD. Crowley JL. Oh SW. Luna EJ. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J Biol Chem. 2002;277:43399–43409. doi: 10.1074/jbc.M205386200. [DOI] [PubMed] [Google Scholar]
- 272.Ng G. Chau EMT. Shi Y. Recent developments in immune activation by uric acid crystals. Archivum Immunologiae Et Therapiae Experimentalis. 2010;58:273–277. doi: 10.1007/s00005-010-0082-1. [DOI] [PubMed] [Google Scholar]
- 273.Ng G. Sharma K. Ward SM. Desrosiers MD. Stephens LA. Schoel WM. Li T. Lowell CA. Ling CC. Amrein MW. Shi Y. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ni X. Morales CR. The lysosomal trafficking of acid sphingomyelinase is mediated by sortilin and mannose 6-phosphate receptor. Traffic. 2006;7:889–902. doi: 10.1111/j.1600-0854.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 275.Nicco C. Laurent A. Chereau C. Weill B. Batteux F. Differential modulation of normal and tumor cell proliferation by reactive oxygen species. Biomed Pharmacother. 2005;59:169–174. doi: 10.1016/j.biopha.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 276.Nichols BJ. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat Cell Biol. 2002;4:374–378. doi: 10.1038/ncb787. [DOI] [PubMed] [Google Scholar]
- 277.Nicolau DV., Jr. Burrage K. Parton RG. Hancock JF. Identifying optimal lipid raft characteristics required to promote nanoscale protein-protein interactions on the plasma membrane. Mol Cell Biol. 2006;26:313–323. doi: 10.1128/MCB.26.1.313-323.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Nieminen J. Kuno A. Hirabayashi J. Sato S. Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J Biol Chem. 2007;282:1374–1383. doi: 10.1074/jbc.M604506200. [DOI] [PubMed] [Google Scholar]
- 279.Nika K. Charvet C. Williams S. Tautz L. Bruckner S. Rahmouni S. Bottini N. Schoenberger SP. Baier G. Altman A. Mustelin T. Lipid raft targeting of hematopoietic protein tyrosine phosphatase by protein kinase C theta-mediated phosphorylation. Mol Cell Biol. 2006;26:1806–1816. doi: 10.1128/MCB.26.5.1806-1816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Nini L. Waheed AA. Panicker LM. Czapiga M. Zhang JH. Simonds WF. R7-binding protein targets the G protein beta 5/R7-regulator of G protein signaling complex to lipid rafts in neuronal cells and brain. BMC Biochem. 2007;8:18. doi: 10.1186/1471-2091-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Novgorodov SA. Gudz TI. Ceramide and mitochondria in ischemia/reperfusion. J Cardiovasc Pharmacol. 2009;53:198–208. doi: 10.1097/FJC.0b013e31819b52d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.O'Reilly MS. Boehm T. Shing Y. Fukai N. Vasios G. Lane WS. Flynn E. Birkhead JR. Olsen BR. Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 283.Oakley FD. Abbott D. Li Q. Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal. 2009;11:1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Oakley FD. Smith RL. Engelhardt JF. Lipid rafts and caveolin-1 coordinate interleukin-1beta (IL-1beta)-dependent activation of NFkappaB by controlling endocytosis of Nox2 and IL-1beta receptor 1 from the plasma membrane. J Biol Chem. 2009;284:33255–33264. doi: 10.1074/jbc.M109.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ochsendorf FR. Infection and reactive oxygen species. Andrologia. 1998;30(Suppl 1):81–86. doi: 10.1111/j.1439-0272.1998.tb02830.x. [DOI] [PubMed] [Google Scholar]
- 286.Odani K. Kobayashi T. Ogawa Y. Yoshida S. Seguchi H. ML-7 inhibits exocytosis of superoxide-producing intracellular compartments in human neutrophils stimulated with phorbol myristate acetate in a myosin light chain kinase-independent manner. Histochem Cell Biol. 2003;119:363–370. doi: 10.1007/s00418-003-0531-6. [DOI] [PubMed] [Google Scholar]
- 287.Oh KI. Kim BK. Ban YL. Choi EY. Jung KC. Lee IS. Park SH. CD99 activates T cells via a costimulatory function that promotes raft association of TCR complex and tyrosine phosphorylation of TCR zeta. Exp Mol Med. 2007;39:176–184. doi: 10.1038/emm.2007.20. [DOI] [PubMed] [Google Scholar]
- 288.Ong SL. Zhang Y. Whitworth JA. Reactive oxygen species and glucocorticoid-induced hypertension. Clin Exp Pharmacol Physiol. 2008;35:477–482. doi: 10.1111/j.1440-1681.2008.04900.x. [DOI] [PubMed] [Google Scholar]
- 289.Oshikawa J. Urao N. Kim HW. Kaplan N. Razvi M. McKinney R. Poole LB. Fukai T. Ushio-Fukai M. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS One. 2010;5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ostrom RS. Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Otahal P. Angelisova P. Hrdinka M. Brdicka T. Novak P. Drbal K. Horejsi V. A new type of membrane raft-like microdomains and their possible involvement in TCR signaling. J Immunol. 2010;184:3689–3696. doi: 10.4049/jimmunol.0902075. [DOI] [PubMed] [Google Scholar]
- 292.Oyagbemi AA. Azeez OI. Saba AB. Interactions between reactive oxygen species and cancer: the roles of natural dietary antioxidants and their molecular mechanisms of action. Asian Pac J Cancer Prev. 2009;10:535–544. [PubMed] [Google Scholar]
- 293.Pani B. Ong HL. Liu X. Rauser K. Ambudkar IS. Singh BB. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE) J Biol Chem. 2008;283:17333–17340. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Paravicini TM. Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(Suppl 2):S170–S180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 295.Park SJ. Kim HY. Kim H. Park SM. Joe EH. Jou I. Choi YH. Oxidative stress induces lipid-raft-mediated activation of Src homology 2 domain-containing protein-tyrosine phosphatase 2 in astrocytes. Free Radic Biol Med. 2009;46:1694–1702. doi: 10.1016/j.freeradbiomed.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 296.Parton RG. Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 297.Patel RP. Moellering D. Murphy-Ullrich J. Jo H. Beckman JS. Darley-Usmar VM. Cell signaling by reactive nitrogen and oxygen species in atherosclerosis. Free Radic Biol Med. 2000;28:1780–1794. doi: 10.1016/s0891-5849(00)00235-5. [DOI] [PubMed] [Google Scholar]
- 298.Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta. 2008;1785:182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 299.Patterson SI. Posttranslational protein S-palmitoylation and the compartmentalization of signaling molecules in neurons. Biol Res. 2002;35:139–150. doi: 10.4067/s0716-97602002000200005. [DOI] [PubMed] [Google Scholar]
- 300.Pelkmans L. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim Biophys Acta. 2005;1746:295–304. doi: 10.1016/j.bbamcr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 301.Pelkmans L. Burli T. Zerial M. Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 302.Pelkmans L. Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–320. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 303.Pelkmans L. Kartenbeck J. Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 304.Perrone GG. Tan SX. Dawes IW. Reactive oxygen species and yeast apoptosis. Biochim Biophys Acta. 2008;1783:1354–1368. doi: 10.1016/j.bbamcr.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 305.Perry G. Castellani RJ. Hirai K. Smith MA. Reactive Oxygen Species Mediate Cellular Damage in Alzheimer Disease. J Alzheimers Dis. 1998;1:45–55. doi: 10.3233/jad-1998-1103. [DOI] [PubMed] [Google Scholar]
- 306.Peshavariya H. Dusting GJ. Di Bartolo B. Rye KA. Barter PJ. Jiang F. Reconstituted high-density lipoprotein suppresses leukocyte NADPH oxidase activation by disrupting lipid rafts. Free Radic Res. 2009;43:772–782. doi: 10.1080/10715760903045304. [DOI] [PubMed] [Google Scholar]
- 307.Pi J. Zhang Q. Fu J. Woods CG. Hou Y. Corkey BE. Collins S. Andersen ME. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 2010;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Piccoli C. Ria R. Scrima R. Cela O. D'Aprile A. Boffoli D. Falzetti F. Tabilio A. Capitanio N. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J Biol Chem. 2005;280:26467–26476. doi: 10.1074/jbc.M500047200. [DOI] [PubMed] [Google Scholar]
- 309.Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem J. 2004;378:281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Plowman SJ. Muncke C. Parton RG. Hancock JF. H-ras, K-ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc Natl Acad Sci U S A. 2005;102:15500–15505. doi: 10.1073/pnas.0504114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Plyte S. Majolini MB. Pacini S. Scarpini F. Bianchini C. Lanfrancone L. Pelicci P. Baldari CT. Constitutive activation of the Ras/MAP kinase pathway and enhanced TCR signaling by targeting the Shc adaptor to membrane rafts. Oncogene. 2000;19:1529–1537. doi: 10.1038/sj.onc.1203451. [DOI] [PubMed] [Google Scholar]
- 312.Pollard TD. Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 313.Poteser M. Graziani A. Rosker C. Eder P. Derler I. Kahr H. Zhu MX. Romanin C. Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- 314.Pralle A. Keller P. Florin EL. Simons K. Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Pritchard KA. Ackerman AW. Ou J. Curtis M. Smalley DM. Fontana JT. Stemerman MB. Sessa WC. Native low-density lipoprotein induces endothelial nitric oxide synthase dysfunction: role of heat shock protein 90 and caveolin-1. Free Radic Biol Med. 2002;33:52–62. doi: 10.1016/s0891-5849(02)00851-1. [DOI] [PubMed] [Google Scholar]
- 316.Puddu P. Puddu GM. Cravero E. Rosati M. Muscari A. The molecular sources of reactive oxygen species in hypertension. Blood Press. 2008;17:70–77. doi: 10.1080/08037050802029954. [DOI] [PubMed] [Google Scholar]
- 317.Qin XS. Tsukaguchi H. Shono A. Yamamoto A. Kurihara H. Doi T. Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol. 2009;20:2534–2545. doi: 10.1681/ASN.2009010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Qiu H. Edmunds T. Baker-Malcolm J. Karey KP. Estes S. Schwarz C. Hughes H. Van Patten SM. Activation of human acid sphingomyelinase through modification or deletion of C-terminal cysteine. J Biol Chem. 2003;278:32744–32752. doi: 10.1074/jbc.M303022200. [DOI] [PubMed] [Google Scholar]
- 319.Rajagopalan S. Kurz S. Munzel T. Tarpey M. Freeman BA. Griendling KK. Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Rao Malla R. Raghu H. Rao JS. Regulation of NADPH oxidase (Nox2) by lipid rafts in breast carcinoma cells. Int J Oncol. 2010;37:1483–1493. doi: 10.3892/ijo_00000801. [DOI] [PubMed] [Google Scholar]
- 321.Ravetch JV. Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 322.Razani B. Engelman JA. Wang XB. Schubert W. Zhang XL. Marks CB. Macaluso F. Russell RG. Li M. Pestell RG. Di Vizio D. Hou H., Jr. Kneitz B. Lagaud G. Christ GJ. Edelmann W. Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 323.Rehn M. Pihlajaniemi T. Alpha 1(XVIII), a collagen chain with frequent interruptions in the collagenous sequence, a distinct tissue distribution, and homology with type XV collagen. Proc Natl Acad Sci U S A. 1994;91:4234–4238. doi: 10.1073/pnas.91.10.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Rentsch D. Boorer KJ. Frommer WB. Structure and function of plasma membrane amino acid, oligopeptide and sucrose transporters from higher plants. J Membr Biol. 1998;162:177–190. doi: 10.1007/s002329900355. [DOI] [PubMed] [Google Scholar]
- 325.Resh MD. Use of analogs and inhibitors to study the functional significance of protein palmitoylation. Methods. 2006;40:191–197. doi: 10.1016/j.ymeth.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Rikihisa Y. Molecular events involved in cellular invasion by Ehrlichia chaffeensis and Anaplasma phagocytophilum. Vet Parasitol. 2010;167:155–166. doi: 10.1016/j.vetpar.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Rinia HA. de Kruijff B. Imaging domains in model membranes with atomic force microscopy. FEBS Lett. 2001;504:194–199. doi: 10.1016/s0014-5793(01)02704-1. [DOI] [PubMed] [Google Scholar]
- 328.Rinia HA. Snel MM. van der Eerden JP. de Kruijff B. Visualizing detergent resistant domains in model membranes with atomic force microscopy. FEBS Lett. 2001;501:92–96. doi: 10.1016/s0014-5793(01)02636-9. [DOI] [PubMed] [Google Scholar]
- 329.Riteau B. Barber DF. Long EO. Vav1 phosphorylation is induced by beta2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J Exp Med. 2003;198:469–474. doi: 10.1084/jem.20021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Rodighiero S. De Simoni A. Formenti A. The voltage-dependent nonselective cation current in human red blood cells studied by means of whole-cell and nystatin-perforated patch-clamp techniques. Biochim Biophys Acta. 2004;1660:164–170. doi: 10.1016/j.bbamem.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 331.Rosen GM. Pou S. Ramos CL. Cohen MS. Britigan BE. Free radicals and phagocytic cells. FASEB J. 1995;9:200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 332.Rossin A. Kral R. Lounnas N. Chakrabandhu K. Mailfert S. Marguet D. Hueber AO. Identification of a lysine-rich region of Fas as a raft nanodomain targeting signal necessary for Fas-mediated cell death. Exp Cell Res. 2010;316:1513–1522. doi: 10.1016/j.yexcr.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 333.Saitoh S. Zhang C. Tune JD. Potter B. Kiyooka T. Rogers PA. Knudson JD. Dick GM. Swafford A. Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006;26:2614–2621. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 334.Salzer U. Hinterdorfer P. Hunger U. Borken C. Prohaska R. Ca(++)-dependent vesicle release from erythrocytes involves stomatin-specific lipid rafts, synexin (annexin VII), and sorcin. Blood. 2002;99:2569–2577. doi: 10.1182/blood.v99.7.2569. [DOI] [PubMed] [Google Scholar]
- 335.Samhan-Arias AK. Garcia-Bereguiain MA. Martin-Romero FJ. Gutierrez-Merino C. Clustering of plasma membrane-bound cytochrome b5 reductase within “lipid raft” microdomains of the neuronal plasma membrane. Mol Cell Neurosci. 2009;40:14–26. doi: 10.1016/j.mcn.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 336.San Martin A. Griendling KK. Redox control of vascular smooth muscle migration. Antioxid Redox Signal. 2010;12:625–640. doi: 10.1089/ars.2009.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sasaki H. Yamamoto H. Tominaga K. Masuda K. Kawai T. Teshima-Kondo S. Rokutan K. NADPH oxidase-derived reactive oxygen species are essential for differentiation of a mouse macrophage cell line (RAW264.7) into osteoclasts. J Med Invest. 2009;56:33–41. doi: 10.2152/jmi.56.33. [DOI] [PubMed] [Google Scholar]
- 338.Sauer H. Wartenberg M. Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 339.Say YH. Hooper NM. Contamination of nuclear fractions with plasma membrane lipid rafts. Proteomics. 2007;7:1059–1064. doi: 10.1002/pmic.200600849. [DOI] [PubMed] [Google Scholar]
- 340.Scheel-Toellner D. Wang K. Assi LK. Webb PR. Craddock RM. Salmon M. Lord JM. Clustering of death receptors in lipid rafts initiates neutrophil spontaneous apoptosis. Biochem Soc Trans. 2004;32:679–681. doi: 10.1042/BST0320679. [DOI] [PubMed] [Google Scholar]
- 341.Schenck M. Carpinteiro A. Grassme H. Lang F. Gulbins E. Ceramide: physiological and pathophysiological aspects. Arch Biochem Biophys. 2007;462:171–175. doi: 10.1016/j.abb.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 342.Schengrund CL. Lipid rafts: keys to neurodegeneration. Brain Res Bull. 2010;82:7–17. doi: 10.1016/j.brainresbull.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 343.Schilling T. Eder C. Importance of lipid rafts for lysophosphatidylcholine-induced caspase-1 activation and reactive oxygen species generation. Cell Immunol. 2010;265:87–90. doi: 10.1016/j.cellimm.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 344.Schlegel A. Volonte D. Engelman JA. Galbiati F. Mehta P. Zhang XL. Scherer PE. Lisanti MP. Crowded little caves: structure and function of caveolae. Cell Signal. 1998;10:457–463. doi: 10.1016/s0898-6568(98)00007-2. [DOI] [PubMed] [Google Scholar]
- 345.Schmitz G. Grandl M. Role of redox regulation and lipid rafts in macrophages during Ox-LDL-mediated foam cell formation. Antioxid Redox Signal. 2007;9:1499–1518. doi: 10.1089/ars.2007.1663. [DOI] [PubMed] [Google Scholar]
- 346.Sengupta P. Baird B. Holowka D. Lipid rafts, fluid/fluid phase separation, and their relevance to plasma membrane structure and function. Semin Cell Dev Biol. 2007;18:583–590. doi: 10.1016/j.semcdb.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Shao D. Segal AW. Dekker LV. Lipid rafts determine efficiency of NADPH oxidase activation in neutrophils. FEBS Lett. 2003;550:101–106. doi: 10.1016/s0014-5793(03)00845-7. [DOI] [PubMed] [Google Scholar]
- 348.Sharma DK. Brown JC. Choudhury A. Peterson TE. Holicky E. Marks DL. Simari R. Parton RG. Pagano RE. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol Biol Cell. 2004;15:3114–3122. doi: 10.1091/mbc.E04-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Sheppard FR. Kelher MR. Moore EE. McLaughlin NJ. Banerjee A. Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 350.Shiose A. Kuroda J. Tsuruya K. Hirai M. Hirakata H. Naito S. Hattori M. Sakaki Y. Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 351.Siafakas AR. Wright LC. Sorrell TC. Djordjevic JT. Lipid rafts in Cryptococcus neoformans concentrate the virulence determinants phospholipase B1 and Cu/Zn superoxide dismutase. Eukaryot Cell. 2006;5:488–498. doi: 10.1128/EC.5.3.488-498.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Silva JP. Proenca F. Coutinho OP. Protective role of new nitrogen compounds on ROS/RNS-mediated damage to PC12 cells. Free Radic Res. 2008;42:57–69. doi: 10.1080/10715760701787719. [DOI] [PubMed] [Google Scholar]
- 353.Silvius JR. Nabi IR. Fluorescence-quenching and resonance energy transfer studies of lipid microdomains in model and biological membranes. Mol Membr Biol. 2006;23:5–16. doi: 10.1080/09687860500473002. [DOI] [PubMed] [Google Scholar]
- 354.Simons K. Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110:597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Simons K. Gruenberg J. Jamming the endosomal system: lipid rafts and lysosomal storage diseases. Trends Cell Biol. 2000;10:459–462. doi: 10.1016/s0962-8924(00)01847-x. [DOI] [PubMed] [Google Scholar]
- 356.Simons K. Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 357.Simons K. Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 358.Simons K. Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 359.Simons K. van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 360.Singer SJ. A fluid lipid-globular protein mosaic model of membrane structure. Ann N Y Acad Sci. 1972;195:16–23. [PubMed] [Google Scholar]
- 361.Singer SJ. Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–31. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 362.Siow YL. Au-Yeung KK. Woo CW O K. Homocysteine stimulates phosphorylation of NADPH oxidase p47phox and p67phox subunits in monocytes via protein kinase Cbeta activation. Biochem J. 2006;398:73–82. doi: 10.1042/BJ20051810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Slaughter N. Laux I. Tu X. Whitelegge J. Zhu X. Effros R. Bickel P. Nel A. The flotillins are integral membrane proteins in lipid rafts that contain TCR-associated signaling components: implications for T-cell activation. Clin Immunol. 2003;108:138–151. doi: 10.1016/s1521-6616(03)00097-4. [DOI] [PubMed] [Google Scholar]
- 364.Smythies J. Redox aspects of signaling by catecholamines and their metabolites. Antioxid Redox Signal. 2000;2:575–583. doi: 10.1089/15230860050192332. [DOI] [PubMed] [Google Scholar]
- 365.Song KS. Li S. Okamoto T. Quilliam LA. Sargiacomo M. Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 366.Sorice M. Manganelli V. Matarrese P. Tinari A. Misasi R. Malorni W. Garofalo T. Cardiolipin-enriched raft-like microdomains are essential activating platforms for apoptotic signals on mitochondria. FEBS Lett. 2009;583:2447–2450. doi: 10.1016/j.febslet.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 367.Soubias O. Gawrisch K. Nuclear magnetic resonance investigation of oriented lipid membranes. Methods Mol Biol. 2007;400:77–88. doi: 10.1007/978-1-59745-519-0_6. [DOI] [PubMed] [Google Scholar]
- 368.Sowa G. Pypaert M. Sessa WC. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci U S A. 2001;98:14072–14077. doi: 10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Stadtman ER. Role of oxidant species in aging. Curr Med Chem. 2004;11:1105–12. doi: 10.2174/0929867043365341. [DOI] [PubMed] [Google Scholar]
- 370.Stan RV. Structure and function of endothelial caveolae. Microsc Res Tech. 2002;57:350–364. doi: 10.1002/jemt.10089. [DOI] [PubMed] [Google Scholar]
- 371.Stancevic B. Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 2010;584:1728–1740. doi: 10.1016/j.febslet.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Steinbauer B. Mehnert T. Beyer K. Hydration and lateral organization in phospholipid bilayers containing sphingomyelin: a 2H-NMR study. Biophys J. 2003;85:1013–1024. doi: 10.1016/S0006-3495(03)74540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Stuven E. Porat A. Shimron F. Fass E. Kaloyanova D. Brugger B. Wieland FT. Elazar Z. Helms JB. Intra-Golgi protein transport depends on a cholesterol balance in the lipid membrane. J Biol Chem. 2003;278:53112–53122. doi: 10.1074/jbc.M300402200. [DOI] [PubMed] [Google Scholar]
- 374.Sumimoto H. Miyano K. Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- 375.Sun G. Xu X. Wang Y. Shen X. Chen Z. Yang J. Mycoplasma pneumoniae infection induces reactive oxygen species and DNA damage in A549 human lung carcinoma cells. Infect Immun. 2008;76:4405–4413. doi: 10.1128/IAI.00575-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Suzuki KG. Fujiwara TK. Sanematsu F. Iino R. Edidin M. Kusumi A. GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J Cell Biol. 2007;177:717–730. doi: 10.1083/jcb.200609174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Sverdlov M. Shajahan AN. Minshall RD. Tyrosine phosphorylation-dependence of caveolae-mediated endocytosis. J Cell Mol Med. 2007;11:1239–1250. doi: 10.1111/j.1582-4934.2007.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Szanto I. Rubbia-Brandt L. Kiss P. Steger K. Banfi B. Kovari E. Herrmann F. Hadengue A. Krause KH. Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol. 2005;207:164–176. doi: 10.1002/path.1824. [DOI] [PubMed] [Google Scholar]
- 379.Szocs K. Endothelial dysfunction and reactive oxygen species production in ischemia/reperfusion and nitrate tolerance. Gen Physiol Biophys. 2004;23:265–295. [PubMed] [Google Scholar]
- 380.Szoke E. Borzsei R. Toth DM. Lengl O. Helyes Z. Sandor Z. Szolcsanyi J. Effect of lipid raft disruption on TRPV1 receptor activation of trigeminal sensory neurons and transfected cell line. Eur J Pharmacol. 2010;628:67–74. doi: 10.1016/j.ejphar.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 381.Tang XL. Takano H. Rizvi A. Turrens JF. Qiu Y. Wu WJ. Zhang Q. Bolli R. Oxidant species trigger late preconditioning against myocardial stunning in conscious rabbits. Am J Physiol Heart Circ Physiol. 2002;282:H281–H291. doi: 10.1152/ajpheart.2002.282.1.H281. [DOI] [PubMed] [Google Scholar]
- 382.Tani M. Ito M. Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell Signal. 2007;19:229–237. doi: 10.1016/j.cellsig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 383.Tian W. Li XJ. Stull ND. Ming W. Suh CI. Bissonnette SA. Yaffe MB. Grinstein S. Atkinson SJ. Dinauer MC. Fc{gamma}R-stimulated activation of the NADPH oxidase: phosphoinositide-binding protein p40phox regulates NADPH oxidase activity after enzyme assembly on the phagosome. Blood. 2008;112:3867–3877. doi: 10.1182/blood-2007-11-126029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Toledo-Pereyra LH. Lopez-Neblina F. Toledo AH. Reactive oxygen species and molecular biology of ischemia/reperfusion. Ann Transplant. 2004;9:81–83. [PubMed] [Google Scholar]
- 385.Touyz RM. Lipid rafts take center stage in endothelial cell redox signaling by death receptors. Hypertension. 2006;47:16–18. doi: 10.1161/01.HYP.0000196730.13216.f3. [DOI] [PubMed] [Google Scholar]
- 386.Ueda N. Kaushal GP. Shah SV. Apoptotic mechanisms in acute renal failure. Am J Med. 2000;108:403–415. doi: 10.1016/s0002-9343(00)00311-9. [DOI] [PubMed] [Google Scholar]
- 387.Ueno N. Takeya R. Miyano K. Kikuchi H. Sumimoto H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J Biol Chem. 2005;280:23328–23339. doi: 10.1074/jbc.M414548200. [DOI] [PubMed] [Google Scholar]
- 388.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 389.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Ushio-Fukai M. Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 391.Ushio-Fukai M. Hilenski L. Santanam N. Becker PL. Ma Y. Griendling KK. Alexander RW. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem. 2001;276:48269–48275. doi: 10.1074/jbc.M105901200. [DOI] [PubMed] [Google Scholar]
- 392.Utermohlen O. Herz J. Schramm M. Kronke M. Fusogenicity of membranes: the impact of acid sphingomyelinase on innate immune responses. Immunobiology. 2008;213:307–314. doi: 10.1016/j.imbio.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 393.Valensin S. Paccani SR. Ulivieri C. Mercati D. Pacini S. Patrussi L. Hirst T. Lupetti P. Baldari CT. F-actin dynamics control segregation of the TCR signaling cascade to clustered lipid rafts. Eur J Immunol. 2002;32:435–446. doi: 10.1002/1521-4141(200202)32:2<435::AID-IMMU435>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 394.van Meer G. Simons K. Lipid polarity and sorting in epithelial cells. J Cell Biochem. 1988;36:51–58. doi: 10.1002/jcb.240360106. [DOI] [PubMed] [Google Scholar]
- 395.Van Wart HE. Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.van Zanten TS. Cambi A. Koopman M. Joosten B. Figdor CG. Garcia-Parajo MF. Hotspots of GPI-anchored proteins and integrin nanoclusters function as nucleation sites for cell adhesion. Proc Natl Acad Sci U S A. 2009;106:18557–18562. doi: 10.1073/pnas.0905217106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Vetrivel KS. Cheng H. Lin W. Sakurai T. Li T. Nukina N. Wong PC. Xu H. Thinakaran G. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Vilhardt F. van Deurs B. The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO J. 2004;23:739–748. doi: 10.1038/sj.emboj.7600066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Wan J. Roth AF. Bailey AO. Davis NG. Palmitoylated proteins: purification and identification. Nat Protoc. 2007;2:1573–1584. doi: 10.1038/nprot.2007.225. [DOI] [PubMed] [Google Scholar]
- 400.Wang HD. Johns DG. Xu S. Cohen RA. Role of superoxide anion in regulating pressor and vascular hypertrophic response to angiotensin II. Am J Physiol Heart Circ Physiol. 2002;282:H1697–702. doi: 10.1152/ajpheart.00914.2001. [DOI] [PubMed] [Google Scholar]
- 401.Wang L. Sapuri-Butti AR. Aung HH. Parikh AN. Rutledge JC. Triglyceride-rich lipoprotein lipolysis increases aggregation of endothelial cell membrane microdomains and produces reactive oxygen species. Am J Physiol Heart Circ Physiol. 2008;295:H237–H244. doi: 10.1152/ajpheart.01366.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Wang T. Gu J. Wu PF. Wang F. Xiong Z. Yang YJ. Wu WN. Dong LD. Chen JG. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-kappaB pathways and inhibition of intracellular ROS/RNS generation. Free Radic Biol Med. 2009;47:229–240. doi: 10.1016/j.freeradbiomed.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 403.Wang X. Tian QB. Okano A. Sakagami H. Moon IS. Kondo H. Endo S. Suzuki T. BAALC 1–6-8 protein is targeted to postsynaptic lipid rafts by its N-terminal myristoylation and palmitoylation, and interacts with alpha, but not beta, subunit of Ca/calmodulin-dependent protein kinase II. J Neurochem. 2005;92:647–659. doi: 10.1111/j.1471-4159.2004.02902.x. [DOI] [PubMed] [Google Scholar]
- 404.Wang YX. Zheng YM. ROS-dependent signaling mechanisms for hypoxic Ca(2+) responses in pulmonary artery myocytes. Antioxid Redox Signal. 2010;12:611–623. doi: 10.1089/ars.2009.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Watanabe M. Wake H. Moorhouse AJ. Nabekura J. Clustering of neuronal K+-Cl- cotransporters in lipid rafts by tyrosine phosphorylation. J Biol Chem. 2009;284:27980–27988. doi: 10.1074/jbc.M109.043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Waugh MG. Minogue S. Anderson JS. dos Santos M. Hsuan JJ. Signalling and non-caveolar rafts. Biochem Soc Trans. 2001;29:509–511. doi: 10.1042/bst0290509. [DOI] [PubMed] [Google Scholar]
- 407.Weaver AK. Olsen ML. McFerrin MB. Sontheimer H. BK channels are linked to inositol 1,4,5-triphosphate receptors via lipid rafts: a novel mechanism for coupling [Ca(2+)](i) to ion channel activation. J Biol Chem. 2007;282:31558–31568. doi: 10.1074/jbc.M702866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Wei XF. Zhou QG. Hou FF. Liu BY. Liang M. Advanced oxidation protein products induce mesangial cell perturbation through PKC-dependent activation of NADPH oxidase. Am J Physiol Renal Physiol. 2009;296:F427–F437. doi: 10.1152/ajprenal.90536.2008. [DOI] [PubMed] [Google Scholar]
- 409.Weinberg F. Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66:3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Weintraub NL. Nox response to injury. Arterioscler Thromb Vasc Biol. 2002;22:4–5. [PubMed] [Google Scholar]
- 411.Whaley-Connell A. Habibi J. Nistala R. Cooper SA. Karuparthi PR. Hayden MR. Rehmer N. DeMarco VG. Andresen BT. Wei Y. Ferrario C. Sowers JR. Attenuation of NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension. 2008;51:474–480. doi: 10.1161/HYPERTENSIONAHA.107.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.White BH. Sidhu A. Increased oxidative stress in renal proximal tubules of the spontaneously hypertensive rat: a mechanism for defective dopamine D1A receptor/G-protein coupling. J Hypertens. 1998;16:1659–1665. doi: 10.1097/00004872-199816110-00013. [DOI] [PubMed] [Google Scholar]
- 413.Wolf G. Role of reactive oxygen species in angiotensin II-mediated renal growth, differentiation, and apoptosis. Antioxid Redox Signal. 2005;7:1337–1345. doi: 10.1089/ars.2005.7.1337. [DOI] [PubMed] [Google Scholar]
- 414.Wolin MS. Subcellular localization of Nox-containing oxidases provides unique insight into their role in vascular oxidant signaling. Arterioscler Thromb Vasc Biol. 2004;24:625–627. doi: 10.1161/01.ATV.0000117201.14603.5d. [DOI] [PubMed] [Google Scholar]
- 415.Wolin MS. Ahmad M. Gao Q. Gupte SA. Cytosolic NAD(P)H regulation of redox signaling and vascular oxygen sensing. Antioxid Redox Signal. 2007;9:671–678. doi: 10.1089/ars.2007.1559. [DOI] [PubMed] [Google Scholar]
- 416.Wolin MS. Ahmad M. Gupte SA. Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol. 2005;289:L159–L173. doi: 10.1152/ajplung.00060.2005. [DOI] [PubMed] [Google Scholar]
- 417.Wolin MS. Ahmad M. Gupte SA. The sources of oxidative stress in the vessel wall. Kidney Int. 2005;67:1659–1661. doi: 10.1111/j.1523-1755.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 418.Woudenberg J. Rembacz KP. van den Heuvel FA. Woudenberg-Vrenken TE. Buist-Homan M. Geuken M. Hoekstra M. Deelman LE. Enrich C. Henning RH. Moshage H. Faber KN. Caveolin-1 is enriched in the peroxisomal membrane of rat hepatocytes. Hepatology. 2010;51:1744–1753. doi: 10.1002/hep.23460. [DOI] [PubMed] [Google Scholar]
- 419.Wu H. Mahmood A. Lu D. Jiang H. Xiong Y. Zhou D. Chopp M. Attenuation of astrogliosis and modulation of endothelial growth factor receptor in lipid rafts by simvastatin after traumatic brain injury. J Neurosurg. 2010;113:591–597. doi: 10.3171/2009.9.JNS09859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Xia F. Gao X. Kwan E. Lam PP. Chan L. Sy K. Sheu L. Wheeler MB. Gaisano HY. Tsushima RG. Disruption of pancreatic beta-cell lipid rafts modifies Kv2.1 channel gating and insulin exocytosis. J Biol Chem. 2004;279:24685–24691. doi: 10.1074/jbc.M314314200. [DOI] [PubMed] [Google Scholar]
- 421.Yamamoto S. Shimizu S. Mori Y. Involvement of TRPM2 channel in amplification of reactive oxygen species-induced signaling and chronic inflammation. Nippon Yakurigaku Zasshi. 2009;134:122–126. doi: 10.1254/fpj.134.122. [DOI] [PubMed] [Google Scholar]
- 422.Yang B. Oo TN. Rizzo V. Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. Faseb J. 2006;20:1501–1503. doi: 10.1096/fj.05-5359fje. [DOI] [PubMed] [Google Scholar]
- 423.Yang B. Rizzo V. TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H954–H962. doi: 10.1152/ajpheart.00758.2006. [DOI] [PubMed] [Google Scholar]
- 424.Yang Z. Asico LD. Yu P. Wang Z. Jones JE. Escano CS. Wang X. Quinn MT. Sibley DR. Romero GG. Felder RA. Jose PA. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R96–R104. doi: 10.1152/ajpregu.00434.2005. [DOI] [PubMed] [Google Scholar]
- 425.Yao Y. Hong S. Zhou H. Yuan T. Zeng R. Liao K. The differential protein and lipid compositions of noncaveolar lipid microdomains and caveolae. Cell Res. 2009;19:497–506. doi: 10.1038/cr.2009.27. [DOI] [PubMed] [Google Scholar]
- 426.Yaqoob P. The nutritional significance of lipid rafts. Annu Rev Nutr. 2009;29:257–282. doi: 10.1146/annurev-nutr-080508-141205. [DOI] [PubMed] [Google Scholar]
- 427.Yasunari K. Kohno M. Kano H. Minami M. Yoshikawa J. Dopamine as a novel antioxidative agent for rat vascular smooth muscle cells through dopamine D(1)-like receptors. Circulation. 2000;101:2302–2308. doi: 10.1161/01.cir.101.19.2302. [DOI] [PubMed] [Google Scholar]
- 428.Yi F. Chen QZ. Jin S. Li PL. Mechanism of homocysteine-induced Rac1/NADPH oxidase activation in mesangial cells: role of guanine nucleotide exchange factor Vav2. Cell Physiol Biochem. 2007;20:909–918. doi: 10.1159/000110451. [DOI] [PubMed] [Google Scholar]
- 429.Yi F. Jin S. Li PL. Lipid raft-redox signaling platforms in plasma membrane. Methods Mol Biol. 2009;580:93–107. doi: 10.1007/978-1-60761-325-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Yi F. Jin S. Zhang F. Xia M. Bao JX. Hu J. Poklis JL. Li PL. Formation of lipid raft redox signalling platforms in glomerular endothelial cells: an early event of homocysteine-induced glomerular injury. J Cell Mol Med. 2009;13:3303–3314. doi: 10.1111/j.1582-4934.2009.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Yi F. Xia M. Li N. Zhang C. Tang L. Li PL. Contribution of guanine nucleotide exchange factor Vav2 to hyperhomocysteinemic glomerulosclerosis in rats. Hypertension. 2009;53:90–96. doi: 10.1161/HYPERTENSIONAHA.108.115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Yi F. Zhang AY. Janscha JL. Li PL. Zou AP. Homocysteine activates NADH/NADPH oxidase through ceramide-stimulated Rac GTPase activity in rat mesangial cells. Kidney Int. 2004;66:1977–1987. doi: 10.1111/j.1523-1755.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- 433.Yi XY. Li VX. Zhang F. Yi F. Matson DR. Jiang MT. Li PL. Characteristics and actions of NAD(P)H oxidase on the sarcoplasmic reticulum of coronary artery smooth muscle. Am J Physiol Heart Circ Physiol. 2006;290:H1136–H1144. doi: 10.1152/ajpheart.00296.2005. [DOI] [PubMed] [Google Scholar]
- 434.Yin T. Sandhu G. Wolfgang CD. Burrier A. Webb RL. Rigel DF. Hai T. Whelan J. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. J Biol Chem. 1997;272:19943–19950. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- 435.Ying M. Grimmer S. Iversen TG. Van Deurs B. Sandvig K. Cholesterol loading induces a block in the exit of VSVG from the TGN. Traffic. 2003;4:772–784. doi: 10.1034/j.1600-0854.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- 436.Young RM. Zheng X. Holowka D. Baird B. Reconstitution of regulated phosphorylation of FcepsilonRI by a lipid raft-excluded protein-tyrosine phosphatase. J Biol Chem. 2005;280:1230–1235. doi: 10.1074/jbc.M408339200. [DOI] [PubMed] [Google Scholar]
- 437.Yu P. Yang Z. Jones JE. Wang Z. Owens SA. Mueller SC. Felder RA. Jose PA. D1 dopamine receptor signaling involves caveolin-2 in HEK-293 cells. Kidney Int. 2004;66:2167–2180. doi: 10.1111/j.1523-1755.2004.66007.x. [DOI] [PubMed] [Google Scholar]
- 438.Yuan T. Hong S. Yao Y. Liao K. Glut-4 is translocated to both caveolae and non-caveolar lipid rafts, but is partially internalized through caveolae in insulin-stimulated adipocytes. Cell Res. 2007;17:772–782. doi: 10.1038/cr.2007.73. [DOI] [PubMed] [Google Scholar]
- 439.Zabrocki P. Bastiaens I. Delay C. Bammens T. Ghillebert R. Pellens K. De Virgilio C. Van Leuven F. Winderickx J. Phosphorylation, lipid raft interaction and traffic of alpha-synuclein in a yeast model for Parkinson. Biochim Biophys Acta. 2008;1783:1767–1780. doi: 10.1016/j.bbamcr.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 440.Zeng C. Villar VA. Yu P. Zhou L. Jose PA. Reactive oxygen species and dopamine receptor function in essential hypertension. Clin Exp Hypertens. 2009;31:156–178. doi: 10.1080/10641960802621283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Zhai J. Strom AL. Kilty R. Venkatakrishnan P. White J. Everson WV. Smart EJ. Zhu H. Proteomic characterization of lipid raft proteins in amyotrophic lateral sclerosis mouse spinal cord. FEBS J. 2009;276:3308–3323. doi: 10.1111/j.1742-4658.2009.07057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Zhang AY. Teggatz EG. Zou AP. Campbell WB. Li PL. Endostatin uncouples NO and Ca2+ response to bradykinin through enhanced O2*- production in the intact coronary endothelium. Am J Physiol Heart Circ Physiol. 2005;288:H686–H694. doi: 10.1152/ajpheart.00174.2004. [DOI] [PubMed] [Google Scholar]
- 443.Zhang AY. Yi F. Jin S. Xia M. Chen QZ. Gulbins E. Li PL. Acid sphingomyelinase and its redox amplification in formation of lipid raft redox signaling platforms in endothelial cells. Antioxid Redox Signal. 2007;9:817–828. doi: 10.1089/ars.2007.1509. [DOI] [PubMed] [Google Scholar]
- 444.Zhang AY. Yi F. Zhang G. Gulbins E. Li PL. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension. 2006;47:74–80. doi: 10.1161/10.1161/01.HYP.0000196727.53300.62. [DOI] [PubMed] [Google Scholar]
- 445.Zhang C. Hu JJ. Xia M. Boini KM. Brimson C. Li PL. Redox signaling via lipid raft clustering in homocysteine-induced injury of podocytes. Biochim Biophys Acta. 2010;1803:482–491. doi: 10.1016/j.bbamcr.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Zhang C. Li PL. Membrane raft redox signalosomes in endothelial cells. Free Radic Res. 2010;44:831–842. doi: 10.3109/10715762.2010.485994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Zhang F. Zhang G. Zhang AY. Koeberl MJ. Wallander E. Li PL. Production of NAADP and its role in Ca2+ mobilization associated with lysosomes in coronary arterial myocytes. Am J Physiol Heart Circ Physiol. 2006;291:H274–H282. doi: 10.1152/ajpheart.01064.2005. [DOI] [PubMed] [Google Scholar]
- 448.Zhang G. Zhang F. Muh R. Yi F. Chalupsky K. Cai H. Li PL. Autocrine/paracrine pattern of superoxide production through NAD(P)H oxidase in coronary arterial myocytes. Am J Physiol Heart Circ Physiol. 2007;292:H483–H495. doi: 10.1152/ajpheart.00632.2006. [DOI] [PubMed] [Google Scholar]
- 449.Zhang Y. Li X. Becker KA. Gulbins E. Ceramide-enriched membrane domains—structure and function. Biochim Biophys Acta. 2009;1788:178–183. doi: 10.1016/j.bbamem.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 450.Zheng L. McQuaw CM. Ewing AG. Winograd N. Sphingomyelin/phosphatidylcholine and cholesterol interactions studied by imaging mass spectrometry. J Am Chem Soc. 2007;129:15730–15731. doi: 10.1021/ja0741675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Zheng YZ. Berg KB. Foster LJ. Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. J Lipid Res. 2009;50:988–998. doi: 10.1194/jlr.M800658-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Zuo L. Ushio-Fukai M. Hilenski LL. Alexander RW. Microtubules regulate angiotensin II type 1 receptor and Rac1 localization in caveolae/lipid rafts: role in redox signaling. Arterioscler Thromb Vasc Biol. 2004;24:1223–1228. doi: 10.1161/01.ATV.0000132400.25045.2a. [DOI] [PubMed] [Google Scholar]
- 453.Zuo L. Ushio-Fukai M. Ikeda S. Hilenski L. Patrushev N. Alexander RW. Caveolin-1 is essential for activation of Rac1 and NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells: role in redox signaling and vascular hypertrophy. Arterioscler Thromb Vasc Biol. 2005;25:1824–1830. doi: 10.1161/01.ATV.0000175295.09607.18. [DOI] [PubMed] [Google Scholar]
- 454.Zurzolo C. van't Hof W. van Meer G. Rodriguez-Boulan E. VIP21/caveolin, glycosphingolipid clusters and the sorting of glycosylphosphatidylinositol-anchored proteins in epithelial cells. EMBO J. 1994;13:42–53. doi: 10.1002/j.1460-2075.1994.tb06233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]