Abstract
Various types of cardiomyocytes undergo changes in automaticity and electrical properties during fetal heart development. Human embryonic stem cell-derived cardiomyocytes (hESC-CMs), like fetal cardiomyocytes, are electrophysiologically immature and exhibit automaticity. We used hESC-CMs to investigate developmental changes in mechanisms of automaticity and to determine whether electrophysiological maturation is driven by an intrinsic developmental clock and/or is regulated by interactions with non-cardiomyocytes in embryoid bodies (EBs). We isolated pure populations of hESC-CMs from EBs by lentivirus-engineered Puromycin resistance at various stages of differentiation. Using pharmacological agents, calcium (Ca2+) imaging, and intracellular recording techniques, we found that intracellular Ca2+-cycling mechanisms developed early and contributed to dominant automaticity throughout hESC-CM differentiation. Sarcolemmal ion channels evolved later upon further differentiation within EBs and played an increasing role in controlling automaticity and electrophysiological properties of hESC-CMs. In contrast to the development of intracellular Ca2+-handling proteins, ion channel development and electrophysiological maturation of hESC-CMs did not occur when hESC-CMs were isolated from EBs early and maintained in culture without further interaction with non-cardiomyocytes. Adding back non-cardiomyocytes to early-isolated hESC-CMs rescued the arrest of electrophysiological maturation, indicating that non-cardiomyocytes in EBs drive electrophysiological maturation of early hESC-CMs. Non-cardiomyocytes in EBs contain most cell types present in the embryonic heart that are known to influence early cardiac development. Our study is the first to demonstrate that non-cardiomyocytes influence electrophysiological maturation of early hESC-CMs in cultures. Defining the nature of these extrinsic signals will aid in the directed maturation of immature hESC-CMs to mitigate arrhythmogenic risks of cell-based therapies.
Introduction
Animal models and human clinical trials have suggested the promising therapeutic potential of stem and progenitor cell (SPC)-based therapies for myocardial replacement [1] and for generating biological pacemaker cells [2]. However, most SPC-derived cardiomyocytes (SPC-CMs) possess automaticity despite differences in their action potential properties [3–5] and are reminiscent of primitive cardiomyocytes isolated from various regions of embryonic hearts [3,6]. Since implantation of immature cardiomyocytes carries potential arrhythmogenic risks [1], defining mechanisms that govern automaticity and electrophysiological maturation during SPC-CM differentiation is important for developing safe replacement therapies. Since the molecular natures of signals that stimulate electrical maturation during cardiomyogenesis are largely unknown, we evaluated the electrophysiological development of early human embryonic stem cell-derived cardiomyocytes (hESC-CMs) and addressed whether an intrinsic developmental timer versus extrinsic signals directs electrophysiological maturation.
The physiological mechanisms that underlie automaticity in early-differentiated human or mouse ESC-CMs, as in mature sinus nodal cells (SNCs), are complex and remain controversial [5–11]. Various sarcolemmal ion channels, ion exchangers, and intracellular calcium (Ca2+) cycling machinery have been implicated in impulse generation of ESC-CMs [5,9–13]. However, intracellular Ca2+ handling machinery has been reported to be immature in some studies of hESC-CMs [12,13]. Additionally, ion channels, such as T-type Ca2+ [10], sodium (Na+) [5], and hyperpolarization-activated cyclic nucleotide-gated (HCN) channels [10,11] of ESC-CMs, also display developmental changes during ESC-CM differentiation. Whether development of intracellular Ca2+ handling machinery and ion channels in hESC-CMs requires extrinsic cues for further maturation has been largely unexplored. In mouse embryonic hearts, ion channels are known to undergo developmental changes during cardiogenesis [14,15], but little is known about the molecular pathways or developmental cues that control electrical maturation of early human fetal cardiomyocytes. Differentiating embryoid bodies (EBs) derived from mouse ESCs have been used as a model system to study cardiogenesis, in part because they contain many non-cardiomyocytes, such as endodermal, endothelial, neural crest, and epicardial cells, which influence development of cardiomyocytes [3,9,16]. Therefore, we used human ESC-derived beating EBs, containing similar non-cardiomyocytes, to study developmental cues of inducing electrophysiological maturation of hESC-CMs.
In this study, we isolated hESC-CMs from EBs with a Puromycin-selection method [17] and compared their electrical maturation in isolation, to maturation in the presence of non-cardiomyocytes. Puromycin selection of hESC-CMs efficiently removed non-cardiomyocytes and overcame the limitations of commonly used methods, such as impurity (manual microdissection and density sedimentation) or poor viability (fluorescence-activated cell sorting) [5,18]. We then studied developmental changes in mechanisms of dominant automaticity of purified hESC-CMs by Ca2+ imaging and pharmacological intervention as well as investigated their electrical maturation by intracellular recordings during hESC-CM differentiation in EBs. We found that intracellular Ca2+-mediated mechanisms developed early and depicted similar contribution to automaticity during 20–60 days of hESC-CM differentiation without significant influences from neighboring non-cardiomyocytes in EBs. Sarcolemmal ion channels developed later with maturation in EBs and displayed an increasing contribution to automaticity and electrical properties of hESC-CMs. Importantly, hESC-CMs isolated at early stages of differentiation without further contacts with non-cardiomyocytes showed arrested electrical maturation and ion channel development. This arrested electrical maturation can be rescued by adding non-cardiomyocytes back to hESC-CM cultures. These results suggest that non-cardiomyocytes in developing EBs provide signals that direct electrophysiological maturation of early hESC-CMs.
Methods
Generation of Puromycin-selected hESC-derived cardiomyocyte spheroids
Details of the protocols to establish stable lines of uniformly transduced H9 hESCs [17] and to purify hESC-CMs are provided in Supplementary Materials (available online at www.liebertonline.com/scd). In brief, cardiomyocyte spheroids (CSs) of hESC-CMs were purified from EBs harboring the α-MHC promoter-driven Puromycin-resistant (α-MHC-Puror) cassette by treatment with 1.8 μg/mL Puromycin for 36–48 h and were analyzed on day 20 (early CS) and day 60 (60D) (Fig. 1). We operationally define cells in EBs that are killed by Puromycin treatment as “non-cardiomyocytes (non-CMs)” because they do not express α-MHC-Puror. To evaluate whether developmental changes resulted from intrinsic versus extrinsic cues, 20D CSs were maintained in culture for an additional 40 days (20+40D) without non-CMs. The Puromycin purification process did not affect the rate of primitive hESC-CM proliferation (Supplementary Fig. 1). We also added parental H9 hESC-derived EBs, containing mostly non-CMs without Puromycin resistance, back to 20D CSs to rescue the maturational deficit (add-back experiments, see below and Supplementary Materials for details).
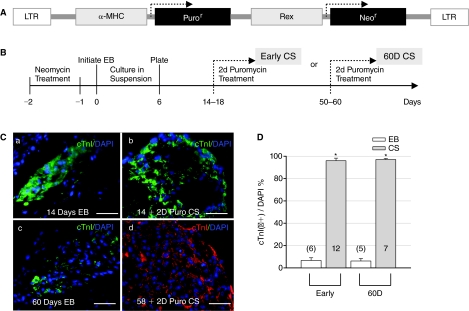
FIG. 1.
Culture, differentiation, and Puromycin selection of human embryonic stem cell (hESC)-derived cardiomyocyte spheroids (CSs). (A) Diagram of the lentivirus used to stably integrate α-MHC-Puror and Rex-Neor genes into hESCs. (B) Scheme of culturing procedures and Puromycin selection at 2 different stages of hESC-derived CSs. (C) Staining with cTnI antibodies (a) of an embryoid body (EB) at day 14 of differentiation (14D); (b) of a CS following 2 days of Puromycin (Puro) selection after 14D (early CS); (c) of an EB at 60 days; and (d) of a CS after 2 days of Puromycin selection at 58 days (60D CS). DAPI stains cell nuclei (blue). Scale bars are 50 μM. (D) Puromycin selection resulted in 95.99% ± 2.29% and 97.02% ± 0.96% purity of cardiomyocytes from early (6.67% ± 2.41%) and 60D (6.15% ± 1.24%) EBs. The number in each column and in parentheses represents the number of CS or EB clusters tested in this and following figures. Asterisks denote statistically significant difference between CSs and EBs.
Ca2+ imaging, intracellular recordings, and pharmacological applications
Standard techniques were used for intracellular recording and Ca2+ imaging with Fluo-3AM (Biotium, Inc., CA) of hESC-CSs. In brief, beating CSs were incubated with 5 μM Fluo-3AM and 0.1% DMSO in knockout (KO)-Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) for 30 min at 37°C. After the dye loading process, coverslips containing these cells were transferred to the recording chamber of an inverted microscope (Zeiss, Oberkochen, Germany) and were constantly superfused with pre-oxygenated KO-DMEM. Fluorescent signals were obtained by a cooled CCD camera (Cooke Sensicam; PCO, Kelheim, Germany) using SlideBook software (Intelligent Imaging Innovations, Santa Monica, CA). The sources and the final concentrations (in KO-DMEM) of pharmacological agents used were (in pM): 1 ZD-7899, 10 KB-R7943, and 10 ryanodine from Tocris (Ellisville, MO); 1 tetrodotoxin (TTX) from Tocris; and 10 2-APB from Sigma (St. Louis, MO) and Tocris. Beating CSs were stabilized for 10 min and the Ca2+ signal was then recorded for 10 min as the baseline control. All solutions, with or without drugs, were perfused into the chamber by a temperature-controlled perfusion system (ALA Scientific Instrument Inc., NY) at a rate of 1–2 mL/min and Ca2+ signals recorded after 10 min of drug applications were used for comparison (see Supplementary Figs. 2 and 3).
For intracellular recordings of beating CSs, sharp glass microelectrodes with resistances of 50–150 MΩ, when filled with 3 M KCl, were used. The intracellular recordings of action potentials (APs) were obtained using an AxoPatch 200B amplifier in current clamp mode and pCLAMP-10 software (Molecular Devices, Sunnyvale, CA). Data were sampled at 10 kHz using Clampex 10 software (Molecular Devices) and low-pass filtered at 5 kHz. The following parameters of APs with >10 s of stable baselines were measured: AP amplitude (APA), maximum diastolic potential (MDP), maximal upstroke velocity (Vmax), AP duration at 90% of the repolarization (APD90), and the cycle length between 2 spontaneous APs (RR). There is no known formula that can precisely correct the influences of beating rates (BRs) on the APD (restitution effect). In order to obtain the mean value of APD90 from different CSs, the value of APD90 was corrected by heart rates with commonly used Bazett formula (APD/square root of RR) to avoid restitution effects on ADP90. Details of these protocols are provided in Supplementary Materials. All experiments were conducted at 37°C and with 95% O2/5% CO2.
Quantitative RT-PCR and immunostaining of frozen sections
Standard techniques were used for quantitative RT-PCR, immunostaining, and cryosections of EBs and CSs. In brief, total RNA was prepared using Qiagen RNeasy Lipid Tissue Kit (Qiagen) from 5 to 10 Puromycin-selected CSs at various stages of hESC-CM differentiation. cDNA was synthesized by using a poly-dT primer and Superscript II Reverse Transcriptase (Invitrogen, Carlsbad, CA). Quantitative real-time polymerase chain reaction (PCR) was performed on a Roche Light Cycler using the Light Cycler FastStart DNA master SYBR Green I kit (Roche Applied Science, Indianapolis, IN). Quantification was carried out by correcting for amplification efficiency of the primer using a standard curve and followed by normalizing transcript levels to the amount of total ubiquitously expressed GAPDH or PGK transcripts to control for variations in total amount of cDNA. Three to four respective sets of experiments from various stages of CSs were used to construct the histogram shown in the Results section. Detailed protocols of quantitative RT-PCR and immunostaining of frozen sections as well as PCR primer sequences and antibodies used are provided in Supplementary Materials.
Statistical analysis
Data were presented as the mean ± standard error of the mean. Using the StatView program (SAS institute, Cary, NC), statistical difference was analyzed by an ANOVA procedure with post hoc Tukey/Kramer test for multiple comparisons and by the unpaired Student's t-test for pairwise comparisons. A difference with a P value <0.05 was considered statistically significant and was labeled with an asterisk (*) in all figures.
Results
Puromycin-purified hESC-derived cardiomyocytes (hESC-CMs)
H9 cells stably transduced with the α-MHC-Puror_Rex-Neor (Rex promoter-driven neomycin resistance) tandem cassette were used to form EBs (Fig. 1A and 1B). Beating foci expressing cardiac α-MHC markers generally started to appear on day 9 of differentiation (9D). Early (<20D) and 60D CSs purified by Puromycin treatment were plated on coverslips for immunostaining and Ca2+ imaging studies. By immunostaining, the number of cardiac-specific Troponin I (cTnI)-positive cells relative to total cells (DAPI-labeled) in beating EBs was usually <7% and Puromycin selection resulted in >95% pure cardiomyocytes (Fig. 1C and 1D).
Contribution of intracellular Ca2+ signaling to automaticity developed early and was unchanged during 20–60 days of hESC-CMs differentiation
The intracellular Ca2+ handling machinery in cardiac cells, including type 2 inositol 1,4,5-trisphosphate (IP3) receptor (IP3R2) [19], type 2 ryanodine receptor (RyR2) [9], and type 1 sodium (Na+)/Ca2+ exchanger (NCX1) [8], had been implicated in the generation of pacemaker rhythms in adult SNCs and fetal cardiomyocytes. However, due to lack of specific markers to distinguish or isolate pacemaker cells from working cardiomyocytes at early embryonic stages [20], it has not been possible to apply single-cell recordings to evaluate the mechanisms of automaticity in early pacemaker cells. Nonetheless, dominant pacemaker cells in CSs would be expected to dictate the BR and a change of BR by applied drugs, in turn, would implicate the mechanism of dominant automaticity. Therefore, Ca2+ imaging of purified CSs combined with pharmacological characterization would be a better method to experimentally determine drug effects on intracellular calcium ([Ca2+]i) transients of hESC-CMs and dominant BRs of CSs simultaneously. Puromycin-purified CSs were used to avoid potential confounding effects, such as neurohormonal influences, on automaticity. Of note, this study was not designed to address the controversial topic of whether intracellular Ca2+ cycling proteins or sarcolemmal ion channels “initiate” cardiac rhythm, which would require single pacemaker cell recordings [7,8]. However, without a specific pacemaker cell marker at these early stages of hESC-CM differentiation, applying interpretation of results from non-selective single-cardiomyocyte recordings to pacemaker cells is very contentious.
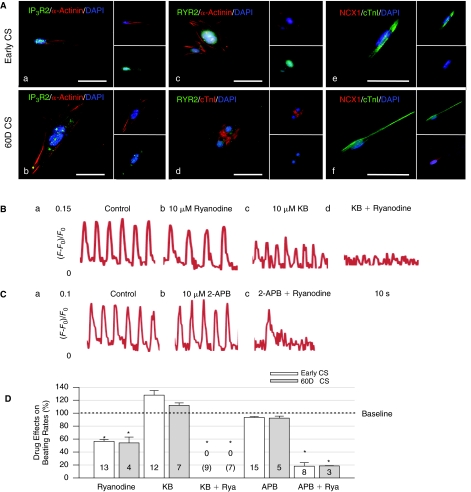
We first used specific immunostaining to indicate that IP3R2, RyR2, and NCX1, which handle [Ca2+]i, co-existed in cells with cTnI (or α-actinin) and displayed minor changes in their levels from early to 60D CSs (Fig. 2A). Next, we applied various antagonists to determine the degree of contribution of these [Ca2+]i handling proteins to automaticity during hESC-CM differentiation. For both early and 60D CSs, 10 μM ryanodine, a RyR2 blocker, decreased BRs by ∼50% and reduced the intensity of [Ca2+]i signals in CSs by ∼14% (Fig. 2Ba-b). KB-R7943 at 10 μM should block Ca2+ influx via NCX1 (the reverse mode) and slightly inhibit the inward current through NCX1 (the forward mode) at this concentration [21]. Application of 10 μM KB-R7943 decreased the intensity of [Ca2+]i signals in CSs by ∼47% but slightly increased BRs of CSs (Fig. 2Bc). Furthermore, co-application of 10 μM KB-R7943 and ryanodine after either KB-R7943 or ryanodine blockade completely abolished BRs of CSs (Fig. 2Bd). Since 10 μM KB-R7943 slightly increased the BRs at baseline when RyRs are fully functional yet significantly blocked the BRs when RyRs are deficient, this result suggests that NCX1 acts as an automaticity reserve to compensate the loss of RyRs for maintaining the beating rhythm (or vice versa). The 10 μM 2-APB (2-aminoethoxydiphenyl borate), a membrane-permeable IP3R blocker [22], insignificantly decreased BRs of CSs and did not affect the intensity of [Ca2+]i signals in CSs (Fig. 2Ca-b). However, after RyR blockade, 10 μM 2-APB decreased BRs of CSs by additional 20%–30%, indicating that IP3R also plays a part in the automaticity reserve when RyRs are mostly unavailable. Most importantly, these pharmacological agents exerted similar effects on the automaticity and intensity of [Ca2+]i transient of both early and 60D CSs during differentiation (results are summarized in Fig. 2D). These results suggest that intracellular Ca2+ handling proteins developed early and contributed to automaticity with similar magnitudes at 20–60 days of hESC-CM differentiation.
FIG. 2.
Constant roles of intracellular Ca2+ cycling on automaticity during human embryonic stem cell-derived cardiomyocyte (hESC-CM) differentiation. (A) Representative hESC-CMs from early and 60D cardiomyocyte spheroids (CSs) revealed cytoplasmic localization of IP3R2 (a, b) and RyR2 (c, d) as well as co-expression of NCX1 proteins (e, f) with cTnI (or α-actinin). (B) Control (a) and ryanodine-treated (b) early CSs showed that ryanodine blocked beating rates (BRs) with minimal effects on the intensity of [Ca2+]i. KB-R7943 (KB, c) slightly increased BRs and reduced [Ca2+]i by about 50%. Co-application of ryanodine and KB (d) completely blocked BRs and [Ca2+]i of early CSs. (C) 2-APB displayed minimal effects on BRs and [Ca2+]i of early CSs (a, b). In (c), combination of ryanodine and 2-APB significantly blocked the BRs of early CSs and slightly decreased the [Ca2+]i. Concentrations of all drugs are 10 μM. (D) Summary of effects of ryanodine (Rya), KB and 2-APB on BRs of early and 60D CSs. For CSs at early and 60D differentiation, ryanodine blocked BRs to 56.49% ± 3.00% and 54.09% ± 9.02% of control BRs; KB slightly increase BRs to 127.82% ± 7.06% and 112.06% ± 3.81%; and 2-APB minimally changed the BRs to 93.43% ± 1.32% and 92.39% ± 3.03%, respectively. Co-application of ryanodine and KB completely arrested beating. Also, co-application of ryanodine and 2-APB dramatically decreased BRs to 18.20% ± 5.42% and 18.57% ± 0.38%, respectively. Asterisks indicate statistically significant blockade relative to controls (P < 0.05, analysis of variance [ANOVA]). There was no significant difference for effects of these drugs on BRs between early and 60D CSs.
Increasing role of HCN4 channels in the automaticity of hESC-CMs during differentiation by developmental cues from non-cardiomyocytes
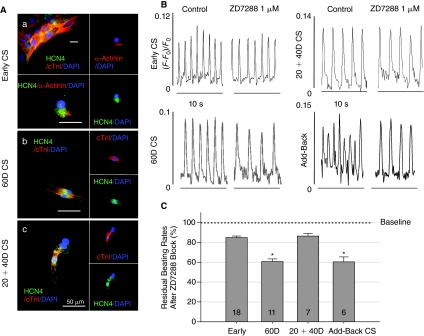
HCN channels, especially the type 4 isoform (HCN4), are thought to mediate automaticity of SNCs [23], murine ESC-CMs [10], and human ESC-CMs [11]. ZD7288 at 1 μM is a specific HCN channel blocker and was therefore used to inhibit the HCN-mediated current (If) and automaticity [24]. Immunostaining with the HCN4 antibody revealed substantial presence of membrane HCN4 channels in most hESC-CMs at early and 60D differentiation (>99% of cells, Fig. 3A). However, ZD7288 at 1 μM blocked BRs of early CSs by only 15% yet significantly reduced BRs of 60D CSs by 39% [Fig. 3B (top and bottom left panels) and 3C]. Furthermore, when CSs were isolated at 20D and cultured for another 40 days in isolation of influences from non-cardiomyocytes in EBs (labeled as 20+40D CS in Fig. 3B top right panel), 1 μM ZD7288 blocked the BRs by only 14%, which is the same degree of blockade by ZD7288 on early CSs. This lack of ZD7288 sensitivity could be corrected by adding non-cardiomyocytes back to early CS cultures (add-back CS, Fig. 3B, bottom right panel and summarized in Fig. 3C). These results indicate that HCN channel-mediated currents play an increasing role in automaticity from early to 60D CSs, potentially reflecting ongoing differentiation and/or maturation. Furthermore, this mechanistic maturation of automaticity with increasing ZD7288 sensitivity appears to depend on cues from non-cardiomyocytes in EBs.
FIG. 3.
Effects of the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel blocker, ZD7288, and non-cardiomyocytes on the automaticity of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) during differentiation. (A) Immunostaining showed the presence of membrane HCN4 channels on isolated early (a), 60D (b), and 20+40D (c) hESC-CMs. An early cardiomyocyte spheroid (CS) cluster is shown in the left upper panel of (a) as an example indicating that >99% of purified hESC-CMs were positive for both cTnI and HCN4 staining. (B) The 1 μM ZD7288 reduced beating rates (BRs) of 60D CSs (left lower panel) and add-back CSs (right lower panel) more than the BR of early and 20+40D CSs (top 2 panels). Horizontal scale bars are 10 s. (C) Summary of effects of ZD7288 on BRs. The 1 μM ZD7288 decreased BRs to 84.87% ± 1.50%, 60.70% ± 2.62%, and 86.35% ± 2.85% of control BRs at early, 60D, and 20+40D of differentiation, respectively. Adding non-cardiomyocytes back to 20+40D CS cultures rescued the sensitivity of BRs to ZD7288 (60.36% ± 4.99%). Reduction of BRs at every stages of differentiation was statistically different from the baseline BRs and is shown as gray bars. The BR reduction by ZD7288 at 60D and add-back CSs is statistically different (P < 0.05, analysis of variance [ANOVA], asterisks) from the BR reduction at early and 20+40D CSs.
For add-back experiments, the protocol is shown in Figure 4A and detailed in Supplementary Materials. In brief, parental H9 hESC lines without Puromycin resistance were used to derive beating EBs, which contained <7% of cardiomyocytes (Fig. 1C and 1D). These parental H9-derived beating EBs were dissociated into small, isolated cell clusters with collagenase to obtain sufficient numbers of non-cardiomyocytes for efficient rescue of electrical maturation of early purified CSs. Dissociated small clusters of cells from beating EBs contained only <1% of parental cardiomyocytes due to cell loss during enzymatic dissociations (Fig. 4B). Cells with ∼99% non-cardiomyocytes from parental EBs were then mixed with a Puromycin-purified early hESC-CS (<20D, >95% pure cardiomyocytes) for add-back experiments. The add-back clusters were maintained for an additional 40 days and then treated with a second Puromycin treatment to eliminate parental H9-derived cells and to recover Puromycin-resistant hESC-CMs (with >97% purity of hESC-CMs determined by the method shown in Fig. 1) for subsequent electrophysiological and molecular studies (Fig. 4B). This add-back protocol would have, in worse cases, <0.05% contamination of non-Puromycin-resistant H9 hESC-CMs for final add-back experiments after 2 sequential treatments with Puromycin.
FIG. 4.
Protocol of the add-back experiment with non-cardiomyocytes from parental H9-derived beating embryoid bodies (EBs). (A) Scheme of culturing procedures and Puromycin selection for obtaining 20+40D cardiomyocyte spheroids (CSs) and add-back CSs. Add-back CSs were treated with Puromycin twice to allow experiments on purified human embryonic stem cell-derived cardiomyocytes (hESC-CMs) with α-MHC-Puror. (B) Images (a and b) of cell clusters of non-cardiomyocytes (non-CMs) obtained from 2 separate parental H9-derived beating EBs after enzymatic dissociations. Horizontal scale bars are 200 μm. (c) The percentage of cardiomyocytes (cardiac α-actinin-positive) in add-back CSs after the second treatment of Puromycin is 97.51% ± 0.85% (n = 10). Hoechst stains cell nuclei (blue). A magnified area of hESC-CMs from add-back CSs is shown as the inset (right lower corner).
Non-cardiomyocytes promote the role of sodium channels in automaticity of hESC-CMs during differentiation
Cardiac Na+ currents (INa), mainly mediated by NaV1.5 subunit, have been suggested to underlie the automaticity in hESC-CMs [5]. We found that the intensity of immunostaining of NaV1.5 Na+ channel subunit in most hESC-CMs increases substantially from early to 60D CSs but not in 20+40D CS (Fig. 5A). We used tetrodotoxin (TTX) at 1 μM to determine the role of INa in automaticity. At 1 μM, TTX should block >50% of TTX-resistant NaV1.5 channels and completely block TTX-sensitive Na+ channels (such as NaV1.1) that mediate cardiac Na+ currents [5,25]. The 1 μM TTX blocked BRs of early CSs by ∼20% and BRs of 60D CSs by 79% [Fig. 5B (top and second panels) and 5C]. In contrast to 60D CSs, 1 μM TTX blocked BRs of 20+40D CSs by ∼30%, which is not statistically different (ANOVA) from blockade by TTX on BRs of early CSs (Fig. 5B, the third panel and 5C). Additionally, this low TTX sensitivity of automaticity of early-isolated CSs was partially recovered (Fig. 5B, bottom panel and Fig. 5C) by co-cultures with non-cardiomyocytes (add-back) and the levels of Na+ channel transcripts in working cardiomyocytes of add-back CSs were also corrected (see below). These results suggest that the dependence of automaticity on Na+ channels increases with hESC-CM differentiation in EBs and non-cardiomyocytes appear to promote this developmental progression.
FIG. 5.
Effects of the Na+ channel blocker, tetrodotoxin (TTX), and non-cardiomyocytes on the automaticity of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) during differentiation. (A) Immunostaining showed increasing levels of NaV1.5 channels on hESC-CMs from early (a and b) to 60D (c and d) but not in 20+40D cardiomyocyte spheroids (CSs) (e and f). Adding non-cardiomyocytes to early CS restored the protein levels of NaV1.5 channels (g and h). (B) The 1 μM TTX reduced the beating rates (BRs) of 60D CSs much more than the BRs of early and 20+40D CSs. Adding non-CMs back to 20+40D CS cultures rescued the TTX sensitivity of automaticity. Horizontal scale bars are 10 s. (C) Summary of effects of TTX on the BRs. The 1 μM TTX decreased the BRs to 82.85% ± 1.96%, 21.21% ± 4.74%, 68.06% ± 6.90%, and 43.32% ± 8.00% of control BRs in early, 60D, 20+40D, and add-back CSs, respectively. Gray bars have the same meaning as in Figure 4. The reduction of BRs by TTX on either 60D or add-back CSs is statistically different (asterisks, analysis of variance [ANOVA]) from its effects on other CSs.
Of note, it is not possible to investigate the development of every ion channels in a single study. However, many ion channels, other than HCN and Na+ channels, also display developmental changes during hESC-CM and cardiac differentiation [9–11,14,15]. We therefore examined the influence of non-cardiomyocytes on the expression of ion channel transcripts and overall electrophysiological properties of hESC-CMs during 20 to 60 days of differentiation with our Puromycin-selection and non-cardiomyocyte add-back methods.
Non-cardiomyocytes influence the maturation of electrophysiological properties of primitive working hESC-CMs
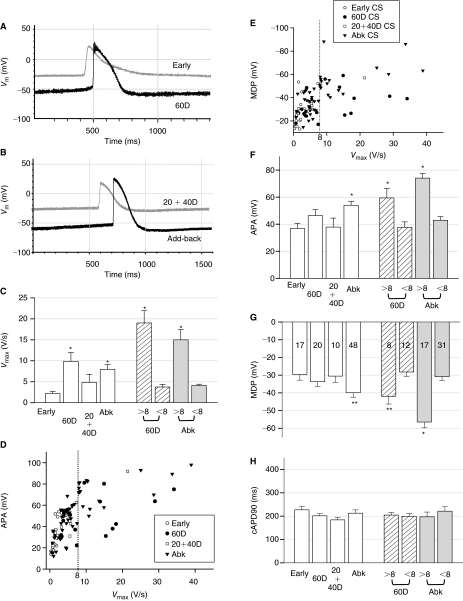
The preceding experiments suggest that non-cardiomyocytes in the EB influence some of ion channel maturation during hESC-CM differentiation. Next, we compared electrophysiological properties of cardiomyocytes (>95% purity) in early CSs (<20D) with those of 60D and 20+40D CSs by intracellular recording techniques. Since only a very small percentage of cells evolved into pacemaker cells [3] and we could not isolate pacemaker cells at these early stages of hESC-CM differentiation, the majority of recordings are likely to be of primitive working cardiomyocytes instead of early pacemaker cells. As previously reported [3,4], electro-physiological properties of hESC-CMs are heterogeneous and variable. We speculated that inhomogeneous contact of hESC-CMs with non-cardiomyocytes might account for the variation. The measured action potential (AP) duration at the 90% of repolarization (APD90) was corrected by BRs (cAPD90 = APD90/square root of RR, see Supplementary Materials) to minimize restitution effects [26]. Figure 6A–6H shows that the AP amplitude (APA), maximum diastolic potential (MDP), maximal upstroke velocity (Vmax), and cAPD90 were compared between hESC-CMs from early, 60D, and 20+40D CSs. Among all parameters measured (summarized in Table 1), Vmax was the only value that was statistically different between early and 60D hESC-CMs (Fig. 6C). After a detailed examination, values of Vmax of all early hESC-CMs were <8 V/s. We therefore divided the cardiomyocytes from 60D CSs into 2 groups: Immature hESC-CMs with Vmax < 8 V/s (60%) and relatively matured CMs with Vmax > 8 V/s (40%). The mature subgroup of 60D hESC-CMs possessed more hyperpolarized MDP, higher APA, and faster Vmax than the immature group (P < 0.05, Fig. 6D–6G). Importantly, these parameters of electrophysiological maturation were lost when the hESC-CMs were isolated at <20D (early CSs) and cultured without further interaction with non-cardiomyocytes (20+40D CSs) (Fig. 6C–6H). These immature electrophysiological properties could be rescued by co-culturing early CSs with non-cardiomyocytes (add-back CSs, Fig. 6C–6H), indicating that non-cardiomyocytes also stimulate the overall electrophysiological maturation of working cardiomyocytes.
FIG. 6.
Characteristics of action potentials from various stages of cardiomyocyte spheroids (CSs) by intracellular recordings. (A) Representative tracings of action potentials (APs) of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) from early (gray) and 60D (black) CSs as well as (B) from 20+40D CS (gray) and a mature hESC-CM (black) in add-back CSs. (C) The Vmax (= dVm/dtmax) values of hESC-CMs from 60D and add-back (Abk) CSs were statistically faster than those of early CSs. They were divided into 2 groups based on Vmax was >8 V/s or < 8 V/s (shaded columns at right, see text). The statistically difference of Vmax between early and 60D hESC-CMs (or add-back hESC-CMs) was from the group of mature cells with Vmax >8 V/s. (D) The action potential amplitude (APA) versus Vmax plot demonstrated a mature population of hESC-CMs with Vmax > 8 V/s (the dotted line) and higher APAs in 60D and add-back CSs. (E) The maximum diastolic potential (MDP) versus Vmax plot also showed a mature population of hESC-CMs with Vmax > 8 V/s (the dotted line) and more hyperpolarized MDPs in 60D and add-back CSs. (F) Compared to early CSs, the values of APA were statistically different in add-back CSs as well as in the subgroup of 60D and add-back CSs with Vmax > 8 V/s, which had a higher APA. (G) The values of MDP were variable at every stage of differentiation. Compared to early CSs, only hESC-CMs from add-back CSs displayed statistical difference. Also, 60D and add-back hESC-CMs with Vmax > 8 V/s had significantly more hyperpolarized MDPs (** indicates P < 0.05 by Student's t-test only). (H) The values of cAPD90 display no statistical difference between all groups and subgroup analysis. Importantly, adding non-CMs back to early CSs rescued all electrophysiological phenotypes. The number of hESC-CMs tested is indicated in each column of (G). The values used to construct this figure are listed in Table 1. Asterisks in all figures indicate P < 0.05 with analysis of variance (ANOVA).
Table 1.
Electrophysiological Properties of hESC-CMs
| |
Early CS |
60D CS |
20+40D CS |
Add-back CS |
60D CS |
Add-back CS |
||
|---|---|---|---|---|---|---|---|---|
| n = 17 | n = 20 | n = 10 | n = 48 | Vmax > 8 (n = 8) | <8 (n = 12) | Vmax > 8 (n = 17) | <8 (n = 31) | |
| Vmax (V/s) | 2.21 ± 0.46 | 9.86 ± 2.09 | 4.83 ± 1.94 | 7.96 ± 1.15 | 19.03 ± 2.93 | 3.74 ± 0.63 | 15.00 ± 2.43 | 4.10 ± 0.32 |
| APA (mV) | 36.91 ± 3.78 | 46.41 ± 4.33 | 37.88 ± 6.65 | 53.99 ± 3.00 | 59.41 ± 6.99 | 37.73 ± 4.01 | 74.11 ± 3.28 | 42.96 ± 2.68 |
| MDP (mV) | −29.68 ± 3.15 | −33.68 ± 2.70 | −30.52 ± 3.52 | −39.93 ± 2.49 | −41.90 ± 4.39 | −28.19 ± 2.44 | −56.44 ± 3.29 | −30.87 ± 2.03 |
| cAPD90 (ms) | 227.27 ± 14.97 | 201.58 ± 8.57 | 184.18 ± 10.62 | 212.91 ± 14.12 | 204.75 ± 11.69 | 199.46 ± 12.35 | 197.36 ± 20.40 | 221.44 ± 18.84 |
| Raw APD90 (ms) | 353.11 ± 38.65 | 249.63 ± 16.96 | 201.30 ± 13.59 | 388.58 ± 18.74 | 222.85 ± 16.24 | 267.48 ± 25.38 | 347.72 ± 29.96 | 410.98 ± 23.28 |
Abbreviations: hESC-CMs, human embryonic stem cell-derived cardiomyocytes; CS, cardiomyocyte spheroid; APA, action potential amplitude; MDP, maximum diastolic potential.
Effects of non-cardiomyocytes on the developmental regulation of ion channels and [Ca2+]i handling proteins at the level of transcript expression
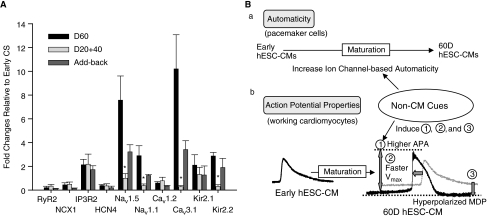
In order to provide molecular insight into the basis of electrical maturation of early working hESC-CMs, quantitative RT-PCR was performed on various stages of CSs to determine if electrical maturation is controlled by pre- or post-translational regulation (Fig. 7A). Minimal changes in transcript levels of RyR2 and NCX1 from 20 to 60D hESC-CM differentiation were detected. Relative to early CSs, IP3R2 mRNA abundance increased slightly in 60D CS and this increase is not dependent on non-cardiomyocytes. HCN4 levels changed only slightly and were consistent with the immunostaining results in Figure 3. Since we could not isolate pacemaker cells for a mechanistic study, one of the possible explanations for the increased ZD7288 sensitivity of automaticity in 60D CSs is that more hyper-polarized MDP with maturation increases If currents due to the voltage dependence of HCN channel activation profiles [5]. In addition to HCN4, other isoforms of HCN channels may also be involved in early automaticity [5,10,11]. Both NaV1.5 mRNA level (Fig. 7A) and immunostaining (Fig. 5) were up-regulated with maturation, which is dependent on cues from non-cardiomyocytes. Since 1 μM TTX significantly blocked BRs of 60D and add-back CSs, NaV1.1 mRNA levels of various CSs were also measured. The transcript levels of NaV1.1 mRNA also increased with maturation that required the presence of non-cardiomyocytes. The T-type Ca2+ channel subunit (Cav3.1) mRNA was up-regulated and non-cardiomyocytes also influenced this process. L-Type Ca2+ channel (Cav 1.2) mRNA did not change dramatically during 60D of hESC-CM differentiation. Transcripts of IK1 channel subunits (Kir2.1/2.2) and IK1-mediated currents (see (Supplementary Fig. 4), responsible for maintaining resting membrane potentials, also increased with maturation. Consistent with the electrophysiological finding reported in Figure 6G, non-cardiomyocytes also influenced the expression of IK1 channel transcripts, particularly Kir2.2, which resulted in the hyperpolarized MDP of mature 60D hESC-CMs (see Discussion). Thus, signals from non-cardiomyocytes had a profound influence on transcript levels of several channels, such as NaV1.5.
FIG. 7.
Quantitative RT-PCR results of ion channels and [Ca2+]i handling proteins from various stages of cardiomyocyte spheroids (CSs) relative to early CSs. (A) Relative to early CSs, quantitative RT-PCR results of ion channels and [Ca2+]i handling proteins from 3 to 4 respective sets of various stages of CSs indicated that non-cardiomyocytes (CMs) present during the culture period restored the mRNA levels of Nav1.5, NaV1.1, Cav3.1, and Kir2.2 of 20+40D CSs. mRNA levels were normalized to that for GAPDH. Normalization to PGK mRNA resulted in a similar trend of RT-PCR data (not shown). The levels of NaV1.1, NaV1.5, CaV3.1, and Kir2.2 mRNA in either 60D or add-back CSs are statistically different (asterisks, analysis of variance (ANOVA) from those in 20+40D CSs. (B) A schematic summary is shown to demonstrate effects of non-CMs on the development of automaticity and electrophysiological properties of human embryonic stem cell-derived cardiomyocytes (hESC-CMs). Intracellular Ca2+-based mechanisms developed early and contributed to automaticity throughout the first 60D of hESC-CM development. Non-CMs induced the development of sarcolemmal ion channel-mediated automaticity of CSs from 20 to 60 days of hESC-CMs differentiation in embryoid bodies (EBs) (a). For electrophysiological properties of working cardiomyocytes, non-CMs in EBs induced faster Vmax, higher action potential amplitude (APA) and more hyperpolarized maximum diastolic potentials (MDPs) of hESC-CMs during differentiation (b, bottom tracings).
Discussion
Human or murine ESC-CMs provide a model system to study the development of cardiomyocytes [9–11], in part because they are a heterogeneous and immature population of cardiomyocytes [3–5,9] that have been shown to be able to mature in the EB environment [3–5]. Elucidating molecular pathways governing the functional development of early hESC-CMs would help in creating directed myocardiogenesis for myocardial repair or replacement therapies. Both intracellular [Ca2+]i handling proteins and sarcolemmal ion channels play significant roles in the functional maturation of early hESC-CMs and contribute to their automaticity and electrophysiological properties. Prior studies have shown that various sarcolemmal ion channels, such as HCN [10,11] and Na+ [5] channels displayed developmental changes during ESC-CM differentiation. The developmental status of intracellular [Ca2+]i handling apparatus of hESC-CMs is less clear with reports varying from the presence of a fully functional intracellular [Ca2+]i handling apparatus [27] to defective [12] or immature [13] apparatus in hESC-CMs. These differences in functional development of hESC-CMs have been attributed to different culture and experimental conditions as well as different sources of human ESC lines [13]. Although not mutually exclusive, another possibility suggested by our results is that non-cardiomyocytes regulate electrophysiological maturation, and differential exposure of hESC-CMs to non-cardiomyocytes in EBs might cause cell-to-cell and EB-to-EB variation in automaticity and electrophysiological properties. More importantly, we found that intracellular Ca2+ handling proteins developed early and contributed to automaticity with similar degrees during 20–60 days of hESC-CM differentiation regardless of further maturation in the EB milieu. In contrast, non-cardiomyocytes in EBs heavily influenced the development of sarcolemmal ion channels, resulting in electrical maturation of hESC-CMs. Non-cardiomyocytes have been implicated in the induction and differentiation of various types of cardiomyocytes during embryonic development [16,28]. Our results suggest for the first time that the electrical maturation of developing pacemaking and working cardiomyocytes also depends upon signals from neighboring non-cardiomyocytes in stem cell cultures.
Intracellular Ca2+ handling proteins developed early and their contributions to automaticity were not changed during 60D of hESC-CM differentiation
We found that RyR, IP3R2, and NCX1 played similar roles in handling [Ca2+]i and controlling pacemaker rhythm in early and 60D CSs (Fig. 2). Expression levels of mRNA transcripts and immunostaining of these [Ca2+]i handling machineries are also unchanged between early and 60D CSs (Figs. 2 and 7), suggesting further maturation or development of these proteins, if any, occurred after 60D of hESC-CM differentiation. We found that RyR contributed significantly to automaticity in early and 60D CSs, consistent with the role of RyRs in [Ca2+]i handling in other hESC-CMs [13] and early murine ESC-CMs [9]. In early and 60D CSs, NCX is responsible for a portion of Ca2+ influx and IP3Rs play a minor role in automaticity and [Ca2+]i transients at the basal state. However, when BRs of CSs were decreased by 50% from RyR blockade, both NCX1 and IP3R acted as a backup system and play compensatory roles in maintaining automaticity. The interplay between IP3R, NCX1, and RyR for generating pacemaker activities in early hESC-CMs is consistent with the intracellular Ca2+ store-mediated automaticity reported in mouse ESC-CMs [9,29]. Importantly, the mechanisms underlying automaticity become more complex and ion channels play increasing roles in automaticity with further cardiac development.
Non-cardiomyocytes influence the development of HCN and Na+ channel-dependent automaticity and electrical properties
A major implication of this study is that non-cardiomyocytes in EBs promote the acquisition of HCN-based automaticity (Fig. 3) and the development of more hyperpolarized MDP in primitive cardiomyocytes (Fig. 6) during hESC-CM differentiation. Since molecular levels of HCN channels did not change significantly and they only activate at membrane potentials more negative than −40 mV [5,10,11,15], induction of HCN channel-based automaticity is most likely due to the development of the hyperpolarized MDP that allows preexisting HCN channels to be operational.
Non-cardiomyocytes also appear to regulate Na+ channel-dependent automaticity and Na+ channel expression in primitive hESC-CMs. Notably, TTX sensitivity of automaticity was partially recovered when non-cardiomyocytes were added back to culture, and we interpret this as indicating that a subset of non-cardiomyocytes is needed for close contact with the rare pacemaker cells for this induction. The Vmax and Nav1.5 transcripts of primitive working hESC-CMs also increased in the presence of non-cardiomyocytes but not in their absence (Figs. 6 and 7). It is likely that increased expression of Na+ channels and their availability associated with more hyperpolarized MDP [5] account for the induction of Na+ channel-based automaticity and Vmax during these stages of differentiation.
Other ion channel development
Since many ion channels, such as Ca2+ [30] and K+ [31] channels, also undergo developmental changes during cardiogenesis and it is not feasible to report functional studies on all relevant ion channels in one study, we include only data of expression levels of molecular transcripts pertaining to Ca2+ and K+ channels during the first 60D of hESC-CM development in this report. However, some preliminary predictions could be drawn from our molecular and electrophysiological studies. Ca2+ channels have been implicated in the excitability and conduction of murine ESC-CMs [10,30]. The mRNA level of CaV1.2, the major L-type Ca2+ channel α subunit in adult hearts, did not change during 20–60 days of hESC-CM differentiation. The mRNA level of Cav3.1, a major T-type Ca2+ channel α subunit in adult hearts, increased with hESC-CM differentiation and non-cardiomyocytes appeared to promote this process, consistent with the up-regulation of CaV3.1 sub-units reported during cardiac development [30]. In terms of K+ channel development, MDPs of hESC-CMs become more hyperpolarized with maturation and in the presence of non-cardiomyocytes (Fig. 6G). However, only Kir2.2 but not Kir2.1 mRNA level increased with non-cardiomyocytes (Fig. 7A), suggesting that IK1 (Kir2.x) development is intrinsically as well as extrinsically regulated. Future functional studies with our Puromycin-selection and add-back methods will shed light on how non-cardiomyocytes exert their influence on development of various ion channel subunits in hESC-CMs.
Implications for deriving mature pacemaking and/or working cardiomyocytes
Our result demonstrates that non-cardiomyocytes influence the electrophysiological maturation of hESC-CMs. A summary of the proposed role of non-cardiomyocytes on ion channel maturation is presented in Figure 2B. Different lines of human SPCs might vary in their capacity to generate specific subtypes of non-cardiomyocytes that provide the developmental signals and, consequently, lead to cell line-to-cell line variation of the electrical phenotype of hESC-CMs. Moreover, inhomogeneous exposure to non-cardiomyocytes within EBs could explain variation within cultures as demonstrated by our add-back experiments. Lastly, implantation of heterogeneous and immature populations of hESC-CMs into hearts for cell replacement therapy may carry arrhythmogenic risks. Therefore, research to elucidate the identity of non-cardiomyocytes and the molecular nature of signals (or cell–cell contacts) that promote electrophysiological maturation could facilitate safe application of pacemaking or working cardiomyocytes for cell-based therapies.
Supplementary Material
Acknowledgments
This work was supported by an Early Career Development Award from American College of Cardiology Foundation, Bechtel Trusts and Foundation Grant, and California Institute of Regenerative Medicine (CIRM) Grants (RS1-00171-1 and RT1-01143) (to H-S.V.C.); and by NIH grants (R37 HL059502), CIRM (RC1-00132-1) and the Mathers Charitable Foundation (to M. M.)
Author Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Smith RR. Barile L. Messina E. Marbán E. Stem cells in the heart: what's the buzz all about? Part 2: Arrhythmic risks and clinical studies. Heart Rhythm. 2008;5:880–887. doi: 10.1016/j.hrthm.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kehat I. Khimovich L. Caspi O. Gepstein A. Shofti R. Arbel G. Huber I. Satin J. Itskovitz-Eldor J. Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 3.Mummery C. Ward-van Oostwaard D. Doevendans P. Spijker R. van den Brink S. Hassink R. van der Heyden M. Opthof T. Pera M. de la Riviere AB. Passier R. Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 4.JQ He. Ma Y. Lee Y. Thomson JA. Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 5.Satin J. Kehat I. Caspi O. Huber I. Arbel G. Itzhaki I. Magyar J. Schroder EA. Perlman I. Gepstein L. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol (Lond) 2004;559(Pt 2):479–496. doi: 10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janse MK. Anderson RH. van Capelle FJ. Durrer D. A combined electrophysiological and anatomical study of the human fetal heart. Am Heart J. 1976;91:556–562. doi: 10.1016/s0002-8703(76)80139-1. [DOI] [PubMed] [Google Scholar]
- 7.Dobrzynski H. Boyett MR. Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921–1932. doi: 10.1161/CIRCULATIONAHA.106.616011. [DOI] [PubMed] [Google Scholar]
- 8.Lakatta EG. Vinogradova T. Lyashkov A. Sirenko S. Zhu W. Ruknudin A. Maltsev VA. The integration of spontaneous intracellular Ca2+ cycling and surface membrane ion channel activation entrains normal automaticity in cells of the heart's pacemaker. Ann N Y Acad Sci. 2006;1080:178–206. doi: 10.1196/annals.1380.016. [DOI] [PubMed] [Google Scholar]
- 9.Boheler KR. Czyz J. Tweedie D. Yang HT. Anisimov SV. Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res. 2002;91:189–201. doi: 10.1161/01.res.0000027865.61704.32. [DOI] [PubMed] [Google Scholar]
- 10.Yanagi K. Takano M. Narazaki G. Uosaki H. Hoshino T. Ishii T. Misaki T. Yamashita JK. Hyperpolarization-activated cyclic nucleotide-gated channels and T-type calcium channels confer automaticity of embryonic stem cell-derived cardiomyocytes. Stem Cells. 2007;25:2712–2719. doi: 10.1634/stemcells.2006-0388. [DOI] [PubMed] [Google Scholar]
- 11.Sartiani L. Bettiol E. Stillitano F. Mugelli A. Cerbai E. Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25:1136–1144. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 12.Dolnikov K. Shilkrut M. Zeevi-Levin N. Gerecht-Nir S. Amit M. Danon A. Itskovitz-Eldor J. Binah O. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006;24:236–245. doi: 10.1634/stemcells.2005-0036. [DOI] [PubMed] [Google Scholar]
- 13.Liu J. Fu JD. Siu CW. Li RA. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 2007;25:3038–3044. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- 14.Davies MP. An RH. Doevendans P. Kubalak S. Chien KR. Kass RS. Developmental changes in ionic channel activity in the embryonic murine heart. Circ Res. 1996;78:15–25. doi: 10.1161/01.res.78.1.15. [DOI] [PubMed] [Google Scholar]
- 15.Yasui K. Liu W. Opthof T. Kada K. Lee JK. Kamiya K. Kodama I. I(f) current and spontaneous activity in mouse embryonic ventricular myocytes. Circ Res. 2001;88:536–542. doi: 10.1161/01.res.88.5.536. [DOI] [PubMed] [Google Scholar]
- 16.Eisenberg LM. Markwald RR. Cellular recruitment and the development of the myocardium. Dev Biol. 2004;274:225–232. doi: 10.1016/j.ydbio.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Kita-Matsuo H. Barcova M. Prigozhina N. Salomonis N. Wei K. Jacot JG. Nelson B. Spiering S. Haverslag R. Kim C. Talantova M. Bajpai R. Calzolari D. Terskikh A. McCulloch AD. Price JH. Conklin BR. Chen HS. Mercola M. Lentiviral vectors and protocols for creation of stable hESC lines for fluorescent tracking and drug resistance selection of cardiomyocytes. PLoS ONE. 2009;4:e5046. doi: 10.1371/journal.pone.0005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber I. Itzhaki I. Caspi O. Arbel G. Tzukerman M. Gepstein A. Habib M. Yankelson L. Kehat I. Gepstein L. Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. FASEB J. 2007;21:2551–2563. doi: 10.1096/fj.05-5711com. [DOI] [PubMed] [Google Scholar]
- 19.Méry A. Aimond F. Ménard C. Mikoshiba K. Michalak M. Pucéat M. Initiation of embryonic cardiac pacemaker activity by inositol 1,4,5-trisphosphate-dependent calcium signaling. Mol Biol Cell. 2005;16:2414–2423. doi: 10.1091/mbc.E04-10-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viswanathan S. Burch JB. Fishman GI. Moskowitz IP. Benson DW. Characterization of sinoatrial node in four conduction system marker mice. J Mol Cell Cardiol. 2007;42:946–953. doi: 10.1016/j.yjmcc.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe Y. Koide Y. Kimura J. Topics on the Na+/Ca2+ exchanger: pharmacological characterization of Na+/Ca2+ exchanger inhibitors. J Pharmacol Sci. 2006;102:7–16. doi: 10.1254/jphs.fmj06002x2. [DOI] [PubMed] [Google Scholar]
- 22.Gysembergh A. Lemaire S. Piot C. Sportouch C. Richard S. Kloner RA. Przyklenk K. Pharmacological manipulation of Ins(1,4,5)P3 signaling mimics preconditioning in rabbit heart. Am J Physiol. 1999;277(6 Pt 2):H2458–H2469. doi: 10.1152/ajpheart.1999.277.6.H2458. [DOI] [PubMed] [Google Scholar]
- 23.Barbuti A. DiFrancesco D. Control of cardiac rate by “funny” channels in health and disease. Ann N Y Acad Sci. 2008;1123:213–223. doi: 10.1196/annals.1420.024. [DOI] [PubMed] [Google Scholar]
- 24.Briggs I. BoSmith RE. Heapy CG. Effects of Zeneca ZD7288 in comparison with alinidine and UL-FS 49 on guinea pig sinoatrial node and ventricular action potentials. J Cardiovasc Pharmacol. 1994;24:380–387. doi: 10.1097/00005344-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Guo XT. Uehara A. Ravindran A. Bryant SH. Hall S. Moczydlowski E. Kinetic basis for insensitivity to tetrodotoxin and saxitoxin in sodium channels of canine heart and denervated rat skeletal muscle. Biochemistry. 1987;26:7546–7556. doi: 10.1021/bi00398a003. [DOI] [PubMed] [Google Scholar]
- 26.Molnar J. Weiss JS. Rosenthal JE. The missing second: what is the correct unit for the Bazett corrected QT interval? Am J Cardiol. 1995;75:537–538. doi: 10.1016/s0002-9149(99)80603-1. [DOI] [PubMed] [Google Scholar]
- 27.Satin J. Itzhaki I. Rapoport S. Schroder EA. Izu L. Arbel G. Beyar R. Balke CW. Schiller J. Gepstein L. Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem Cells. 2008;26:1961–1972. doi: 10.1634/stemcells.2007-0591. [DOI] [PubMed] [Google Scholar]
- 28.High FA. Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet. 2008;9:49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- 29.Kapur N. Banach K. Inositol-1,4,5-trisphosphate-mediated spontaneous activity in mouse embryonic stem cell-derived cardiomyocytes. J Physiol (Lond) 2007;581(Pt 3):1113–1127. doi: 10.1113/jphysiol.2006.125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangoni ME. Couette B. Marger L. Bourinet E. Striessnig J. Nargeot J. Voltage-dependent calcium channels and cardiac pacemaker activity: from ionic currents to genes. Prog Biophys Mol Biol. 2006;90:38–63. doi: 10.1016/j.pbiomolbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Nerbonne JM. Regulation of voltage-gated K+ channel expression in the developing mammalian myocardium. J Neurobiol. 1998;37:37–59. doi: 10.1002/(sici)1097-4695(199810)37:1<37::aid-neu4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.