Abstract
Intravascular delivery of adeno-associated virus (AAV) vector is commonly used for liver-directed gene therapy. In humans, the high prevalence of neutralizing antibodies to AAV-2 capsid and the wide cross-reactivity with other serotypes hamper vector transduction efficacy. Moreover, the safety of gene-based approaches depends on vector biodistribution, vector dose, and route of administration. Here we sought to characterize the safety of AAV-5 and AAV-6 for liver-mediated human factor IX (hFIX) expression in rabbits at doses of 1 × 1012 or 1 × 1013 viral genomes/kg. Circulating therapeutic levels of FIX were observed in both cohorts of AAV-6-hFIX, whereas for AAV-5-hFIX only the high dose was effective. Long-lasting inhibitory antibodies to hFIX were detected in three of the 10 AAV-6-injected animals but were absent in the AAV-5 group. Overall, vector shedding in the semen was transient and vector dose-dependent. However, the kinetics of clearance were remarkably faster for AAV-5 (3–5 weeks) compared with AAV-6 (10–13 weeks). AAV-6 vector sequences outside the liver were minimal at 20–30 weeks post-injection. In contrast, AAV-5 exhibited relatively high amounts of vector DNA in tissues other than the liver. Together these data are useful to further define the safety and potential for clinical translation of these AAV vectors.
In this study, Favaro and colleagues characterize the safety of AAV-5 and AAV-6 for liver-mediated human factor IX (hFIX) expression in rabbits at doses of 1 × 1012 and 1 × 1013 viral genomes/kg. Circulating therapeutic levels of FIX were observed in both AAV-6-hFIX cohorts, whereas for the AAV-5-hFIX cohorts only the high dose was effective. The authors also report that the kinetics of vector clearance from the semen were remarkably faster for AAV-5 (3–5 weeks) compared with AAV-6 (10–13 weeks).
Introduction
Recent clinical successes using adeno-associated virus (AAV)-2 vectors for the treatment of genetic disease present the opportunity to expand the use of this vector to a myriad of diseases and population demographics (Bainbridge et al., 2008; Hauswirth et al., 2008; Maguire et al., 2008, 2009). However, the high prevalence of antibodies to AAV-2 capsid is a major impediment for its application to the majority of the human population. Direct tissue delivery of AAV-2 in the presence of circulating neutralizing antibodies (NAB) is feasible in humans and has been demonstrated by reports of the safety and efficacy of subretinal delivery of AAV-2 (Bainbridge et al., 2008; Hauswirth et al., 2008; Maguire et al., 2009) and by evidence of local expression of the transgene at sites of the intramuscular injection of AAV-2 (Manno et al., 2003; Jiang et al., 2006c). However, a more clinically challenging scenario is the delivery of AAV via an intravascular route.
Because of the high cross-reactivity among several serotypes, many humans exhibit NAB to AAV serotypes other than AAV-2, the only known serotype to be ubiquitous in the human population. Data from several publications of the most tested serotypes (AAV-1, - 2, - 5, - 6, - 8, - 7, and - 9) suggest that in humans, lower prevalence and intensity of NAB titers are found against AAV-5, followed by AAV-6 and AAV-8. Typically these studies use NAB titers of < 1:20 as the threshold defining low titer (Halbert et al., 2006; Calcedo et al., 2009; Boutin et al., 2010). However, these data probably underestimate the ability of these NAB to prevent in vivo gene delivery. Studies in murine models of passive immunization demonstrate that the efficiency of in vivo inhibition of transduction by NAB following systemic administration of AAV-2, AAV-6, and AAV-8 by human immunoglobulin is markedly higher than inhibition observed in in vitro experiments (Scallan et al., 2006). Early data from non-human primates showed that NAB titers to AAV-8 as low as 1:5 could prevent gene transfer to the liver of the cognate serotype (Jiang et al., 2006a), and more recently these findings were confirmed (Wang et al., 2010).
The efficacy of AAV-5 for liver-directed gene therapy as single-stranded or self-complementary vectors has been demonstrated in non-human primates, whether naive or with preexisting immunity to AAV-8 capsid (Nathwani et al., 2002, 2007; Davidoff et al., 2005). Moreover, in dogs with hemophilia B previously exposed to AAV-2, injection of AAV-5 resulted in successful gene transfer and transgene expression (Wang et al., 2005).
Recently we demonstrated that AAV-6 vectors could overcome the presence of NAB to AAV-2 following intravascular delivery to skeletal muscle in a hemophilia dog model, whereas attempts using AAV-2 were unsuccessful (Arruda et al., 2010). AAV-6 is also effective by direct injection into skeletal or cardiac muscle in dogs (Bish et al., 2008; Wang et al., 2009). The experience with AAV-6 in large animals for liver-directed gene transfer is limited. In the hemophilia A dog model, long-term sustained expression of canine factor VIII from AAV-6 was comparable to that from AAV-8 (Jiang et al., 2006b).
Because AAV-5 and AAV-6 vectors are effective in gene transfer in the presence of NAB to AAV-2 in large animals, we sought to determine the safety of these vectors following peripheral vein injection in a rabbit model. These studies include the risk of vector dissemination to the gonads and semen as these are safety issues not yet defined for these serotypes.
Materials and Methods
Production of recombinant AAV vectors
Recombinant AAV–human factor IX (hFIX) vectors were produced as described (Matsushita et al., 1998). The plasmid encodes the hFIX gene under the control of the human α1-antitrypsin promoter, one copy of the apolipoprotein A enhancer (hAAT/ApoE), and a hepatocyte control region, which results in liver-specific transgene expression (Le et al., 1997; Miao et al., 2000). The transgene cassette was flanked by AAV-2 inverted terminal repeats and was packaged in capsid from either AAV-5 or AAV-6 (Gao et al., 2002). AAV vectors were purified by combined chromatography and repeated cesium chloride density gradient centrifugation, resulting in empty capsid-free fractions. Vector titers were determined by quantitative polymerase chain reaction (PCR) using hFIX-specific primers and probes (Sommer et al., 2003).
Intravenous injection of AAV-hFIX vector
Adult male (n = 20) New Zealand White rabbits (weighing 3–4 kg) were purchased from Covance Research Products (Denver, PA). The vector (AAV-hFIX) was administered by a single injection into the marginal ear vein at doses of AAV-5 or AAV-6 vector of 1 × 1012 viral genomes (vg)/kg or 1 × 1013 vg/kg.
Determination of factor IX antigen levels and antibodies to factor IX transgene
Peripheral blood was obtained by marginal ear vein puncture prior to vector injection and weekly thereafter during long-term follow-up. The circulating factor IX (FIX) concentration was determined by using an antigen-specific FIX assay in which a monoclonal antibody to hFIX, clone HIX-1 (Sigma, St. Louis, MO), was used as a capture antibody at a dilution of 1:800, whereas the detecting antibody was a peroxidase-conjugated polyclonal goat anti-hFIX (Affinity Biologicals, Hamilton, ON, Canada) at a dilution of 1:2,500 (Schuettrumpf et al., 2006). Levels of anti-hFIX IgG antibodies were determined using an enzyme-linked immunosorbent assay where the plate was coated with purified hFIX (Wyeth, Madison, NJ) at a concentration of 1 μg/ml. Samples were diluted in Low Cross buffer (Candor Biosciences, Wangen, Germany), and the detecting antibody is a peroxidase-conjugated polyclonal goat anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA) at a dilution of 1:3,000. Bethesda assays were performed using citrated plasma samples following heat inactivation at 56°C for 1 hr to eliminate the endogenous rabbit FIX. Serial dilutions of heat-inactivated rabbit plasma were then incubated with human plasma for 2 hr at 37°C (Favaro et al., 2009). Residual hFIX clotting activity was determined by one-stage activated partial thromboplastin time and compared with a standard curve. Results are expressed in Bethesda units (BU), in which 1 BU is the amount of antibody that neutralizes 50% of FIX clotting activity present in normal plasma.
Detection of antibodies against AAV capsid proteins
For detection of anti-AAV capsid antibodies, enzyme-linked immunosorbent assay plates were coated with empty capsid particles (AAV-5 or AAV-6) at 1 μg/ml. Plasma samples were diluted in Low Cross buffer (Candor Bioscience), and a peroxidase-conjugated polyclonal goat anti-rabbit IgG (Jackson Immunoresearch) was used as a secondary antibody at a dilution of 1:3,000.
Detection of vector sequences in semen and tissue samples
Semen was collected with the aid of an artificial vagina as reported previously (Arruda et al., 2001) before and at several time points following vector injection. Biodistribution studies were carried out at 20–30 weeks post-vector injection. All major tissues such as liver, spleen, testes, prostate, accessory glands, bladder, kidney, lung, and heart were harvested using fresh sterile instruments for each tissue sample. For quantitative determination of vg count in DNA in all tissues, a quantitative PCR assay was performed. A total of 200 ng of genomic DNA extracted from each sample was used for TaqMan real-time PCR (Applied Biosystems, Foster City, CA). The primers (forward, 5′-ttcgatctacaaagttcaccatctataac-3′; reverse, 5′-aaactggtcccttccacttcag-3′) and the fluorescein aminohexylamidite–labeled probe (5′-aatctctacctccttcatggaagccagca-3′) were designed to detect the AAV-hFIX16 vector sequences. The lowest sensitivity of the quantitative PCR was 25 copies per 1 μg of genomic DNA (Favaro et al., 2009).
Vector DNA analysis
DNA from serum samples was isolated with the QIAamp Blood Kit (Qiagen, Chatsworth, CA). The procedure for DNA extraction from semen and tissues consisted of overnight incubation with proteinase K before isolation of total genomic DNA using the QIAamp Tissue Kit (Qiagen). DNA was resuspended in 50 ml of 10 mM Tris/0.1 mM EDTA. Triplicate PCR assays were carried out using 1 μg of genomic DNA as a template per reaction for semen samples. A fragment of 647 bp of the AAV-hFIX16 vector was amplified by PCR as previously described (Schuettrumpf et al., 2006).
Liver function test
Serum levels of alanine aminotransferase (ALT) were determined using a kit from TECO Diagnostics (Anaheim, CA). Samples were assayed undiluted (following the manufacturer's instructions), and a calibrator sample was used to standardize all of the values.
Statistical analysis
Comparison of data between experimental groups was analyzed by unpaired Student's t test, Z-test, or analysis of variance, using JMP version 4.0.2 (SAS Institute Inc., Cary, NC) (Schuettrumpf et al., 2005).
Results
Liver-directed gene expression by AAV-6 is superior to that by AAV-5
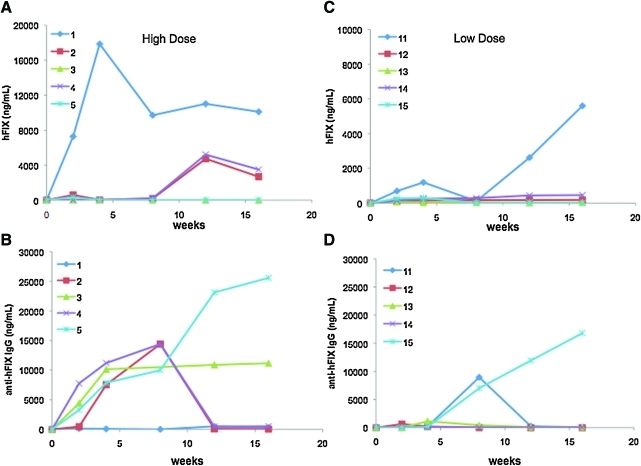
Adult rabbits weighing 3–4 kg are very close in size to non-human primates. We injected AAV-6 or AAV-5 encoding the hFIX gene under the control of a liver-specific promoter at doses of 1 × 1012 vg/kg (low-dose) or 1 × 1013 vg/kg (high-dose) via the peripheral intravenous route. In the high-dose cohort AAV-6-hFIX group, circulating FIX levels initially increased at week 2 and then returned to the baseline level in four of five animals (Fig. 1A and B). This was due to the formation of NAB (inhibitory antibodies) to hFIX that slowly diminished in two out of four rabbits after week 8, with a concomitant increase in the hFIX antigen levels ranging from 2,600 to 3,400 ng/ml (52–68% of normal). In two animals (number 5 and number 3) the NAB to hFIX remained detectable for the duration of the experiment, at a titer of 12 BU and 13 BU, respectively (Table 1). One animal (number 1) did not develop antibodies to hFIX, and hFIX levels were continuously detectable throughout the study with plateau levels of approximately 200%.
FIG. 1.
Time course of expression of hFIX antigen and formation of antibodies to hFIX in rabbits following delivery of AAV-6 vectors. Rabbits were injected via the peripheral vein with AAV-6 at doses of 1 × 1013 vg/kg (high-dose, A and B) or 1 × 1012 vg/kg (low-dose, C and D). Data are shown for each individual animal. Note the differences in the scales from (A) and (C). Color images available online at www.liebertonline.com/hum.
Table 1.
Prevalence of Neutralizing Antibodies to hFIX Following Liver Gene Delivery by AAV Vectors
| |
|
Vector dose/kg |
|||
|---|---|---|---|---|---|
| |
|
AAV-5 |
AAV-6 |
||
| Inhibitory antibody | Week | 1 × 1012 | 1 × 1013 | 1 × 1012 | 1 × 1013 |
| Prevalence | 4–8 | 0/5 | 0/5 | 2/5 | 4/5 |
| Inhibitor titer range (BU)a | 4–8 | NA | NA | 6.8–8.6 | 1.4–13 |
| Inhibitor eradication | 16 | NA | NA | 1/2 | 2/4 |
| Inhibitor titers (BU) | 16 | NA | NA | 34 | 12.5–13.8 |
| FIX levels (%)a | |||||
| Mean | 16 | <1% | 8.7% | 6.4% | 63% |
| Range | (0–0.76%) | (5–20%) | (4–9%) | (58–69%) | |
Excluding one animal each from the low- and high-dose cohort injected with AAV-6 and expressing FIX levels of 100% and 200%, respectively.
BU, Bethesda unit, where 1 BU is defined as the amount of antibody that neutralizes the clotting activity of FIX by 50%; NA, not applicable.
In the AAV-6-FIX low-dose cohort, hFIX levels reached plateaus of 4–9% of normal (with no antibody to FIX). In one rabbit (number 13), hFIX levels reached approximately 1.5% by week 4 and subsequently dropped to levels < 1% concurrent with the appearance of non-NAB to hFIX (Fig. 1C and D). Inhibitory antibodies to FIX were detected in animals number 11 and number 15 and peaked at levels of 8.6 BU and 6.8 BU, respectively. These antibodies were transient in one rabbit (number 11), with spontaneous disappearance by inhibitory and anti-hFIX IgG assays, accompanied by a rise in circulating hFIX levels to 100% of normal in this animal. Together, these data showed that, although inhibitory antibodies developed in six of the 10 rabbits administered AAV-6-hFIX, long-lasting inhibitors remain in only three of the 10, which is comparable to the approximately 20% of inhibitor formation rates to hFIX data observed in non-human primate models following injection with AAV-hFIX vectors of distinct serotypes (Nathwani et al., 2002, 2007; Davidoff et al., 2005).
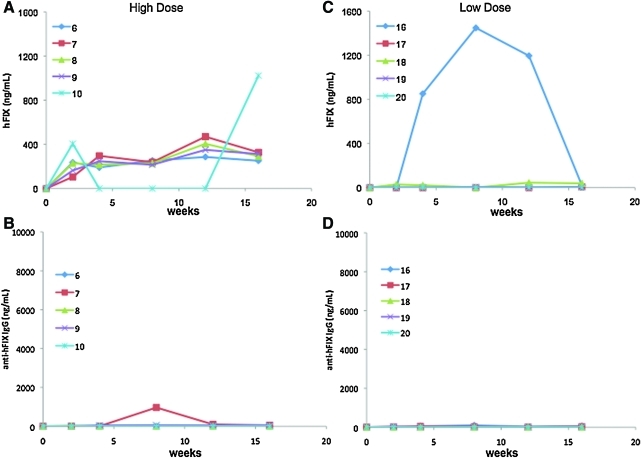
In the AAV-5 high-dose cohort, circulating FIX levels increased over time, reaching plateau levels of 4–10% (n = 4), with no increase of anti-hFIX IgG above the baseline values (Fig. 2). In one animal (rabbit number 10), hFIX expression peaked at week 2 (approximately 200 ng/ml) and remained undetected until week 12, when circulating FIX levels rose above 20% of normal without detectable antibodies to FIX (Fig. 2A and B). In the low-dose cohort, FIX levels were less than 1% of normal with no formation of antibodies to hFIX using specific enzyme-linked immunosorbent or inhibitory assays (Fig. 2C and D and Table 1). One animal (rabbit number 16) showed transient expression that reached levels > 1,000 ng/ml followed by a slowly decline to undetectable levels with no evidence of antibodies to FIX. In this animal, the value for gene copy number (gcn) in the liver at week 30, the time that the circulating FIX was no longer detected, was within the range of vector content detected in other animals in this cohort (see below). The alanine aminotransferase levels in this animal were unchanged from the baseline as measured at several time points up to week 16, and they were comparable to the levels of animals injected with AAV-5 or AAV-6 (data not shown).
FIG. 2.
Circulating FIX antigen levels and anti-hFIX IgG titers in rabbits injected with AAV-5 vectors. Rabbits were injected via the peripheral vein with AAV-5 at 1 × 1013 vg/kg (high-dose, A and B) or 1 × 1012 vg/kg (low-dose, C and D). Data are shown for each individual animal. Color images available online at www.liebertonline.com/hum.
In contrast to AAV-6-injected rabbits, no inhibitory antibodies to FIX were found in the animals from the AAV-5 groups, and the levels of non-NAB were remarkably lower for both dose cohorts compared with AAV-6 (Figs. 1 and 2 and Table 1).
Vector DNA content in the liver samples at the end of the experiment demonstrated a high variability for both vectors. In the high-dose cohort, the gene copy number (gcn) per cell for AAV-6 was higher than for those injected with AAV-5 (3.9 ± 2.6 vs. 1.3 ± 1.6 gcn/cell, p < 0.05). No differences were observed in the vector DNA in the liver of animals injected with low doses of AAV-6 or AAV-5 (0.37 ± 0.1 vs. 0.25 ± 0.33 gcn/cell, respectively). Together with the data showing higher levels of circulating FIX, these findings demonstrate that AAV-6-mediated liver transduction efficiency is superior to that of AAV-5 in this model. Because of the wide range of circulating FIX levels, especially in the AAV-6 cohorts, the determination of the exact fold difference between AAV-5 and AAV-6 is not feasible. By excluding the single highest expression in each cohort, we could speculate that AAV-6 is approximately six- to sevenfold more efficient than AAV-5 (Table 1).
Kinetics of humoral responses to AAV-6 and AAV-5 capsid proteins
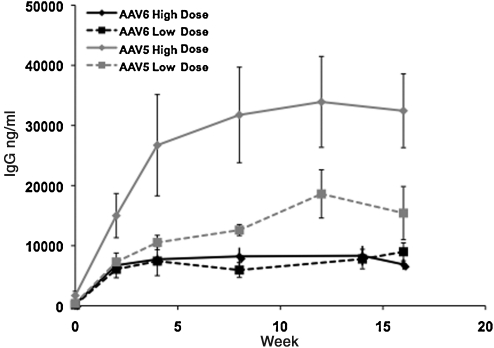
The time course and magnitude of the humoral immune response as a function of vector serotype and dose were determined by monitoring the levels of capsid-specific IgG. All animals developed antibodies to AAV capsid proteins. In both dose cohorts, the anti-AAV-6 IgG levels reached plateaus at week 5 and remained stable up to week 16 (Fig. 3). In contrast, the levels of anti-AAV-5 IgG continued to increase, albeit modestly, to reach plateau levels after week 12. The levels of capsid-specific IgG in the groups injected with AAV-5 showed dose dependence, but no dose dependence was seen for the AAV-6 cohorts. In the high-dose cohort, the levels of AAV-5-specific IgG were two- to four-fold higher (p < 0.009) than the antibodies to AAV-6 capsid at all time points tested (Fig. 3).
FIG. 3.
Humoral responses to AAV capsid protein. Rabbits were injected with AAV-5 or AAV-6 at doses of 1 × 1012 vg/kg (low-dose) or 1 × 1013 vg/kg (high-dose). Titers of specific IgG to each dose cohort (n = 5 animals per dose cohort) are shown. Values of p < 0.05 (by analysis of variance) reflect comparison between groups of the high-dose cohorts.
Clearance of AAV sequences in semen is faster in rabbits injected with AAV-5
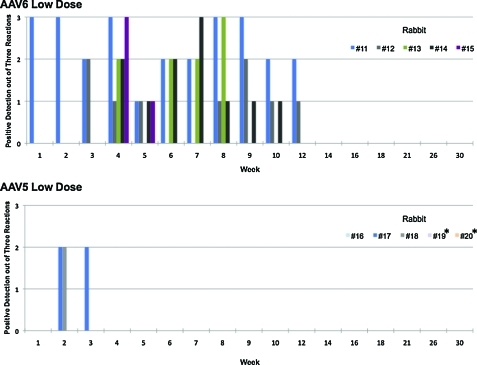
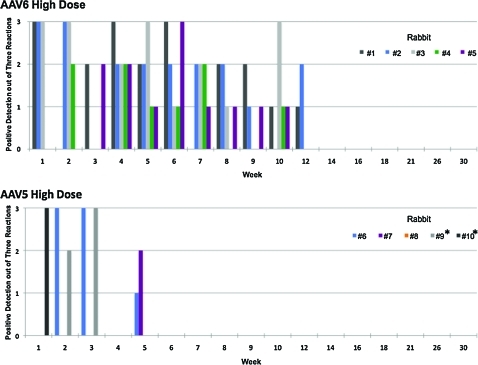
Previous data demonstrated that the duration of detectable vector sequences in the semen of rabbits injected with AAV-2 and AAV-8 was vector dose-dependent (Favaro et al., 2009). Here we documented similar findings for AAV-6. Triplicate assays in samples collected at 11 time points post-vector injection from five animals per dose resulted in a total of 165 PCR procedures per cohort; the number of semen samples testing positive with the high-dose were higher than with the low-dose cohort (47% vs. 34%, respectively; p < 0.05). Initial AAV-6 vector shedding to the semen was present in one or two animals, and only after week 4 did all animals test positive. However, the last semen samples that tested positive were from week 12 for both cohorts (Figs. 4 and 5).
FIG. 4.
Kinetics of appearance of vector DNA sequences in total semen from the low-dose cohort rabbits: (top panel) animals injected with AAV-6 and (bottom panel) animals injected with AAV-5 at doses of 1 × 1012 vg/kg. The numbers of triplicate PCR signals from each individual animal are indicated. *Denotes animals followed up to week 20 after vector injection. Color images available online at www.liebertonline.com/hum.
FIG. 5.
Kinetics of appearance of vector DNA sequences in total semen from the high-dose cohort rabbits: (top panel) animals injected with AAV-6 and (bottom panel) animals injected with AAV-5 at doses of 1 × 1013 vg/kg. The numbers of triplicate PCR signals from each individual animal are indicated. *Denotes animals followed up to week 20 after vector injection. Color images available online at www.liebertonline.com/hum.
Notably, among rabbits injected with AAV-5 the kinetics were remarkably different from the previous AAV serotype tested (Figs. 4 and 5). In the low-dose cohort, vector sequences were detected in only two of five animals and cleared within 2 or 3 weeks post-injection. In the high-dose cohort, although the vector sequences were found in four of five animals, there was a rapid clearance within 5 weeks.
We demonstrated no late recurrence of vector DNA in the semen of AAV-5- and AAV-6-dosed rabbits. The long-term follow-up design of this study allowed the assessment of several rabbit spermatogenesis cycles (42 days/cycle) (Adams, 1987). This period covered three to seven consecutive cycles of spermatogenesis per animal for each serotype (Figs. 4 and 5). This represents a total of 23 and 37 cycles of spermatogenesis for AAV-6 and AAV-5 cohorts, respectively.
Biodistribution of vector DNA in tissues differs between AAV-5 and AAV-6
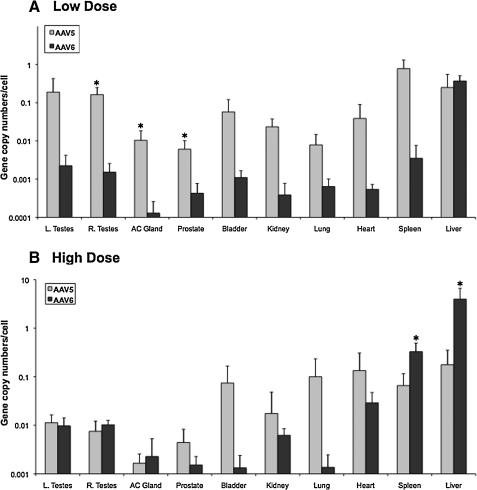
We compared vector-DNA content using real-time quantitative PCR (Fig. 6) in rabbits injected with AAV-5 (n = 10) or AAV-6 (n = 8). In the AAV-5 cohort, two rabbits from each dose cohort were studied at week 20. Because there was no difference in the vector content in these rabbits, we are showing the data obtained at 20 and 30 weeks together in Fig. 6. The highest number of vector copies per cell was found in the liver for both vector serotypes, followed by the spleen. One animal in the low-dose group of AAV-5 presented high levels of vector DNA in the liver and spleen (4.0 and 1.7 gcn/cell, respectively), which explains the apparent higher amounts of DNA in the spleen compared with the liver in this cohort.
FIG. 6.
Biodistribution of AAV DNA in rabbits following intravenous injection of AAV vectors. (A) Rabbits received a low dose (1 × 1012 vg/kg) of AAV-5 (n = 3) or AAV-6 (n = 4) vectors, and the indicated tissues were harvested at week 30. Three animals were sacrificed at week 20 (two for AAV-5 and one for AAV-6). (B) Rabbits were injected with a high dose (1 × 1013 vg/kg) of AAV-5 (n = 5) or AAV-6 (n = 5) vectors, and the indicated tissues were harvested at week 30. Three animals (AAV-5, n = 2; AAV-6, n = 1) were sacrificed at week 20. Vector DNA content was obtained by real-time quantitative PCR assay. Combined data from the week 20 or 30 time points are shown as mean ± SD values. *p < 0.05 between serotype groups. AC Gland, accessory gland; L. and R. Testes, left and right testes, respectively.
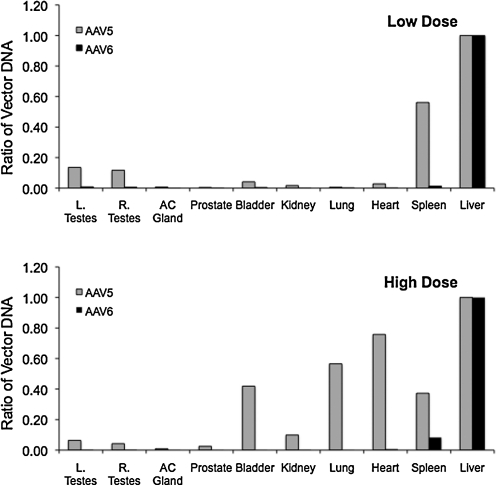
The vector DNA content in the tissues harvested from several organs showed similar or higher gcn values for AAV-5 compared with AAV-6, with the exception of the liver and spleen in the high-dose cohorts. For AAV-6 the extrahepatic tissues contain minimal amounts of vector genomes. The vector DNA content from the spleen was 12-fold and 76-fold lower than the liver for the high- and low-dose cohorts, respectively. In the gonadal tissues, vector DNA content was 200- (low dose) to 400-fold (high dose) lower than that in the liver (Fig. 7). In contrast, in the high-dose cohort the relative concentration of AAV-5 vector DNA was considerably higher in the spleen, heart, lung, and bladder compared with the liver (Fig. 7). In the low-dose cohort, spleen and testes contain relative high amounts of residual vector. In the gonadal tissue, vector DNA was eightfold and 15–20-fold lower than in the liver for the low- and high-dose cohorts, respectively.
FIG. 7.
Relative concentration of vector DNA in tissues compared with the liver. Data show the difference in the mean concentration of vector DNA in low-dose (top panel) and high-dose (bottom panel) cohorts. AC Gland, accessory gland; L. and R. Testes, left and right testes, respectively.
Discussion
A recent study has shown that only 27% of humans did not demonstrate NAB to the capsid of the most commonly tested AAV serotypes (Boutin et al., 2010). The development of an AAV vector based on an alternate serotype that can overcome the high prevalence of NAB to AAV capsid is of fundamental interest for gene therapy or vaccination using these vectors. Here we compared AAV-5 and AAV-6 in a rabbit model that allowed simultaneous characterization of gene transfer efficacy and acute and long-term safety. We chose a peripheral vein injection model of vector administration because this route is advantageous for clinical studies for liver-target gene delivery and provides a stringent assessment of vector biodistribution. Overall, AAV-6 vectors were superior to AAV-5 in increasing the circulating levels of hFIX in a dose-dependent manner. This is consistent with the observation that AAV-6 gcn values were higher in the liver than those of AAV-5. The efficacy of the AAV-6 vectors is comparable and potentially superior (in the high-dose cohort) to AAV-8 vector, as has been previously reported using this model (Favaro et al., 2009).
The use of a xeno-transgene resulted in transient expression of hFIX in the AAV-6-injected animals due to the formation of antibodies to hFIX in six of the 10 rabbits. In three animals, this was followed by spontaneous disappearance of the antibodies with a concurrent increase of circulating FIX levels. Thus, antibodies persisted in only three (30%) of all AAV-6-injected rabbits, which is comparable to the rates of 20% of long-lasting inhibitors in rabbits injected with AAV-2 or AAV-8 (Favaro et al., 2009) and in non-human primates following liver-directed injection of AAV-5 or AAV-8 (Nathwani et al., 2002, 2007; Davidoff et al., 2005).
Data on the efficacy of liver-directed transfer using AAV-5 vectors in murine models are conflicting (Mingozzi et al., 2002; Grimm et al., 2003; Zincarelli et al., 2008). In this study, although FIX levels were subtherapeutic at the low dose of AAV-5, at the high dose circulating FIX reached therapeutic levels that could potentially improve the disease phenotype to moderate–mild, without the formation of inhibitory antibodies to hFIX. We did not anticipate these findings because early data suggested that AAV-5 could present an increased risk of immune responses because of its tropism for dendritic cells compared with other AAV serotypes (Xin et al., 2006). However, in non-human primates, liver-directed gene expression by AAV-5 at doses comparable to that of the high-dose cohort in this study resulted in sustained expression of rhesus erythropoietin or of the β subunit of the rhesus choriogonadotropic hormone (Gao et al., 2006), with no immune responses to the transgene. On the other hand, in this model, initial expression of macaque erythropoietin was followed by sustained immune response to the transgene following intramuscular injection of AAV of distinct serotypes, including AAV-5. Thus, AAV-5 may not be as immunogenic in a liver-specific approach as suggested earlier in other experimental models (Xin et al., 2006). Humoral immune responses to the vector capsid were commonly found, and the highest antibody titers were observed for AAV-5. We speculate that the increased tropism of AAV-5 for dendritic cells, T cells, and macrophages compared with other alternate AAV serotypes (Xin et al., 2006) would increase the amount of AAV-5 capsid antigen available for antigen presentation and thus lead to enhanced anti-AAV-5 humoral immune responses. However, the levels of antibodies were significantly lower than in our previous report in rabbits following intravascular delivery of AAV-2 or AAV-8 at similar doses (Favaro et al., 2009).
In one animal (rabbit number 16, AAV-5 low-dose cohort) we observed an unusual time course of transgene expression. Circulating hFIX levels were initially detected at 2 weeks, peaked at 28% (week 8), and then returned to undetectable levels at week 16 without changes in the liver enzymes. There was no formation of antibodies to FIX, and the liver gcn value at week 30 was low (0.16 gcn/cell). The humoral response to the vector capsid in this animal was indistinguishable from that of the other rabbits in the same dose cohort. To date, we have injected AAV-hFIX of four distinct serotypes in 45 rabbits (including the groups reported here), and this pattern of expression was never observed (Favaro et al., 2009). It is unclear whether a cellular immune response or other hepatotoxicity is the underlying mechanism.
The use of the rabbit model has the advantage of allowing determination of AAV vector shedding to the semen, as initially documented in humans following hepatic artery delivery of AAV-2 (Manno et al., 2006; Schuettrumpf et al., 2006). Previously, we have determined that in rabbits injected with AAV-2 or AAV-8, the kinetics of vector clearance were dose-dependent. These findings were also confirmed for both AAV-5 and AAV-6. The duration of vector detection in the semen of animals injected with AAV-6 was similar to that seen with AAV-2 or AAV-8 (Favaro et al., 2009; Schuettrumpf et al., 2006). Notably, AAV-5 vector exhibits unique kinetics of clearance from the semen compared with all three previously tested serotypes delivered by a similar route and at a similar dose. Vector DNA in the testes was found in low amounts with the exception of the low-dose cohort of AAV-5. Together, the limited vector shedding to the semen and the relatively high vector DNA per cell in the testes suggest a high affinity of AAV-5 for gonadal tissue. Platelet-derived growth factor receptors have been identified as AAV-5 cellular receptors (Di Pasquale et al., 2003). Platelet-derived growth factor receptors and platelet-derived growth factor are involved in several developmental processes, including male testicular development and spermatogenesis, and their mRNAs are present in human fetus and adult human testicular samples (Mariani et al., 2002). Thus, it is possible that AAV-5 vector has high tissue tropism for male gonadal tissue and that the vector dissemination to the semen is minimized. Recently, the epidermal growth factor receptor was identified as a co-receptor for AAV-6 (Weller et al., 2010). These receptors are commonly found in several anatomic structures of the genitorurinary tract of mammals as well as in human spermatozoa (Damjanov et al., 1993; Oliva-Hernandez and Perez-Gutierrez, 2008). Thus, it is possible that AAV-6 binding to mature spermatozoa is, at least in part, the mechanism of transient vector shedding to the semen. However, the lack of late recurrence of vector in the semen for several consecutive spermatogenesis cycles for both the AAV-5 and AAV-6 experimental groups suggests that these vectors are inefficient in transducing the male germ cell, as previously observed for AAV-2 and AAV-8 (Favaro et al., 2009).
Biodistribution analyses of vector genomes in several tissues showed that AAV-6 genomes are found in very low amounts outside the liver and spleen. In contrast, the AAV-5 genomes were found in several tissues and other organs compared with the liver. Extrahepatic distribution was almost uniformly higher for AAV-5 compared with AAV-6 at both low dose and high dose, with the exception of the spleen. These differences probably reflect distinct cellular receptor distributions and/or intracellular vector processing for distinct serotypes (Wu et al., 2000; Di Pasquale et al., 2003; Seiler et al., 2006). In addition, there are relatively high proportions of AAV-5 vector genomes in several tissues compared with the liver. Notably, the findings in rabbits are comparable to those of non-human primates injected with AAV-5 (Gao et al., 2006; Nathwani et al., 2007) and thus unlikely to represent a species-specific bias in the vector biodistribution.
Data on biodistribution of AAV-6 by systemic intravascular delivery in large animals are limited. One study reported that early systemic dissemination (7–10 days) following percutaneous transendocardial delivery by AAV-6 resulted in high vg counts in the heart, followed by the liver (Bish et al., 2008). Overall, the data from AAV-6 in rabbits are comparable to those of intravascular delivery of AAV-2 or AAV-8 vectors; thus the long-term safety profile of AAV-6 is encouraging for systemic delivery. The use of AAV-5 vectors in large animals is associated with a distinct biodistribution compared with other serotypes and with negligible vector shedding to the semen. Together with our previous work using AAV-2 or AAV-8 vectors we concluded that biodistribution is serotype- and dose-dependent. Thus, the safety of a given AAV serotype cannot be extrapolated from studies based on distinct serotypes. The development of novel AAV genomes (McCarty et al., 2001; Wang et al., 2003), capsid modifications (Stemmer, 1994; Zhong et al., 2008), and use of transgenes with more efficient transduction or enhanced biological activity are all attractive strategies to further lower the therapeutic dose of intravascular delivery of vector to liver and other target tissues. This could facilitate the clinical translation of AAV of alternate serotypes by designing more efficacious protocols that are not limited by the high prevalence of NAB to AAV capsid.
Acknowledgments
We thank Aaron Weilerstein (Charles River Laboratories, Horsham, PA) and Michael Maiden for excellent technical assistance and Linda B. Couto, Bernd Hauck, and Olga Zelenaia (Center for Cell and Molecular Therapeutics, The Children's Hospital of Philadelphia) for scientific discussion. We thank Junwei Sun for expert assistance. This work was supported by National Institutes of Health grants RO1 HL084220 (to V.R.A.) and P01 HL064910 (to V.R.A., Project 1; and to K.A.H., Project 2).
Author Disclosure Statement
J.F.W. and K.A.H. are consultants for companies that are developing AAV-based therapeutics not in the field of hemophilia and hold patents related to AAV gene therapy. All other authors declare no competing financial interests.
References
- Adams C.E. The laboratory rabbit. In: Poole TB, editor. In The UFAW Handbook on the Care and Management of Laboratory Animals. Churchill Livingstone; New York: 1987. pp. 415–435. [Google Scholar]
- Arruda V.R. Fields P.A. Milner R., et al. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol. Ther. 2001;4:586–592. doi: 10.1006/mthe.2001.0491. [DOI] [PubMed] [Google Scholar]
- Arruda V.R. Stedman H.H. Haurigot V., et al. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood. 2010;115:4678–4688. doi: 10.1182/blood-2009-12-261156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge J.W. Smith A.J. Barker S.S., et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Bish L.T. Sleeper M.M. Brainard B., et al. Percutaneous transendocardial delivery of self-complementary adeno-associated virus 6 achieves global cardiac gene transfer in canines. Mol. Ther. 2008;16:1953–1959. doi: 10.1038/mt.2008.202. [DOI] [PubMed] [Google Scholar]
- Boutin S. Monteilhet V. Veron P., et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- Calcedo R. Vandenberghe L.H. Gao G., et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanov I. Solter D. Knowles B.B. Functional epidermal growth factor receptor localizes to the postacrosomal region of human spermatozoa. Biochem. Biophys. Res. Commun. 1993;190:901–906. doi: 10.1006/bbrc.1993.1134. [DOI] [PubMed] [Google Scholar]
- Davidoff A.M. Gray J.T. Ng C.Y., et al. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol. Ther. 2005;11:875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Di Pasquale G. Davidson B.L. Stein C.S., et al. Identification of PDGFR as a receptor for AAV-5 transduction. Nat. Med. 2003;9:1306–1312. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- Favaro P. Downey H.D. Zhou J.S., et al. Host and vector-dependent effects on the risk of germline transmission of AAV vectors. Mol. Ther. 2009;17:1022–1030. doi: 10.1038/mt.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Alvira M. Wang L., et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Lu Y. Calcedo R., et al. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol. Ther. 2006;13:77–87. doi: 10.1016/j.ymthe.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Grimm D. Zhou S. Nakai H., et al. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003;102:2412–2419. doi: 10.1182/blood-2003-02-0495. [DOI] [PubMed] [Google Scholar]
- Halbert C.L. Miller A.D. McNamara S., et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2006;17:440–447. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth W.W. Aleman T.S. Kaushal S., et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum. Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. Couto L.B. Patarroyo-White S., et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006a;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. Lillicrap D. Patarroyo-White S., et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006b;108:107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- Jiang H. Pierce G.F. Ozelo M.C., et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia. B. Mol. Ther. 2006c;14:452–455. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Le M. Okuyama T. Cai S.R., et al. Therapeutic levels of functional human factor X in rats after retroviral-mediated hepatic gene therapy. Blood. 1997;89:1254–1259. [PubMed] [Google Scholar]
- Maguire A.M. High K.A. Auricchio A., et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: A phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M. Simonelli F. Pierce E.A., et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C.S. Chew A.J. Hutchison S., et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mariani S. Basciani S. Arizzi M., et al. PDGF and the testis. Trends Endocrinol. Metab. 2002;13:11–17. doi: 10.1016/s1043-2760(01)00518-5. [DOI] [PubMed] [Google Scholar]
- Matsushita T. Elliger S. Elliger C., et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- McCarty D.M. Monahan P.E. Samulski R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- Miao C.H. Ohashi K. Patijin G.A., et al. Inclusion of the hepatic locus control region, an intron, and untranslated region increases and stabilizes hepatic factor IX gene expression in vivo but not in vitro. Mol. Ther. 2000;1:522–532. doi: 10.1006/mthe.2000.0075. [DOI] [PubMed] [Google Scholar]
- Mingozzi F. Schüttrumpf J. Arruda V., et al. Improved hepatic gene transfer by using an adeno-associated virus serotype 5 vector. J. Virol. 2002;76:10497–10502. doi: 10.1128/JVI.76.20.10497-10502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani A.C. Davidoff A.M. Hanawa H., et al. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100:1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- Nathwani A.C. Gray J.T. McIntosh J., et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva-Hernandez J. Perez-Gutierrez J.F. Localization of the epidermal growth factor (EGF) in the epididymis and accessory genital glands of the boar and functional effects on spermatozoa. Theriogenology. 2008;70:1159–1169. doi: 10.1016/j.theriogenology.2008.06.090. [DOI] [PubMed] [Google Scholar]
- Scallan C.D. Jiang H. Liu T., et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- Schuettrumpf J. Herzog R.W. Schlachterman A., et al. Factor IX variants improve gene therapy efficacy for hemophilia B. Blood. 2005;105:2316–2323. doi: 10.1182/blood-2004-08-2990. [DOI] [PubMed] [Google Scholar]
- Schuettrumpf J. Liu J.H. Couto L.B., et al. Inadvertent germline transmission of AAV2 vector: Findings in a rabbit model correlate with those in a human clinical trial. Mol. Ther. 2006;13:1064–1073. doi: 10.1016/j.ymthe.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Seiler M.P. Miller A.D. Zabner J. Halbert C.L. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum. Gene Ther. 2006;17:10–19. doi: 10.1089/hum.2006.17.10. [DOI] [PubMed] [Google Scholar]
- Sommer J.M. Smith P.H. Parthasarathy S., et al. Quantification of adeno-associated virus particles and empty capsids by optical density measurement. Mol. Ther. 2003;7:122–128. doi: 10.1016/s1525-0016(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Stemmer W.P. Rapid evolution of a protein in vitro by DNA shuffling. Nature. 1994;370:389–391. doi: 10.1038/370389a0. [DOI] [PubMed] [Google Scholar]
- Wang L. Calcedo R. Nichols T.C., et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105:3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- Wang L. Calcedo R. Wang H., et al. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol. Ther. 2010;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Chamberlain J.S. Tapscott S.J. Storb R. Gene therapy in large animal models of muscular dystrophy. ILAR J. 2009;50:187–198. doi: 10.1093/ilar.50.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Ma H.I. Li J., et al. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- Weller M.L. Amornphimoltham P. Schmidt M., et al. Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nat. Med. 2010;16:662–664. doi: 10.1038/nm.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. Xiao W. Conlon T., et al. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J. Virol. 2000;74:8635–8647. doi: 10.1128/jvi.74.18.8635-8647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin K.Q. Mizukami H. Urabe M., et al. Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells. J. Virol. 2006;80:11899–11910. doi: 10.1128/JVI.00890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L. Li B. Mah C.S., et al. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zincarelli C. Soltys S. Rengo G. Rabinowitz J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]