Abstract
Cell replacement therapy could be an important treatment strategy for Parkinson's disease (PD), which is caused by the degeneration of dopamine neurons in the midbrain (mDA). The success of this approach greatly relies on the discovery of an abundant source of cells capable of mDAergic function in the brain. With the paucity of available human fetal tissue, efforts have increasingly focused on renewable stem cells. Human induced pluripotent stem (hiPS) cells offer great promise in this regard. If hiPS cells can be differentiated into authentic mDA neuron, hiPS could provide a potential autologous source of transplant tissue when generated from PD patients, a clear advantage over human embryonic stem (hES) cells. Here, we report that mDA neurons can be derived from a commercially available hiPS cell line, IMR90 clone 4, using a modified hES differentiation protocol established in our lab. These cells express all the markers (Lmx1a, Aldh1a1, TH, TrkB), follow the same mDA lineage pathway as H9 hES cells, and have similar expression levels of DA and DOPAC. Moreover, when hiPS mDA progenitor cells are transplanted into 6-OHDA-lesioned PD rats, they survive long term and many develop into bona fide mDA neurons. Despite their differentiation and integration into the brain, many Nestin+ tumor-like cells remain at the site of the graft. Our data suggest that as with hES cells, selecting the appropriate population of mDA lineage cells and eliminating actively dividing hiPS cells before transplantation will be critical for the future success of hiPS cell replacement therapy in PD patients.
Introduction
Currently there is no cure for PD patients, though some pharmaceutical or surgical treatments may alleviate symptoms at early stages. In most cases, however, serious side effects and other PD-related complications appear as the disease progresses. Consequently, cell-based therapy, particularly in late stage disease, remains a promising approach. Cell replacement is especially attractive in PD because neurodegeneration is largely confined to one nucleus, the substantia nigra (SN), which is comprised exclusively of midbrain dopamine (mDA) neurons. Finding a reliable and abundant cell source capable of mDAergic function in the brain is therefore vital for the success of this therapy. With the paucity of available human fetal tissue, efforts have increasingly focused on renewable cells such as human neural progenitor cells (hNPs). Over the past decade, much progress has been made in defining the parameters in culture that promotes the appearance of mDA traits in hNPs derived from any of a number of embryonic and adult tissues [1,2]. Our own studies have shown that hNPs derived from clonal lines [3–5], embryonic cerebral cortex [6], adult human bone marrow [7,8], amniotic fluid [9], and hES cells [10,11] can be induced to express mDA traits by modifying their growth conditions in culture. However, only those hNPs derived from pluripotent cells such as human embryonic stem (hES) cells actually survive and give rise to midbrain-specific DA (mDA) neurons following transplantation in vivo.
Capitalizing on the advances in somatic cell nuclear transfer technology and fueled by a greater understanding of ES cell pluripotency, several seminal studies have now shown that somatic cells can be re-programmed to become pluripotent stem cells, termed induced pluripotent stem (iPS) cells, following the transfection of several key transcription factors, including Oct4 and Sox2, into mouse or human fibroblasts [12–14]. The emergence of iPS cell technology has prompted a plethora of new studies. Of relevance to PD is the recent study by Wernig et al. [15] showing that iPS cells generated from mouse fibroblasts can be differentiated in culture and transplanted into PD rats to produce motor recovery. These studies, taken together with the recent demonstration that human iPS (hiPS) cells can be generated directly from PD patients [16], offer hope that patients may someday supply their own replacement mDA neurons.
In recent months, Wicell has made available lines of hiPS cells, including a clone (IMR90, clone 4) that behaves nearly identically to the H9 hES cell line [14]. Here, we report that we can generate mDA neurons from this hiPS line at a similar efficiency as with H9 hES cell line. The mDA precursor cells were also injected into the striatum of 6-OHDA-lesioned rat PD models and mature DA neurons were found 6 weeks after transplantation. Our data suggested that this hiPS cell line can now be used in proof of principle experiments to study the genes and mechanisms that promote the mDA differentiation of hiPS cells in culture and factors that enhance their survival and function after their transplantation into the PD rat brain. In addition, these studies can provide the critical validation needed before hiPS cells, even those generated directly from patients can be fully embraced for use in PD.
Materials and Methods
Tissue culture
hiPS cells (IMR90, clone 4) were purchased from Wicell Research Institute (University of Wisconsin-Madison, Madison, WI) who generate the cell line through viral infection [14] and maintained according to the supplier's instructions. In brief, cells were grown on a monolayer of primary mouse fibroblasts (MEFs; Millipore #PMEF-CF) in DMEM/F12 media (Invitrogen #11330) supplemented with 20% Knockout Serum Replacer™ (KOSR; Invitrogen #10828), 1% nonessential amino acids (Invitrogen #11140), 1 mM l-glutamine (Invitrogen #25030), 0.1 mM 2-mercaptoethanol, and 100 ng/mL bFGF (R&D systems,Minneapolis, MN, #233-FB/CF). Cell propagation was achieved through manual dissection and transfer of cell colonies once per week (Stg I). The differentiation process was initiated with the formation of embryoid bodies (EBs, Stg II, 4 days). Rosettes of hNPs were generated on Geltrex (Invitrogen #A10480)-coated tissue culture plates in NEP basal medium consisting of DMEM/F12 (Invitrogen #11320), 1 mg/mL BSA (Sigma #A2153), 1× N2 supplements (Invitrogen #17502), 1× B27 supplements (Invitrogen #17504), and 1% Pen-strep (Stg III, 4 days). Rosettes were then expanded in NEP basal medium supplemented with 20 ng/mL bFGF every 2 days (Stg IV, 7 days). After 1 week, rosettes were lifted, seeded onto Ultralow dishes, and carried for 1–3 weeks as NP spheres before further differentiation in culture or transplantation into the brain. For further differentiation down the DA pathway, cells were seeded onto Geltrex-coated slides and incubating for 1 week in 1 mM dibutyryl cAMP (dbcAMP; Sigma, St. Louis, MO), as described in [10] (Stg V, 7 days).
Immunocytochemistry
Cultures were rinsed twice and then fixed with 4% paraformaldehyde as previously detailed [10]. Transplanted rat brains were perfused with 4% cold (4°C) periodate–lysine–paraformaldehyde, sectioned at 30 μm on a freezing microtome, and processed for immunocytochemistry using the immunofluorescence staining method described previously [6]. Cultures or sections were stained with rabbit antibodies to Lmx1a (kind gift of Dr. M. German, UCSF); rabbit anti-Nestin from Millipore AB5922, 1:200; mouse anti-Sox2 from R&D Systems MAB2018, 1:1,000; mouse anti-TrkB from R&D Systems MAB397, 1:100; rabbit anti-TH from Pel-Freez P40101–0, 1:200 or sheep anti-TH from Abcam AB113, 1:1,000; Goat anti-Aldh1a1 from Novus Biologicals NB100–2563, 1:200; mouse anti-nuclear human specific (HNA) from Millipore MAB1281 at 1:40; rabbit anti-Ki67 from Abcam AB833 at 1:50. All secondary antibodies were Alexa Fluor antibodies from Invitrogen used at 1:200. Cultures or sections were mounted in ProLong Gold antifade reagent (Invitrogen, Carlsbad, CA) and analyzed using a Nikon-Scanalytics Image System or an Olympus IX81 Image System. Confocal analysis was performed using a laser scanning confocal microscope (Olympus Fluoview, Olympus America Inc.).
RNA isolation and CDNA synthesis
Total RNA was isolated directly from freshly collected Stg IV and Stg V hiPS cells with TRIzol LS (Invitrogen), a modification of the guanidine isothiocyanate-phenol-chloroform extraction method. cDNA was synthesized by using 100 ng total RNA in a 20 μL reaction with Superscript III (Invitrogen) and oligo(dT)12–18 (Invitrogen). One microliter of RNase H (Invitrogen) was added to each reaction tube, and the tubes were incubated for 20 min at 37°C before proceeding to PCR.
PCR amplification
A 0.5 μL cDNA template was used in a 20 μL reaction volume with the PCR master mix (Promega, Madison, WI). The cycling parameters were: 94°C, 30 s; 55°C, 30 s; 72°C, 30 s, for 30 cycles. The PCR cycles were preceded by an initial denaturation of 3 min at 94°C and followed by a final extension of 10 min at 72°C. Primers are listed in Table 1 (CHAT primers were found in [17]).
Table 1.
PCR Amplification Primers
| Gene names | Primer location | Sequence | Product size (bp) |
|---|---|---|---|
| Aldhlal | Sense | GCTTATCAGCAGGAGTGTTTACCA | 150 |
| Antisense | CTCTTCCATTTCCAGACATCTTGA | ||
| CHAT* | Sense | ATCGCTGGTACGACAAGTCC | 151 |
| Antisense | ATCAGCTTCCTGCTGCTCTG | ||
| DBH | Sense | CCACTGGTGATAGAAGGACGAAA | 120 |
| Antisense | GGCCATCACTGGCGTGTAC | ||
| Foxa2 | Sense | AGGAGGAAAACGGGAAAGAATATAA | 150 |
| Antisense | AAGTAAGACTTCCCTGCAACAACA | ||
| GAD67 | Sense | ATTCTTGAAGCCAAACAG | 617 |
| Antisense | TAGCTTTTCCCGTCGTTG | ||
| GAPDH | Sense | GGAGTCAACGGATTTGGTCGTA | 150 |
| Antisense | GAATTTGCCATGGGTGGAAT | ||
| GLAST | Sense | GGCTTACTCATTCACGCAGTCA | 140 |
| Antisense | GGTAGGGTGGCAGAACTTGAAG | ||
| Lmx1a | Sense | CAGCCTCAGACTCAGGTAAAAGTG | 150 |
| Antisense | TGAATGCTCGCCTCTGTTGA | ||
| Msx1 | Sense | CTCCCTGAGTTCACTCTCCG | 192 |
| Antisense | CAGGAGACATGGCCTCTAGC | ||
| Nurrr1 | Sense | GGCTGAAGCCATGCCTTGT | 150 |
| Antisense | GTGAGGTCCATGCTAAACTTGACA | ||
| Pitx3 | Sense | GGACTAGGCCCTACACACAGA | 151 |
| Antisense | TCCGCGCACGTTTATTTC | ||
| TH | Sense | GCACCTTCGCGCAGTTCT | 107 |
| Antisense | CCCGAACTCCACCGTGAA | ||
| TPH1 | Sense | TCTATACCCCAGAGCCAGATACCT | 150 |
| Antisense | AGTAGCACGTTGCCAGTTTTTG |
Detection of dopamine and metabolite
Dopamine and metabolite levels were measured using high pressure liquid chromatography (HPLC) with electrochemical detection (Coulochem III system; ESA Inc., Chelmsford, MA). Cells from 2 different batches were sonicated in 0.4 M perchloric acid, the homogenate was centrifuged at 14,000 rpm for 10 min, and the supernatant was removed for analysis. Dopamine and metabolites were separated by injection of sample onto a C18 solid phase extraction column (ESA Inc.). The mobile phase consisted of 75 mM sodium dihydrogen phosphate, 1.7 mM 1-octanesulfonic acid, 100 μL/L triethylamine, 25 μM EDTA, and 10% acetonitrile. Peak heights of known external standards were used to create a standard curve to quantify dopamine and metabolite levels in each sample (EZChrome Chromatography Software, Scientific Software Inc., San Ramon, CA). Protein was extracted from the remaining cell pellet (NE-PER®, Pierce, Rockford, IL) and cytoplasmic protein levels were measured using a bicinchoninic acid (BCA) reaction (Pierce, Rockford, IL). Final dopamine and metabolite levels were then corrected based on the amount of protein in each sample.
6-OHDA lesions
As described previously [10], 9 Fischer 344 rats (Taconic) were made Parkinsonian for these studies. In brief, rats were anesthetized with sodium pentobarbital (30 mg/kg i.p.) and placed in a stereotaxic apparatus (Kopf Instruments), and a 26-gauge Hamilton syringe containing 6-OHDA (Sigma; 20 μg/mL in 4 μL PBS containing 0.2 mg/mL ascorbate) was lowered into the right median forebrain bundle (AP: −4.4 mm, ML: −1.2 mm, DV: −7.8 mm from bregma). The 6-OHDA solution was gradually injected at a rate of 1 μL/min. All lesions were verified 3 and 6 weeks later by assessment of rotational behavior in an automated rotometer system (Columbus Instruments) following amphetamine challenge (5 mg/kg, i.p.). Only rats with consistent and stable lesions (>10 ipsilateral turns/min on multiple tests) were used for transplantation studies.
Transplantation procedures
Animals with verifiable lesions (>10 ipsilateral turns/min) were implanted with a total of 10 μL of HBSS containing approximately 1 × 106 stage IV hiPS cells, deposited at 3 stereotaxic levels (AP: +1.2 mm, ML: −2.7 mm, DV: −5.4 mm, −4.9 mm and −4.2 mm) in the striatum on the side ipsilateral to the 6-OHDA lesion as described previously [10]. All transplant recipients received cyclosporin A (15 mg/kg IP) daily, beginning 1–2 days before transplantation. Animals were sacrificed at 6 weeks (N = 3).
Results
Human iPS cells behave and differentiate into mDA neurons similarly as HES cells in vitro
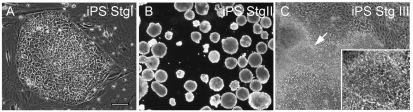
Human iPS cells (IMR90, clone 4) were expanded according to the manufacturer's instruction. When supplemented with 100 ng/mL bFGF, hiPS colonies displayed distinct smooth boundaries, which showed that cells were in the undifferentiated condition (Fig. 1A). Undifferentiated hiPS cells (Stg I) were differentiated further (Stg II to Stg V) using a modified 5-stages DA differentiation protocol developed in the lab as detailed in [10]. These 5 stages of the mDA differentiation process are: Stg I undifferentiated stem cells; Stg II embryonic bodies: Stg III hNPs forming; Stg IV hNPs expansion; and Stg V mDA neuron maturation. As shown in Figure 1B, undifferentiated hiPS cells could be lifted and made into EBs in a very similar way as hES cells. When EBs were replated on Geltrex-coated dishes with NEP basal medium, cells were further differentiated into Stg III hNPs (Fig. 1C). Occasionally rosette-like structures were observed (Fig. 1C, arrow and inset).
FIG. 1.
Bright field pictures of (A) undifferentiated human-induced pluripotent stem (hiPS) cells (Stg I), (B) embryonic bodies (Stg II), and (C) neural differentiating hiPS cells (Stg III). The arrow in C indicates rosettes observed in Stg III cells (inset). Scale bar = 100 μm for A and C, 250 μm for B.
By tracking transcription factor Lmx1a and other cell type (Aldh1a1, TH, TrkB), and stage (Sox2, Nestin)-specific markers, we have delineated individual steps in the mDA fate restriction process as hES cells transition from unspecified hNPs (Aldh1a1+/Lmx1a-) to mDA-specified NPS (mDANPs, Aldh1a1+/Lmx1a+) to mDA-specified precursors (mDA-PCs) to differentiated mDA neurons (Aldh1a1+/TH+).
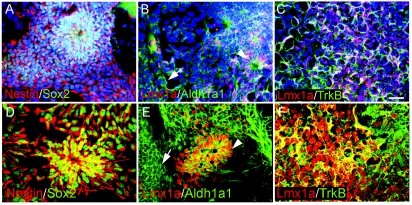
At Stg IV, hNPs labeled with Nestin and Sox2 (Fig. 2A and 2D) were further expanded in the NEP basal medium supplemented with bFGF. Compared to Stg III cells, many more rosettes of Nestin+Sox2+ hNPs were formed at Stg IV (Fig. 2A and 2D), which also expressed mDA-specified hNPs markers Lmx1a and Aldh1a1 (Fig. 2B and 2E, arrowheads). Non-specified Aldh1a1+ only hNPs were also found outside cell aggregates (Fig. 2B and 2E, arrows). As with hES cells, mDA-specified hiPS cells also expressed TrkB on their surface (Fig. 2C and 2F).
FIG. 2.
Expression of neural markers at Stg IV of human-induced pluripotent stem (hiPS) cells. (A) Neural progenitor markers Nestin (red) and Sox2 (green) were detected in many Stg IV hiPS cells, including cells inside rosettes. (B) Dopamine neurons in the midbrain (mDA) lineage markers Aldh1a1 and Lmx1a were expressed in Stg IV hiPS cells. Notice that there were 2 types of Aldh1a1 expressing cells: one was with long processes and inside rosettes or aggregates (arrowhead), the other was flat and outside aggregates (arrow). (C) Similar to human embryonic stem (hES) cells, mDA-specified hiPS cells labeled by Lmx1a also expressed TrkB on their surface. Dapi was used to counterstain all the nuclei in the field (A–C). Scale bar = 50 μm for A–C. (D–F) Higher magnification confocal images of A–C, respectively.
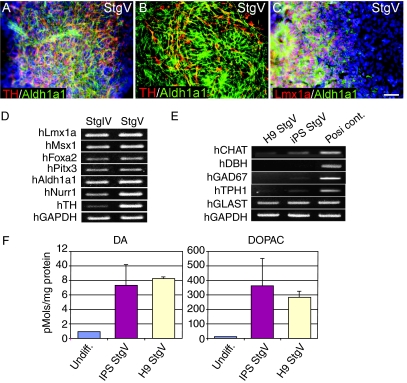
With the addition of 1 mM cAMP in Stage V, cells further differentiated into Aldh1a1+/TH+ mDA neurons (Fig. 3A and 3B). As with hES cells, even in terminally differentiated Stg V hiPS cultures, cells at earlier (Lmx1a+/Aldh1a1+) steps in the mDA differentiation process continued to be present (Fig. 3C). The yield of TH+ cells in total cells, 6.5% ± 1.4%, was also similar to that of hES cells. As expected, these mDA neurons did not co-label with GABA (data not shown). Corroborating our immunocytochemical findings, the mDA lineage markers Lmx1a, Msx1, Foxa2, Pitx3, Aldh1a1, Nurr1, and TH were all detected in later differentiation stages (IV, V) by RT-PCR (Fig. 3D). Meanwhile, RT-PCR analysis also identified that Stg V hiPS cells contained other neurotransmitter types, particularly glutamatergic neurons expressing the glutamate transporter gene (GLAST). However, very few cholinergic, noradrenergic, GABAergic, or serotonergic neurons were found in our Stg V hiPS cells when synthetic enzymes specific for these neurotransmitters (CHAT, DBH, GAD67, and TPH1, respectively) were examined. The expression patterns of these iPS genes were equivalent to Stg V cells of H9 hES cell origin (Fig. 3E).
FIG. 3.
RT-PCR and immunocytochemical analyses of DA-related markers in late stages of human-induced pluripotent stem (hiPS) cells differentiation in culture. Many Aldh1a1+/TH+ dopamine neurons in the midbrain (mDA) neurons [(A) fluorescent microscope image, (B) confocal microscope image] and some Lmx1a+/Aldh1a1+ earlier step cells in the mDA differentiation (C) were observed at Stg V. Dapi was used to counterstain all the nuclei in the field. The yield of TH+ expressing cells in Stg V-differentiated hiPS cells is similar as hES cells. Scale bar = 50 μm for A and C. (D) DA-related genes are differentially expressed at stages IV–V of hiPS cell differentiation detecting by RT-PCR. (E) StgV of both H9 and hiPS cells express GLAST, but not other neurotransmitter system genes. (F) Significantly higher levels of DA and DOPAC were detected in Stg V hiPS cells and H9 cells as well.
Using HPLC, we also detected DA and its metabolite DOPAC in hiPS Stg V cells (Fig. 3F). While DA and DOPAC levels in undifferentiated cells (0.91, 17.32 pmoles/mg protein) were similar to those of other negative control cell lines (such as B35), hiPS Stg V cells expressed much higher levels of DA and DOPAC (7.31 ± 2.79, 357.94 ± 189.19 pmoles/mg protein) indicating that functional DA neurons were differentiated from hiPS cells (The DA level of our positive control SK-N-SH cells is around 3.0 pmoles/mg protein). Interestingly, H9 Stg V cells had comparable levels (8.18 ± 0.26, 283.78 ± 40.37 pmoles/mg protein) to hiPS Stg V cells, which is another characteristic shared by hES cells and hiPS cells.
Partially differentiated hiPS cells survive and give rise to mDA neurons after transplantation into a PD rat model
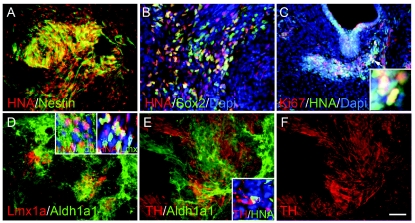
We next examined whether hiPS cells followed the same mDA differentiation process after their transplantation into the 6-OHDA-lesioned rat striatum. Cells were harvested at stage IV and transplanted as described previously [11]. When grafts were analyzed 6 weeks post-transplantation, we again found cells at all stages in the DA fate restriction process (Fig. 4). Thus, the grafts contained abundant hiPS cells identified by the presence of the human nuclear antigen (HNA, Fig. 4A and 4B) and the stem cell markers Nestin (Fig. 4A) or Sox2 (Fig. 4B). In addition, Sox2+/HNA–neural progenitor cells of host origin were also detected (Fig. 4B). Within the grafts, a subset of cells are mDA-specified, including Lmx1a+/Aldh+ mDA-NPs and Lmx1a-/Aldh+ mDA precursor cells (Fig. 4D). In addition, we found many differentiated Aldh1a1+/TH+ mDA neurons (Fig. 4E and 4F) that elicited long complex processes and appeared be well integrated into the parenchyma of the brain. As with hES cells grafts, however, in addition to differentiated cell types, undifferentiated hiPS cells continued to proliferate, as detected by Ki67 staining, with concomitant formation of teratoma-like structures (Fig. 4C). These data indicate that hiPS cells can indeed give rise to mDA neurons in vivo, but like their hES counterparts will require further purification to eliminate cell heterogeneity and unregulated proliferation in the graft. At week 6 of posttransplantation, although we observed a decline in rotation scores for several transplanted animals compared with their respective pre-transplantation scores, these values did not reach statistical significance (data not shown).
FIG. 4.
Immunofluorescence analysis of DA-related markers in stage IV human-induced pluripotent stem (hiPS) cells 6 weeks after transplantation. Most survived transplanted cells in the graft as detected by human nuclear antigen (HNA) are Nestin+ neural progenitor cells (A). Some are still Sox2+ neural progenitor cells (B). Occasionally proliferating Ki67 cells were found in the HNA+ grafted cells (C). Arrowhead pointed area is shown at a higher magnification in the inset. Clusters of Lmx1a+/Aldh1a1+, Aldh1a1+ precursor cells (D) were found in the grafts. Insets of D show the majority of these DA precursor cells were HNA+. Mature TH+/Aldh1a1+ neurons were also detected (E and F). Inset of E shows a grafted TH+/HNA+ neuron was surrounded by host cells (HNA–). Scale bar = 50 μm.
Discussion
Human iPS cells behave similarly with hES cells
These studies establish that the Wicell line IMR90 (clone 4) of hiPS cells behave nearly identically in culture to that of the H9 hES line. Cells are propagated in much the same fashion on media that varies only in its concentration of bFGF. Although hiPS cells require 25-fold higher levels of mitogen for continual cell propagation [14], the rate of cell division was comparable with both hiPS and hES cells requiring passaging one time per week. Moreover, when hiPS cells were differentiated into mDA neurons using the same conditions as those described for hES cells [11], they followed an analogous differentiation pathway: forming EBs, neural differentiation, neural progenitor proliferation and specification, and mDA neuron development. As detected by either RT-PCR or immunocytochemistry, at each stage they also expressed markers typical of their mDA phenotype (Lmx1a+ Aldh1a1+ mDA-specified hNPs, Aldh1a1+ mDA precursors, Aldh1a1+TH+ mDA neurons) and not other (GABAergic, cholinergic, noradrenalinergic, serotonergic) neurotransmitter systems. In addition, these differentiated hiPS cells also manufacture measurable levels of DA and its metabolite DOPAC. When Stg IV neural progenitors/precursors were transplanted into 6-OHDA-lesioned PD rat models, differentiated hiPS cells survived and integrated into host striatum and continued their mDA development into differentiated Aldh1a1+TH+ mDA neurons. Our data confirm that hiPS cells, similar to hES cells, can serve as another potential cell source of authentic mDA neurons for cell replacement therapy in PD patients. Moreover, once procedures for generating hiPS cells from a patient's own somatic cells become standardized, it will be possible to generate autologous mDA neurons for transplantation, overcoming one of the greatest obstacles, immunorejection, facing the hES cell field.
Human iPS cell line IMR90 clone 4 as a standard model for basic research
Since the discovery of iPS technology several years ago, many groups have tried to optimize reprogramming procedures to more efficiently produce safer iPS cell lines. For example, a number of procedures have been developed using different combinations of transcription factors in an effort to eliminate the introduction of oncogene c-Myc [14,18]. In addition, nonintegrating adenovirus vectors [19] or excisable viruses [16] have been used to transiently express stemness genes. More recently, protein cocktails have been developed to avoid viral vectors all together [20]. While these new techniques are being refined, it is important that the stem cell field have access to a reliable commercially available hiPS cell line that can be used to continue to study basic biological questions pertaining to these cells and their potential uses. In the PD research area, for instance, the Wicell clone can be used to effectively and efficiently develop mDA differentiation methods, to identify markers of mDA differentiation stages, to select appropriate mDA cells for transplantations, and to optimize transplantation protocols.
Challenges remaining in hiPS application for cell replacement therapy in PD
Similar to hES cells, the transplantation of differentiated hiPS cells into a rat model of PD resulted in continued cell heterogeneity in the graft as well as unchecked cell proliferation. In our transplantation experiment, a large number of Nestin+ precursor cells existed together with mDA neurons inside the grafts. These undifferentiated cells possessed the potential to continue dividing and to give rise not only to more mDA neurons but also to many other cell types, some of which formed teratoma-like structures. This phenomenon makes the pre-selection of mDA lineage-restricted late precursor cells critical for the future success of this clinical application. Although we now know a great deal about the transcription machinery involved in mDA differentiation process (reviewed in ref. [21–23]), unfortunately there are still no good cell surface markers specific for mDA precursor population that allow for their precise selection. Further characterization of mDA lineage cells will be extremely important in this regard and the availability of hiPS cell line IMR90 clone 4 will be very useful in such studies.
Acknowledgments
This work was supported by NS32519, NS48315, the Tilker Foundation, and the Hassel Foundation. We thank Xiaotao Wei for her technical support.
Author Disclosure Statement
No competing financial interests exist
References
- 1.Lie DC. Colamarino SA. Song HJ. Désiré L. Mira H. Consiglio A. Lein ES. Jessberger S. Lansford H. Dearie AR. Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 2.Lindvall O. Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 3.Iacovitti L. Stull ND. Expression of tyrosine hydroxylase in newly differentiated neurons from a human cell line (hNT) Neuroreport. 1997;8:1471–1474. doi: 10.1097/00001756-199704140-00029. [DOI] [PubMed] [Google Scholar]
- 4.Iacovitti L. Stull ND. Jin H. Differentiation of human dopamine neurons from an embryonic carcinomal stem cell line. Brain Res. 2001;912:99–104. doi: 10.1016/s0006-8993(01)02723-8. [DOI] [PubMed] [Google Scholar]
- 5.Stull ND. Jung JW. Iacovitti L. Induction of a dopaminergic phenotype in cultured striatal neurons by bone morphogenetic proteins. Brain Res Dev Brain Res. 2001;130:91–98. doi: 10.1016/s0165-3806(01)00216-4. [DOI] [PubMed] [Google Scholar]
- 6.Yang M. Donaldson AE. Marshall CE. Shen J. Iacovitti L. Studies on the differentiation of dopaminergic traits in human neural progenitor cells in vitro and in vivo. Cell Transplant. 2004;13:535–547. doi: 10.3727/000000004783983729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suon S. Jin H. Donaldson AE. Caterson EJ. Tuan RS. Deschennes G. Marshall C. Iacovitti L. Transient differentiation of adult human bone marrow cells into neuron-like cells in culture: development of morphological and biochemical traits is mediated by different molecular mechanisms. Stem Cells Dev. 2004;13:625–635. doi: 10.1089/scd.2004.13.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suon S. Yang M. Iacovitti L. Adult human bone marrow stromal spheres express neuronal traits in vitro and in a rat model of Parkinson's disease. Brain Res. 2006;1106:46–511. doi: 10.1016/j.brainres.2006.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donaldson AE. Cai J. Yang M. Iacovitti L. Human amniotic fluid stem cells do not differentiate into dopamine neurons in vitro or after transplantation in vivo. Stem Cells Dev. 2008;18:1003–1012. doi: 10.1089/scd.2008.0300. [DOI] [PubMed] [Google Scholar]
- 10.Iacovitti L. Donaldson AE. Marshall CE. Suon S. Yang M. A protocol for the differentiation of human embryonic stem cells into dopaminergic neurons using only chemically defined human additives: Studies in vitro and in vivo. Brain Res. 2007;1127:19–25. doi: 10.1016/j.brainres.2006.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai J. Donaldson A. Yang M. German MS. Enikolopov G. Iacovitti L. The role of Lmx1a in the differentiation of human embryonic stem cells into midbrain dopamine neurons in culture and after transplantation into a Parkinson's disease model. Stem Cells. 2009;27:220–229. doi: 10.1634/stemcells.2008-0734. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Yu J. Vodyanik MA. Smuga-Otto K. Antosiewicz-Bourget J. Frane JL. Tian S. Nie J. Jonsdottir GA. Ruotti V. Stewart R. Slukvin II. Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–19201. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 15.Wernig M. Zhao JP. Pruszak J. Hedlund E. Fu D. Soldner F. Broccoli V. Constantine-Paton M. Isacson O. Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci USA. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soldner F. Hockemeyer D. Beard C. Gao Q. Bell GW. Cook EG. Hargus G. Blak A. Cooper O. Mitalipova M. Isacson O. Jaenisch R. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lips KS. Wunsch J. Zarghooni S. Bschleipfer T. Schukowski K. Weidner W. Wessler I. Schwantes U. Koepsell H. Kummer W. Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol. 2007;51:1042–1053. doi: 10.1016/j.eururo.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa M. Koyanagi M. Tanabe K. Takahashi K. Ichisaka T. Aoi T. Okita K. Mochiduki Y. Takizawa N. Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 19.Stadtfeld M. Nagaya M. Utikal J. Weir G. Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou H. Wu S. Joo JY. Zhu S. Han DW. Lin T. Trauger S. Bien G. Yao S. Zhu Y. Siuzdak G. Schöler HR. Duan L. Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burbach JP. Smidt MP. Molecular programming of stem cells into mesodiencephalic dopaminergic neurons. Trends Neurosci. 2006;29:601–603. doi: 10.1016/j.tins.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Gale E. Li M. Midbrain dopaminergic neuron fate specification: Of mice and embryonic stem cells. Mol Brain. 2008;1:8. doi: 10.1186/1756-6606-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prakash N. Wurst W. Development of dopaminergic neurons in the mammalian brain. Cell Mol Life Sci. 2006;63:187–206. doi: 10.1007/s00018-005-5387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]