Abstract
Seven patients with acute myeloid leukemia (AML) and two patients with chronic myelogenous leukemia (CML) were transplanted from HLA-identical sibling donors with CD34+ cell-enriched stem cells (HSCTs) without further immunosuppression. The myeloablative standard transplantation protocol was adapted to include transfusion of gene-modified donor T cells after HSCT. Donor T cells were transduced with the replication-deficient retrovirus SFCMM-3, which expresses herpes simplex thymidine kinase (HSV-Tk) and a truncated version of low-affinity nerve growth factor receptor (ΔLNGFR) for selection and characterization of transduced cells. Transduced T cells were detectable in all patients during follow-up for up to 5 years after transfusion. Proteomic screening for development of acute graft-versus-host disease (aGvHD) was applied to five of the seven patients with AML. No positivity for the aGvHD grade II-specific proteomic pattern was observed. Only one patient developed aGvHD grade I. To date, three of the patients with AML relapsed; one responded to three escalating transfusions of lymphocytes from the original donor and is in complete remission. Two were retransplanted with non-T cell-depleted peripheral blood stem cells from their original donors and died after retransplantation of septic complications or relapse, respectively. In one patient with CML, loss of bcr-abl gene expression was observed after an expansion of transduced cells. Seven of nine patients are alive and in complete remission.
Borchers and colleagues provide long-term follow-up data on seven patients with acute myeloid leukemia and two patients with chronic myelogenous leukemia who were transplanted from HLA-identical sibling donors with CD34+ cell-enriched stem cells. Donor-derived retroviral transduced T cells were also infused. The authors report that transduced T cells were detectable in all patients during follow-up for up to 5 years after transfusion. They also use proteomic screening to analyze the development of acute graft-versus-host disease in the research subjects.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative therapy for hematopoietic malignancies and hematopoietic dysfunction syndromes. Despite its curative potential, success of allo-HSCT is limited by complications such as severe graft-versus-host disease (GvHD). Although prophylactic immunosuppressive treatment of patients after transplantation is widely used to prevent GvHD, T cell depletion of the graft by CD34+ cell enrichment is the most effective method to prevent GvHD (Barrett et al., 1998). The major disadvantage of T cell depletion is an increased rate of leukemic relapse, which occurs in about 80% of patients (Horowitz et al., 1990; Gratwohl et al., 2002). Transfusion of donor lymphocytes (DLI) has been used successfully to treat recurrent chronic myeloid leukemia (CML) (Hertenstein et al., 1995; Kolb et al., 1995, 2004) and has provided definitive evidence of an immunologically mediated graft-versus-leukemia (GvL) effect. This has also been shown for patients with acute myeloid leukemia (AML) (Massenkeil et al., 2004). Thus, prophylactic and/or therapeutic transfusion of donor T cells has been included in many protocols, despite being complicated by acute GvHD in about 50% of patients (Kolb et al., 2004). Because acute GvHD contributes significantly to treatment-related morbidity and mortality, better control of this complication is mandatory.
T cells are optimal targets for retroviral gene transfer and thus for somatic gene therapy (Mavilio et al., 1994; Tiberghien, 1994; Contassot et al., 2000) and provide a potential strategy for the control of GvHD. The expression of suicide genes in donor lymphocytes offers the possibility of in vivo elimination of cells responsible for GvHD and thus might render GvHD controllable, while retaining the positive effects of T cells such as GvL activity (Bonini et al., 1998; Verzeletti et al., 1998; Bondanza et al., 2006; Ciceri et al., 2009). Animal experiments using retrovirally transduced cells have shown the possibility to separate GvHD and GvL (Ferrand et al., 2000; Georges et al., 2000; Weissinger et al., 2000; Kornblau et al., 2001). Ganciclovir (GCV)-induced death of T cells expressing herpes simplex thymidine kinase (HSV-Tk) resulted in elimination of GvHD in animals without additional immunosuppression. Clinical trials with HSV-Tk-transduced donor T cells were initiated in Europe and the United States in the 1990s. In Italy, donor T cells transduced with SFCMM-2 (ΔLNGFR-HSV-TK-neomycin resistance [neoR]) and SFCMM-3 were transfused to treat leukemic relapse or infectious complications after allo-HSCT. In France, the retroviral vector G1Tk1SvNa, which expresses HSV-Tk and neoR, was used. Transfusion of transduced donor T cells was performed immediately after HSCT (Ferrand et al., 2000), in the presence of cyclosporine A (CSA) and other immunosuppressants. These studies demonstrated the feasibility of gene therapy in this setting (Bonini et al., 1998; Verzeletti et al., 1998). A clinical benefit with complete or partial clinical remission was observed in patients with relapsed hematological malignancies and Epstein–Barr virus (EBV)-lymphoproliferative disease (Bonini et al., 1998; Ciceri et al., 2007). Six patients from these two studies developed acute (n = 4) or chronic (n = 2) GvHD, the majority of which resolved after GCV treatment alone. Immunization against HSV-Tk epitopes was observed in patients with an active immune system and led to premature elimination of transduced T cells (Traversari et al., 2007).
Here we report transfusion of HSV-Tk-transduced cells (HSV-Tk-DLI) after day +60 post-HSCT. In contrast to the previous prophylactic transfusions accomplished on day 0 (Tiberghien et al., 2001), CD34+ cell enrichment was the only immunosuppression applied in our study. Engraftment, GvHD and GvL effects, as well as signs of leukemic relapse were monitored and correlated with the persistence of the transduced cells. A proteomic screening approach for the early identification of severe acute graft-versus-host disease (aGvHD) (Kaiser et al., 2004a; Weissinger et al., 2007) was used to monitor patients included after 2003.
Patients and Methods
Patients
The myeloablative HSCT protocol with total body irradiation (12 Gy) and cyclophosphamide (CY, 120 mg) was expanded to include gene-modified DLI. The gene therapy protocol was initiated at Hannover Medical School (MHH; Hanover, Germany) in 2002 and included nine adult patients, seven with acute myeloid leukemia in first complete remission (AML, CR1) and two with chronic myeloid leukemia in first chronic phase (CML, CP1) after informed consent had been obtained. The protocol follows all criteria of the Declaration of Helsinki. Clinical characteristics of patients and donors are summarized in Tables 1 and 2. Exclusion criteria for transfusion of genetically modified lymphocytes were no informed consent, acute GvHD and/or severe infections after stem cell transplantation.
Table 1.
Patient Characteristics and Transplantation Details
| |
Patient |
Donor |
Transplantation |
Follow-up posttransplantation |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN | Sex/age | Diagnosis/status before HSCT | CMV status | Sex/age | CMV status | HSCT date | CD34+cells (per kg BW) | CD3plus; cells (per kg BW) | Chimerism Day +25 post-HSCT | Neutrophils >500/μl (days post-HSCT) | Platelets ≥20,000/μl (days post-HSCT) | Clinical condition post-HSCT |
| 914 | f/45 | CML/CP1 | Negative | m/47 | Negative | 18.02.2002 | 4.9 × 106 | 5 × 103 | 75% | 14 | 10 | No major problems, bcr-abl positivity post-HSCT |
| 919 | m/35 | CML/CP1 | Positive | m/39 | Negative | 04.03.2002 | 5.5 × 106 | 1.1 × 103 | 86% | 18 | 15 | No major problems |
| 1021 | f/39 | AML-M4/CR1 | Positive | m/46 | Positive | 10.02.2003 | 6.6 × 106 | 5 × 103 | 100% | 11 | 13 | No major problems |
| 1040 | f/50 | AML-M1/1.CR | Positive | f/48 | Negative | 24.03.2003 | 6 × 106 | 3.4 × 103 | 100% | 20 | 29 | Bone marrow depression; SC boost; cardiomyopathy |
| 1048 | m/38 | AML-M5/1.CR | Positive | f/56 | Positive | 14.04.2003 | 6 × 106 | 3.7 × 103 | 100% | 14 | 13 | CMV reactivation day +14 (persisting for 50 days) |
| 1108 | f/51 | AML-M2/1.CR | Negative | m/53 | Positive | 28.10.2003 | 8.1 × 106 | 5 × 103 | 100% | 13 | 14 | CMV reactivation day +20 (persisting for 21 days) |
| 1159 | m/35 | AML-M1/1.CR | Negative | f/32 | Negative | 26.04.2004 | 3.9 × 106 | 9 × 103 | 100% | 14 | 10 | No major problems |
| 1190 | f/38 | AML-M4/1.CR | Positive | f/50 | Positive | 26.07.2004 | 4.6 × 106 | 2.4 × 103 | 100% | 16 | 12 | No major problems |
| 1208 | f/51 | AML-M1/1.CR | Positive | f/49 | Positive | 20.09.2004 | 6.6 × 106 | 18,6 × 103 | 94% | 14 | 12 | CMV reactivation day +35 |
All patients are identified via their UPN. Six were females and three were males, and their median age at transplantation was 39 years (range, 35–51 years). The diagnosis and disease status at time of transplantation are indicated. CMV status, age, and sex of donors are shown under “donor.” All patients were transplanted with CD34+ cell-enriched stem cells from their HLA-identical siblings, the number of CD34+ cells/kg BW as well as the number of CD3+ cells/kg BW transplanted are indicated. Donor chimerism (day +25 post-HSCT) was 100% in six patients. Engraftment is shown by the reconstitution of neutrophils (>500 cells/μl) and platelets (>20,000/μl) and occurred between days +11 and +29. All patients had engraftment by day +20 for neutrophils and day +29 for platelets. Clinical follow-up post-HSCT is described in the last column.
AML, acute myelogenous leukemia; BW, body weight; CM, complete remission; CML, chronic myelogenous leukemia; CMV, cytomegalovirus; CP, chronic phase; f, female; HSCT, hematopoietic stem cell transplantation; m, male; SC, stem cell; UPN, unique patient number.
Table 2.
Clinical Data for SFCMM3-Donor Lymphocyte Infusion (DLI) and Follow-Up Post-SFCMM3 DLI
| UPN | Lymphapheresis (days before/after HSCT) | Days post-HSCT: SFCMM3-CD3/kg | ΔLNGFR max (in vivo) | ΔLNGFR max (absolute numbers) | Chimerism at last follow-up | Follow-up post-SFCMM-3 DLI and current status | Survival (years post-HSCT) |
|---|---|---|---|---|---|---|---|
| 914 | 24 | +71: 1 × 107 | +182: 1.2% | 1.3 × 104/μl | 100% | bcr-abl negativity after transfusion SCFMM3-DLI; 4 untransduced DLI → GvHD grade IV; CR | Alive, 7.7 years |
| 919 | −101 | +87: 4 × 106 | +118: 2.1% | 4.6 × 103/μl | 100% | bcr-abl positivity 2 × 1 × neg → imatinib → bcr-abl negative, CR, no GvHD | Alive, 7.5 years |
| 1021 | 84 | +113: 1.3 × 107 | +135: 0.8% | 2 × 103/μl | 100% | Relapse 6.7 years after HSCT (post-HSCT) | Alive, 6.9 years |
| 1040 | 98 | +330: 1 × 107 | +336: 0.6% | 1.9 × 103/μl | 12% | Relapse: 2 years post-HSCT; retransplantation, second relapse | Deceased, 5.2 years |
| 1048 | 44 | +100: 1 × 107 | +233: 0.5% | 8 × 103/μl | 92% | CR, no GvHD | Alive, 6.7 years |
| 1108 | 70 | +126: 4 × 106 | +140: 0.9% | 1.9 × 103/μl | 98% | CR, no GvHD | Alive, 5.9 years |
| 1159 | 65 | +136: 7.5 × 106 | +192: 2.1% | 6.9 × 103/μl | 99% | GvHD grade I (skin) day +56 post-DLI, CR | Alive, 5.1 years |
| 1190 | −26 | +129: 1 × 107 | +177: 0.9% | 2.8 × 103/μl | 98% | CR, no GvHD | Alive, 5.2 years |
| 1208 | −17 | +73: 1.3 × 107 | +99: 3.9% | 3.4 × 104/μl | 100% | Relapse: 1 year post-HSCT, retransplantation (septic complications) | Deceased, 2.0 years |
Lymphapheresis was done before HSCT in three patients and after HSCT in six patients. The day of transfusion of the transduced cells is indicated. The actual number of SCFMM3 DLI T cells ranged from 4 × 106 to 1.5 × 107 cells/kg body weight. The highest number of ΔLNGFR-expressing T cells (ΔLNGFR max) in each patient is shown as the percentage of CD3+ cells as well as the actual number of CD3+ cells/μl. Six patients have achieved almost 100% donor chimerism to date (three, >97%), three have mixed chimerism, and two of those experienced relapse during the time of mixed chimerism (<50%). In all patients, mixed chimerism is due to recipient T cells within the CD3+ compartment. To date, two patients have died. One died 6 months after retransplantation with non-T cell-depleted donor cells from her identical sibling donor, due to septic complications, and the other patient had a second relapse after retransplantation and died 5.2 years after the first HSCT. Survival status and time after first allogeneic HSCT are shown in the last column.
CR, complete remission; DLI, donor lymphocyte infusion; GvHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; ΔLNGFR, truncated version of low-affinity nerve growth factor receptor; ΔLNGFR max, highest number of ΔLNGFR-expressing T cells; UPN, unique patient number.
Study protocol
The clinical protocol was approved by the ethics committee of Hannover Medical School (protocol no. 2517) and by the committee for somatic gene therapy of the Bundesärztekammer (no. 53) and the Paul Ehrlich Institute (PEI, no. 1790/01). The study is registered with the DeReG-German Somatic Gene Transfer Clinical Trials Database (DRKS00000211, ZKS-Zentrum Klinische Studien/Center of Clinical Trials).
Conditioning and hematopoietic stem cell transplantation
All patients received total body irradiation (TBI; 12 Gy) and cyclophosphamide (Cy; 60 mg/kg/day) for two consecutive days followed by transplantation of CD34+ cell-enriched peripheral blood progenitor cells from HLA-identical family donors. Donors were treated with granulocyte colony-stimulating factor (G-CSF, 5 μg/kg twice daily) for 4 or 5 days and peripheral blood leukocytes were harvested at the Stem Cell Apheresis Center (MHH). CD34+ cell selection for all patients was performed under Good Manufacturing Practices (GMP) conditions by Cytonet (Hannover, Germany), using the CliniMACS system (Miltenyi Biotec, Bergisch Gladbach, Germany). At least 3.9 × 106 CD34+ cells per kilogram were transfused on day 0, while CD3+ cells were below 2 × 104 cells per kilogram body weight (Table 1). Lymphaphereses were harvested from all donors in the gene therapy trial, after informed consent was obtained, and air shipped to Milan by courier for transduction.
Description of retroviral vector and transduction protocol
The retroviral vector SFCMM-3 encodes the HSV-Tk gene, which confers sensitivity to GCV, and the truncated low-affinity nerve growth factor receptor gene (ΔLNGFR) (Mavilio et al., 1994; Bonini et al., 1998; Ciceri et al., 2007), which allows for rapid in vitro selection of the transduced cells with a monoclonal antibody (mAb) (anti-LNGFR antibody; Roche/Boehringer Mannheim, Mannheim, Germany) and immunomagnetic beads as described previously (Verzeletti et al., 1998). GpenvAM12 was used for retroviral vector packaging (D. Markowitz et al., 1988; D.G. Markowitz et al., 1988). Lymphapheresis samples were obtained from all donors of the gene therapy trial and air shipped to MolMed (Milan, Italy) by courier for transduction. Briefly, cells were stimulated for 72 hr with OKT-3 (30 ng/ml) in RPMI 1640 (Bonini et al., 1998), supplemented with 5% autologous plasma and interleukin-2 (IL-2, 100 U/ml; Chiron, Emeryville, CA). Subsequently, cells were transduced twice with SFCMM-3 supernatant at a multiplicity of infection (MOI) of 1 within 24 hr by spin-inoculation in the presence of protamine sulfate (4 μg/ml). After each round of transduction, cells were washed twice with RPMI 1640, supplemented with autologous plasma, and expanded in the presence of IL-2 (100 U/ml). For the evaluation of transduction efficiencies, cells were expanded for 48 hr and analyzed by flow cytometry (fluorescence-activated cell sorting [FACS]) after incubation with the anti-LNGFR antibody (Roche/Boehringer Mannheim). Cells were selected with immunomagnetic beads as described (Verzeletti et al., 1998) and purities of >90% LNGFR-expressing T cells were obtained. Transduced and selected cells were cryopreserved while safety tests were performed. Safety tests were performed as required by the regulatory authorities. After completion of the safety tests, cells were air shipped to Hannover by courier. The flow chart of the clinical trial is shown in Fig. 1. On arrival, cells were thawed, washed, and transfused at the time points indicated (Fig. 2 and Table 2). Samples from peripheral blood and bone marrow were stored frozen for future analyses.
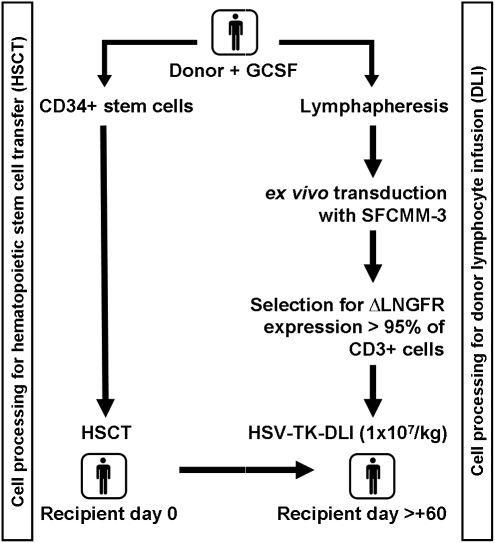
FIG. 1.
Clinical trial: HSV-TK-DLI. The clinical protocol was designed to include SFCMM-3-transduced donor lymphocyte infusion (DLI) in a myeloablative conditioning regimen and CD34+ cell-enriched hematopoietic stem cell transplantation (HSCT). The donor underwent leukapheresis once either before or at least 6 weeks after granulocyte colony-stimulating factor (G-CSF) administration. In a separate leukapheresis, peripheral blood stem cells were collected and transplanted to the recipient on day 0. Transduction of the cells and safety testing were performed at MolMed (Milan, Italy), and cells were shipped via courier to Hannover. Transfer of the transduced cells was scheduled on day +60 after HSCT. ΔLNGFR, truncated version of low-affinity nerve growth factor receptor; HSV-Tk, herpes simplex virus thymidine kinase.
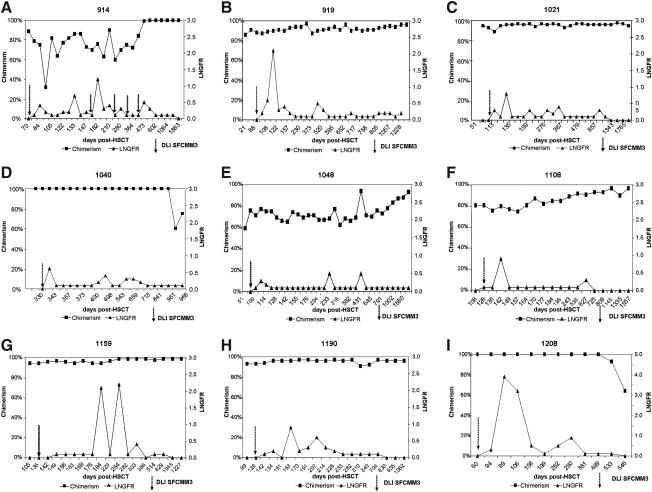
FIG. 2.
Follow-up of transduced donor T cells: (A) UPN 914; (B) UPN 919; (C) UPN 1021; (D) UPN 1040; (E) UPN 1048; (F) UPN 1108; (G) UPN 1159; (H) UPN 1190; (I) UPN 1208. Shown is the percentage of donor chimerism in peripheral blood (solid squares; left y axis) and the percentage of ΔLNGFR-expressing cells (FACS) (triangles; right y axis) plotted against days after HSCT. The time of transfusion of donor lymphocytes is indicated by an arrow (DLI SFCMM-3). UPN 914 received more than one DLI, but only the first one was transduced; all other patients have received only one DLI to date (DLI SFCMM-3). UPN 919 (B) and UPN 1108 (F) received only 4 × 106 SFCMM-3-transduced cells/kg (Table 2). HSCT, hematopoietic stem cell transplantation; LNGFR, low-affinity nerve growth factor receptor.
Screening for the presence of transduced T lymphocytes
Because of long-term follow-up safety issues the patients were screened by FACS and polymerase chain reaction (PCR) as described subsequently. Ex vivo detection and characterization of circulating transduced cells were accomplished at weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24; at 9 and 12 months; and yearly thereafter. FACS analyses were performed to examine the frequency and phenotype of the transferred gene-modified T cells in vivo, using mAbs specific for LNGFR (Roche/Boehringer Mannheim) and for CD3, CD4, and CD8 (BD Biosciences, San Jose, CA). Immunophenotyping studies were performed in all patients to evaluate recovery of B cells, T cells, and natural killer cells, as well as monocyte reconstitution. Samples from each patient included in the study are stored at yearly visits as reference samples (viably frozen cells and serum samples).
Molecular analysis
Transgene expression
To confirm the presence of gene-modified cells, genomic DNA and mRNA were isolated and transcribed into cDNA, and amplified with primers specific for all exogenous genes transduced, as described (Garin et al., 2001). CD3+ cell selection was performed by MACS separation, using the CD3-positive selection kit in accordance with the manufacturer's suggestions (Miltenyi Biotec). A nested PCR was developed to increase the sensitivity of detection from approximately 1 in 104 cells to 1 in 105 transduced cells in the mononuclear cell fraction of the patients. The 1-kb fragment of the HSV-Tk gene was detected with primers HTK2+ and HTK4− (Garin et al., 2001). Primers for the nested PCR (HSV-Tk1797 [forward], 5′-AGA AAA TGC CCA CGC TAC TG-3′; and HSV-Tk2211 [reverse], 5′-ATG CTG CCC ATA AGG TAT CG-3′) generated a 0.4-kb amplicon. The DNA polymerase was HotStarTaq (Quiagen), and PCR products were separated on 1.2% agarose gels and visualized by staining with ethidium bromide. Long-term follow-up PCR analyses for HSV-Tk gene expression were performed for all patients.
Nested RT-PCR for detection of the bcr-abl fusion transcript was performed as described at the BIOMED-1 conference (van Dongen et al., 1999).
T cell receptor-Vβ repertoire studies
T cell receptor (TCR)-Vβ family expression was analyzed in donor cells, after transduction and selection, and in recipient cells isolated 1, 2, and 3 years post-HSCT, using the bifamily multiplex PCR described previously (Fig. 3) (Naumov et al., 1995). Frozen samples were thawed and expanded for 48 hr in RPMI supplemented with 5% human serum albumin (HSA) and IL-2 (100 U/ml). CD3+CD4+ or CD3+CD8+ subpopulations were analyzed separately. Spectratyping was performed with the CEQ 8000 genetic analysis system (Beckman Coulter, Fullerton, CA). The 0.330-kb fragment of the constant region (CR) of the T cell receptor (TCR) and human β-actin served as controls for all PCRs (Naumov et al., 1995).
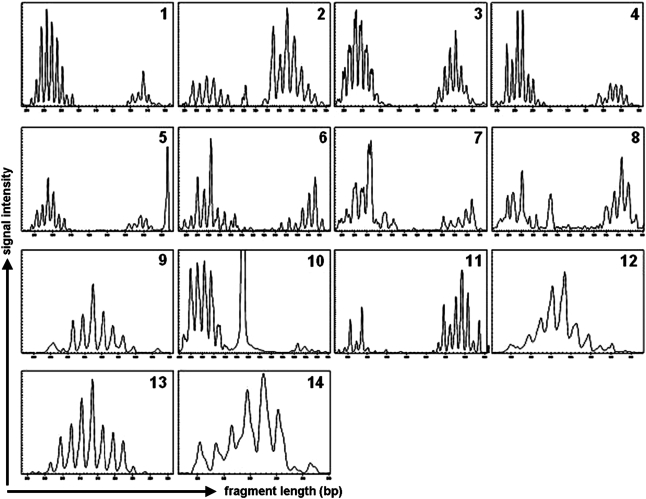
FIG. 3.
T cell receptor repertoire screening. Donor (after transduction and enrichment for LNGFR expression) and patient T cells were isolated, RNA and cDNA were prepared and spectratyping was performed as described in Patients and Methods. Data for all donors and recipients are shown in Supplementary Table S1. Fourteen bifamily primer sets were used to analyze the Vβ family TCR repertoire for each donor and the corresponding patient. Here results are shown for one donor (for patient UPN 1280). Multiplex primers used are indicated: 1, Vβ1 and Vβ12; 2, Vβ13 and Vβ3; 3, Vβ5.1 and Vβ1; 4, Vβ4 and Vβ5.3; 5, Vβ8 and Vβ7; 6, Vβ9 and Vβ14; 7, Vβ11 and Vβ20; 8, Vβ16 and Vβ22; 9, Vβ18 and Vβ23; 10, Vβ17 and Vβ15; 11,Vβ24 and Vβ22; 12, Vβ6.1; 13, Vβ6.2; 14, Vβ12 (single).
Proteomic screening for development of aGvHD after DLI
Urine samples were collected from five patients with AML included after 2003 and were screened by capillary electrophoresis (CE) coupled on-line to mass spectrometry (MS; electrospray ionization time of flight [ESI-TOF]) as described (Kaiser et al., 2004b; Weissinger et al., 2007). Ten milliliters of midstream urine was obtained from each participant, and samples were immediately frozen and either stored at −20°C until analysis or prepared as described previously (Kaiser et al., 2004b). All samples were analyzed by CE-MS, as described (Wittke et al., 2003; Weissinger et al., 2004; Theodorescu et al., 2006).
Chimerism studies
Molecular analysis of the engraftment was performed by PCR amplification of highly polymorphic short tandem repeat (PCR-STR) sequences in peripheral blood and bone marrow samples as described earlier (Briones et al., 1998). Donor chimerism in peripheral blood is shown over time post-HSCT in Fig. 2 and is described in Tables 2 and 3. Subset analyses of CD3+CD4+ and CD3+CD8+ T cells are performed yearly in all patients as an additional safety follow-up after allo-HSCT and transduced DLI.
Table 3.
Chimerism Analyses After-HSCT and HSV-Tk DLI
| UPN | Day +25 | Day +100 | Day +200 | 1 year | 2 years | CD4+cells, >2 years | CD8+cells, >2 years | Last follow-up |
|---|---|---|---|---|---|---|---|---|
| 914 | 99% | 82% | 54% | 72% | 100% | ND | ND | 100% |
| 919 | 86% | 87% | 94% | 87% | 96% | 100% | 78% | 97% |
| 1021 | 100% | 96% | 97% | 95% | 96% | 68% | 33% | Relapse/100% |
| 1040 | 100% | 100% | 100% | 100% | 58% | 100% | 100% | Relapse |
| 1048 | 87% | 71% | 67% | 66% | 78% | 77% | 54% | 92% |
| 1108 | 100% | 81% | 85% | 85% | 93% | 96% | 81% | 98% |
| 1159 | 100% | 95% | 99% | 99% | 99% | ND | ND | 99% |
| 1190 | 100% | 93% | 96% | 94% | 96% | 93% | 95% | 95% |
| 1208 | 94% | 100% | 100% | 100% | 64% | NA | NA | Relapse |
Peripheral blood of all patients was screened routinely for donor chimerism by PCR. Here the percent (%) donor chimerism in peripheral blood mononuclear cells (PBMCs) is shown for days +25, +100, and +20; 1 and 2 years post-HSCT; and at follow-up (most recent visit, up to 7 years post-DLI). Subgroup chimerism analyses (CD4+ and CD8+ cells, >2 years) were performed in all patients with mixed chimerism 2 years post-HSCT.
DLI, donor lymphocyte infusion; HSCT, hematopoietic stem cell transplantation; HSV-Tk, herpes simplex virus thymidine kinase; NA, not applicable; UPN, unique patient number. ND, not detectable.
Ex vivo detection of transgene-specific immune responses
In case of unexpected loss of circulating HSV-Tk-transduced cells, patient peripheral blood mononuclear cells (PBMCs) were collected and stimulated with irradiated (3000 rad) autologous SFCMM-3-transduced donor T lymphocytes at a 1:1 effector-to-substrate (E/S) ratio, in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% human serum (Cambrex, Milan, Italy). To verify a patient's immune competence, PBMCs were also stimulated with irradiated allogeneic T cells. To determine whether an immune response to transgene products had occurred after SFCMM-3-transduced T cell transfusions, or was already detectable in the HSCT donor, donor PBMCs were also stimulated with the same targets. On day 3, IL-2 at a final concentration of 60 IU/ml was added to all cultures. Stimulations were repeated every 10 to 11 days. Specific immune responses to transgene products and to the allogeneic targets were measured in a standard 51Cr release assay using the following targets: SFCMM3-transduced and untransduced donor lymphocytes and the allogeneic T cells previously used as stimulators.
Results
To modulate GvL effects, nine patients received HSV-Tk DLIs from their HLA-identical sibling donors after myeloablative conditioning and CD34+ cell-selected HSCT without further immunosuppressive treatment. Patient and donor characteristics are summarized in Table 1.
TCR repertoire is normal in transduced, selected donor T cells
All transduced and anti-LNGFR-selected donor T cells were screened for transgene expression by RT-PCR, PCR, and FACS analysis. In addition, the TCR repertoire was analyzed for diversity in the donor DLI after selection. Figure 3 shows the TCR repertoire analyses of transduced and selected donor T cells as well as cells from all patients collected 1 to 3 years post-HSCT. Normal TCR repertoires were observed for all selected donor T cells and skewing was a rare event, even after selection.
Transfusion of genetically modified T cells promotes stable donor chimerism
Clinically stable patients exhibiting no signs of infections or GvHD were scheduled to receive transduced and selected donor T cells post-HSCT. HSV-Tk-expressing T cells were transfused at a median of day +107 after HSCT (range, days 71–330; Table 2). Transfusions at later time points were either due to cytomegalovirus (CMV) reactivations (n = 3) or to myelosuppression requiring a stem cell boost (UPN 1040). The follow-up after transduced DLI is summarized in Table 2. At the time of DLI, the immune reconstitution for lymphocytes was 640 CD3+ cells/μl (median) (range, 150–1000 cells/μl; data not shown). Subgroup chimerism analyses of MACS-sorted CD3+CD4+ and CD3+CD8+ cells were performed for patients (UPN 919, 1021, 1048, 1108, 1190, 1208) with mixed chimerism at 2 years post-HSCT. As shown in Table 3, mixed chimerism was observed in PBMCs. For example, in UPN 1048 chimerism in PBMCs constantly increased after the DLI to reach 78% donor chimerism in peripheral blood lymphocytes (PBLs) after 2 years; at this time the CD3+CD4+ cells and CD3+CD8+ cells were 77 and 54% for of donor origin, respectively. At last follow-up, the chimerism increased to 92% in PBLs on average. In all six patients, the mixed chimerism detected in PBL leukocytes could be correlated with mixed T cell chimerism, a common finding after T cell-depleted HSCT (Table 3) (Rodriguez-Luaces et al., 2004).
Clinical and molecular follow-up after HSV-Tk DLI
Figure 2 summarizes the FACS data (ΔLNGFR expression) and the development of donor chimerism over time post-HSCT for all patients included in the study. The results of chimerism studies and the analyses of ΔLNGFR expression are shown in Fig. 2. Patients UPN 1048, UPN 1108, and UPN 1208 experienced cytomegalovirus (CMV) reactivation on days +14, +20, and +35 post-HSCT, respectively, and were treated with GCV infusions, thus delaying the transfusion of transduced cells. Patient UPN 1040 received the transduced DLI almost 1 year after the HSCT, because of insufficient engraftment of the donor cells, requiring a stem cell boost. This patient then developed cardiomyopathy, thus further delaying transfusion of HSV-Tk-transduced donor T cells. Six patients developed donor chimerism of more than 90% post-DLI (Table 2 and Fig. 3). FACS analyses for LNGFR expression and PCR for transgene expression were done in parallel with chimerism PCRs. In all patients, transduced donor T cells were detectable by FACS and PCR analysis for at least 1.5 years. ΔLNGFR-expressing T cells became undetectable by FACS in most patients (Fig. 2), whereas transduced cells were still detectable by PCR. In patient UPN 919 transduced cells were no longer detectable by PCR on day +600 (20 months) after HSCT. The follow-up for all patients by PCR is shown in Fig. 4.
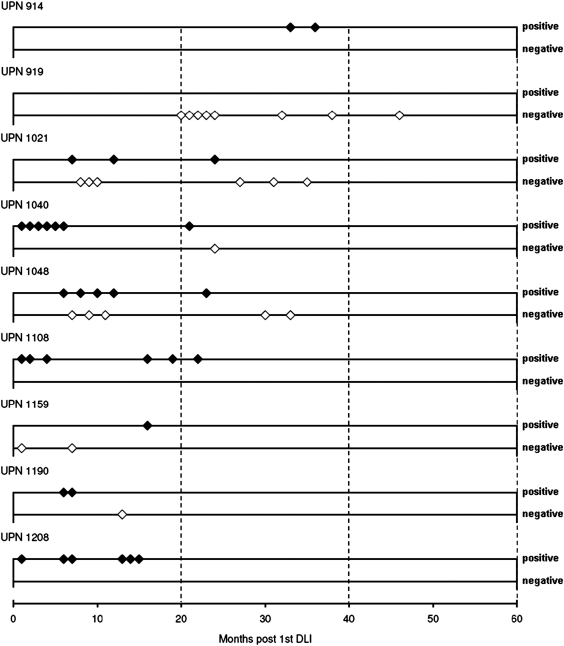
FIG. 4.
Long-term follow-up of transgene expression in all patients. PCR analyses were performed routinely for all patients in order to ensure transgene expression. To date, six of nine patients have shown HSV-Tk and ΔLNGFR gene expression, three experienced relapse and had no transgene expression at that time, and one (UPN 919) lost expression of the transgenes at about 20 months post-DLI. The x axis of the plot shows months posttransfusion of SFCMM-3 DLI, and positivity or negativity for HSV-Tk gene expression is indicated by diamonds on the y axis in the positive (+) or negative (−) direction.
Transduced T cells expanded in correlation with clinical events
In patient UPN 914 transduced T cells expanded on bcr-abl positivity. bcr-abl had been negative for two consecutive RT-PCRs after HSCT, but on day +112 was detected again by RT-PCR (data not shown). Transduced T cells expanded in vivo from a level undetectable by FACS to 0.7% of CD3+ T cells, corresponding to an increase from 0/μl up to 1 × 104 ΔLNGFR-positive (ΔNGFR+) T cells/μl. Within 2 weeks bcr-abl expression was no longer detected in the bone marrow of the patient, and at the same time ΔLNGFR+ T cells declined. Despite consistent negativity for minimal residual disease, UPN 914 received another DLI of 1 × 107 unmodified donor cells per kilogram body weight because of impending graft rejection on day +178. Interestingly, the genetically modified T cells expanded by day +182 to 1.2% of the CD3+ cells (equivalent to 1.3 × 104 ΔLNGFR+ cells/μl; Table 2). The patient received untransduced cells, because the clinical study had been temporarily stopped because of reports of leukemia development in a murine model and in two children after transplantation of transduced stem cells (Li et al., 2002; Hacein-Bey-Abina et al., 2003). The patient was taken off study, while monitoring for safety and persistence of transduced cells was continued. ΔLNGFR-expressing T cells also expanded on occasions of infections; for example, in UPN 919 the peak expression level of transduced cells, 2.1% (4.6 × 103 ΔLNGFR+CD3+ cells), was observed concurrently with tonsillitis. In patient UPN 1159, transduced cells became detectable by FACS analysis and peaked at 2.1% (equivalent to 6.9 × 103 ΔLNGFR+ T cells/μl; Table 2) during an episode of mild GvHD of the skin. The last patient of this series, UPN 1208, received transduced T cells 73 days after HSCT with a peak expression level of about 4% ΔLNGFR-expressing T cells by FACS on day +99 (Fig. 2).
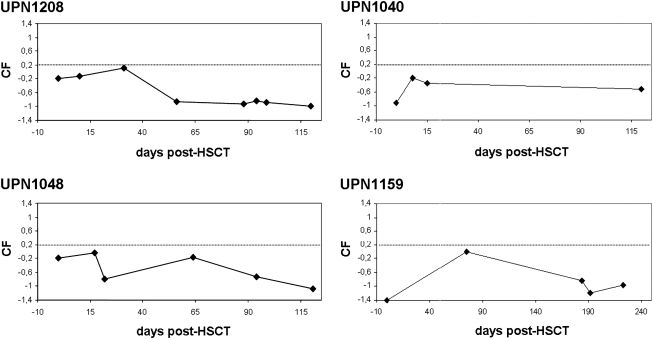
To monitor development of aGvHD early on, a urinary screening test for early diagnosis of aGvHD was used for five patients included into the study after 2003. Urine samples were collected and screened for the aGvHD proteomic pattern described earlier (Kaiser et al., 2004a,b; Weissinger et al., 2007). Scoring with the support vector machine (SVM)-based classification factor (CF) yielded no detection of aGvHD between HSCT and DLI, as expected. Patients treated within the CD34+ cell selection protocols were considered negative controls for the aGvHD-specific proteomic pattern. Even after DLI, none of the patients scored positive for the aGvHD proteomic patterns. Nonetheless, screening continued until day +100 after DLI (Fig. 5). Only one patient developed signs of grade I aGvHD of the skin (UPN 1159). The clinical course was mild and transient and the aGvHD resolved without further treatment within 2 days.
FIG. 5.
Proteomic screening of urine collected post-HSCT. The days post-HSCT (x axis) are plotted against the classification factor (CF) (y axis) as described (Weissinger et al., 2007b). The cutoff for diagnosis of acute GvHD was set to CF = +0.2 and is indicated as a dotted line. Proteomic screening data were obtained from five of nine patients, and four examples are shown here (UPN 1040, 1048, 1159, and 1208).
In vivo detection of transduced T cells by FACS analysis
Various amounts of LNGFR-positive cells were detectable by FACS, with up to 4% transgene-positive CD3+ T cells. In all patients, only a relatively small percentage of CD3+ T cells was actually transduced cells, whereas the majority were either recipient T cells (mixed chimerism patients) or newly developing T cells from the graft (Table 2). Table 2 shows the actual numbers of circulating CD3+ and ΔLNGFR+ T cells in the patients. Total numbers of ΔLNGFR gene-expressing cells were calculated on the basis of FACS analyses. Between 1.9 × 103 and 9.9 × 104 ΔLNGFR-expressing cells per microliter were detected in the patients at various time points after DLI (Table 2).
Long-term follow-up for transduced T cells
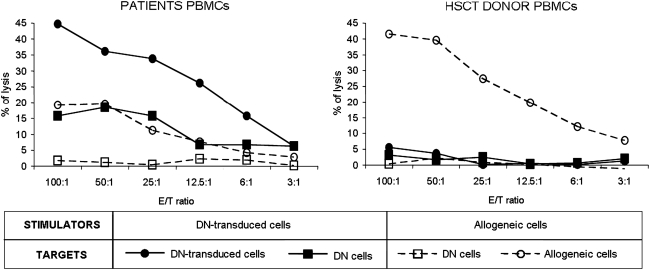
Long-term follow-up of transduced cells by PCR for HSV-Tk gene expression is shown in Fig. 4. Eight of nine patients had low, but detectable, levels of transduced cells within the 2 years after HSV-Tk DLI. Applying nested PCR to CD3+ cell-selected T cells increased the sensitivity of the PCR to more than 1 in 105 cells. Patient UPN 914 showed detectable levels of HSV-Tk gene expression for at least 33 months, although this patient had received four nontransduced DLIs. HSV-Tk gene expression fell below the detection limit after 20 months in the absence of GCV treatment in only one patient (UPN 919). We hypothesized that the patient was immunized against transgene products. To verify this hypothesis, patient and donor PBMCs were stimulated in vitro with SFCMM-3-transduced donor T cells. At the same time, specific immune response against allogeneic targets was tested to assess patient immunocompetence. Results of a 51Cr release assay (Fig. 6) documented the development of a T cell-mediated immune response against the transgene products. This response most likely occurred after DLI, because no lytic response was observed after multiple stimulation of donor PBMCs with transduced lymphocytes. Safety monitoring for HSV-Tk and ΔLNGFR expression is routinely performed for all surviving patients. To date, three patients have had a relapse of the initial AML. UPN 1040 and UPN 1208 were retransplanted from their matched related donors (MRDs) with unmodified donor grafts. Both patients died, one of septic complications 1 year after retransplantation, the second 2 years after retransplantation in a second relapse. UPN 1021 had a late relapse 6.7 years after the first HSCT, and received three escalating DLIs after two cycles of chemotherapy consisting of cytosine arabinoside (AraC), mitoxantrone, and panobinostat. At the last follow-up the chimerism of the patient was 100% donor in peripheral blood cells. To date, seven of nine patients are alive and in complete hematological remission.
FIG. 6.
Detection of an immune response to HSV-Tk in patient UPN 919. After coculture with SFCMM-3-transduced donor cells (straight line) or allogeneic cells (dashed line), patient and donor PBMCs were tested against the same stimulators and untransduced donor cells in a standard 51Cr release assay. Although patient effectors showed a weaker response to allogeneic targets as compared with donor effectors, only patient T cells showed a specific lytic response to transduced lymphocytes.
Discussion
Our data indicate that transfer of suicide genes and selection markers into mature T cells using retroviral gene transfer is feasible and safe. Proteomic screening of urine for the development of aGvHD or other complications after HSCT and DLI showed no aGvHD : grade II in any of the patients. No other immediate toxicity has occurred in any patient and to date all safety tests completed for detection of replication-competent particles have remained negative. The cells were transfused after stable engraftment and in the absence of aGvHD, viral or other infections. At the time of DLI, the lymphocyte counts exceeded more than 100 CD3+ lymphocytes/μl. Stable chimerism was observed in all patients after transfusion of donor T cells. Achievement of full chimerism is particularly difficult in T cell-depleted HSCT without DLI, and mixed chimerism is frequently observed. About 50–60% of patients achieve complete chimerism after CD34+ cell-selected HSCT by day +20, but the majority change to mixed chimerism after a longer follow-up. In accordance with our findings, Rodriguez-Luaces and colleagues (2004) reported that mixed chimerism is often obtained after CD34+ cell-selected HSCT, and doses of about 2 × 105 unmodified T cells transfused on day +28 or day +60 after HSCT did not yield conversion to full donor chimerism.
In our study, the kinetics of the HSV-Tk-expressing cells correlated well with clinical events and reproduced the expected phases of expansion and regression of antigen-specific T cell lines, mimicking results expected after in vitro challenge with minor histocompatibility antigens. For example, expansion of LNGFR-expressing T cells observed in the FACS analysis correlated well with a decrease in bcr-abl expression. bcr-abl negativity was observed and persisted after expansion of the transduced cells, suggesting a direct GVL effect of the transduced cells in patients with chronic myelogenous leukemia.
Although the results of the first clinical trials with genetically modified lymphocytes were promising, a number of questions were raised. Partial resistance to GCV-induced elimination of HSV-Tk-expressing cells was observed in a patient with chronic GvHD (n = 1), most likely due to cell cycle dependence of the HSV-Tk gene. Also, HSV-Tk genes rendered nonfunctional because of alternative splicing were isolated from some patients (Garin et al., 2001). Issues related to immune competence and the alloreactive potential of transduced cells were also raised (Sauce et al., 2002b, 2004; Marktel et al., 2003). ΔLNGFR expression allows rapid selection of the transduced cells, which may be favorable for the phenotype of the cells, as compared with more time-consuming selection methods with antibiotic resistance genes. The safety of the ΔLNGFR cell surface marker has been validated in a cooperative study performed by 17 independent institutions and involving experiments on more than 900 animals and in clinical studies as well (Marktel et al., 2003).
In our study, one of the nine patients treated developed a mild, transient GvHD of the skin, observed on day +185 post-HSCT (day +56 post-SCFMM3 DLI). The GvHD did not require treatment and subsided by 2 weeks after first diagnosis. The persistence of the transduced cells over 1.9 years and up to 6 years suggests that the transduced cells successfully engrafted. Furthermore, expansions of transduced cells correlated with clinical events, suggesting function of transduced cells. After transfusion of the transduced cells, none of the patients developed reactivations of viruses such as CMV or EBV. Normal T cell receptor repertoires were seen in transduced donor T cells after selection with anti-NGFR antibodies. This finding is in contrast to the selection with neomycin, where clonal deletions or expansions of particular Vβ families were described (Sauce et al., 2002a). The TCR repertoire analyzed from patient cells obtained at various times post-HSCT exhibited skewing for at least 1 year after HSCT, but normalized within 3 years post-HSCT in all patients evaluated (Epperson et al., 2001; Bahceci et al., 2003). In the case of G418 selection of transduced T cells significant skewing of the T cell repertoire was observed and the development of EBV lymphomas was reported despite the transfusion of transduced T cells (Sauce et al., 2002a). In our study the T cell receptor repertoire of the HSV-Tk-LNGFR-expressing transduced and selected donor T cells was normal (Fig. 3). In patient UPN 919, immunity against the transgene (HSV-Tk) led to a loss of transduced cells within 20 months post-DLI. The immune reaction against HSV-Tk may have occurred as a result of a herpes simplex virus infection post-DLI.
Three patients (of seven) of the AML cohort had relapse of the leukemia within 2 and 3 years after first HSCT; one had a late relapse, more then 6 years post-HSCT. No genetically modified cells were detected within the leukemic blasts. Two patients died after retransplantation with nondepleted grafts; the third was successfully treated by chemotherapy and three consecutive, dose-escalating untransduced DLIs. To date, seven of nine patients are alive and well, and in hematological or molecular remission.
In summary, the data presented here underline once more that the use of SFCMM-3-transduced cells can be monitored in a clinical setting and is a safe and adequate treatment to limit GvHD while allowing for GvL effects. Furthermore, the expression of suicide genes in combination with other genes of interest will dramatically increase safety for future gene therapy approaches.
Acknowledgments
The authors thank Rüdiger Rüger (Roche Diagnostics; formerly Boehringer Mannheim) for the biotinylated, GMP-grade anti-LNGFR antibody provided to B.H.; and J. Apperley (Hammersmith Hospital, UK), A.J. Barrett (NHLBI, NIH), C. Baum, M. Morgan, R. Stripecke (MHH, Germany), and J.F. Mushinski (NCI, NIH) for critical revision of the manuscript. This work was supported in part by Deutsche Jose Carreras Leukämiestiftung grant DJCLS-R98/11 (to A.G. and B.H.) and DJCLS R05/08 (to E.M.W. and B.H.), and by European Union (European Study Group CB et al.) Suicide Gene Transfer in Stem Cell Transplantation grant QLRT-2000-01265.
Contribution of Authors
E.M.W., B.H., B.C., and A.G. designed the study, recruited patients, discussed the protocols, and performed research. E.M.W. and M.M. performed research and wrote the paper. S.B. performed research, collected data, and did all the FACS and molecular follow-up studies in the HSV-Tk group. E.D. collected data and documented the study patients and data management. A.S., M.R., and C.Be. performed transduction of patient T cells at MolMed, collected data, and performed research. C.G.S. and M.R. collected data and performed documentation of the study at MolMed. F.C. and C.Bo. collected data, discussed patients, and results and helped in the design of the study. H.M. performed research (proteomics) and collected data.
Author Disclosure Statement
A.S., M.R., and C.Be. are employed by MolMed; F.C. and C.Bo. are consultants to MolMed, whose potential product was analyzed in this study.
References
- Bahceci E. Epperson D. Douek D.C., et al. Early reconstitution of the T-cell repertoire after non-myeloablative peripheral blood stem cell transplantation is from post-thymic T-cell expansion and is unaffected by graft-versus-host disease or mixed chimaerism. Br. J. Haematol. 2003;122:934–943. doi: 10.1046/j.1365-2141.2003.04522.x. [DOI] [PubMed] [Google Scholar]
- Barrett A.J. Mavroudis D. Tisdale J., et al. T cell-depleted bone marrow transplantation and delayed T cell add-back to control acute GVHD and conserve a graft-versus-leukemia effect. Bone Marrow Transplant. 1998;21:543–551. doi: 10.1038/sj.bmt.1701131. [DOI] [PubMed] [Google Scholar]
- Bondanza A. Valtolina V. Magnani Z., et al. Suicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytes. Blood. 2006;107:1828–1836. doi: 10.1182/blood-2005-09-3716. [DOI] [PubMed] [Google Scholar]
- Bonini C. Ciceri F. Marktel S., et al. Suicide-gene-transduced T-cells for the regulation of the graft-versus-leukemia effect. Vox Sanguinis. 1998;74(Suppl. 2):341–343. doi: 10.1111/j.1423-0410.1998.tb05440.x. [DOI] [PubMed] [Google Scholar]
- Briones J. Urbano-Ispizua A. Lawler M., et al. High frequency of donor chimerism after allogeneic transplantation of CD34+-selected peripheral blood cells. Exp. Hematol. 1998;26:415–420. [PubMed] [Google Scholar]
- Ciceri F. Bonini C. Marktel S., et al. Antitumor effects of HSV-TK-engineered donor lymphocytes after allogeneic stem-cell transplantation. Blood. 2007;109:4698–4707. doi: 10.1182/blood-2006-05-023416. [DOI] [PubMed] [Google Scholar]
- Ciceri F. Bonini C. Stanghellini M.T.L., et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): A non-randomised phase I–II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- Contassot E. Ferrand C. Angonin R., et al. Ganciclovir-sensitive acute graft-versus-host disease in mice receiving herpes simplex virus-thymidine kinase-expressing donor T cells in a bone marrow transplantation setting. Transplantation. 2000;69:503–508. doi: 10.1097/00007890-200002270-00007. [DOI] [PubMed] [Google Scholar]
- Epperson D.E. Margolis D.A. McOlash L., et al. In vitro T-cell receptor Vβ repertoire analysis may identify which T-cell Vβ families mediate graft-versus-leukaemia and graft-versus-host responses after human leucocyte antigen-matched sibling stem cell transplantation. Br. J. Haematol. 2001;114:57–62. doi: 10.1046/j.1365-2141.2001.02879.x. [DOI] [PubMed] [Google Scholar]
- Ferrand C. Robinet E. Contassot E., et al. Retrovirus-mediated gene transfer in primary T lymphocytes: Influence of the transduction/selection process and of ex vivo expansion on the T cell receptor β chain hypervariable region repertoire. Hum. Gene Ther. 2000;11:1151–1164. doi: 10.1089/10430340050015202. [DOI] [PubMed] [Google Scholar]
- Garin M.I. Garrett E. Tiberghien P., et al. Molecular mechanism for ganciclovir resistance in human T lymphocytes transduced with retroviral vectors carrying the herpes simplex virus thymidine kinase gene. Blood. 2001;97:122–129. doi: 10.1182/blood.v97.1.122. [DOI] [PubMed] [Google Scholar]
- Georges G.E. Storb R. Thompson J.D., et al. Adoptive immunotherapy in canine mixed chimeras after nonmyeloablative hematopoietic cell transplantation. Blood. 2000;95:3262–3269. [PubMed] [Google Scholar]
- Gratwohl A. Brand R. Apperley J., et al. Graft-versus-host disease and outcome in HLA-identical sibling transplantations for chronic myeloid leukemia. Blood. 2002;100:3877–3886. doi: 10.1182/blood.V100.12.3877. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Von Kalle C. Schmidt M., et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hertenstein B. Hampl W. Bunjes D., et al. In vivo/ex vivo T cell depletion for GVHD prophylaxis influences onset and course of active cytomegalovirus infection and disease after BMT. Bone Marrow Transplant. 1995;15:387–393. [PubMed] [Google Scholar]
- Horowitz M.M. Gale R.P. Sondel P.M., et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- Kaiser T. Kamal H. Rank A., et al. Proteomics applied to the clinical follow-up of patients after allogeneic hematopoietic stem cell transplantation. Blood. 2004a;104:340–349. doi: 10.1182/blood-2004-02-0518. [DOI] [PubMed] [Google Scholar]
- Kaiser T. Wittke S. Just I., et al. Capillary electrophoresis coupled to mass spectrometer for automated and robust polypeptide determination in body fluids for clinical use. Electrophoresis. 2004b;25:2044–2055. doi: 10.1002/elps.200305788. [DOI] [PubMed] [Google Scholar]
- Kolb H.J. Schattenberg A. Goldman J.M., et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- Kolb H.J. Schmid C. Barrett A.J., et al. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004;103:767–776. doi: 10.1182/blood-2003-02-0342. [DOI] [PubMed] [Google Scholar]
- Kornblau S.M. Stiouf I. Snell V., et al. Preemptive control of graft-versus-host disease in a murine allogeneic transplant model using retrovirally transduced murine suicidal lymphocytes. Cancer Res. 2001;61:3355–3360. [PubMed] [Google Scholar]
- Li Z. Dullmann J. Schiedlmeier B., et al. Murine leukemia induced by retroviral gene marking. Science. 2002;296:497. doi: 10.1126/science.1068893. [DOI] [PubMed] [Google Scholar]
- Markowitz D. Goff S. Bank A. Construction of a safe and efficient retrovirus packaging cell line. Adv. Exp. Med. Biol. 1988;241:35–40. doi: 10.1007/978-1-4684-5571-7_6. [DOI] [PubMed] [Google Scholar]
- Markowitz D.G. Goff S.P. Bank A. Safe and efficient ecotropic and amphotropic packaging lines for use in gene transfer experiments. Trans. Assoc. Am. Physicians. 1988;101:212–218. [PubMed] [Google Scholar]
- Marktel S. Magnani Z. Ciceri F., et al. Immunologic potential of donor lymphocytes expressing a suicide gene for early immune reconstitution after hematopoietic T-cell-depleted stem cell transplantation. Blood. 2003;101:1290–1298. doi: 10.1182/blood-2002-08-2351. [DOI] [PubMed] [Google Scholar]
- Massenkeil G. Nagy M. Le Coutre P., et al. Nonmyeloablative stem cell transplantation in patients with ALL and AML results in low nonrelapse mortality despite high rate of infections and GVHD. Hematol. J. 2004;5:395–402. doi: 10.1038/sj.thj.6200543. [DOI] [PubMed] [Google Scholar]
- Mavilio F. Ferrari G. Rossini S., et al. Peripheral blood lymphocytes as target cells of retroviral vector-mediated gene transfer. Blood. 1994;83:1988–1997. [PubMed] [Google Scholar]
- Naumov V.Z. Saroiants L.V. Balybin E.S. [Effects of endogenous cortisol on the function of nonspecific T-suppressors and quantitative characteristics of main subpopulations of circulating T-lymphocytes in leprosy] Problemy Tuberkuleza. 1995;2:49–51. [PubMed] [Google Scholar]
- Rodriguez-Luaces M. Ferra C. Martin-Henao G., et al. Mixed chimerism is frequent after allogeneic peripheral blood stem cell transplantation with positive CD34 selection, and is not reverted by low doses of donor T-cells add-back. Eur. J. Haematol. 2004;73:162–168. doi: 10.1111/j.1600-0609.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- Sauce D. Bodinier M. Garin M., et al. Retrovirus-mediated gene transfer in primary T lymphocytes impairs their anti-Epstein–Barr virus potential through both culture-dependent and selection process-dependent mechanisms. Blood. 2002a;99:1165–1173. doi: 10.1182/blood.v99.4.1165. [DOI] [PubMed] [Google Scholar]
- Sauce D. Tonnelier N. Duperrier A., et al. Influence of ex vivo expansion and retrovirus-mediated gene transfer on primary T lymphocyte phenotype and functions. J. Hematother. Stem Cell Res. 2002b;11:929–940. doi: 10.1089/152581602321080592. [DOI] [PubMed] [Google Scholar]
- Sauce D. Mercier P. Battini J.L., et al. Preferential retroviral-mediated transduction of EBV- and CMV-specific T cells after polyclonal T-cell activation. Gene Ther. 2004;11:1019–1022. doi: 10.1038/sj.gt.3302273. [DOI] [PubMed] [Google Scholar]
- Theodorescu D. Wittke S. Ross M.M., et al. Discovery and validation of new protein biomarkers for urothelial cancer: A prospective analysis. Lancet Oncol. 2006;7:230–240. doi: 10.1016/S1470-2045(06)70584-8. [DOI] [PubMed] [Google Scholar]
- Tiberghien P. Use of suicide genes in gene therapy. J. Leukoc. Biol. 1994;56:203–209. doi: 10.1002/jlb.56.2.203. [DOI] [PubMed] [Google Scholar]
- Tiberghien P. Ferrand C. Lioure B., et al. Administration of herpes simplex-thymidine kinase-expressing donor T cells with a T-cell-depleted allogeneic marrow graft. Blood. 2001;97:63–72. doi: 10.1182/blood.v97.1.63. [DOI] [PubMed] [Google Scholar]
- Traversari C. Marktel S. Magnani Z., et al. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood. 2007;109:4708–4715. doi: 10.1182/blood-2006-04-015230. [DOI] [PubMed] [Google Scholar]
- van Dongen J.J. Macintyre E.A. Gabert J.A., et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease: Report of the BIOMED-1 Concerted Action Investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13:1901–1928. doi: 10.1038/sj.leu.2401592. [DOI] [PubMed] [Google Scholar]
- Verzeletti S. Bonini C. Marktel S., et al. Herpes simplex virus thymidine kinase gene transfer for controlled graft-versus-host disease and graft-versus-leukemia: Clinical follow-up and improved new vectors. Hum. Gene Ther. 1998;9:2243–2251. doi: 10.1089/hum.1998.9.15-2243. [DOI] [PubMed] [Google Scholar]
- Weissinger E.M. Franz M. Voss C., et al. Expression of HSV-TK suicide gene in primary T lymphocytes: The dog as a preclinical model. Cytokines Cell. Mol. Ther. 2000;6:25–33. doi: 10.1080/13684730050515886. [DOI] [PubMed] [Google Scholar]
- Weissinger E.M. Wittke S. Kaiser T., et al. Proteomic patterns established with capillary electrophoresis and mass spectrometry for diagnostic purposes. Kidney Int. 2004;65:2426–2434. doi: 10.1111/j.1523-1755.2004.00659.x. [DOI] [PubMed] [Google Scholar]
- Weissinger E.M. Schiffer E. Hertenstein B., et al. Proteomic patterns predict acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood. 2007;109:5511–5519. doi: 10.1182/blood-2007-01-069757. [DOI] [PubMed] [Google Scholar]
- Wittke S. Fliser D. Haubitz M., et al. Determination of peptides and proteins in human urine with capillary electrophoresis-mass spectrometry, a suitable tool for the establishment of new diagnostic markers. J. Chromatogr. A. 2003;1013:173–181. doi: 10.1016/s0021-9673(03)00713-1. [DOI] [PubMed] [Google Scholar]