Abstract
Conditionally replicative adenoviral (CRAd) virotherapy represents a promising therapeutic approach for cancer. We have demonstrated that a serotype chimeric adenoviral 5/3 fiber-knob modification achieves enhanced ovarian cancer infectivity, conditional replication, and oncolytic activity. This study evaluated the safety of intraperitoneal (IP) Ad5/3-Δ24 in advance of a phase I clinical trial in gynecologic cancers. Syrian hamster cohorts were treated with IP Ad5/3-Δ24 or control buffer for 3 consecutive days and euthanized on study days 8, 17, 57, and 89. Blood and tissue samples were harvested from each animal. For biodistribution studies, presence and quantitation of viral levels within samples were determined via quantitative polymerase chain reaction. For safety studies, animals were assessed for adverse vector-related tissue or laboratory effects. In the biodistribution study, low levels of Ad5/3-Δ24 DNA were noted outside of the abdominal cavity. Viral DNA levels in tissues obtained from the peritoneal cavity peaked at day 8 and declined thereafter. In the safety study, no specific histopathologic changes were attributable to virus administration. Hematologic findings noted in the 1 × 1011 viral particles (vp)/dose group on Days 4 and/or 8 were indicative of an Ad5/3-Δ24–specific generalized inflammatory response; these findings resolved by day 56. The no observable adverse effect level was determined to be 1 × 1010 vp/dose. This study elucidates the safety profile of IP administration of the serotype chimeric infectivity-enhanced CRAd, Ad5/3-Δ24, and provides guidance for a planned phase I trial for patients with recurrent gynecologic cancers.
In this study, Kim and colleagues evaluate the biodistribution, safety, and immunological response associated with intraperitoneal administration of a novel adenoviral vector with a 5/3 fiber-knob modification in a hamster model. These studies were done in advance of a planned phase I human clinical trial in ovarian and other select gynecological cancers.
Introduction
With an estimated 21,880 new cases and 13,850 deaths in 2010, ovarian cancer has the fifth highest mortality rate of any cancer among American women, and remains the gynecologic malignancy with the highest mortality (Jemal et al., 2010). Despite ongoing research and development in screening as well as therapeutics, most patients affected by ovarian cancer are diagnosed with late-stage disease and have poor long-term survival. As has been the case for many years, standard treatment of advanced ovarian cancer entails cytoreductive surgery followed by cytotoxic chemotherapy. Upon recurrence, only a finite number of agents are available with wide-ranging clinical efficacy to attempt to control the disease. As such, there remains a perspicuous need for improved therapeutics for this grave disease.
Adenovirus-based virotherapy has lent itself as a novel approach in the treatment of ovarian cancer (Matthews et al., 2009). With its natural progression and spread throughout the peritoneal cavity, an intraperitoneal (IP) approach to treat ovarian cancer may represent the optimal treatment route. Indeed, recent studies have demonstrated improved survival with intraperitoneally based chemotherapy leading to an National Cancer Institute (NCI) alert on this topic (NCI, 2006). Adenovirus-based virotherapies are ideally suited for IP delivery for compartment-based malignancies such as ovarian cancer. For example, investigators have evaluated the safety and potential efficacy of ONYX-015, a CRAd with an E1A modification that allows for conditional replication in cells with p53 deficiencies (Vasey et al., 2002). These studies have demonstrated the feasibility and safety of administering ONYX-015 at reasonable dosages; however, limited clinical responses were noted. ONYX-015 cell entry is dependent upon the coxsackie-adneovirus receptor (CAR). CAR is the natural receptor for adenoviruses but is typically under-expressed in many tumor cells such as ovarian cancer (Douglas et al., 2001; Bauerschmitz et al., 2002; Kanerva et al., 2002a). Diminished viral cell surface receptors may have contributed substantially to the limited clinical responses noted with ONYX-015.
It has subsequently been demonstrated that modifications in the fiber knob of the adenovirus can enhance viral infectivity of cancer cells. These modifications allow adenoviruses to infect cells using cellular entry mechanisms other than the CAR. For example, the adenoviral HI loop of its fiber knob has been modified to incorporate an arginine–glycine–aspartate (RGD-4C) motif which allows for the virus to target cell surface integrins. Studies of ours have demonstrated this modification enhances cellular infectivity in both in vitro and in vivo models of ovarian cancer and efforts to evaluate an RGD-modified CRAd and an RGD-modified suicide gene therapeutic in early phase clinical trials are ongoing (Dmitriev et al., 1998; Vanderkwaak et al., 1999; Krasnykh et al., 2000; Kimball et al., 2010).
We have previously demonstrated the feasibility of replacing the fiber knob of an adenovirus type 5 with an adenovirus type 3 knob (Kanerva et al., 2002b). This Ad 5/3 serotype modification allows for CAR-independent cell entry by utilizing an Ad type 3 receptor and results in significant enhancement in ovarian cancer cellular infectivity. Ad 5/3-Δ24 is a serotype chimeric infectivity-enhanced oncolytic CRAd that has also been developed by our group (Kanerva et al., 2003). This CRAd contains a Δ24 modification that allows for conditional replication in Rb- and p16-deficient cancer cells and incorporates the 5/3 serotype modification in its fiber knob. Prior studies of ours have demonstrated enhanced infectivity and potential therapeutic efficacy of this reagent in both established and primary ovarian cancer models (Kanerva et al., 2002a,b, 2003; Lam et al., 2004; Page et al., 2007). This serotype chimeric CRAd Ad5/3-Δ24, the first of its kind, represents one of the most advanced, potent CRAds developed by our group to date.
The purpose of this study was to evaluate the biodistribution, safety, and immunologic response associated with IP administration of Ad5/3-Δ24 in an appropriate animal model. These studies, designed in collaboration with the U.S. Food and Drug Administration, were done in advance of a planned phase I human clinical trial in ovarian and other select gynecologic cancers.
Materials and Methods
Vectors and controls
Vials of Ad 5/3-Δ24 adenovirus were received from the NCI (Frederick, MD) and stored at approximately −70°C until use. Formulation buffer GST (20 mM Tris, 25 mM NaCl, 2.5% glycerol) was also received from NCI and stored at 4°C until use. Ad5/3Luc adenovirus used for the neutralizing antibody studies was created in the Division of Human Gene Therapy at the University of Alabama at Birmingham.
Animals
Female Syrian hamsters from Charles River Laboratories (Kingston, NY) were used for all studies. Syrian hamsters are an acceptable immunocompetent model allowing for adenoviral replication similar to that in human cancer patients, and they provide excellent support for safety studies of compounds and vectors ultimately intended for human use (Thomas et al., 2006).
Hamsters were 7–8 weeks of age at the time of study commencement with body weights ranging from 111 to 130 g. Animals were individually housed and cared for in accordance with the current Association for Assessment and Accreditation of Laboratory Animal Care recommendations and requirements stated in “Guide for the Care and Use of Laboratory Animals.” Each animal was identified with a unique cage card and ear tag. A certified rodent diet and water, both without evidence of contamination that could affect the study, were given ad libitum.
General design of biodistribution study
A total of 32 hamsters were used for the biodistribution study: 12 hamsters in Group 1 received no Ad5/3-Δ24 and 20 hamsters in Group 2 were treated with 1 × 1011 vp/dose of Ad5/3-Δ24 intraperitoneally three times daily (Table 1). On days 8, 17, 57, and 89, three animals from Group 1 and five animals from Group 2 underwent peripheral blood sampling followed by necropsy with full gross examination. Sixteen distinct blood and tissue samples were taken from each animal. Two blood samples were collected from each hamster prior to euthanization; one sample was frozen and saved for quantitative polymerase chain reaction (PCR) analysis. After necropsy, selected tissues were collected and sent for processing and adenoviral distribution studies.
Table 1.
Animal Groups for Biodistribution Study
| Group | Animal no. | No. of hamsters | Route/schedule | Ad5/3-Δ24 (vp/dose) | Ad5/3-Δ24 (vp/kg/dose) | Equivalent human dose (vp/dose) |
|---|---|---|---|---|---|---|
| 1 | 101–112 | 12 | IP/daily × 3 days | 0 | 0 | |
| 2 | 201–220 | 20 | 1 × 1011 | 7.5 × 1011 | 5 × 1013 |
Determination of end organ viral concentration in biodistribution study
Three animals from Group 1 and five animals from Group 2 were humanely euthanized on study days 8, 17, 57, and 89, and 16 distinct blood and tissue samples were harvested from each animal. Tissue samples included bone marrow, brain, heart, jejunum, kidneys, liver, lungs, lymph nodes (mesenteric and abnormal), ovaries, pancreas, skeletal muscle (diaphragm, bicep femoris), and sternum. Gross examinations of the tissues were noted. After harvesting, the tissue samples were weighed and snap-frozen and stored between −60°C and −80°C. Prior to quantitative PCR, each sample was divided into approximate halves; one half was spiked with adenovirus at 1 × 108 vp/ml, while the other half remained unspiked. The spiked samples served to evaluate nucleic acid isolation efficiency among and across tissue types.
Virus isolation from both blood and tissue samples was performed using the fully automated bioMérieux NucliSENS EasyMAG (bioMérieux, Durham, NC); 50–150 μl of samples were mixed with easyMAG Lysis Buffer and incubated at room temperature for 10 min. EasyMAG Magnetic Silica was then added, and the samples were then incubated for an additional 10 min. Samples were then washed twice with easyMAG Buffer 1, twice with easyMAG Buffer 2, and once with easyMAG Buffer 3. Purified nucleic acid was then eluted, quantified with a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE) and stored at −70°C. Samples were read in triplicate, and the average was used to assign a concentration to each sample.
Quantitative PCR was then carried out with each sample in triplicate with adenoviral DNA positive controls and master-mix-only/no-template controls. PCR amplifications were carried out in 50-μl total volumes containing 1 × TaqMan Universal PCR Master Mix (AmpliTaq Gold DNA Polymerase, AmpErase UNG, dNTPs with dUTP, Passive Reference, and optimized buffer components [Applied Biosystems Inc., Foster City, CA]), 1 × Gene Expression Assay mixture designed for the Hexon gene (900 nM forward primer, 900 nM reverse primer, and 250 nM probe [dual-labeled with FAM at the 5′ end and a nonfluorescent quencher at the 3′ end]) (Applied Biosystems Inc.), nuclease-free distilled water, and either 5 μl of reference standard DNA or various volumes of isolated nucleic acid from each test sample. Primers and probe used are shown in Table 2. Each sample was also analyzed using quantitative PCR with an 18S primer/probe set, which showed consistency between PCR amplifications of differing tissue types. PCR was run on an ABI PRISM 7900HT Fast Sequence Detection System (Applied Biosystems Inc.), and data were analyzed with Sequence Detection System software (Applied Biosystems Inc.).
Table 2.
Primers and Probes Utilized for Biodistribution Study
| Gene | Primer/probe | Oligonucleotide sequence (5′–3′) |
|---|---|---|
| Hex593 | Forward | CCTACTCTGGCACTGCCTACAA |
| Hex593 | Reverse | CATCCCATTCGCAAGGATTT |
| Hex593 | Probe | 6FAM-CCTGGCTCCCAAGGGTGCCC |
General design of safety study
Syrian hamsters were divided into four cohorts of 15 animals each (Table 3). These animals underwent IP administration of Ad5/3-Δ24 at dosages of 1 × 109 to 1 × 1011 vp/dose for three daily treatments. Hematology and serum chemistry samples were drawn from all surviving hamsters on study days 4, 8, 17, and 56, while coagulation panels were drawn only at the time of termination on study days 8, 17, and 56. Samples were obtained from each animal prior to necropsy. On days 8, 17, and 56, five animals from each group were humanely euthanized and subjected to complete gross and microscopic examination.
Table 3.
Animal Groups for Toxicity Study
| Group | Animal no. | No. of hamsters | Route/schedule | Ad5/3-Δ24 (vp/dose) | Ad5/3-Δ24 (vp/kg/dose) | Equivalent human dose (vp/dose) |
|---|---|---|---|---|---|---|
| 1 | 101–115 | 15 | IP/daily × 3 days | 0 | 0 | 0 |
| 2 | 201–215 | 15 | 1 × 109 | 7.5 × 109 | 5 × 1011 | |
| 3 | 301–315 | 15 | 1 × 1010 | 7.5 × 1010 | 5 × 1012 | |
| 4 | 401–415 | 15 | 1 × 1011 | 7.5 × 1011 | 5 × 1013 |
Evaluation of general condition and body weight in toxicity study
On dosing days, hamsters were observed clinically prior to dosing through 1 hr post-dosing, and then once daily thereafter. Body weight was measured at the outset of the study, and again prior to the first dosing; body weights of surviving hamsters were then taken once weekly at approximately the same time of day for the remainder of the study until necropsy.
Analysis of Ad5/3-Δ24–related end organ toxicity
On the specified days, after laboratory samples were drawn, animals were humanely euthanized. A board-certified veterinary pathologist was available at all times during necropsies. The following tissues were fixed in 10% neutral buffered formalin for histopathologic examination: adrenal glands, aorta, bone and bone marrow (femur), bone marrow sheath (sternum), brain, esophagus, eyes, gallbladder, harderian glands, heart, large and small intestines, kidneys, liver, lungs, lymph nodes (mesenteric, mandibular, and any abnormal), ovaries and fallopian tubes, pancreas, pituitary gland, salivary gland, skeletal muscle (biceps femoris), skin/mammary gland, thyroid/parathyroid gland, trachea, uterus, urinary bladder, and vagina. Tissues were fixed, processed to slides, and stained with hemotoxylin and eosin for evaluation.
Analysis of Ad5/3-Δ24–related laboratory toxicity
Serum samples were obtained on the specified study days, prior to necropsy. Hematologic studies included erythrocyte count, hematocrit, hemoglobin, leukocyte count (total and differential), mean corpuscular volume, mean corpuscular hemoglobin, platelet count, and reticulocyte count. Coagulation studies included activated partial thromboplastin time, fibrinogen, and prothrombin time. Serum chemistries included alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, albumin, amylase, total bilirubin, cholesterol, creatinine, gamma glutamyl transferase, globulin, glucose, lipase, total protein, triglycerides, and urea nitrogen.
Evaluation of anti-adenovirus neutralizing antibody response
A separate serum sample was obtained from each animal in the toxicity study cohorts prior to scheduled necropsy on Days 8, 17, and 56 for evaluation of Ad5/3-Δ24–induced anti-adenovirus neutralizing antibody (NAbs). For this study, the Ad5/3Luc was neutralized by sera specimens before infection for SKOV3.ip1 cells, and transduction efficacy was then determined by luciferase assay. Briefly, triplicates of SKOV3.ip1 cells were plated into 96-well plates (10,000/well) and allowed to grow overnight before infection. A twofold dilution of serum of each day point specimen was prepared in Opti-MEM (Media Preparation Shared Facility, University of Alabama at Birmingham), in a normalized volume. Nonreplicative Ad5/3Luc at 100 PFU/cell was mixed with each dilution for 30 min at room temperature before adding into appropriate wells. This infection was allowed to proceed for 48 hr. A luciferase assay was carried out with the luciferase assay system (Promega, Madison, WI) on an Orion microplate luminometer (Berthold, Pforzheim, Germany) with Culturplate-96 (Research Parkway, Meriden, CT) according to manufacturers' protocols.
An enzyme-linked immunosorbent assay (ELISA) was also used to determine the titer of anti-Ad antibodies in human ascites and sera specimens. Each well of a 96-well plate (Nunc-Immuno 96-well plates; Thermo Fisher Scientific) was incubated with 100 μl of phosphate-buffered saline (PBS) containing 100 ng of Ad5/3-Δ24 (NCI) overnight at 4°C. The coated wells were blocked with 1% bovine serum albumin (BSA) (A-7906, Sigma, St. Louis, MO)/TBS-Tween20 (Teknova, Hollister, CA; Thermo Fisher Scientific) for 1 hr at room temperature. After washing three times with TBS-Tween20, ascites or sera specimens were diluted two times with 1% BSA/TBS-Tween20, added to the appropriate well in triplicate, and then incubated overnight at 4°C. After washing four times with TBS-Tween20, the second antibody (goat anti-Human IgA, IgG, and IgM, G, M; Millipore, Billerica, MA; Thermo Fisher Scientific) diluted 1:5000 with 1% BSA/TBS-Tween20 was then added into each well for 2 hr at room temperature, and then put into 4°C for overnight. AP substrate (Kirkegaard and Perry PhosphaGLO AP Substrate, Kirkegaard and Perry Laboratories, Gaithersburg, MD; Thermo Fisher Scientific) was added and color developed at room temperature for 2 hr after washing four times with TBS-Tween20. Color intensity was quantified by measuring the absorbance at 405 nm in a 96-well plate reader (PowerWave HT 340, BioTek, Winooski, VT) and the data were analyzed by KC4.
Statistical analysis
All data were analyzed for effects by analysis of variance. For homogenous data determined by Bartlett's test for homogeneity at an α level of 0.05, differences between control and comparison groups were made with Dunnett's test. Conversely, for nonhomogeneous data, differences were made using the Cochran and Cox's modified two-sample t-test. Statistical significance for each comparison was reported at a level of 0.05.
Results
PCR quantitation
A reference standard dilution series was created via 10-fold serial dilutions of reference DNA from 6 × 105 to 6 × 10–1 copies/μl. Adenovirus Reference Material (ARM) DNA was also used to prepare positive controls (Table 4). All reactions were performed in triplicate, and each run contained two ARM positive controls at concentrations of both 6 × 103 copies/μl and 6 × 102 copies/μl. A negative control using master mix only was also used with each run.
Table 4.
Adenovirus DNA and Positive Control
| Material | Name | Concentration (ng/ml) | Copies/ml |
|---|---|---|---|
| Reference standard | Ad5/3-Δ24 | 18.91 | 4.844 × 108 |
| Positive control (ARM) | wtAd5 | 11.46 | 2.935 × 108 |
ARM, adenovirus reference material.
Biodistribution associated with IP administration of Ad5/3-Δ24
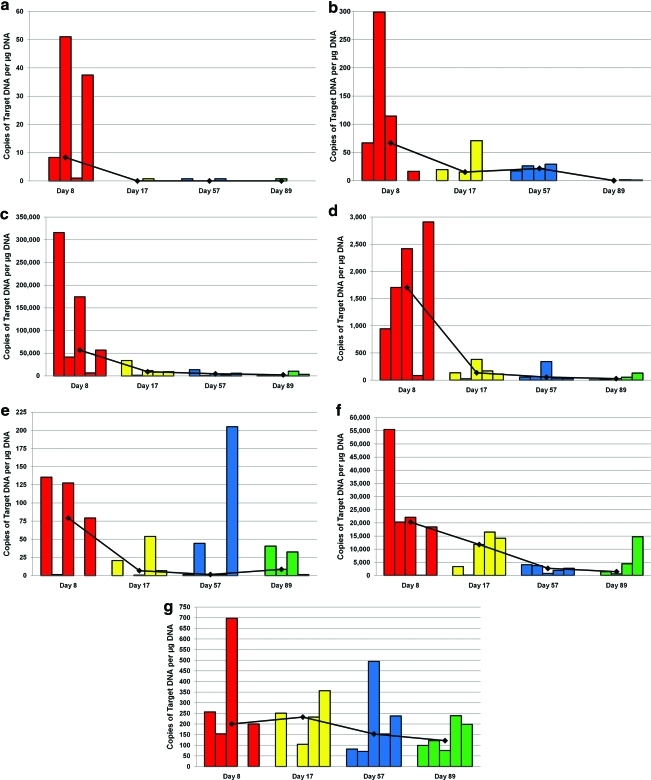
Levels of Ad5/3-Δ24 CRAd varied in different organs and among individual animals in each treatment group. In general, high levels of virus were demonstrated in some tissues, including peritoneal lining, jejunum, diaphragm, pancreas, spleen, and sternum, while low levels of virus were found in other tissues including blood, heart, kidney, lung, ovaries, and biceps femoris. There was no virus detected in the brain. Notable findings are depicted in Figure 1. Levels of Ad5/3-Δ24 in blood and tissue outside of the abdominal cavity were generally low, indicating low systemic absorption.
FIG. 1.
Biodistribution of viral DNA in (a) blood, (b) lung, (c) peritoneal lining, (d) liver, (e) kidney, (f) spleen, and (g) ovaries, respectively. Each bar represents viral DNA levels measured in one animal. Each time point had a total of eight animals. Number of bars depicted reflects only those animals that had measurable viral DNA. Red bars: day 8; yellow bars: day 17; blue bars: day 57; green bars: day 89. Color images available online at www.liebertonline.com/hum.
Among tissue samples from within the peritoneal cavity, the highest detection levels of viral DNA were found on study day 8; subsequent decline in DNA detection was consistent thereafter, indicating vector elimination. In most tissues, the virus was cleared completely or to low (but detectable) levels by the end of the study; in a few tissue types (sternum, ovaries), the virus persisted through the final day of the study.
Effect of IP Ad5/3-Δ24 on general condition and body weight
Of note, there were no unscheduled deaths during the study. There were no significant changes in body weight or food/water consumption related to virus administration. One animal in the control group was noted to have a thin appearance for approximately 5 days (between study days 39 and 43), while another animal in the control group was observed to have ventral body swelling for approximately 10 days (between study days 8 and 17). In the vector group, two animals were noted to have ventral body swelling for 1 day and 4 days (on day 8 and between days 8 and 11, respectively), while another animal in this cohort was noted to have a ventral body abrasion lasting approximately 10 days (between days 8 and 17). These observations were not attributed to vector administration; there were no other abnormal observations noted in any animal from either group.
Effect of IP Ad5/3-Δ24 on end organ pathologic findings
No gross abnormal findings were noted at the time of necropsy that were attributed to the administration of Ad5/3-Δ24. Furthermore, there were no adverse toxicological or biological changes noted in the microscopic end organ histopathologic examination. All changes that were observed, such as mild cellular hyperplasia in some tissues such as skin and lymph nodes, an occasional mononuclear cell infiltration in the liver or mild myodegeneration in skeletal muscle, were noted to be typical of background inflammatory changes found in untreated hamsters.
Effect of IP Ad5/3-Δ24 on laboratory findings
Increased white blood cells, specifically neutrophils, monocytes, and lymphocytes were observed in the group receiving 1 × 1011 vp/dose, which was attributed to Ad5/3-Δ24 (Table 5). On study day 4, the lymphocyte counts were slightly elevated, while total white cells, neutrophils, and monocytes were more significantly elevated compared with the control group. On study day 8, neutrophil and lymphocyte counts were significantly increased. Also on day 8, an increase in reticulocyte count was noted. These findings were most likely due to a generalized inflammatory response and had cleared by day 17. Other hematologic and coagulation data revealed no major differences between treated and control groups.
Table 5.
Group Mean Absolute White Blood Cell Count and Relevant Differential Data
| |
|
Laboratory value (mean) |
|||
|---|---|---|---|---|---|
| Dose (vp/dose) | Day | WBC (103ml) | Neutrophil (103ml) | Lymphocyte (103ml) | Reticulocyte (103ml) |
| 0 | 4 | 6.83 | 2.32 | 4.2 | 164.9 |
| 8 | 6.11 | 2.17 | 3.66 | 549.8 | |
| 17 | 5.1 | 1.36 | 3.46 | 242.7 | |
| 56 | 5.7 | 1.29 | 4.15 | 141.7 | |
| 1 × 109 | 4 | 7.43 | 2.46 | 4.55 | 186.8 |
| 8 | 6.89 | 2.21 | 4.40* | 492.3 | |
| 17 | 5.33 | 1.32 | 3.77 | 264.4 | |
| 56 | 5.61 | 1.17 | 4.21 | 124.7 | |
| 1 × 1010 | 4 | 7.99 | 3.27 | 4.24 | 191.2 |
| 8 | 6.61 | 1.97 | 4.32 | 629.7 | |
| 17 | 5.75 | 1.95 | 3.52 | 230.8 | |
| 56 | 6.55 | 2.27 | 4.02 | 167.2 | |
| 1 × 1011 | 4 | 10.78* | 4.84* | 4.54 | 154 |
| 8 | 11.60* | 5.83* | 5.35* | 353.8* | |
| 17 | 5.18 | 1.56 | 3.36 | 251.3 | |
| 56 | 5.39 | 1.25 | 3.89 | 140.5 | |
Statistically significant (p ≤ 0.05) difference from the comparison group (i.e. 0 vp/dose) when N > 2.
WBC: white blood cell count.
In the serum chemistry analysis, changes attributed to the vector were mainly found in the group receiving 1 × 1011 vp/dose (Table 6). The only exception to this was elevated cholesterol on day 4 and 8 in the group receiving 1 × 1010 vp/dose. On study day 4, there were significantly elevated levels of alkaline phosphatase, cholesterol, triglycerides, and serum globulin and significantly decreased levels of albumin when compared with control groups. Alkaline phosphatase levels remained elevated through day 17, but by day 56 were similar to control values. All other changes in chemistry panels had resolved by the end of the study.
Table 6.
Group Mean Serum Chemistry Data
| |
|
Laboratory value (mean) |
|||
|---|---|---|---|---|---|
| Dose (vp/dose) | Day | Alkaline phosphatase (U/L) | Cholesterol (mg/dl) | Triglycerides (mg/dl) | Globulin (g/dl) |
| 0 | 4 | 179 | 130 | 153 | 3 |
| 8 | 156 | 132 | 128 | 2.9 | |
| 17 | 157 | 155 | 162 | 2.9 | |
| 56 | 136 | 183 | 257 | 3 | |
| 1 × 109 | 4 | 161 | 140 | 130 | 2.9 |
| 8 | 154 | 147 | 133 | 3.1 | |
| 17 | 158 | 174 | 156 | 3.1 | |
| 56 | 132 | 169 | 263 | 3 | |
| 1 × 1010 | 4 | 177 | 154* | 180 | 3.1 |
| 8 | 149 | 149* | 136 | 3.1 | |
| 17 | 153 | 160 | 153 | 3.1 | |
| 56 | 126 | 187 | 245 | 3.2 | |
| 1 × 1011 | 4 | 250* | 180* | 385* | 3.3* |
| 8 | 283* | 144 | 135 | 3.1 | |
| 17 | 180* | 157 | 163 | 3.1 | |
| 56 | 152 | 185 | 217 | 3 | |
Statistically significant (p ≤ 0.05) difference from the comparison group (i.e. 0 vp/dose) when N > 2.
Effect of anti-adenovirus NAbs response
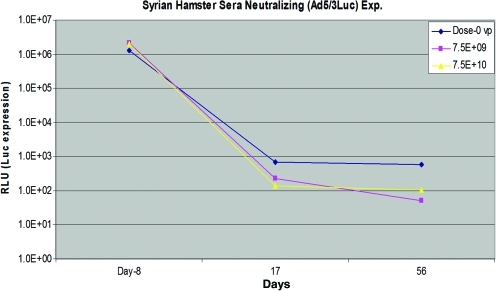
To analyze the neutralizing antibodies present, serum was obtained from blood samples immunized with virus prior to infection of SKOV3.ip1 cells. Forty-eight hours after incubation, the reporter gene luciferase was measured and is indicated as RLU in Figure 2. From this assay, neutralizing antibodies against Ad5/3-Δ24 were detected in each of the dose cohorts. The presence of neutralizing antibodies increased from day 8 to 17 and remained at relatively constant levels. In the ELISA-based analysis, neutralizing antibodies were also detected; expression of neutralizing antibodies appears greater in the 1 × 1010 vp/dose group; however, this was not statistically significant (data not shown).
FIG. 2.
Anti-adenoviral neutralizing antibody response in serum. Luciferase expression decreased from day 8 to 17, reflecting an increase in neutralizing antibodies from day 8 to 17. Neutralizing antibodies then remained at relatively constant levels. Color images available online at www.liebertonline.com/hum.
Discussion
Herein we describe the preclinical safety profile of the serotype chimeric modified CRAd, Ad5/3-Δ24. As previously noted, Ad5/3-Δ24 contains a Δ24 modification that allows for conditional replication in Rb and p16 deficient cancer cells (Fueyo et al., 2000; Heise et al., 2000) and incorporates the 5/3 serotype modification in its fiber knob (Dmitriev et al., 1998; Vanderkwaak et al., 1999; Krasnykh et al., 2000; Page et al., 2007). This Ad5/3 serotype modification allows for CAR-independent cell entry by utilizing an Ad type 3 receptor and results in significant enhancement in ovarian cancer cellular infectivity. Prior studies of ours have demonstrated potent oncolysis in various established ovarian cancer cells in vitro (Kanerva et al., 2002a). Other studies have demonstrated the ability of Ad5/3-Δ24 to replicate in spheroid models of primary ovarian cancer cells (Kanerva et al., 2003; Lam et al., 2004). Lastly, a significant impact on survival has been noted in an orthotopic model of ovarian cancer treated with single and three times daily IP injection of Ad5/3-Δ24 compared with controls (Kanerva et al., 2002b; Raki et al., 2008).
The current study validates the safety of IP administration of Ad5/3-Δ24. In this study, we utilized Syrian hamsters, an immunocompetent model that allows for replication of human adenoviruses within normal and tumor tissues (Thomas et al., 2006). The maximum dosage of Ad5/3-Δ24 employed in this study was 1 × 1011 vp/dose daily for 3 days. Using standard conversion factors, this hamster dosage corresponds to a human dosage of approximately 5 × 1013 vp/dose per day, based on a human weight of 65 kg. Our biodistribution analysis revealed the highest levels of Ad5/3-Δ24 viral DNA within the peritoneal cavity. Viral levels peaked at day 8 following IP administration and diminished thereafter. Ad5/3-Δ24 was cleared by day 56 in all tissues. With the exception of high viral levels noted in the sternum, low systemic absorption outside of the peritoneal cavity was noted.
Our safety studies noted no adverse clinical effects on weight and food and water consumption of animals. No gross abnormalities were seen at the time of euthanization and only nonspecific inflammatory and other findings were noted on histopathologic analysis of harvested tissues. Potential Ad5/3-Δ24 specific laboratory findings were noted, in general, only in animals treated with the highest dose (1 × 1011 vp/dose). This included hematologic findings (i.e., increased white blood cell count) attributed to a general inflammatory response and increases in serum alkaline phosphatase, cholesterol, triglyceride, and serum globulin. Any noted abnormalities ultimately resolved by day 56. Lastly, a potent neutralizing antibody response was noted.
While no other formal biodistribution or toxicity studies were done specifically for the purposes of gaining U.S. Food and Drug Administration investigational new drug approval, Kanerva et al. (2002b) did assess biodistribution and liver toxicity of a 5/3 modified adenovirus expressing a reporter gene in a SCID mouse model. Their study demonstrated a biodistribution pattern and histologic finding in the liver similar to that noted in the current study after IP administration of this reporter gene expressing 5/3 modified adenovirus. The results of the current study are also similar to those noted in preclinical studies evaluating other infectivity enhanced conditionally replicative and nonreplicative adenoviruses developed by our group (Page et al., 2007; Matthews et al., 2009). Specifically, preclinical studies in appropriate animal models have demonstrated high viral concentrations within the abdominal cavity, low systemic absorption, and limited clinical and laboratory inflammatory changes associated with IP administration of either the CRAd, Ad5-Δ24-RGD, or the bicistronic suicide gene therapeutic, Ad5.SSTR/TK.RGD, both infectivity enhanced via incorporation of an RGD-4C motif in the HI loop of the adenoviral fiber.
Clinical trial experience with a 5/3 modified armed CRAd, Ad 5/3-Δ24-GMCSF, has recently been reported (Koski, 2010). In this trial, 21 patients with advanced solid tumors refractory to standard therapies were treated with up to 4 × 1011 vp/dose Ad 5/3-Δ24-GMCSF for one treatment and 16 of these patients also received oral cyclophosphamide (50 mg/d) to reduce regulatory T cells. At least 20% of the dose of Ad 5/3-Δ24-GMCSF was administered intravenously and the remainder was administered intratumorally (or intraperitoneally in four ovarian cancer patients). The most commonly experienced side effects included grade 1–2 flu-like symptoms, injection site discomfort, and abdominal pain, and no grade 4 or 5 adverse events were noted. Though the dose, route of delivery, and schedule utilized in this recently reported trial differed from our planned phase I trial, the results are in line with safety results of the current study and do demonstrate the potential safety of utilizing a 5/3 serotype modified CRAd. In addition, clinical trials evaluating IP administration of two other infectivity enhanced adenoviral agents (Ad5-Δ24-RGD and Ad5.SSTR/TK.RGD) in patients with recurrent ovarian and other select gynecologic cancers have demonstrated no dose-limiting toxicity to date (Kimball, 2010; Alvarez, personal communication). Preclinical data currently exist for other disease sites as well, including breast cancer, renal cancer, and gastric cancer (Kangasniemi et al., 2006; Guse et al., 2007; Ranki et al., 2007), potentially enabling the utilization of serotype chimeric or other infectivity enhanced modified adenoviral based therapies for the treatment of other tumor types.
In summary, this is the first study to demonstrate the preclinical safety associated with IP administration of a novel serotype chimeric infectivity enhanced CRAd. Ad5/3-Δ24 is an advanced, highly potent CRAd developed by our group. The current study provides further justification for clinical translation and a phase I trial evaluating intraperitoneal administration of Ad5/3-Δ24 in patients with recurrent ovarian and other select gynecologic cancers is currently being pursued.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant 5R01CA121187 and has been funded in whole or in part with federal funds from the National Cancer Institute, NIH, under Contract No. N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
This research was supported in part by the Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute under Contract No. N01-CM-42200.
Author Disclosure Statement
Each author declares that there are no conflicts of interest.
References
- Bauerschmitz G.J. Lam J.T. Kanerva A., et al. Treatment of ovarian cancer with a tropism modified oncolytic adenovirus. Cancer Res. 2002;62:1266–1270. [PubMed] [Google Scholar]
- Dmitriev I. Krasnykh V. Miller C.R., et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J. Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J.T. Kim M. Sumerel L.A., et al. Efficient oncolysis by a replicating adenovirus (Ad) in vivo is critically dependent on tumor expression of primary Ad receptors. Cancer Res. 2001;61:813–817. [PubMed] [Google Scholar]
- Fueyo J. Gomez-Manzano C. Alemany R., et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 19, 2–12. Erratum in Oncogene. 2000;19:5038. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Guse K. Ranki T. Ala-Opas M., et al. Treatment of metastatic renal cancer with capsid-modified oncolytic adenoviruses. Mol. Cancer Ther. 2007;6:2728–2736. doi: 10.1158/1535-7163.MCT-07-0176. [DOI] [PubMed] [Google Scholar]
- Heise C. Hermiston T. Johnson L., et al. An adenovirus E1A mutant that demonstrates potent and selective systemic antitumoral efficacy. Nat. Med. 2000;6:1134–1139. doi: 10.1038/80474. Erratum in: Nat. Med. 6, 1412. [DOI] [PubMed] [Google Scholar]
- Jemal A. Siegel R. Xu J., et al. Cancer statistics, 2010. CA. Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Kanerva A. Mikheeva G.V. Krasnykh V., et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin. Cancer Res. 2002a;8:275–280. [PubMed] [Google Scholar]
- Kanerva A. Wang M. Bauerschmitz G.J., et al. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol. Ther. 2002b;5:695–704. doi: 10.1006/mthe.2002.0599. [DOI] [PubMed] [Google Scholar]
- Kanerva A. Zinn K.R. Chaudhuri T.R., et al. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol. Ther. 2003;8:449–458. doi: 10.1016/s1525-0016(03)00200-4. [DOI] [PubMed] [Google Scholar]
- Kangasniemi L. Kiviluoto T. Kanerva A., et al. Infectivity-enhanced adenoviruses deliver efficacy in clinical samples and orthotopic models of disseminated gastric cancer. Clin. Cancer Res. 2006;12:3137–3144. doi: 10.1158/1078-0432.CCR-05-2576. [DOI] [PubMed] [Google Scholar]
- Kimball K.J. Preuss M.A. Barnes M.N., et al. A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases. Clin Cancer Res. 2010;6:5277–5287. doi: 10.1158/1078-0432.CCR-10-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski A. Kangasniemi L. Escutenaire S., et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther. 2010;18:1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnykh V. Dmitriev I. Navarro J.G., et al. Advanced generation adenoviral vectors possess augmented gene transfer efficiency based upon coxsackie adenovirus receptor-independent cellular entry capacity. Cancer Res. 2000;60:6784–6787. [PubMed] [Google Scholar]
- Lam J.T. Kanerva A. Bauerschmitz G.J., et al. Inter-patient variation in efficacy of five oncolytic adenovirus candidates for ovarian cancer therapy. J. Gene Med. 2004;6:1333–1342. doi: 10.1002/jgm.635. [DOI] [PubMed] [Google Scholar]
- Matthews K. Noker P.E. Tian B., et al. Identifying the safety profile of Ad5.SSTR/TK.RGD, a novel infectivity-enhanced bicistronic adenovirus, in anticipation of a phase I clinical trial in patients with recurrent ovarian cancer. Clin. Cancer Res. 2009;15:4131–4137. doi: 10.1158/1078-0432.CCR-08-3354. [DOI] [PubMed] [Google Scholar]
- Matthews K.S. Alvarez R.D. Curiel D.T. Advancements in adenoviral based virotherapy for ovarian cancer. Adv. Drug. Deliv. Rev. 2009;61:836–841. doi: 10.1016/j.addr.2009.04.012. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute (NCI) NCI issues clinical announcement for preferred method of treatment for advanced ovarian cancer. 2006. www.cancer.gov/newscenter/pressreleases/IPchemotherapyrelease. [Mar 10;2010 ]. www.cancer.gov/newscenter/pressreleases/IPchemotherapyrelease
- Page J.G. Tian B. Schweikart K., et al. Identifying the safety profile of a novel infectivity-enhanced conditionally replicative adenovirus, Ad5-24-RGD, in anticipation of a phase I trial for recurrent ovarian cancer. Am. J. Obstet. Gynecol. 2007;196:389e1–e10. doi: 10.1016/j.ajog.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Raki M. Sarkioja M. Desmond R.A., et al. Oncolytic adenovirus Ad5/3-delta24 and chemotherapy for treatment of orthotopic ovarian cancer. Gynecol. Oncol. 2008;108:166–172. doi: 10.1016/j.ygyno.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Ranki T. Sarkioja M. Hakkarainen T., et al. Systemic efficacy of oncolytic adenoviruses in imagable orthotopic models of hormone refractory metastatic breast cancer. Int. J. Cancer. 2007;121:165–174. doi: 10.1002/ijc.22627. [DOI] [PubMed] [Google Scholar]
- Thomas M.A. Spencer J.F. La Regina M.C., et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- Vanderkwaak T.J. Wang M. Gomez-Navarro J., et al. An advanced generation of adenoviral vectors selectively enhances gene transfer for ovarian cancer gene therapy approaches. Gynecol. Oncol. 1999;74:227–234. doi: 10.1006/gyno.1999.5432. [DOI] [PubMed] [Google Scholar]
- Vasey P. Shulman L.N. Campos S., et al. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus, ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J. Clin. Oncol. 2002;20:1562–1569. doi: 10.1200/JCO.2002.20.6.1562. [DOI] [PubMed] [Google Scholar]