Abstract
To test if manipulating TCR complex-mediated signaling (TCR signaling) could treat autoimmune disease, we generated the double SKG Src-like adapter protein (SLAP) knockout (DSSKO) mouse model. The SKG mutation in ZAP70 and SLAP have opposing functions on the regulation of TCR signaling. The combination of these two mutations alters TCR signaling in the context of a defined genetic background, uniform environmental conditions, and a well-characterized signaling disruption. In contrast to SKG mice, DSSKO mice do not develop zymosan-induced chronic autoimmune arthritis. This arthritis prevention is not due to significant alterations in thymocyte development or repertoire selection but instead enhanced numbers of regulatory T cells (Tregs) and decreased numbers of Th17 cells skewing the ratio of Tregs to autoreactive effector T cells. Treg depletion and/or functional blockade led to the development of arthritis in DSSKO mice. In vitro suppression of effector T cell proliferation was also enhanced, demonstrating that DSSKO mice have increased numbers of Tregs with increased function. Understanding how TCR signals influence development, expansion, and function of Tregs in DSSKO mice could advance our ability to manipulate Treg biology to treat ultimately autoimmune disease.
Manipulating T cell function by altering TCR signaling could be a viable strategy to treat autoimmune disease. Evidence that alterations in TCR complex signaling play a critical role in autoimmunity comes from genome-wide association studies in humans and mouse models involving both spontaneous and engineered mutations in important TCR signal transduction proteins (reviewed in Refs. 1, 2). The SKG mouse has a point mutation in ZAP70 rendering it hypomorphic with decreased signaling through the TCR complex. This decreased signaling in developing SKG thymocytes results in the selection of highly autoreactive Th17 cells and autoimmune arthritis upon exposure to zymosan, an environmental trigger (3–5). An obstacle in manipulating T cells to treat autoimmune disease is the gap in our understanding of the TCR-associated signaling networks that are required to eliminate or modulate autoreactive T cells. We tested the hypothesis that increased signaling through the TCR complex in SKG mice could prevent autoimmunity. To enhance TCR signaling, we crossed SKG mice with Src-like adapter protein (SLAP)-deficient mice to generate double SKG SLAP knockout (DSSKO) mice. SLAP is a negative regulator of TCR signaling that adapts the E3 ubiquitin ligase c-Cbl to the ζ-chain of the TCR (TCRζ), targeting it for degradation. SLAP-deficient double-positive (DP) thymocytes have increased levels of the TCR on their surfaces and enhanced signaling (6–8). In addition, SLAP deficiency partially restores thymocyte development in ZAP70-deficient mice (6), possibly due in part to the fact that SLAP and ZAP70 converge on the same signaling molecule, TCRζ, to regulate TCR complex signaling. The combination of these two mutations provides a unique opportunity to determine how alterations in proximal TCR signaling can modulate autoimmune disease in the context of a defined genetic background, uniform environmental conditions, and a well-characterized signaling disruption.
In this report, we show that SLAP deficiency prevented chronic arthritis in DSSKO mice injected with zymosan, which induces arthritis in SKG mice. SLAP deficiency partially rescued positive selection of thymocytes and had minimal effects on negative thymocyte selection in naive SKG mice; the most dramatic effect was the increased number of regulatory T cells (Tregs) in both thymus and spleen of naive DSSKO mice. After zymosan treatment, DSSKO mice had a further expansion of splenic Tregs and a decrease in Th17 cells, the autoreactive effectors in SKG mice (5), skewing the ratio of Tregs to autoreactive effectors. Depletion and/or functional blockade of Tregs using a mixture of anti-CD25 Abs in zymosan-treated mice unleashed the autoreactive T cells, resulting in arthritis development in DSSKO mice. In vitro, DSSKO Tregs displayed enhanced suppressive capacity upon activation through their TCRs. Thus, increasing signal strength through the TCR complex in autoreactive T cells enhanced development and function of Tregs, preventing arthritis development.
Materials and Methods
Mice
BALB/c (wild type [WT]) and DO-11.10 mice were bred in house. SLAP-deficient (SLAP−/−) mice on a mixed genetic background have previously been described (6) and have been back-crossed 10 generations onto a BALB/c background. SLAP−/− mice were crossed into the SKG mouse line to generate DSSKO mice. To assess effects on positive selection, SLAP−/−, SKG, and DSSKO mice were crossed with BALB/c mice expressing the DO-11.10 TCR transgene. All mice were maintained in specific pathogen-free conditions according to the guidelines of the National Jewish Health Institutional Animal Care and Use Committee.
Induction and scoring of arthritis
Eight- to ten-week-old mice were injected i.p. with 2 mg zymosan A. Mice were weighed and observed for clinical signs weekly for 10 wk. Arthritis signs were assessed according to criteria established and published by Sakaguchi et al. (3): 0 = no joint swelling; 0.1 = swelling in one digit; 0.5 = mild to moderate swelling of wrist or ankle; and 1 = moderate to severe swelling of wrist or ankle. Scores for all digits, wrists, and ankles were combined for each mouse.
Histology
Mice were euthanized and joints fixed in 10% formalin solution, decalcified in 0.5 M EDTA, and embedded in paraffin. Four-micrometer-thick tissue sections were stained with toluidine blue (Premier Laboratories, Boulder, CO).
Abs
Monoclonal Abs against the following Ags were purchased from eBio-science: CD3ε (17A2), CD5 (53.7.3), CD8α (53-6.7), CD25 (PC61), CD24 (M1/69), CD44 (IM7), CD62L (MEL-14), CD69 (H1.2F3), DO-11.10 TCR (KJ1-26), Foxp3 (FJK-16a), IFN-γ (XMG1.2), IL-4 (11B11), IL-17A (eBio17B7), and TCRζ (H57-597) conjugated to FITC, PE, PE-Cy5, PE-Cy7, PB, allophycocyanin, or allophycocyanin–Alexa Fluor 750. Streptavidin-allophycocyanin and monoclonal Abs against the following Ags were purchased from BD Biosciences: CD4 (RMA4-5), CD8α (53-6.7), Vβ3 (KJ25), Vβ5.1/5.2 (MR9-4), Vβ6 (RR4-7), Vβ7 (TR310), Vβ8.1/8.2 (MR5-2), Vβ8.3 (1B3.3), Vβ9 (MR10-2), Vβ10b (B21.5), Vβ11 (RR3-15), and Vβ12 (MR11-1) conjugated to FITC, PE, PerCP, or PB. Anti-CD4–allophycocyanin–Cy7 (GK1.5) was purchased from Bio-Legend. Biotin-conjugated anti–DO-11.10 TCR (KJ1-26) and anti-Vβ13–PE were kindly provided by the Kappler and Marrack laboratory (National Jewish Health, Denver, CO).
Cell stimulation and Western blotting
Thymocytes were stimulated with a mixture of 5 μg/ml anti-CD3 (2C11; eBioscience) and 5 μg/ml anti-CD4 (GK1.5; eBioscience) Abs, washed, and then the TCR complex and CD4 were cross-linked to induce signaling with 20 μg/ml goat anti-Armenian hamster IgG (Jackson ImmunoResearch Laboratories) and 20 μg/ml donkey anti-rat IgG (Jackson ImmunoResearch Laboratories) as previously described (8). At the indicated time points, cells were lysed and immunoblots performed as previously described (3, 8, 9). Immunoblot analysis was performed on 2.5 × 106 cell equivalents/lane with an anti-phosphotyrosine Ab (4G10; Millipore) to detect global changes in tyrosine phosphorylation. Duplicate immunoblots were performed to determine the phosphorylation status and total levels of ZAP70 (65E4 and D1C10E; Cell Signaling), anti-pp42/44 MAPK (ERK; Cell Signaling), and TCRζ (BD Biosciences).
Intracellular cytokine staining
Lymph node (LN) cells were stimulated with 20 ng/ml PMA and 1 μM ionomycin in the presence of GolgiStop (BD Biosciences) for 5 h, stained for surface Ags, fixed and permeabilized using Caltag Cytofix/Cytoperm, and stained with anti–IL-17 and anti–IFN-γ or anti–IL-4. In some experiments, cells were stained for both intracellular cytokines and Foxp3 expression using the eBioscience Foxp3 Staining Buffer Set. Data were collected on a CyAn flow cytometer (Dako Cytomation) and analyzed using FlowJo software (Tree Star).
Cell purification and generation of inducible Tregs
Naive T cells (CD4+CD25 −) were isolated from spleens and LNs of BALB/c mice by enriching for untouched CD4+ T cells using magnetic bead separation (Miltenyi Biotec) and then depleting CD25+ cells by incubating with biotin-conjugated anti-CD25 (clone 7D4; BD Biosciences) followed by a second magnetic bead separation using anti-biotin magnetic beads (Miltenyi Biotec). Purified CD4+CD25− cells were plated at 2 × 106/ ml in 24-well flat-bottom plates coated with 10 μg/ml anti-CD3 (2C11; eBioscience) in X-Vivo15 serum-free media supplemented with 10% FBS (Hyclone) in the presence of 2 μg/ml anti-CD28 (37.51; eBioscience), 5 ng/ml TGF-β1 (R&D Systems), 20 ng/ml IL-2 (eBioscience), and 10 ng/ml retinoic acid (Sigma-Aldrich) at 37°C for 4 d (10). After 4 d of incubation, an aliquot of cells from the culture was stained with anti-CD4, fixed and permeabilized using the eBioscience Foxp3 Staining Buffer Set, and stained with anti-Foxp3 to assess the induction of Foxp3+ inducible Tregs (iTregs). Data were collected and analyzed as described above.
Immunoprecipitation of SLAP
Thymocytes, peripheral Tregs, iTregs, and activated peripheral T cells were lysed in 1% Nonidet P-40 lysis buffer plus protease and phosphatase inhibitors, solubilized, and immunoprecipitated as described previously (6, 11). Lysates were immunoprecipitated using SLAP-specific rabbit polyclonal antisera covalently coupled to staphylococcal protein A–Sepharose. Protein retained during the immunoprecipitation was fractionated by SDS-PAGE, transferred to a polyvinylidene fluoride membrane, and blotted for SLAP using SLAP-specific rabbit polyclonal antisera and subsequently incubated in Rabbit TrueBlot HRP conjugated IgG secondary (eBioscience).
Anti-CD25 treatment
Eight- to ten-week-old mice were injected with 2 mg zymosan A i.p. On days 5, 7, and 9 post-zymosan injection, mice were injected with 200 μg of an anti-CD25 Ab mixture (100 μg PC6.1 + 100 μg 3C7) or 200 μg Rat Ig (12–14). Mice were weighed weekly and observed for clinical signs every 2–3 d for the first 6 wk, then weekly until 10 wk after zymosan injection. Arthritis signs were assessed as described above.
Suppression assay
CD4+CD25− effector T cells were isolated as described above. CD4+ CD25+ Tregs were purified from spleens and LNs of WT, SLAP−/−, SKG, and DSSKO mice by magnetic bead separation using a CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec). Purified WT CD4+ CD25− responder cells (3 × 104) were loaded with CFSE (Invitrogen) and cultured with the indicated numbers of CD4+CD25+ Tregs, CD4+ T cell-depleted mitomycin C-treated APCs, 1 μg/ml anti-CD3 (2C11; eBio-science), 2 μg/ml anti-CD28 (37.51; eBioscience), and 4 μg/ml anti-Armenian hamster (Jackson ImmunoResearch Laboratories) for 72 h. CD4+CD25+ Tregs were activated by culturing them in wells coated with 10 μg/ml anti-CD3 (2C11; eBioscience) in the presence of 2 μg/ml soluble anti-CD28 (37.51; eBioscience) for 72 h. At 72 h, activated CD4+CD25+ Tregs were harvested and counted and the indicated numbers of activated cells were used in the suppression assays. Proliferation was assessed by CFSE dilution on a CyAn flow cytometer (Dako Cytomation) and analyzed using FlowJo software (Tree Star).
Proliferation assay
CD4+ T cells were isolated from spleens and LNs of WT, SLAP–/–, SKG, and DSSKO mice by magnetic bead separation using a CD4+ T Cell Isolation Kit (Miltenyi Biotec) with >90% purity. Purified WT CD4+ T cells (1 × 105) were loaded with CFSE (Invitrogen) and cultured with media alone or in wells coated with 10 μg/ml anti-CD3 (2C11; eBio-science) in the presence or absence of 2 μg/ml soluble anti-CD28 (37.51; eBioscience). Cells were harvested 48 and 72 h after stimulation, stained with anti-CD4 and anti-CD8, and proliferation assessed and analyzed in CD4+ T cells as described above.
Statistical analysis
Unpaired two-tailed Student t tests were performed using Prism 5.0 (GraphPad Software). Differences were considered statistically significant for p values <0.05.
Online supplemental material
Supplemental Fig. 1 shows enhanced positive selection and signaling in thymocytes from DSSKO DO-11.10 TCR transgenic mice. Supplemental Fig. 2 shows peripheral T cell composition and proliferation in WT, SLAP−/−, SKG, and DSSKO mice. Supplemental Fig. 3 shows peripheral T cell composition in WT, SLAP−/−, SKG, and DSSKO mice expressing the DO-11.10 TCR transgene. Supplemental Fig. 4 shows expansion of splenic Tregs in zymosan-treated mice. Supplemental Fig. 5 shows that Tregs have returned to normal levels 8 wk after cessation of Ab injections.
Results
SLAP deficiency prevents the development of chronic arthritis in SKG mice
In SKG mice, decreased TCR complex signaling in developing thymocytes results in the selection of highly autoreactive Th17 cells and autoimmune arthritis upon exposure to zymosan, an environmental trigger (3–5). We hypothesized that increasing signaling through the TCR complex in SKG mice would prevent autoimmunity. To enhance TCR signaling, we crossed SKG mice with SLAP-deficient mice. DSSKO mice and controls were challenged with zymosan and monitored for the development of arthritis. SLAP deficiency dramatically reduced both the incidence and severity of arthritis, preventing development of chronic arthritis in the DSSKO mice (Fig. 1A, 1B). Twenty-seven of 41 (66%) DSSKO mice exhibited no signs of arthritis compared with 100% in SKG mice. In 13 of 41 (32%) DSSKO mice there were transient signs of arthritis that resolved, often by week 5 (Fig. 1A, 1B). Only one DSSKO mouse developed more than mild arthritis (maximum score of 4.2), but this resolved by the end of the observation period. Only one DSSKO mouse had an arthritis score at the end of the 10-wk observation period. Thus, some DSSKO mice treated with zymosan developed transient arthritis that resolved within a few weeks of onset. Notably, spontaneous arthritis remission was also evident in 3 of 18 (17%) SKG mice injected with zymosan, and 3 of 13 (23%) SLAP−/− and 3 of 15 (20%) WT mice developed arthritis around 7 wk postzymosan treatment (Fig. 1A, 1B). Collectively, these data show that SLAP deficiency dramatically reduced both the incidence and severity of arthritis and prevented development of chronic arthritis in the DSSKO mice.
Figure 1.
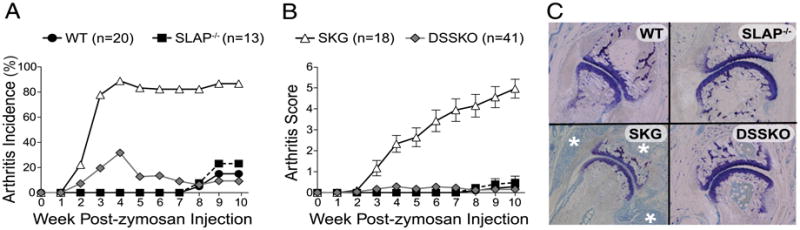
SLAP deficiency prevents the development of chronic arthritis in SKG mice. A and B, Incidence (A) and severity (B) of arthritis in WT, SLAP−/−, SKG, and DSSKO mice treated with 2 mg zymosan. Data are plotted as the average ± SEM. C, Representative histology of joints from WT, SLAP−/−, SKG, and DSSKO mice 10 wk after zymosan treatment (toluidine blue staining) (original magnification ×10). White asterisks identify synovial proliferation and pannus formation.
At the end of the 10-wk observation period, zymosan-treated mice were euthanized, and histologic evaluation was performed. Consistent with clinical scores, DSSKO mice had no synovial inflammation (Fig. 1C). However, despite the lack of pathology in the joints of zymosan-treated DSSKO, not all systemic inflammation was eradicated as both SKG and DSSKO mice had extra-articular inflammation (data not shown). These data demonstrate that SLAP-dependent signaling has a dramatic effect on autoimmune arthritis development in SKG mice.
SLAP deficiency partially rescues positive selection and enhances signaling through the TCR in SKG mice
In SKG mice, inefficient thymocyte signaling and development leads to selection of autoreactive T cells that cause arthritis (3). We targeted this inefficient selection by generating DSSKO mice. SLAP deficiency increased both frequency and absolute number of CD4+ thymocytes in DSSKO mice compared with those in SKG mice (Fig. 2A, 2B), indicating partial restoration of positive selection. Restoration in CD4+ thymocytes was associated with increased TCR levels (TCRβ) on, and enhanced signaling (CD5) in, DP thymocytes (Fig. 2C). SLAP deficiency has previously been shown to enhance positive selection in mice expressing the DO-11.10 TCR transgene (6). Enhanced positive selection of CD4+ thymocytes as well as increased CD5 and TCRβ expression in DP thymocytes were observed in DSSKO mice compared with those in SKG mice in the presence of the DO-11.10 TCR transgene (Supplemental Fig. 1A, 1B). Notably, more CD4+ thymocytes from DSSKO DO-11.10 mice expressed the TCR transgene supporting a role for SLAP in enhancing TCR signaling and selection of DP thymocytes (Supplemental Fig. 1C).
Figure 2.
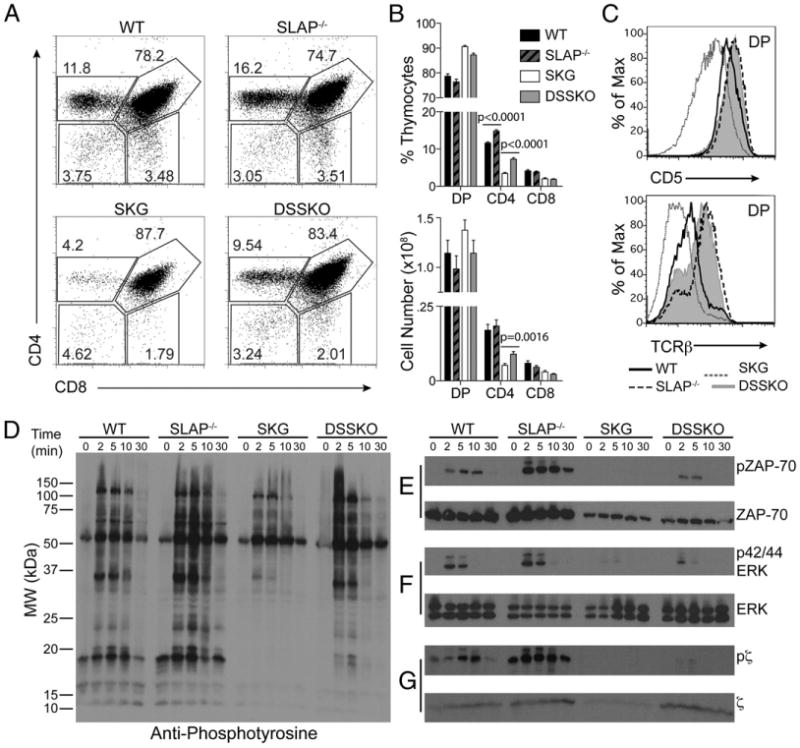
Enhanced thymocyte development and signaling in DSSKO mice. A, Thymocyte composition in WT, SLAP−/−, SKG, and DSSKO mice. B, Frequency and absolute number of thymocyte populations in each group as shown in A. C, CD5 and TCRβ expression on WT, SLAP−/−, SKG, and DSSKO DP thymocytes. Data represent the average of 13 to 17 mice per genotype (±SEM) from 5 independent experiments. D–G, Immunoblot of overall tyrosine phosphorylation (D), total and phospho-ZAP70 (E), total and phospho-p42/44 ERK (F), and total and phospho-TCRζ (G) upon TCR complex-mediated stimulation of total thymocytes from WT, SLAP−/−, SKG, and DSSKO mice. Data shown are from one mouse per genotype and representative of three independent experiments.
To investigate further alterations in TCR signal transduction in DSSKO mice, we evaluated tyrosine phosphorylation of TCR signaling molecules in thymocytes in response to stimulation through the TCR complex. After stimulation, global tyrosine phosphorylation was lowest in SKG thymocytes. In contrast, DSSKO thymocytes exhibited levels similar to WT (Fig. 2D). Lower steady-state levels of ZAP70 were observed in both SKG and DSSKO thymocytes. However, DSSKO thymocytes demonstrated more ZAP70 phosphorylation after stimulation (Fig. 2E). DSSKO thymocytes also displayed increased ERK phosphorylation compared with that in SKG mice, another indication of increased signaling through the TCR (Fig. 2F). Enhancement of ZAP70 and ERK phosphorylation supports the hypothesis that SLAP deficiency improves signaling through the TCR complex in SKG thymocytes. Surprisingly, TCRζ levels were decreased in SKG thymocytes (Fig. 2G). Thus, in SKG thymocytes, SLAP may have enhanced function, and its deficiency in DSSKO thymocytes led to increased TCRζ levels and normalized TCR signaling. Collectively, these data demonstrate that SLAP deficiency increases TCR levels on, and enhances signaling in, SKG DP thymocytes resulting in rescue of positive selection in SKG mice, which could contribute to arthritis prevention in DSSKO mice.
SLAP deficiency has minimal effects on peripheral T cells in naive SKG mice
Because SLAP deficiency enhanced positive selection and signaling in DSSKO thymocytes, we assessed whether it would also alter composition or function in the resulting pool of peripheral T cells. DSSKO mice displayed frequencies and absolute numbers of peripheral CD4+ and CD8+ T cells similar to those in SKG mice but decreased compared with those in WT and SLAP−/− controls (Supplemental Fig. 2A–C). Similar results were observed in mice expressing the DO-11.10 TCR transgene (Supplemental Fig. 3A–C).
SLAP protein is reexpressed in peripheral T cells upon stimulation with PMA and ionomycin or anti-CD3 and anti-CD28 (6) (Fig. 3F). To determine if SLAP reexpression in activated T cells affects function, CD4+ T cells were stimulated with anti-CD3 or anti-CD3 and anti-CD28 (Supplemental Fig. 2D). Decreased proliferation was observed in SKG CD4+ T cells compared with that in WT in response to anti-CD3 alone after both 48 and 72 h of stimulation, as well as when soluble anti-CD28 was present at the 48-h time point. In contrast, frequency of proliferation of DSSKO CD4+ T cells in response to both anti-CD3 and anti-CD3 plus anti-CD28 stimulation was similar to that of WT cells, with a significant difference observed only after 48 h of stimulation with anti-CD3 alone. Similar results were observed for IL-2 production (data not shown). Thus, despite the minimal impact of SLAP deficiency on the naive peripheral T cell composition, enhanced proliferation of SLAP −/− CD4+ T cells and improved proliferative capacity of DSSKO T cells indicates a role for SLAP in regulating TCR signaling in activated peripheral T cells. Despite evidence of increased thymic output, increased TCR transgene usage, and enhanced proliferative capacity, there was no significant difference between DSSKO and SKG mice in the repertoire of peripheral CD4+ or CD8+ T cells expressing particular Vβs (Supplemental Fig. 2E, 2F). These data suggest that SLAP deficiency does not significantly shift the TCR repertoire selected in DSSKO mice compared with that in SKG mice, which could account for the difference in arthritis induction between strains.
Figure 3.
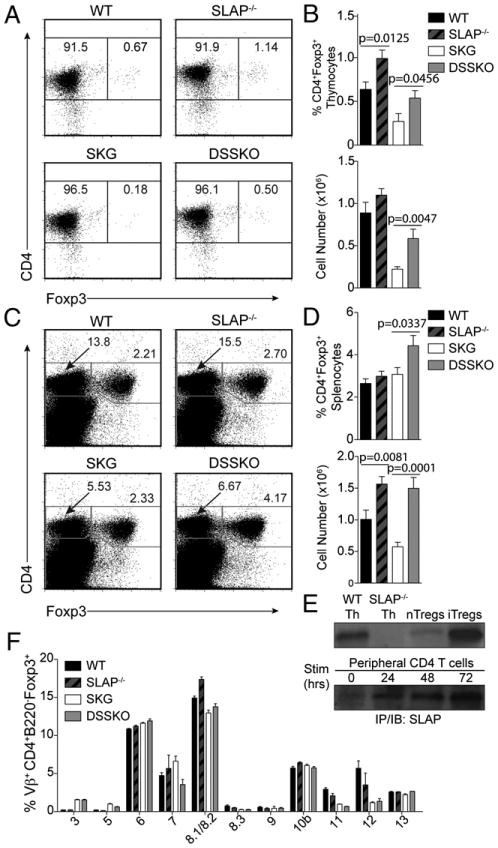
Increased numbers of thymic and splenic Tregs in DSSKO mice compared with those in SKG mice. A and C, Representative dot plots of thymic (A) and splenic (C) Tregs from WT, SLAP−/−, SKG, and DSSKO mice. B and D, Frequency and absolute number of thymic (B) and splenic (D) Tregs in each group as shown in A and C, respectively. Data represent the average of 11 mice per genotype (±SEM) from 3 independent experiments. E, SLAP expression assessed by immunoprecipitation and Western blotting on thymocytes from WT and SLAP−/− mice, and WT nTregs (isolated from pooled spleen and lymph node peripheral lymphocytes from BALB/c mice), iTregs, and peripheral T cells before and after stimulation with anti-CD3 and anti-CD28. F, Analysis of Vβ usage in lymph node CD4+Foxp3+ T cells from WT, SLAP−/−, SKG, and DSSKO mice. Data represent the average of nine mice per genotype (±SEM) from three independent experiments.
Increased numbers of Tregs in SLAP-deficient SKG mice
High-avidity TCR–peptide interactions increase thymic Treg numbers, supporting a role for TCR signal strength in Treg development (15–17). SLAP deficiency had no significant effect on the repertoire of peripheral T cells, suggesting that elimination of autoreactive T cells is not likely responsible for arthritis prevention or amelioration in DSSKO mice (Supplemental Fig. 2E, 2F). Thus, we examined whether SLAP deficiency increases development of Tregs capable of suppressing arthritogenic T cells. Indeed, both the frequency and absolute number of CD4+Foxp3+ Tregs was significantly increased in both thymus and spleen of DSSKO mice compared with those in SKG mice (Fig. 3A–D). In the spleen, DSSKO mice had approximately a 2- to 3-fold increase in the number of Tregs compared with that in SKG mice; DSSKO mice had a similar number of Tregs as that of WT and SLAP−/− mice (Fig. 3D). Despite differences in both thymic and splenic Treg numbers, the frequency of peripheral Tregs expressing particular TCR Vβs was similar between DSSKO and SKG mice with the exception of Vβ7 (Fig. 3E), whose relevance to arthritis suppression remains to be determined. These data suggest that the repertoire of the Tregs is not significantly altered in DSSKO mice compared with that in SKG mice.
The increased number of Tregs in SLAP−/− and DSSKO mice led us to determine whether SLAP is constitutively expressed in Tregs and if its deficiency contributes to increased Treg development. Tregs can develop either centrally in the thymus (natural Tregs; nTregs) or in the periphery upon T cell activation (iTregs) (18). Recently, expression of SLAP mRNA in various Treg populations has been shown by microarray analysis of Treg subphenotypes (Ref. 19; see http://cbdm.hms.harvard.edu/TregSubphenotypes/heatmap.html). Immunoprecipitation and immunoblot analysis revealed that SLAP protein is expressed in both nTregs and in vitro-generated iTregs (Fig. 3F). The decreased SLAP expression in nTregs compared with that in iTregs likely reflects fewer purified nTregs used for the immunoprecipitation and immunoblot analysis. Thus, SLAP is expressed in Tregs, and in its absence enhanced TCR signaling results in increased numbers of Tregs, which could suppress and/or ameliorate chronic arthritis in DSSKO mice.
Increased peripheral Tregs and decreased Th1 and Th17 cells in DSSKO mice after zymosan injection
Because we detected increased steady-state numbers of thymic and splenic Tregs in DSSKO mice, we assessed whether SLAP deficiency increases Tregs upon zymosan exposure, which could contribute to arthritis prevention or amelioration. Ten weeks after zymosan injection, WT, SLAP−/−, and SKG mice had similar frequencies of Tregs in both spleens and LNs, despite only SKG mice having severe arthritis (Fig. 4A, 4B, Supplemental Fig. 4A, 4B). In contrast, DSSKO mice had a significant expansion of Tregs with an almost 3-fold increase in peripheral Treg frequency (Fig. 4A, 4B, Supplemental Fig. 4A, 4B). However, because SKG mice had increased cellularity of both their spleens (2-fold) and LNs (8-fold) compared with that of DSSKO mice, likely due to inflammation associated with arthritis development in SKG mice, the difference in frequency did not translate into a significant increase in peripheral Treg numbers in DSSKO mice compared with that in SKG mice (Fig. 4B, Supplemental Fig. 4B, 4C).
Figure 4.
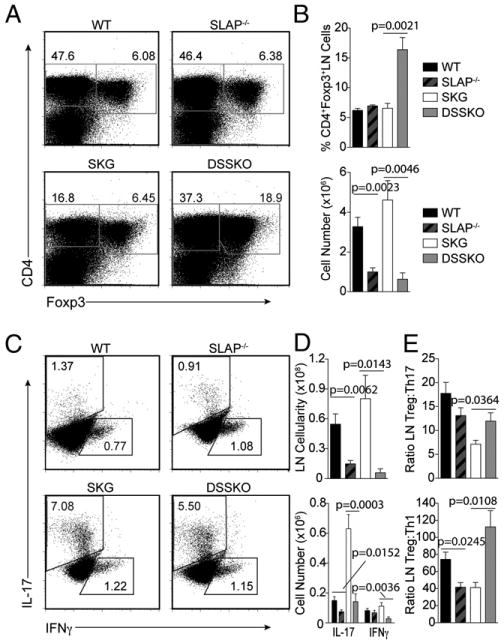
Increased numbers of Tregs and decreased numbers of Th1 and Th17 cells in DSSKO mice after zymosan exposure. A, Representative flow plots of Tregs in the LNs of WT, SLAP−/−, SKG, and DSSKO mice 10 wk after zymosan exposure. B, Frequency and absolute number of LN Tregs in each group as shown in A. C, Intracellular staining of IL-17 and IFN-γ in CD4+ cells from the LNs of WT, SLAP−/−, SKG, and DSSKO mice 10 wk after zymosan exposure. D, Frequency and absolute number of IL-17 and IFN-γ expressing CD4+ LN cells from each group as shown in C. E, Ratio of Tregs compared with Th17 or Th1 cells in zymosan-treated mice. Data represent the average of 7 to 10 mice per genotype (±SEM) from three independent experiments.
Zymosan-treated SKG mice have an increased frequency of Th17 effector T cells, required for arthritis development (5). Because DSSKO mice did not develop chronic arthritis, we examined whether the alterations in TCR signaling due to SLAP deficiency altered differentiation and function of peripheral effector T cells upon zymosan exposure. DSSKO mice had smaller LNs than those of SKG mice due to the lack of arthritis development and associated inflammation in draining LNs and exhibited decreased frequencies and absolute numbers of both Th17 and Th1 cells (Fig. 4C, 4D). Few, if any, IL-4–producing Th2 CD4+ T cells were detected in any strains 10 wk after zymosan treatment (data not shown). Thus, the cytokine milieu necessary for Th17 and Th1 cell development was present in the DSSKO mice, but there was a decrease in generation of effector T cells. These results also translated into a dramatic increase in the ratio of Tregs to both Th17 and Th1 cells (Fig. 4E). A recent report demonstrated protection from arthritis in SKG mice after treatment with nondepleting CD4 due to an increase in the ratio of Tregs to Th17 cells (20). Arthritis amelioration in DSSKO mice is likely due to increased steady-state numbers of Tregs and their expansion upon zymosan treatment without an accompanying expansion of autoreactive Th17 cells leading to alterations in the Treg to Th17 ratio, preventing chronic arthritis development.
Functional blockade of Tregs in DSSKO mice leads to development of arthritis
DSSKO mice have higher numbers of Tregs in both thymus and spleen compared with those in naive SKG mice (Fig. 3) and an expansion of Tregs upon zymosan exposure (Fig. 4A, 4B, Supplemental Fig. 4). Decreasing the number of Tregs in SKG mice through neonatal thymectomy or adoptive transfer of CD4+CD25− T cells from SKG mice into nude mice accelerates the development and increases the severity of arthritis (21). However, whether increasing the number of Tregs could ameliorate arthritis remained to be determined. To test whether Tregs are required for suppression of arthritis in DSSKO mice, mice were treated with a mixture of depleting/blocking anti-CD25 Abs (PC6.1 and 3C7; Refs. 12–14). On days 5, 7, and 9 after zymosan injection, mice were treated with anti-CD25 Abs or control IgG. The onset and magnitude of arthritis induction was not altered in IgG-treated mice that received zymosan (Fig. 5A). Fifty percent (3 of 6) of the DSSKO IgG-treated mice developed a mild transient arthritis, with only one achieving an arthritis score over 0.5, a pattern similar to that observed in mice treated with zymosan alone (Fig. 1). In contrast, all DSSKO mice treated with the anti-CD25 mixture developed arthritis with onset and severity similar to that of SKG mice (Figs. 5A, 1B). Approximately 3 wk after the final anti-CD25 Ab injection, arthritis severity in DSSKO mice began to decrease with complete resolution in 78% (7 of 9) during the 10-wk observation period. At the end of the observation period, the frequency of peripheral Tregs in mice treated with the anti-CD25 mixture had returned to a level similar to that of IgG-treated mice for each of the strains (Supplemental Fig. 5). Development of arthritis in DSSKO mice after depletion of Tregs provides in vivo functional data that SLAP deficiency does not eliminate arthritogenic T cells in DSSKO mice and demonstrates that Tregs were responsible for the prevention of chronic arthritis. This is further supported by arthritis remission in the DSSKO mice once the anti-CD25 Abs were cleared. These data indicate that increasing the number and/or function of Tregs in SKG mice suppresses arthritis development. However, this experiment did not address whether the number of Tregs, the function of Tregs, or both were responsible for arthritis prevention.
Figure 5.
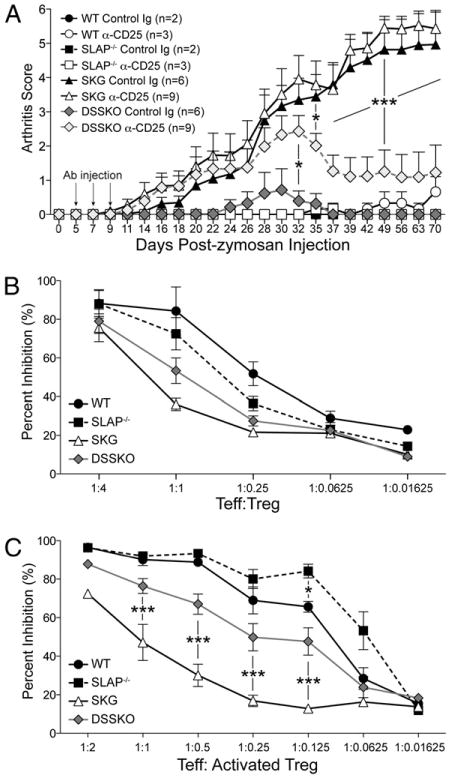
Functional blockade of Tregs in DSSKO mice leads to the development of arthritis. A, Arthritis development in mice injected i.v. with a mixture of anti-CD25 Abs or control rat Ig (black arrows) on days 5, 7, and 9 after zymosan treatment. Data represent the average arthritis score (±SEM) from three independent experiments for SKG and DSSKO mice. Data for WT and SLAP knockout mice are from one experiment. B and C, CFSE-labeled WT CD4+CD25− peripheral T cells were stimulated with anti-CD3 and anti-CD28 in the presence of the indicated ratios of either freshly isolated (B) or activated (C) CD4+CD25+ Tregs from WT, SLAP−/−, SKG, or DSSKO mice and proliferation determined by dilution of CFSE using flow cytometry. Data represent the average of three mice per genotype (±SEM) from three independent experiments.
To determine if SLAP deficiency enhances the suppressive capacity of Tregs, we performed in vitro suppression assays using either freshly isolated or in vitro-activated CD4+CD25+ Tregs from WT, SLAP−/−, SKG, and DSSKO mice to suppress proliferation of WT CD4+CD25− effector T cells in vitro (Fig. 5B, 5C). Activation of Tregs by TCR stimulation augments their suppressive capacity 4- to 6-fold compared with that of freshly isolated Tregs (22) and may reflect the situation in activated Tregs from zymosan-injected mice. Freshly isolated Tregs from all four strains were capable of inducing a similar dose-dependent inhibition of proliferation (Fig. 5B). Activated WT, SLAP−/−, and DSSKO Tregs exhibited enhanced patterns of inhibition, in contrast to SKG Tregs, which had nearly identical capacity to suppress as that of the freshly isolated Tregs (Fig. 5C). Four-fold more activated SKG Tregs were required to achieve the same level of inhibition as that of DSSKO Tregs. Notably, activated SLAP−/− Tregs exhibited the greatest suppressive capacity, with a similar level of inhibition obtained using 4-fold fewer SLAP−/− Tregs compared with WT Tregs. This provides compelling evidence that SLAP deficiency increases the suppressive capacity of activated Tregs. Collectively, these data demonstrate a Treg intrinsic role for SLAP and signaling through the TCR in determining both number and function of Tregs and suggest that the increased number of Tregs and their enhanced suppressive capacity both contribute to chronic arthritis prevention.
A model depicting how SLAP deficiency affects development and effector function of T cells in SKG mice is illustrated in Fig. 6.
Figure 6.
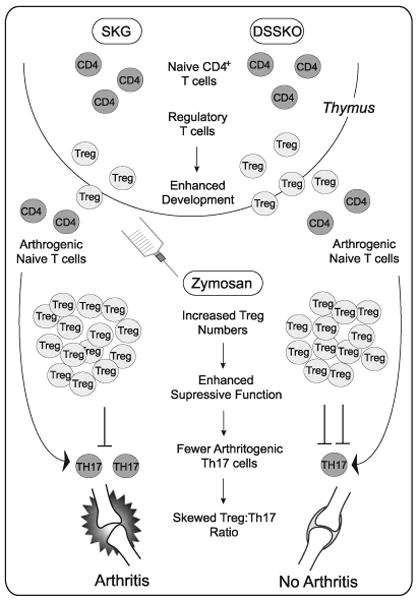
Model depicting how SLAP deficiency affects development and effector function of T cells in SKG mice. In the thymus, SLAP deficiency has a minimal effect on selection of CD4+ T cells yet significantly enhances the development of nTregs. This leads to DSSKO mice having a pool of peripheral T cells with specificity or autoreactive potential similar to that of an SKG mouse, but more of which are Tregs. In SKG mice, both Tregs and autoreactive Th17 cells expand upon zymosan exposure. However, the Tregs are not sufficient to control the autoreactive Th17 cells, and arthritis develops. In contrast, in DSSKO mice zymosan exposure leads to a greater expansion of Tregs that have enhanced suppressive capacity. Increased expansion and function of Tregs in DSSKO mice accompanied by a diminished expansion of autoreactive Th17 cells renders the Tregs sufficient to control the autoreactive T cells, preventing the development of arthritis.
Discussion
In this study, we show that SLAP deficiency increased TCR complex signaling and prevented development of chronic auto-immune arthritis in SKG mice treated with zymosan. SLAP deficiency partially rescues positive selection and has a minimal effect on negative selection of thymocytes in naive SKG mice; the most dramatic effect was the increased number of Tregs in both thymus and spleen of naive DSSKO mice. After zymosan treatment, DSSKO mice had a further expansion of peripheral Tregs but did not have the increase in effector Th17 cells seen in SKG mice, thus preventing arthritis development (Fig. 6). Depletion and/or functional blockade of Tregs in zymosan-treated mice unleashed the autoreactive T cells resulting in development of arthritis in the DSSKO mice. In vitro, DSSKO Tregs displayed enhanced suppressive capacity upon TCR activation. Taken together, these data demonstrate that increasing TCR signal strength can enhance development and function of Tregs and prevent development of autoimmunity.
SLAP is a negative regulator of TCR signaling that adapts the E3 ubiquitin ligase c-Cbl to the ζ-chain of the TCR (TCRζ), targeting it for degradation (6–8). SLAP is highly expressed in DP thymocytes (6). Thus, our initial predictions on how SLAP deficiency would affect selection and development of T cells were based primarily on the thymus. The original prediction was that SLAP deficiency would enhance negative selection of autoreactive thymocytes thus eliminating arthritogenic thymocytes in SKG mice. Surprisingly, the data show a minimal effect of SLAP deficiency in negative selection and support a more significant role for SLAP in agonist selection of Tregs in the thymus. However, SLAP expression is dynamic in peripheral T cells with upregulation occurring upon activation (6) (Fig. 3F). In addition, a recent report demonstrated that signals delivered hours after T cell activation are capable of influencing T cell function. Upregulation of Crtam (an MHC class I-restricted T cell-associated molecule) on a subset of peripheral CD4+ T cells more than 6 h after TCR activation was shown to control polarity, proliferation, and cytokine secretion, thus affecting adaptive immunity to infection (23). Thus, we examined the role of SLAP deficiency in peripheral T cells upon activation. In vivo SLAP deficiency altered the differentiation and/or proliferation of both Th17 cells and Tregs, skewing the ratio between the two in favor of Tregs. In vitro SLAP deficiency enhanced both the proliferation of CD4+ T cells and the suppressive capacity of CD4+CD25+ Tregs after stimulation through the TCR complex. Our data provide the first evidence that SLAP plays a role in autoimmune disease pathogenesis and that regulated expression of SLAP upon TCR activation in peripheral T cells plays a role in determining T cell responses. These responses include effector differentiation of Th17 cells, differentiation and/or proliferation of Tregs, the suppressive capacity of Tregs, and the balance between Tregs and effector Th17 cells.
ZAP70 plays a critical role in TCR signaling during both thymocyte selection and in mature T cell responses (24). Hypomorphic mutations of ZAP70 have been generated that span the spectrum of immune dysregulation from autoantibody production to immunodeficiency (3, 21, 25, 26). A recent study on one of these mutants suggested that reduction in the number and function of Tregs was not sufficient for arthritis to develop (26). Mice with the YYAA mutation of ZAP70 had fewer Tregs with diminished suppressive capacity compared with that of SKG Tregs but failed to develop arthritis after zymosan injection. However, there were significant differences in the repertoire of T cells between these mice, with the likely elimination of many arthritogenic cells as well, thus preventing the development of arthritis. The effects of changing the copy number and/or functional capacity of ZAP70 was examined in a recent report in which the intensity of signaling through the TCR complex was altered by using different combinations of WT, SKG, or null alleles of ZAP70 (21). This allelic series of ZAP70 mutations demonstrated that alterations in TCR signaling can affect thymic selection, effector function of peripheral T cells, and development or function of Tregs. These studies have extended our understanding, but it remains to be determined whether the affected processes cause or prevent autoimmunity independently or only in combination. Although differences in number and/or function of Tregs were observed, prevention of autoimmune arthritis in ZAP70 mutants was always associated with a shift in the T cell repertoire compared with that of SKG, as was development of spontaneous arthritis in the SKG/null mice (26, 21).
Intellectually, the logic behind the aforementioned experiments and those presented in this study was the same with the prediction that increasing signaling through the TCR complex would lead to enhanced negative selection of arthritogenic T cells with a potential increase in Tregs resulting in prevention of arthritis in SKG mice. Yet, strikingly different mechanisms were responsible for prevention of arthritis in these studies. Both mice heterozygous for the SKG allele of ZAP70 and mice homozygous for the YYAA allele of ZAP70 have a different repertoire of T cells containing fewer arthritogenic T cells thus preventing the development of arthritis, regardless of the increased or decreased number and/or function of Tregs, respectively. In contrast, instead of preventing arthritis by eliminating the arthritogenic T cells, DSSKO mice possess a TCR repertoire similar to that of SKG mice that is held in check by more Tregs with enhanced suppressive capacity. Collectively, these studies demonstrate that the balance between induction of and protection from autoimmune disease can be affected by altering the sensitivity of autoreactive thymocytes to selection, by changing their effector function, and by altering the development and function of Tregs (21, 26). In addition, these studies demonstrate that altering TCR signaling not only changes the repertoire and function of peripheral effector T cells and Tregs but also the balance between the two. Preventing autoimmunity by altering thymic selection, as was likely the case in YYAA and SKG heterozygous mice, is not possible in a patient with overt autoimmunity. However, increasing the number or function of Tregs, as in DSSKO mice, might be.
Several non-mutually exclusive mechanisms could explain how SLAP deficiency in the context of SKG affects Treg numbers and function, preventing or ameliorating arthritis. First, enhanced signaling in thymocytes could lead to more nTregs. Second, enhanced signaling in activated peripheral T cells could improve their capacity to convert into iTregs. Third, altered signaling upon zymosan exposure could enhance the suppressive capacity of Tregs. Fourth, increasing the stability of Tregs could affect both their number and function, as it has been shown that Tregs can lose Foxp3 expression, loss of which eliminated their suppressive capacity (27). In addition, alterations in TCR signaling could affect survival and/or expansion of Tregs. Further experiments are under way to examine the effects of SLAP deficiency on these processes in nTregs, as well as whether SLAP deficiency also affects these processes in iTregs, as most of the data on Tregs in this report focuses on nTregs.
Enhancement of both number and function of Tregs as a consequence of increased TCR complex-mediated signaling has important implications for the treatment of autoimmunity. Accumulating evidence implicates Tregs as a therapeutic target for autoimmunity, but the lack of good manufacturing procedures for Ag-specific Tregs limits the translation of this approach to the clinic (28). Technical hurdles to the manufacturing process include the identification of suitable target Ags, purity of Tregs versus potentially autoreactive T cells as Foxp3 is intracellular, and that individualized generation of Ag-specific Tregs may be cost prohibitive (29). Thus, stimulating endogenous Tregs to increase in number and/or efficacy by qualitatively enhancing TCR complex-mediated signaling in a manner similar to SLAP deficiency represents a more practical and useful alternative. Greater understanding of the effects of SLAP deficiency on the generation and function of Tregs will provide insight into the pathogenesis of autoimmunity, as well as information on how Tregs might be generated and/or manipulated to enhance their function in the treatment of autoimmunity.
Supplementary Material
Acknowledgments
We thank M. Taussig for technical assistance and P. Marrack, J. Kemp, and A. Weiss for critical reading of the manuscript.
This work was supported by a Within Our Reach grant from the American College of Rheumatology (to L.L.D.), an Arthritis Foundation postdoctoral fellowship (to L.K.P.), and by National Institutes of Health Grant AI-77609 (to E.W.G.).
Abbreviations used in this article
- DP
double-positive
- DSSKO
double SKG SLAP knockout
- iTreg
inducible Treg
- LN
lymph node
- nTreg
natural Treg
- SLAP
Src-like adapter protein
- Treg
regulatory T cell
- WT
wild type
Footnotes
The online version of this article contains supplemental material.
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Liston A, Enders A, Siggs OM. Unravelling the association of partial T-cell immunodeficiency and immune dysregulation. Nat Rev Immunol. 2008;8:545–558. doi: 10.1038/nri2336. [DOI] [PubMed] [Google Scholar]
- 2.Zikherman J, Weiss A. Antigen receptor signaling in the rheumatic diseases. Arthritis Res Ther. 2009;11:202–210. doi: 10.1186/ar2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, Sakihama T, Matsutani T, Negishi I, Nakatsuru S, Sakaguchi S. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 4.Yoshitomi H, Sakaguchi N, Kobayashi K, Brown GD, Tagami T, Sakihama T, Hirota K, Tanaka S, Nomura T, Miki I, et al. A role for fungal beta-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J Exp Med. 2005;201:949–960. doi: 10.1084/jem.20041758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sosinowski T, Killeen N, Weiss A. The Src-like adaptor protein downregulates the T cell receptor on CD4+CD8+ thymocytes and regulates positive selection. Immunity. 2001;15:457–466. doi: 10.1016/s1074-7613(01)00195-9. [DOI] [PubMed] [Google Scholar]
- 7.Myers MD, Dragone LL, Weiss A. Src-like adaptor protein down-regulates T cell receptor (TCR)-CD3 expression by targeting TCRzeta for degradation. J Cell Biol. 2005;170:285–294. doi: 10.1083/jcb.200501164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers MD, Sosinowski T, Dragone LL, White C, Band H, Gu H, Weiss A. Src-like adaptor protein regulates TCR expression on thymocytes by linking the ubiquitin ligase c-Cbl to the TCR complex. Nat Immunol. 2006;7:57–66. doi: 10.1038/ni1291. [DOI] [PubMed] [Google Scholar]
- 9.Dragone LL, Myers MD, White C, Gadwal S, Sosinowski T, Gu H, Weiss A. Src-like adaptor protein (SLAP) regulates B cell receptor levels in a c-Cbl-dependent manner. Proc Natl Acad Sci USA. 2006;103:18202–18207. doi: 10.1073/pnas.0608965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fantini MC, Dominitzki S, Rizzo A, Neurath MF, Becker C. In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat Protoc. 2007;2:1789–1794. doi: 10.1038/nprot.2007.258. [DOI] [PubMed] [Google Scholar]
- 11.Dragone LL, Myers MD, White C, Sosinowski T, Weiss A. SRC-like adaptor protein regulates B cell development and function. J Immunol. 2006;176:335–345. doi: 10.4049/jimmunol.176.1.335. [DOI] [PubMed] [Google Scholar]
- 12.Couper KN, Blount DG, de Souza JB, Suffia I, Belkaid Y, Riley EM. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. J Immunol. 2007;178:4136–4146. doi: 10.4049/jimmunol.178.7.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelchtermans H, De Klerck B, Mitera T, Van Balen M, Bullens D, Billiau A, Leclercq G, Matthys P. Defective CD4+CD25+ regulatory T cell functioning in collagen-induced arthritis: an important factor in pathogenesis, counter-regulated by endogenous IFN-gamma. Arthritis Res Ther. 2005;7:R402–R415. doi: 10.1186/ar1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHugh RS, Shevach EM. Cutting edge: depletion of CD4+ CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002;168:5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 15.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 16.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J, Yamamoto K. Generation of CD4(+)CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002;168:4399–4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 18.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci USA. 2010;107:5919–5924. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duarte J, Agua-Doce A, Oliveira VG, Fonseca JE, Graca L. Modulation of IL-17 and Foxp3 expression in the prevention of autoimmune arthritis in mice. PLoS ONE. 2010;5:e10558. doi: 10.1371/journal.pone.0010558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka S, Maeda S, Hashimoto M, Fujimori C, Ito Y, Teradaira S, Hirota K, Yoshitomi H, Katakai T, Shimizu A, et al. Graded attenuation of TCR signaling elicits distinct autoimmune diseases by altering thymic T cell selection and regulatory T cell function. J Immunol. 2010;185:2295–2305. doi: 10.4049/jimmunol.1000848. [DOI] [PubMed] [Google Scholar]
- 22.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 23.Yeh JH, Sidhu SS, Chan AC. Regulation of a late phase of T cell polarity and effector functions by Crtam. Cell. 2008;132:846–859. doi: 10.1016/j.cell.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 25.Siggs OM, Miosge LA, Yates AL, Kucharska EM, Sheahan D, Brdicka T, Weiss A, Liston A, Goodnow CC. Opposing functions of the T cell receptor kinase ZAP-70 in immunity and tolerance differentially titrate in response to nucleotide substitutions. Immunity. 2007;27:912–926. doi: 10.1016/j.immuni.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu LY, Tan YX, Xiao Z, Malissen M, Weiss A. A hypomorphic allele of ZAP-70 reveals a distinct thymic threshold for autoimmune disease versus autoimmune reactivity. J Exp Med. 2009;206:2527–2541. doi: 10.1084/jem.20082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207:1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh CS, Bautista JL. Sliding setpoints of immune responses for therapy of autoimmunity. J Exp Med. 2010;207:1819–1823. doi: 10.1084/jem.20101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


