Abstract
Infection of mice with murine gammaherpesvirus-68 (γHV-68) serves as a model to understand the pathogenesis of persistent viral infections, including the potential for co-infections to modulate viral latency. We have previously found that infection of neonates (8-day-old mice) with γHV-68 resulted in a high level of persistence of the virus in the lungs as well as the spleen, in contrast to infection of adult mice, for which long-term latency was only readily detected in the spleen. In this study we investigated whether stimulation of toll-like receptor (TLR)9 would modulate viral latency in mice infected with γHV-68 in an age-dependent manner. Pups and adult mice were injected with the synthetic TLR9 ligand CpG ODN at 30 dpi, at which time long-term latency has been established. Three days after CpG injection, the lungs and spleens were removed, and a limiting dilution assay was done to determine the frequency of latently infected cells. RNA was extracted to measure viral transcripts using a ribonuclease protection assay. We observed that CpG injection resulted in an increase in the frequency of latently-infected cells in both the lungs and spleens of infected pups, but only in the spleens of infected adult mice. No preformed virus was detected, suggesting that TLR9 stimulation did not trigger complete viral reactivation. When we examined viral gene expression in these same tissues, we observed expression only of the immediate early lytic genes, rta and K3, but not the early DNA polymerase gene or late gB transcript indicative of an abortive reactivation in the spleen. Additionally, mice infected as pups had greater numbers of germinal center B cells in the spleen following CpG injection, whereas CpG stimulated the expansion of follicular zone B cells in adult mice. These data suggest that stimulation of TLR9 differentially modulates gammaherpesvirus latency via an age-dependent mechanism.
Introduction
Gammaherpesviruses establish life-long latency in lymphocytes. Whether viral latency is modulated by stimulation of lymphocytes following subsequent heterologous infections is an area of intense investigation. Epidemiological evidence from studies of the human gammaherpesvirus Epstein-Barr virus (EBV), and the malaria parasite Plasmodium falciparum, suggests that co-infections modulate viral latency (26). P. falciparum contains a toll-like receptor (TLR)9 ligand, which consists of both malaria DNA and parasite hemozoin, that increases the efficiency by which malaria DNA is transported to the endosomal location of TLR9 (29,31). TLR9 is found on memory B cells in humans, where EBV is latent, and stimulation of TLR9 can result in B-cell expansion and/or plasma cell differentiation; in mice TLR9 is expressed on all B cells (17). We have proposed that one model for the interaction between EBV and malaria is through TLR9 stimulation of latently-infected memory B cells (35).
In vitro studies have found that HIV infection can trigger another human gammaherpesvirus, Kaposi's sarcoma-associated herpesvirus (KSHV), to reactivate from primary effusion lymphoma cell lines (25,42). Furthermore, lytic reactivation was found to occur following infection of latently-infected endothelial cells and fibroblasts with human cytomegalovirus (44). Using the murine gammaherpesvirus-68 (γHV-68) as a model for human gammaherpesvirus infection, it has previously been reported that splenocytes harboring latent virus can be induced to produce lytic virus by stimulation with anti-Ig, anti-CD40, or LPS (27). More recently this has been modeled in vivo using infection of mice with γHV-68, and subsequent injection of ligands for TLR4 and TLR9 (14).
In Africa, EBV infection occurs early in life and most are infected by the age of 2 years (2,26). Infection with P. falciparum can also occur in infants and young children. The age of the host then needs to be considered when modeling these host-pathogen interactions. Eight-day-old mice are equivalent to approximately a 11/2-year-old infant (12), and thus can be used to model how early age of infection with EBV influences viral pathogenesis. We have previously found that the pathogenesis of γHV-68 infection of mice is age-dependent, such that infection of pups (8-day-old mice) results in enhanced persistence of the virus in the lungs, whereas no viral DNA is detected in the lungs following infection of adult mice (32). In contrast, in both adult mice and pups, once latency is established in the B cells in the spleen, no differences in viral load are seen. However, the long-term effects of neonatal gammaherpesvirus infection on maintaining latency remain to be determined, including how subsequent heterologous infections may affect viral latency.
In this study, we asked whether injection of mice latently infected with γHV-68 with CpG-ODN, a known TLR9 ligand, would modulate latency. In addition, we asked if this modulation was different depending on the site of latency (lung or spleen), and the age of infection with γHV-68 (pup or adult). Our data suggest that there is a potential for heterologous infections through TLR9 stimulation to lead to abortive viral reactivation and transient expansion of the latently-infected cell pool, which has implications for the pathogenesis of gammaherpesvirus-associated diseases. Furthermore, our data indicate that external factors may affect viral latency in an age-dependent manner.
Materials and Methods
Cell lines and virus preparation
All cell culture reagents were purchased from Cellgro (Mediatech, Inc., Herndon, VA). Murine embryonic fibroblast (MEF) cells were grown in Dulbecco modified Eagle's medium (DMEM) supplemented with 4.5 g/L glucose, 1 mM sodium pyruvate, 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Owl monkey kidney (OMK) cells were grown in RPMI supplemented with 10% FBS, L-glutamine, penicillin, and streptomycin. γHV-68 virus stocks were grown as previously described (4).
Mice and infections
C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME), and bred in-house under specific pathogen-free conditions. Male or female mice were used at 8 d old (pups) or at 6–8 wk old (adults). The pups were infected intranasally with 400 pfu γHV-68 in 5 μL HBSS. Adult mice were infected with 4 × 104 pfu in 50 μL of HBSS. For in vivo experiments, latently-infected mice (30 dpi) were injected intravenously with either 10 nmol CpG (Coley Pharmaceutical Group, Wellesley, MA), or with HBSS. For all experiments, at least four mice were used per group, and each experiment was repeated twice.
Tissue preparation
Tissues were removed from the mice and either processed immediately or frozen at −80°C. Mouse lungs were perfused with 10 mL HBSS containing 10 mM ethylenediaminetetraacetic acid (EDTA) before removal. Lung tissues were then processed as previously described, with minor modifications (15). Briefly, the lungs were minced and digested in RPMI supplemented with 5% FBS, 2 mg/mL collagenase D, and 20 μg/mL DNase I for 90 min at 37°C with constant rocking. The cells were then filtered through a 70-μm cell strainer and red blood cells were lysed in a hypotonic solution. The cells were resuspended in 2 mL HBSS and overlaid onto 3 mL Ficoll-Paque PLUS (Amersham Biosciences, Piscataway, NJ), then centrifuged at 800 g for 20 min at room temperature. The cells were harvested from the interface and resuspended in DMEM containing 2.5% FBS, L-glutamine, penicillin, and streptomycin, for limiting dilution assay, or in phosphate-buffered saline containing 0.3% bovine serum albumin (Fisher Scientific, Pittsburgh, PA), and 10 mM HEPES buffer. Spleen cells were isolated as previously described (45).
DNA extraction and real-time quantitative PCR
All DNA extraction procedures were done using the QIAamp DNA Mini Kit, following the manufacturer's protocols for either cultured cells or crude lysates (Qiagen, Valencia, CA). Real-time quantitative PCR was performed using primers and probe against γHV-68 viral DNA as previously described (46). Mouse β-actin was used as an internal control with primers and probe as previously described (5). The reactions were performed using iQ Supermix (Bio-Rad Laboratories, Hercules, CA).
Viral titers of infected tissues
Murine embryonic fibroblasts were seeded in 2 mL in 12-well plates at a concentration of 1 × 105 cells/mL in DMEM. The cells were allowed to adhere overnight. Tissue samples that had been flash-frozen were thawed and weighed, then homogenized in 2 mL complete DMEM. Homogenates were diluted 1:10 for the highest concentration, and 1:10 serial dilutions were made. The remaining homogenates were stored at −80°C. For the plaque assay, the supernatants were aspirated off MEF monolayers, and homogenate dilutions were plated in 1 mL complete DMEM. The plates were incubated for 2 h at 37°C, and the plaque assay procedure was continued as previously described (4).
Limiting dilution assay
Murine embryonic fibroblasts were seeded in 200 μL in 96-well plates at a concentration of 5 × 104 cells/mL in DMEM containing 2.5% FBS (Invitrogen, Carlsbad, CA), L-glutamine, penicillin, and streptomycin. Spleen or lung cells were resuspended at a concentration of 106 cells/mL, and a limiting dilution assay was performed as previously described (41,45), with the exception that threefold serial dilutions were made. To determine the levels of preformed lytic virus, a duplicate aliquot of cells was subjected to three cycles of freeze-thaw, and the lysates were plated in 100 μL volume onto the MEF monolayers. The percentage of wells exhibiting cytopathic effect was calculated after 21 d.
Flow cytometry
Single-cell suspensions were prepared as described above, and all reagents and methods have been previously described (18), with the exception that the cells were first blocked using FcγIII/II receptor (BD Biosciences, San Jose, CA) on ice. The cells were stained with the following antibodies: PE-labeled B220 (BD Biosciences), FITC-labeled PNA (Vector Laboratories, Burlingame, CA), PerCP-Cy5.5-labeled CD19 (BioLegend, San Diego, CA), PE-Cy7-labeled CD23 (eBiosciences, San Diego, CA), and APC-labeled CD21 (BioLegend). The cells were collected on an LSRII flow cytometer (Becton Dickinson, Rutherford, NJ), and live cells were analyzed by gating on forward-side scatter. Data were acquired using FACSDIVA software (BD Biosciences), and the populations were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
RNA extraction and ribonuclease protection assay
RNA was extracted from tissue as previously described (8,36). Viral gene expression was determined using a riboprobe recognizing both lytic (K3, rta, M8, DNA polymerase, and gB), and latent (M2, M3, M9, M11, ORF73, and ORF74) γHV-68 transcripts (36). Riboprobe synthesis was driven by T7 bacteriophage RNA polymerase with [α-32P]UTP as the labeling nucleotide. The ribonuclease protection assay (RPA) and quantitation was done as previously described (18,36). Probe bands were visualized and quantified by a PhosphorImager 445 SI and ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Results
TLR9 stimulation leads to an increased frequency of latently-infected cells in the lungs and spleens of neonates, and the spleens of adult mice
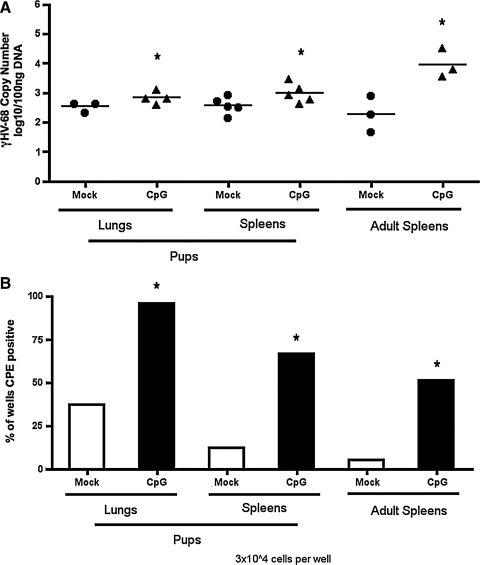
The use of synthetic CpG-ODN to stimulate TLR9 on B cells has been shown to lead to proliferation and differentiation into plasma cells (20–22,24). Because γHV-68 establishes latency in B cells (39), we wanted to determine whether CpG-ODN had an effect on viral load in B cells latently infected with γHV-68. We had previously reported that infection of pups with γHV-68 at 8 d old does not affect splenic latency 30 d after infection; however, infected pups fail to completely clear virus from the lungs, and maintain a latent infection (32). We therefore wanted to determine the effect that CpG injection had on latent virus in both pups and adult mice. Eight-day-old (pups) and 6- to 8-week-old (adults) C57BL/6 mice were infected intranasally with γHV-68. Thirty days later, the mice were injected intravenously with 10 nmol CpG 1826, and control mice were injected with PBS. Three days after injection, the lungs and spleens were removed and viral load was determined by quantitative PCR. Both pups and adult mice injected with CpG had higher viral copy numbers in the spleen than mock-infected mice (Fig. 1A). The pups also had an increased frequency of viral load in the lungs; however, no viral DNA was detected in the lungs of adult mice injected with either PBS or CpG (data not shown).
FIG. 1.
Injection of infected mice leads to a differential increase in viral load and the frequency of latently-infected cells in pups and adult mice. Mice were infected with γHV-68 and at 30 dpi were injected with 10 nmol CpG-ODN or with PBS as a control. Three days following injection, the spleens and lungs were removed. DNA was extracted from a portion, while the rest was processed to make a single-cell suspension. (A) DNA was extracted from whole spleen or lung tissues and viral load was measured by quantitative PCR. (B) The frequency of latently-infected cells that could reactivate virus was measured by limiting dilution assay (*p < 0.05 compared to mock-infected animals; CPE, cytopathic effect).
Because of the increase in viral load, we wanted to determine whether this was due to an increase in the frequency of latently-infected cells following CpG injection. Frequency was measured by a limiting dilution reactivation assay, in which cells were plated onto a monolayer of MEFs. The MEFs were analyzed after 3 wk for cytopathic effect (CPE), indicating reactivation from latency. We found that injection of CpG 1826 led to an increase in the frequency of latently-infected cells in the lungs and spleens of mice infected as neonates, as well as those infected as adults (Fig. 1B). To determine whether CPE was due to reactivated latent virus, we plated duplicate aliquots of cells that had been lysed, thereby preventing any reactivation from latency. Any CPE observed would therefore be due to preformed lytic virus. We did not detect any CPE in wells plated with lysates (data not shown), indicating that no preformed virus was present in the lysates, suggesting that CpG-ODN increased the frequency of latently-infected cells rather than inducing lytic replication.
CpG injection of latently-infected mice leads to viral gene expression in pups and adult mice
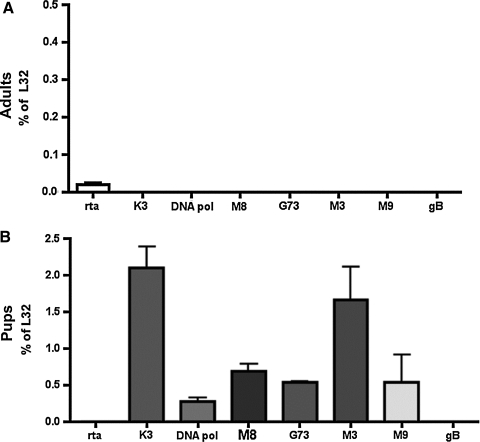
We next wanted to determine whether there were changes in viral gene expression associated with the increased number of latently-infected cells. To do this, we utilized an RPA that measured transcripts for both lytic (K3, rta, M8, DNA polymerase, and gB) and latency-associated (M2, M3, M9, M11, ORF73, and ORF74) transcripts. In the spleens of mice infected as adults, we detected expression of the viral gene rta (Fig. 2A), which is the viral transactivator that initiates reactivation to the lytic cycle (30,48,49). However, no other viral gene expression was detected, and rta expression was no longer detected at 6 d after CpG injection (data not shown), indicating an abortive reactivation. In contrast, in the spleens of mice infected as pups, we were unable to measure rta; however, we detected expression of a variety of γHV-68 genes associated with latent and lytic infection (Fig. 2B), including the early gene K3. Additionally, these genes were detected even though lytic reactivation was not observed. Because increased viral load and frequency of latently-infected cells were also seen in the lungs of mice infected as pups, we analyzed viral gene expression by RPA, but surprisingly we were unable to detect any viral gene expression in this tissue (data not shown).
FIG. 2.
Differential γHV-68 gene expression in mice infected as pups and as adults following injection with CpG. The mice were infected with γHV-68 and at 30 dpi they were injected with 10 nmol CpG-ODN. The spleens were removed 3 d after injection and RNA was extracted from whole tissue. Viral gene expression was measured by ribonuclease protection assay in (A) adult mice and (B) pups (DNA pol, DNA polymerase).
CpG injection of latently-infected mice results in differences in splenic B-cell populations between pups and adult mice
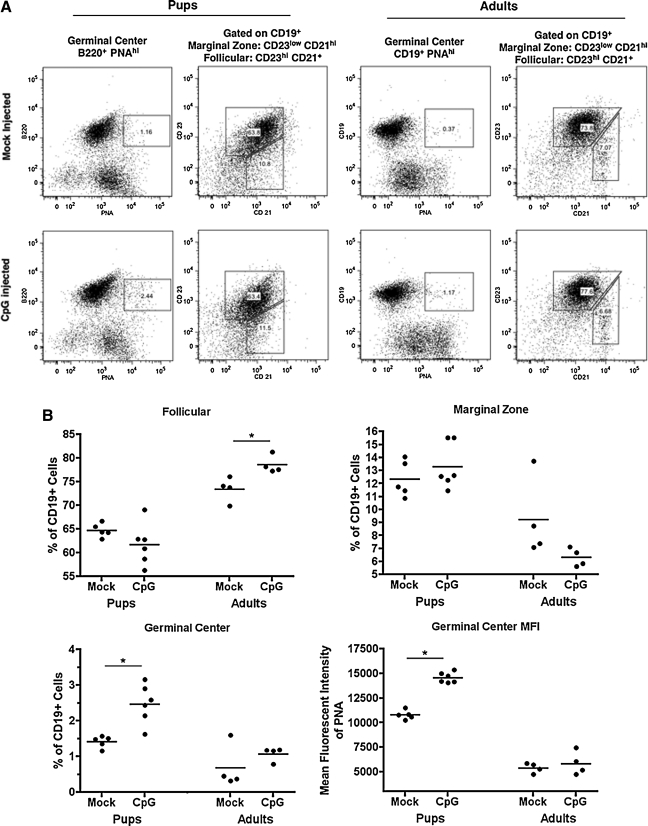
We found that the pool of latently-infected B cells is expanded in response to TLR9 stimulation; however, the phenotype of the expanded B-cell population is unknown. To determine how TLR9 stimulation affected B-cell phenotype in latently-infected mice, or if the age of infection altered the response, we determined the B-cell phenotype in the spleens of latently-infected pups and adults injected with PBS or CpG-ODN and harvested 3 d post-injection. The marginal zone (CD19+, CD23low, and CD21hi), follicular (CD19+, CD23hi, and CD21+), and germinal center (CD19+, or B220+, PNAhi) B-cell subsets were examined by flow cytometry, and for each subset the percentage of the total B-cell population was determined. Fig. 3A shows representative graphs for mice infected as pups or adults, and then injected with PBS or CpG; averages are shown in Fig. 3B. There was a significant increase in the percentage of germinal center B cells in CpG-treated compared to PBS control mice that were infected as pups, but this increase was not seen in mice infected as adults. Additionally, PNA expression on B cells from mice infected as pups was higher following CpG injection, as measured by mean fluorescence intensity, indicating increased activation, whereas this increase was not noted in mice infected as adults. In contrast, the adult mice injected with CpG showed an increase in follicular B cells that was not seen in pups. No differences were seen in marginal zone B cells in either age group.
FIG. 3.
Mice infected as pups or adults have different proportions of B-cell subsets in the spleen following injection with CpG. Eight-day-old mice were infected with γHV-68 and at 30 dpi were injected with 10 nmol CpG-ODN. After 3 d the spleens were removed and put into a single-cell suspension. The cells were stained with either FITC-PNA and PE-anti B220, or PerCP/Cy5.5-CD19, PE/Cy7-CD23, and APC-CD21, to determine the B-cell phenotype. (A) Representative flow cytometry plots of B-cell subsets in mice infected as pups or adults. (B) Percentage of germinal center, follicular zone, and marginal zone B cells and mean fluorescent intensity of PNA on germinal center B cells (control: n = 4, CpG: n = 5; *p < 0.05 compared to mock-injected animals).
Discussion
In this study, we used a mouse model to investigate how polymicrobial interactions can affect gammaherpesvirus persistence. The interaction between EBV, a human gammaherpesvirus, and P. falciparum malaria has been hypothesized to alter viral latency in children in equatorial Africa (7,26). We used γHV-68 infection of mice as a model for latent herpesvirus infection. Because P. falciparum DNA contains CpG motifs, a TLR9 ligand, we used CpG-ODN to study how TLR9 stimulation affects gammaherpesvirus pathogenesis. We found that injection of CpG into latently-infected mice leads to differences in viral gene expression between the spleens of neonates and adult mice, suggesting an age-dependent effect of CpG on γHV-68 latency.
Stimulation of human B cells via TLR9 leads to proliferation and differentiation into plasma cells (20–22,24). When latently-infected B cells differentiate into plasma cells, the EBV lytic cycle reactivates (23). We have also found that stimulating murine B cells with CpG ODN in vitro leads to proliferation and plasma cell differentiation (data not shown). Thus we hypothesized that CpG injection of latently-infected mice would reactivate virus to the lytic cycle. However, while we were able to measure an increase in the frequency of latently-infected cells following CpG injection of infected mice, we did not observe any lytic reactivation in vivo. Recently it has been reported that plasma cell differentiation only occurs in vitro following TLR9 stimulation, but does not occur in vivo (33). Indeed, we did observe an increase in the frequency of latently-infected cells that can reactivate virus when those cells were cultured in vitro. Others have also demonstrated in vitro reactivation using LPS (a TLR4 ligand) and cross-linking of Ig and CD40 (14,27). Previously, none of these agents have been known to induce reactivation in vivo. However, it was recently demonstrated that injection of LPS and CpG into latently-infected mice leads to an increase in the frequency of latently-infected cells, as well as viral reactivation (14). It is unclear why Gargano et al. (14) were able to induce lytic reactivation in vivo while we were not; it may be that the route of inoculation affected the capacity of CpG to reach latently-infected cells. They injected CpG intraperitoneally, while we injected CpG intravenously. Intraperitoneal injection could have resulted in reactivation of γHV-68 in peritoneal macrophages. Alternatively, a different class of CpG-ODN may have been used in their studies; we used a class B CpG-ODN, which has been shown to drive B-cell proliferation (21,43). It is also possible that the slight difference in the weight of mice infected as pups or infected as adults may have altered the dose response to CpG. Additionally, there were differences in the dose of virus and volume of inoculum used. We used a larger volume (50 μL) to inoculate the mice, compared to 20 μL used by Gargano et al. Differences in the volume of inoculation can affect how viruses induce disease, with smaller volumes inducing an upper respiratory tract infection, and larger volumes inducing a lower respiratory tract infection (38). This can alter the pathogenesis of the virus, and may affect the establishment and maintenance of latency, as well as reactivation.
Further evidence for the importance of immune control of viral reactivation in vivo comes from our observation that CpG injection leads to rta expression in the spleens of mice latently-infected as adults. This expression is transient, and is lost by 6 d post-injection. rta is the viral transactivator needed to reactivate latent virus and initiate the lytic cycle (30,48,49), but because we do not observe lytic reactivation in vivo, this suggests that an abortive infection occurred. Interestingly, TLR9 stimulation activates NF-κB, which suppresses rta expression (3,34). This may be another reason why rta expression is lost 6 d after CpG injection. The age-dependent differences in viral gene expression that we observed could likely stem from the fact that mice infected with γHV-68 as pups are not likely to have the same degree of protective immunity as mice infected as adults. This is supported by our earlier observations that there is a higher level of viral persistence in the lungs when mice are infected early in life (32). In addition, others have found that γHV-68 reactivation does not readily occur in an immunocompetent host (4). It is unclear why we didn't see rta in the spleens of mice infected as pups, yet we did detect other lytic transcripts. One possibility could be that the level of rta expression was below the level of detection. However, since we did not observe any preformed virus in this tissue, our results are more consistent with an abortive lytic reactivation.
We found a dramatic increase in the frequency of latently-infected cells in the lungs of mice infected as pups. Because TLR9 expression is increased on memory cells (1), if these cells harbor latently-infected virus, they would be a target for proliferation induced by CpG, thereby increasing the overall frequency of latently-infected cells. Therefore, it would be of interest to know the cell type infected in the lungs of pups. Recently, it has been noted that TLR9 is important in the antiviral immune response to γHV-68, and that this response is organ-specific (16). We have previously reported that immature dendritic cells are a target for γHV-68 infection in the lungs, and that these cells also express TLR9 (19). Thus it may be that CpG injection into mice modulates viral latency in an organ-specific manner. Interestingly, even though we observed an increased frequency of latently-infected cells in the lungs, we did not detect any viral gene expression. It is possible that the ORF73 transcript, which is necessary for episomal amplification (13), was transiently expressed or expressed below the level of detection of the RPA assay.
We observed that CpG injection into mice latently infected with γHV-68 leads to the expansion of different populations of B cells, depending on the age at which the mouse was infected. Indeed, not only were different subsets increased following injection of CpG in pups and adult mice, we also observed that different subsets of B cells were more prevalent, even in mock-infected mice. This suggests that the age of infection affects the proportion of latently-infected B-cell subsets in the spleen, which may lead to one subset being preferentially stimulated by CpG. CpG is surprisingly potent, such that no differences are seen in its effects on B cells when given at a 10- to 50-fold range of doses (9). But it is possible that one reason for the differences in response we observed between the two groups of mice was that the actual dose of CpG per gram of mouse had a differential effect on the response to CpG. Additionally, the immune response to γHV-68 can be driven in part by the presence of TLR9 on cells. It has been demonstrated that mice deficient in TLR9 have a greater viral load in the spleen following intraperitoneal, but not intranasal, infection, compared to WT mice (16). Although we infected mice intranasally, this potentially direct role for TLR9 in the immune response to γHV-68 may be important when TLR9 on infected B cells is stimulated with CpG. Preferential stimulation of different subsets may lead to changes in viral latency. Alternatively, the B-cell population initially infected with γHV-68 could be different in pups and adult mice. This may partially explain the differences in viral gene expression seen in the spleen between the two age groups following CpG injection. The germinal center reaction seen in the spleens of mice infected as pups could be active due to the persistence and altered kinetics of infection that lead to a lower threshold of activation when CpG is injected, and therefore an increase in germinal center B cells in pups but not adults. Whether this is associated with an increase in Ig levels in the blood is unknown.
In our model, we used infection of 8-day-old pups to mimic infection of infants less than 1.5 years old with EBV. The lungs of neonates continue to develop after birth, and inflammation during this stage of development could cause damage to the tissue. It is well established that neonates do not mount a strong proinflammatory response to viral infection; in the neonatal lung, the environment is unable to support an inflammatory response (15). It has been shown that loss of the CD8+ T-cell response in adult mice leads to viral persistence in the lungs (11), and we have found that neonates infected with γHV-68 have fewer CD8+ T cells in the lungs (Ptaschinski and Rochford, unpublished observations). The IFN-γ response is also suppressed in neonates, while the production of Th-2 cytokines is increased (37,47). Because of the decreased inflammatory response in the lungs of neonatal mice in response to viral infection, it is likely that mice infected as neonates do not develop a proper memory response, and thus the immune response is slower to respond following injection of CpG into latently-infected pups. Furthermore, immune tolerance to viral antigens has been reported in HBV (6) and LCMV (28,40), when infection occurs early in life.
Treatment with the TLR9 ligand mimics one aspect of P. falciparum infection, namely signaling of B cells through TLR9 via P. falciparum hemozoin/DNA complexes. Although it has been reported that P. falciparum can induce viral reactivation in children suffering from an episode of acute malaria (10), this was shown in an in vitro system to be due to another component of P. falciparum—the PfEMP-1 protein—which acts as a polyclonal activator of B cells (7). Thus the absence of lytic reactivation in our in vivo model system is consistent with the results of Chene et al., and suggests that it is likely that TLR9 signaling is not responsible for the increased viral DNA seen in children with acute malaria. In summary, our data suggest that the age of primary infection with a gammaherpesvirus can alter the subsequent response of latently-infected cells to heterologous infections. This has important implications for gammaherpesvirus pathogenesis, as in developing countries most people are infected early in life.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bernasconi NL. Onai N. Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 2.Biggar RJ. Henle G. Bocker J. Lennette ET. Fleisher G. Henle W. Primary Epstein-Barr virus infections in African infants. II. Clinical and serological observations during seroconversion. Int J Cancer. 1978;22:244–250. doi: 10.1002/ijc.2910220305. [DOI] [PubMed] [Google Scholar]
- 3.Brown HJ. Song MJ. Deng H. Wu TT. Cheng G. Sun R. NF-kappaB inhibits gammaherpesvirus lytic replication. J Virol. 2003;77:8532–8540. doi: 10.1128/JVI.77.15.8532-8540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardin RD. Brooks JW. Sarawar SR. Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casteels K. Waer M. Laureys J. Valckx D. Depovere J. Bouillon R. Mathieu C. Prevention of autoimmune destruction of syngeneic islet grafts in spontaneously diabetic nonobese diabetic mice by a combination of a vitamin D3 analog and cyclosporine. Transplantation. 1998;65:1225–1232. doi: 10.1097/00007890-199805150-00014. [DOI] [PubMed] [Google Scholar]
- 6.Chen M. Sallberg M. Hughes J, et al. Immune tolerance split between hepatitis B virus precore and core proteins. J Virol. 2005;79:3016–3027. doi: 10.1128/JVI.79.5.3016-3027.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chene A. Donati D. Guerreiro-Cacais AO, et al. A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathog. 2007;3:e80. doi: 10.1371/journal.ppat.0030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomczynski P. Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 9.Davis HL. Weeratna R. Waldschmidt TJ. Tygrett L. Schorr J. Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 10.Donati D. Espmark E. Kironde F, et al. Clearance of circulating Epstein-Barr virus DNA in children with acute malaria after antimalaria treatment. J Infect Dis. 2006;193:971–977. doi: 10.1086/500839. [DOI] [PubMed] [Google Scholar]
- 11.Ehtisham S. Sunil-Chandra NP. Nash AA. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J Virol. 1993;67:5247–5252. doi: 10.1128/jvi.67.9.5247-5252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flurkey K. Currer JM. Harrison DE. Mouse models in aging research. In: Fox JG, editor; Barthold S, editor; Davisson M, editor; Newcomer CE, editor; Quimby FW, editor; Smith A, editor. The Mouse in Biomedical Research; Normative Biology, Husbandry, and Models. Academic Press; New York: 2006. pp. 644–645. [Google Scholar]
- 13.Fowler P. Marques S. Simas JP. Efstathiou S. ORF73 of murine herpesvirus-68 is critical for the establishment and maintenance of latency. J Gen Virol. 2003;84:3405–3416. doi: 10.1099/vir.0.19594-0. [DOI] [PubMed] [Google Scholar]
- 14.Gargano LM. Forrest JC. Speck SH. Signaling through toll-like receptors induces murine gammaherpesvirus 68 reactivation in vivo. J Virol. 2009;83:1474–1482. doi: 10.1128/JVI.01717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garvy BA. Qureshi MH. Delayed inflammatory response to Pneumocystis carinii infection in neonatal mice is due to an inadequate lung environment. J Immunol. 2000;165:6480–6486. doi: 10.4049/jimmunol.165.11.6480. [DOI] [PubMed] [Google Scholar]
- 16.Guggemoos S. Hangel D. Hamm S. Heit A. Bauer S. Adler H. TLR9 contributes to antiviral immunity during gammaherpesvirus infection. J Immunol. 2008;180:438–443. doi: 10.4049/jimmunol.180.1.438. [DOI] [PubMed] [Google Scholar]
- 17.Gururajan M. Jacob J. Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One. 2007;2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobbs MV. Weigle WO. Noonan DJ, et al. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993;150:3602–3614. [PubMed] [Google Scholar]
- 19.Hochreiter R. Ptaschinski C. Kunkel SL. Rochford R. Murine gammaherpesvirus-68 productively infects immature dendritic cells and blocks maturation. J Gen Virol. 2007;88:1896–1905. doi: 10.1099/vir.0.82931-0. [DOI] [PubMed] [Google Scholar]
- 20.Jegerlehner A. Maurer P. Bessa J. Hinton HJ. Kopf M. Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007;178:2415–2420. doi: 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- 21.Jiang W. Lederman MM. Harding CV. Rodriguez B. Mohner RJ. Sieg SF. TLR9 stimulation drives naive B cells to proliferate and to attain enhanced antigen presenting function. Eur J Immunol. 2007;37:2205–2213. doi: 10.1002/eji.200636984. [DOI] [PubMed] [Google Scholar]
- 22.Krieg AM. Yi AK. Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 23.Laichalk LL. Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol. 2005;79:1296–1307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin L. Gerth AJ. Peng SL. CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur J Immunol. 2004;34:1483–1487. doi: 10.1002/eji.200324736. [DOI] [PubMed] [Google Scholar]
- 25.Merat R. Amara A. Lebbe C. de The H. Morel P. Saib A. HIV-1 infection of primary effusion lymphoma cell line triggers Kaposi's sarcoma-associated herpesvirus (KSHV) reactivation. Int J Cancer. 2002;97:791–795. doi: 10.1002/ijc.10086. [DOI] [PubMed] [Google Scholar]
- 26.Moormann AM. Chelimo K. Sumba OP, et al. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J Infect Dis. 2005;191:1233–1238. doi: 10.1086/428910. [DOI] [PubMed] [Google Scholar]
- 27.Moser JM. Upton JW. Gray KS. Speck SH. Ex vivo stimulation of B cells latently infected with gammaherpesvirus 68 triggers reactivation from latency. J Virol. 2005;79:5227–5231. doi: 10.1128/JVI.79.8.5227-5231.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oldstone MB. Viral persistence: mechanisms and consequences. Curr Opin Microbiol. 1998;1:436–441. doi: 10.1016/s1369-5274(98)80062-3. [DOI] [PubMed] [Google Scholar]
- 29.Parroche P. Lauw FN. Goutagny N, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to toll-like receptor 9. Proc Natl Acad Sci USA. 2007;104:1919–1924. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavlova IV. Virgin HWT. Speck SH. Disruption of gammaherpesvirus 68 gene 50 demonstrates that Rta is essential for virus replication. J Virol. 2003;77:5731–5739. doi: 10.1128/JVI.77.10.5731-5739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pichyangkul S. Yongvanitchit K. Kum-arb U, et al. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a toll-like receptor 9-dependent pathway. J Immunol. 2004;172:4926–4933. doi: 10.4049/jimmunol.172.8.4926. [DOI] [PubMed] [Google Scholar]
- 32.Ptaschinski C. Rochford R. Infection of neonates with murine gammaherpesvirus 68 results in enhanced viral persistence in lungs and absence of infectious mononucleosis syndrome. J Gen Virol. 2008;89:1114–1121. doi: 10.1099/vir.0.83470-0. [DOI] [PubMed] [Google Scholar]
- 33.Richard K. Pierce SK. Song W. The agonists of TLR4 and 9 are sufficient to activate memory B cells to differentiate into plasma cells in vitro but not in vivo. J Immunol. 2008;181:1746–1752. doi: 10.4049/jimmunol.181.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rickabaugh TM. Brown HJ. Martinez-Guzman D, et al. Generation of a latency-deficient gammaherpesvirus that is protective against secondary infection. J Virol. 2004;78:9215–9223. doi: 10.1128/JVI.78.17.9215-9223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rochford R. Cannon MJ. Moormann AM. Endemic Burkitt's lymphoma: a polymicrobial disease? Nat Rev Microbiol. 2005;3:182–187. doi: 10.1038/nrmicro1089. [DOI] [PubMed] [Google Scholar]
- 36.Rochford R. Lutzke ML. Alfinito RS. Clavo A. Cardin RD. Kinetics of murine gammaherpesvirus 68 gene expression following infection of murine cells in culture and in mice. J Virol. 2001;75:4955–4963. doi: 10.1128/JVI.75.11.4955-4963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose S. Lichtenheld M. Foote MR. Adkins B. Murine neonatal CD4+ cells are poised for rapid Th2 effector-like function. J Immunol. 2007;178:2667–2678. doi: 10.4049/jimmunol.178.5.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smee DF. Gowen BB. Wandersee MK, et al. Differential pathogenesis of cowpox virus intranasal infections in mice induced by low and high inoculum volumes and effects of cidofovir treatment. Int J Antimicrob Agents. 2008;31:352–359. doi: 10.1016/j.ijantimicag.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sunil-Chandra NP. Efstathiou S. Nash AA. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J Gen Virol. 1992;73(Pt 12):3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- 40.Tishon A. Borrow P. Evans C. Oldstone MB. Virus-induced immunosuppression. 1. Age at infection relates to a selective or generalized defect. Virology. 1993;195:397–405. doi: 10.1006/viro.1993.1389. [DOI] [PubMed] [Google Scholar]
- 41.van Dyk LF. Virgin HWT. Speck SH. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J Virol. 2000;74:7451–7461. doi: 10.1128/jvi.74.16.7451-7461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varthakavi V. Browning PJ. Spearman P. Human immunodeficiency virus replication in a primary effusion lymphoma cell line stimulates lytic-phase replication of Kaposi's sarcoma-associated herpesvirus. J Virol. 1999;73:10329–10338. doi: 10.1128/jvi.73.12.10329-10338.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verthelyi D. Ishii KJ. Gursel M. Takeshita F. Klinman DM. Human peripheral blood cells differentially recognize, respond to two distinct CPG motifs. J Immunol. 2001;166:2372–2377. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- 44.Vieira J. O'Hearn P. Kimball L. Chandran B. Corey L. Activation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J Virol. 2001;75:1378–1386. doi: 10.1128/JVI.75.3.1378-1386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weck KE. Barkon ML. Yoo LI. Speck SH. Virgin HI. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J Virol. 1996;70:6775–6780. doi: 10.1128/jvi.70.10.6775-6780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinberg JB. Lutzke ML. Alfinito R. Rochford R. Mouse strain differences in the chemokine response to acute lung infection with a murine gammaherpesvirus. Viral Immunol. 2004;17:69–77. doi: 10.1089/088282404322875467. [DOI] [PubMed] [Google Scholar]
- 47.White GP. Watt PM. Holt BJ. Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J Immunol. 2002;168:2820–2827. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 48.Wu TT. Tong L. Rickabaugh T. Speck S. Sun R. Function of Rta is essential for lytic replication of murine gammaherpesvirus 68. J Virol. 2001;75:9262–9273. doi: 10.1128/JVI.75.19.9262-9273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu TT. Usherwood EJ. Stewart JP. Nash AA. Sun R. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J Virol. 2000;74:3659–3667. doi: 10.1128/jvi.74.8.3659-3667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]