Abstract
The zinc finger transcription factor Gli3 is an essential mediator of hedgehog signaling. Gli3 has a dynamic expression pattern during embryonic development. In the neural tube, Gli3 transcripts are patterned along the anteroposterior and dorsoventral axes such that the initial broad expression in the posterior neural tube becomes dorsally restricted as neurogenesis takes place. Little is known about the molecular mechanisms that regulate this dynamic expression. Here, we report on a phylogenetic analysis of the Gli3 locus that uncovered a novel regulatory element, HCNE1. HCNE1 contains a compound Pbx/Meis binding site that binds Pbx and Meis/Prep proteins in vitro and in vivo. We show that HCNE1 recapitulates Gli3 expression in the developing neural tube and that mutations in the Pbx/Meis binding site affect the spatiotemporal control of HCNE1 transcriptional activity. Ectopic expression or loss of function of Pbx and Meis/Prep proteins in the chick and mouse embryo results in aberrant expression of endogenous Gli3 transcripts. We propose a novel role for TALE proteins in establishing the correct spatiotemporal expression pattern of Gli3 in the vertebrate spinal cord, thus implicating TALE transcription factors in early embryonic patterning events controlled by Sonic hedgehog signaling.
INTRODUCTION
Sonic hedgehog (Shh) is an essential signaling molecule for embryonic and fetal development. In the absence of Shh signaling, embryonic tissues and organs, including the ventral neural tube, limb, somites, eye, kidneys, and lungs, fail to develop or are abnormally patterned (48). Sonic hedgehog signals are transduced in receiving cells through the Gli proteins, which are zinc finger-containing transcription factors. Three vertebrate Gli genes have been identified, encoding Gli1, Gli2, and Gli3 (36, 37, 59). In vitro and in vivo studies indicate that while Gli2 and Gli3 are primary mediators of immediate-early response to Shh signals, Gli1 acts as a secondary mediator (33). Consistent with this, Gli1 is a transcriptional target of Shh signaling (26, 40, 41). Moreover, while Gli2 and Gli3 have retained the evolutionarily conserved transcriptional duality present in their Drosophila counterpart, Cubitus interruptus, Gli1 acts only as a transcriptional activator whose activity is mostly dispensable during embryonic development (6, 7, 55, 61, 69, 71). Among Gli proteins, Gli3 has unique characteristics. First, genetic studies have established that Gli3 acts primarily as a repressor in many Shh-controlled organs, including the neural tube, limb, and somites, although an inducing activity is revealed in the absence of Gli2 (7, 42, 47, 56). Second, there is evidence that Gli3 functions also in a hedgehog-independent manner in tissue patterning during embryogenesis (65). One possibility is that Gli3 may interfere directly or indirectly with the activity of other signaling pathways, such as Wnt and BMP (43, 52, 67). Finally, Gli3 dosage is critical as mouse and human heterozygous individuals display phenotypic defects. Indeed, a number of human syndromes resulting in limb deformity and craniofacial abnormalities are associated with autosomal dominant mutations in the Gli3 locus, including Greig cephalopolysyndactyly syndrome (GCPS) (34, 70, 72), Pallister-Hall syndrome (PHS) (35), postaxial polydactyly type A (PAPA) (58), and preaxial polydactyly type IV (PPD-IV) (57).
Thus, correct spatiotemporal expression of Gli3 is critical to ensure accurate hedgehog signaling levels and normal organogenesis. This is evidenced by the near-normal phenotype of the Shh−/−; Gli3−/− spinal cord compared to the Shh−/− spinal cord, which indicates that the primary role of Shh in the neural tube is to antagonize Gli3 repressor activity and that excessive Gli3 repressor activity is deleterious for ventral neural cell-type formation (42). Consistent with this, ectopic Gli3 expression inhibits the formation of ventral neural cell types (49, 56). Gli3 repressor activity is normally confined to the dorsal neural tube, because Gli3 is dynamically expressed and subject to spatiotemporal control during neural tube formation. Initially expressed throughout the neural tube, Gli3 becomes restricted to the dorsal neural tube as development proceeds (3, 14). This dorsal restriction appears to be controlled by Shh signaling, although there is no evidence for a direct regulation of Shh on Gli3 expression (14). While significant progress has been made on the biochemistry and biological function of the Gli proteins, the molecular mechanisms controlling Gli gene expression during embryogenesis remain to be elucidated. Gli1 transcription, with its restricted expression adjacent to Shh sources, requires Gli2 and Gli3 activity (26, 41, 47, 68). In contrast, Gli2 and Gli3 dynamic expression patterns in the vertebrate embryo (14, 40, 45, 47, 50) suggest that multiple regulatory elements and combinatorial mechanisms may be at play to control their transcription. Consistent with this hypothesis, previous studies have identified several intragenic sequences that are conserved among vertebrates (1–3, 54). Although several of the elements identified previously drive expression in the neural tube, none of them appear to fully reproduce Gli3 dynamic expression pattern, in particular its dorsal restriction. Therefore, additional elements not reported so far must exist and participate in the control of the Gli3 spatiotemporal expression pattern. Here, we used a similar phylogenetic footprinting approach and identified 11 novel highly conserved noncoding elements (HCNEs) in the Gli3 locus (64). Using a reporter gene expression assay in the chick neural tube, we show that a novel uncharacterized HCNE, HCNE1, generates a reporter gene expression pattern similar to that of Gli3. HCNE1 contains two conserved binding sites for TALE homeodomain transcription factors, and our in vitro and in vivo studies show that TALE proteins of the Pbx and Prep family bind one of these sites and shape HCNE1-derived reporter gene expression as well as endogenous Gli3 expression in the neural tube. Conversely, mutations in the TALE binding sites and misexpression of dominant-negative TALE constructs or analysis of Pbx1 mutant mouse embryos indicate that TALE protein binding and activity are required to control the onset of Gli3 expression and generate the Gli3 dynamic expression pattern. These results suggest that the correct spatiotemporal pattern of Gli3 and HCNE1-mediated reporter gene expression is directly controlled by members of the TALE homeodomain protein family. Together, these data provide novel insights into the regulation of Gli3 and suggest a mechanism by which TALE proteins may integrate control of hedgehog response with control of cell fate specification.
MATERIALS AND METHODS
Cell culture.
The rat pheochromocytoma PC12 cell line was grown in RPMI 1640 (Sigma) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 5% fetal horse serum (FHS; Invitrogen), and 1% l-glutamine (Invitrogen). The human medulloblastoma DAOY cell line was grown in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% FBS (Invitrogen) and 1% l-glutamine (Invitrogen). Cells were grown at 37°C in the presence of 5% CO2.
Mice.
Pbx1+/− mice were maintained as heterozygotes and genotyped as previously described (62). Heterozygous animals were crossed to produce Pbx1−/− embryos. Embryonic day 0.5 (E0.5) was the day that vaginal plugs were found.
5′ RACE.
One microgram of total E9.5 mouse embryo RNA was used to prepare 5′ rapid-amplification-of-cDNA-ends (5′ RACE)-ready cDNA using a Smart RACE cDNA amplification kit (Clontech). RACE was carried out according to the manufacturer's protocol, using Gli3-specific primers in exon 3 and exon 2 (data available on request). Fragments amplified were cloned into the pCR-TOPOII vector (Invitrogen) and sequenced.
Genomic sequence analyses.
Genomic sequences at the Gli3 locus for various vertebrate species were retrieved from the Ensembl database (www.ensembl.org/) (32). Pairwise alignments were generated using the AVID alignment tool (15, 24) and visualized on the VISTA genome server (www-gsd.lbl.gov/vista/) and the ECR genome browser (http://ecrbrowser.dcode.org/). Transcription factor binding sites were identified using the MatInspector tool from Genomatix (www.genomatix.de/).
Reporter constructs.
Putative enhancers (HCNEs) were amplified by PCR from mouse genomic DNA using Expand high-fidelity Taq polymerase (Roche) and subcloned into the LacZ reporter construct nP1230, which expresses β-galactosidase (β-Gal) under the control of the human β-globin promoter. Sequences were chosen on the basis of their conserved homology in human, mouse, and chick genomes. nP1230 was a modified version of p1230 (74), in which a nuclear localization signal (3xnls) from pCIG was introduced upstream of β-galactosidase. In our hands, the human β-globin promoter gave lower basal transcriptional levels than similar constructs driven by the thymidine kinase promoter. Site-directed mutagenesis was carried out in accordance with the protocol described in the QuikChange site-directed mutagenesis kit (Stratagene), using Phusion Hot Start high-fidelity DNA polymerase (Finnzymes). Primers for site-directed mutagenesis were designed using the QuikChange primer design program (Stratagene). All constructs were verified by sequencing using a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems) and run on an ABI 3730 capillary sequencer (Applied Biosystems) by the University of Sheffield Core Genomics service. The primers used are available on request.
Electromobility shift assay (EMSA).
Nuclear extract was prepared from Hamburger-Hamilton (HH) stage 12 chicken embryos (150 embryos) or from cell lines (10 75-cm2 flasks), pelleted, resuspended, and homogenized in 0.4 ml buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], 2 μg aprotinin, 2 μg leupeptin, 750 μM phenylmethylsulfonyl fluoride [PMSF]). After 15 min of incubation on ice, lysis was triggered by the addition of 10% NP-40, and following centrifugation at 4,000 rpm, cytoplasmic extracts present in the supernatant were collected and frozen in liquid nitrogen. Pellets were resuspended in 50 ml buffer C (20 mM HEPES [pH 7.9], 0.1 mM EDTA, 0.1 mM EGTA, 0.4 M NaCl, 1 mM DTT, 5 μg aprotinin, 2 μg leupeptin, 2 mM PMSF). After centrifugation at 4,000 rpm, nuclear extracts present in the supernatant were collected and stored in liquid nitrogen. Proteins (Pbx1b, Prep1, and Prep2) were prepared using a TnT quick coupled transcription/translation system (Promega) in accordance with the manufacturer's instructions. Oligonucleotides were radiolabeled by fill-in using [32P]dCTP (3,000 Ci/mmol; NEN) and Klenow (Roche). Labeled oligonucleotides were purified on a Sephadex G25 column (Roche). Nuclear extracts (1.5 μg) or in vitro-translated proteins were incubated with 400,000 cpm of 32P-labeled oligonucleotides and 2 μg of poly(dI-dC) in BC100 buffer (20 mM HEPES [pH 7.9], 100 mM KCl, 5 mM MgCl2, 1 mM DTT, 17% glycerol) for 15 min at room temperature. Where indicated, cold oligonucleotides and/or antibodies were added to the binding reaction. The antibodies used were anti-Pbx1/2/3/4 (H-260; sc-25411X), anti-Pbx1b (41.1; sc101852), anti-Prep1 (B-2; sc25282X), and anti-Prep2 (P-20; sc55889X) (Santa Cruz Biotechnology). Complexes were analyzed by electrophoresis on a 5.5% polyacrylamide gel run in 0.25× Tris-borate-EDTA buffer. Following electrophoresis, gels were dried and exposed to Kodak Biomax XAR film at −80°C.
Chick neural tube electroporation.
Fertilized white Leghorn chicken eggs (Winter egg farm) were incubated at 39°C and staged according to the Hamburger-Hamilton (HH) staging method (30). HH stage 10 and 11 chicken embryos were coelectroporated with a solution containing reporter constructs and the control plasmid pMES-EGFP (21) at a ratio of 3:1. The En-Meis1a and Meis1a-VP16 constructs (kindly donated by R. Maas) are N-terminal fusion proteins of the Drosophila En repressor domain and C-terminal fusion proteins of the activation domain of virion protein 16 (VP16) of herpes simplex virus with mouse Meis1a, respectively (75). Electrodes (5 mm L-shaped gold; Genetronics, Inc.) were placed on each side of the neural tube, and 5 pulses of 24 V and 30 ms each were delivered at 500-ms intervals using a BTX ECM 830 square wave generator. Green fluorescent protein (GFP) levels were examined at 12 to 24 h postelectroporation under a Leica MZ160F fluorescence stereomicroscope. Embryos with strong GFP expression throughout the anteroposterior axis were retained for analysis.
LacZ staining.
Embryos were fixed in 1% paraformaldehyde (PFA) for 30 to 60 min at room temperature, washed in LacZ rinse solution (5 mM EGTA, 0.01% sodium deoxycholate, 0.02% NP-40 [Igepal], 2 mM MgCl2 in phosphate-buffered saline [PBS]), and stained for 8 h in LacZ rinse solution containing 10 mM K3Fe(CN)6, 10 mM K4Fe(CN)6, and 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; Invitrogen).
Reverse transcription-PCR (RT-PCR).
Total RNA was isolated from HH stage 12 chicken embryos and DAOY and PC12 cells by use of TRIzol reagent (Gibco) in accordance with the manufacturer's protocol. cDNAs were prepared using a Superscript first-strand synthesis kit (Invitrogen). All products were amplified by PCR using BioMix Red (Bioline) in an Eppendorf PCR machine. The primers used are available on request. The identity of the PCR products was confirmed by cloning them into the TOPO-pCRII vector (Invitrogen) and sequencing them.
In situ hybridization.
Embryos were fixed in 4% paraformaldehyde. They were then either embedded in 2% agarose in PBS, in which case transverse sections were obtained using a Vibratome 1500 (Vibratome), or directly processed for whole-mount in situ hybridization. In situ hybridization was essentially performed as previously described (47). A 900-bp cDNA fragment of quail Gli3, a 926-bp cDNA fragment of mouse Gli3, a 788-bp cDNA fragment of chick Pbx1 (25), a 9,901-bp cDNA fragment of chick Prep1 (25), and a 1,059-bp cDNA fragment of chick Prep2 (25) were used as probes and labeled with digoxigenin (DIG) using a DIG RNA labeling kit (Roche) according to the manufacturer's manual. Mouse embryos of different genotypes were marked with small cuts and then processed together in the same tube to ensure equal treatment during the hybridization procedure. Stained embryos were photographed on an MZ12.5 stereomicroscope (Leica) using a Spot Insight camera (Diagnostic Instruments) with Spot Advanced digital image capture software. Following whole-mount in situ hybridization, transverse sections were generated using a Vibratome 1500 and mounted in Glycergel (Dako Cytomation). Images were captured on a DMR microscope (Leica) using a DC300FX digital camera (Leica) and the Leica IM50 image capture software program.
Immunohistochemistry.
Embryos were embedded and processed for immunofluorescence as described previously (4). The antibodies used in this study were mouse anti-β-galactosidase (Promega) at 1:1,000 and Alexa Fluor 594-conjugated goat anti-mouse IgG (A11005; Molecular Probes) at 1:2,000. Images were captured on an SP1 laser-scanning confocal microscope (Leica) and processed using the ImageJ and Photoshop (Adobe) software programs.
ChIP.
E10.5 mouse embryo chromatin was cross-linked for 30 min at room temperature in PBS containing 1.85% formaldehyde. Fixation was quenched by the addition of a 1/20 volume of glycine. Chromatin immunoprecipitation (ChIP) was performed as described previously (31), with the following modifications. Sonication buffer contained 1% sodium deoxycholate and 1% SDS, and lysates were cleared by incubating the samples overnight with 30 μl protein G beads previously washed in PBS containing 0.5% BSA. For the ChIP reaction, 9.25 μg chromatin and 3 μg antibody were incubated at 4°C for 2 h in 450 μl sonication buffer to allow binding. The antibodies were anti-Pbx1/2/3/4 (H-260; sc-25411X) and anti-Meis1/2 (H-80; sc-25412 X) (Santa Cruz Biotechnology). Immune complexes were collected by adsorption to protein G beads for 6 h at 4°C. Beads were washed twice with sonication buffer, twice with sonication buffer containing 500 mM NaCl, twice with 20 mM Tris (pH 8.0)–1 mM EDTA–250 mM LiCl–0.5% NP-40–0.5% sodium deoxycholate, and twice with Tris-EDTA buffer. Immunocomplexes were eluted with 200 μl elution buffer (50 mM [pH 8], 1 mM EDTA, 1% SDS, 50 mM NaHCO3) by incubation in a rotating oven at 65°C for 10 min. Beads were pelleted by centrifugation at 13,000 rpm for 1 min, and supernatants containing chromatin were recovered. This elution process was repeated such that the final volume of recovered supernatant was 400 μl. Twenty microliters of 4 M NaCl was added to the supernatant, and samples were incubated at 65°C overnight. Finally, the DNA was successively treated with 10 μg RNase A for 1 h at 37°C with 20 μg proteinase K for 2 h at 42°C, extracted with phenol-chloroform, and precipitated. Pellets were resuspended in 50 μl H2O. Immunoprecipitated DNA and input DNA were analyzed by PCR using HCNE1-specific primers (data available on request). Amplifications (35 cycles) were performed in the presence of 1 μl dimethyl sulfoxide (DMSO) to generate a 441-bp product.
Transactivation assay.
HELA cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, penicillin-streptomycin (Sigma), and 4 mM glutamine (Sigma) in 5% CO2 at 37°C. Before transient transfection, cells were transferred into Opti-MEM medium in 24-well plates and transfected using Lipofectamine 2000 (Invitrogen). For each transfection, 300 ng of a β-Gal reporter construct and 200 ng of a pGL3 reporter construct were used, alongside 250 ng mPbx1b and mPrep2 in the pCDNA3 vector. The final amount of DNA in the transfection mix was made up to 1 μg by the addition of empty pCDNA3 vector. All transfections were performed in triplicate. Transactivation assays were performed 24 h after transfection, using the dual-light combined reporter gene assay system (Applied Biosystems), using 50 μl lysis solution per well, and chemiluminescence detection was performed using a Sirius luminometer. The values shown are the fold inductions of the average β-galactosidase activity, normalized to the level for firefly luciferase activity from the pGL3 reporter plasmid, following transfection in the presence of Prep2 and Pbx1b expression vectors, compared with the average obtained in the absence of Prep2 and Pbx1b expression vectors.
RESULTS
Identification of HCNEs in the Gli3 locus.
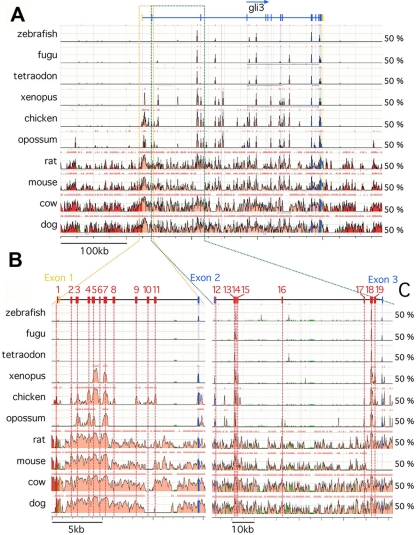
Previous phylogenetic footprinting studies compared human with fugu intronic Gli3 sequences and identified a number of highly conserved noncoding elements (1–3, 54). These elements are likely to control Gli3 expression in domains that are conserved between amniotes and fishes. Indeed, in vivo assays showed that some of these HCNEs drive reporter gene expression in conserved sites of expression such as the central nervous system (CNS) (1, 3, 54). The zebrafish Gli3 temporal expression pattern and its role in the control of spinal cord neuronal differentiation diverge significantly from that of amniote Gli3 (14, 46, 47, 66), which raised the possibility that searches including amphibian and fish genomes would miss important elements that are critical for the spatiotemporal pattern and thus the function of Gli3 in amniotes. This prompted us to compare the sequence alignments of mammalian, bird, amphibian, and fish Gli3 sequences and focus our study on 170 kb surrounding the start codon, with the view of including in our study HCNEs conserved in amniotes but not in the teleost and amphibian genomes. In line with other studies (23, 44), a minimum criterion of 60% (for chicken, xenopus frog, and zebrafish) or 65% (for human, rat, and mouse) homology over 100 bp was applied to identify potential HCNEs. This allowed us to identify a total of 19 elements, including 1 HCNE immediately upstream of exon 1, 10 HCNEs in intron 1, and 8 HCNEs in intron 2 (Fig. 1). Comparing our findings with those in previous studies shows that 11 HCNEs, including HCNEs 1 to 3, HCNEs 8 to 12, HCNEs 16 and 17, and HCNE19, were not previously reported (Table 1). In contrast, HCNEs 4 to 7 correspond to HCNR1 as identified by Alvarez-Medina et al. (3), and HCNE5 corresponds to CNE12 as described by Abassi et al. (2). Likewise, HCNEs 13 to 15 correspond to HCNR2 as identified by Alvarez-Medina et al. (3), while HCNEs 13 and 14 correspond to CNE1 as described by Abassi et al. (2). Finally, HCNE18 corresponds to HCNR3 as reported by Alvarez-Medina et al. (3) and to CNE2 as described by Abassi et al. (2).
Fig. 1.
Sequence comparison of vertebrate Gli3 genomic loci. (A) ECR representations of sequence alignments covering 70 kb upstream of Gli3 exon 2 from human, compared with those for dog, cow, mouse, rat, opossum, chicken, Xenopus frog, tetraodon, zebrafish, and fugu. (B) VISTA representations of sequence alignments covering 70 kb between exons 2 and 3 from human, compared with those for dog, cow, mouse, rat, opossum, chicken, Xenopus frog, tetraodon, zebrafish, and fugu. Conserved coding regions are indicated in blue, 5′ and 3′ untranslated regions are indicated in yellow, and intronic and exogenic highly conserved noncoding elements (HCNE) are indicated and numbered in pink and red, respectively. HCNEs were defined as having a minimum homology of 60% over 100 bp.
Table 1.
HCNEs
| Region | Human/chicken alignment |
Transcriptional activitya | Corresponding region in: |
No. of embryos showing Lacz expression/total no. examined | ||
|---|---|---|---|---|---|---|
| Length (bp) | % identity | Alvarez-Medina et al. (3) | Abassi et al. (2) | |||
| 1 | 229 | 61 | Strong; DV gradient | 40/53 | ||
| 2 | 132 | 83.3 | Strong; DV gradient | 10/10 | ||
| 3 | 406 | 68.5 | Negative | 5/6 | ||
| 4 | 455 | 79.8 | Strong; continuous | HCNR1; weak expression in neural tube | 17/23 | |
| 5 | 720 | 87.4 | Moderate; DV gradient | HCNR1; weak expression in neural tube | CNE12; not tested in vivo | 9/14 |
| 6 | 244 | 61.5 | Strong; DV gradient | HCNR1; weak expression in neural tube | 12/20 | |
| 7 | 542 | 79.9 | Moderate; AP gradient | HCNR1; weak expression in neural tube | 3/9 | |
| 8 | 214 | 64.5 | Moderate; DV gradient | 6/8 | ||
| 9 | 226 | 61.9 | Strong; DV gradient | 13/34 | ||
| 10 | 203 | 80.3 | Negative | 1/6 | ||
| 11 | 111 | 62.2 | No activity | 11/12 | ||
| 12 | 355 | 69 | No activity | 5/8 | ||
| 13 | 483 | 89.6 | Strong; DV gradient | HCNR2; strong expression in neural tube | CNE1; anterior CNS in zebrafish | 6/9 |
| 14 | 487 | 90.1 | Strong; DV gradient | HCNR2; strong expression in neural tube | CNE1; anterior CNS in zebrafish | 6/9 |
| 15 | 229 | 76.9 | Moderate; DV gradient | HCNR2; strong expression in neural tube | 10/13 | |
| 16 | 271 | 67.5 | Weak; posterior | 4/5 | ||
| 17 | 98 | 78.6 | Weak; dorsal | 5/7 | ||
| 18 | 1,090 | 93.1 | Strong; excluded from floor plate | HCNR3; moderate expression in neural tube | CNE2; anterior CNS in zebrafish and mouse | 11/11 |
| 19 | 433 | 71.1 | Weak | 3/7 | ||
AP, anteroposterior; DV, dorsoventral.
In vivo characterization of HCNE transcriptional activity in the chick neural tube.
To test their putative transcriptional activity, mouse HCNEs were subcloned into a reporter plasmid (nP1230, derived from the plasmid P1230) that contains the β-galactosidase (LacZ) reporter gene under the control of the human β-globin promoter (74) and displays low basal transcriptional activity following in ovo electroporation in the chick neural tube (n = 13/38) (see Fig. S1B in the supplemental material). Constructs were coelectroporated with the pMES plasmid, which contains enhanced GFP (EGFP) under the control of the chicken β-actin promoter (63), into the neural tube of HH stage 10 and 11 chicken embryos. LacZ activity was assessed for embryos showing high levels of GFP expression throughout the neural tube after 12 h (at HH stages 13 and 14) (Table 1; see Fig. S1 in the supplemental material) and 24 h (HH stages 15 and 16) (data not shown) of incubation. All regions except four (HCNEs 3 [n = 6], 10 [n = 6], 11 [n = 12], and 12 [n = 8]) showed enhanced transcriptional activity, although five previously reported elements (HCNEs 4 [n = 17/23], 6 [n = 12/20], 13 and 14 [n = 6/9], and 18 [n = 11/11]) and three novel elements (HCNEs 1 [n = 40/53], 2 [n = 10/10], and 9 [n = 13/34]) displayed the strongest increases in LacZ activity compared to that observed in embryos electroporated with nP1230 (see Fig. S1B to D, F, H, K, O, and S in the supplemental material). Interestingly, a number of the putative enhancers tested, including the novel HCNE1 and HCNE9 regions, exhibited a dynamic transcriptional activity along the anteroposterior axis of the embryo, with strong expression in the posterior neural tube and dorsally restricted expression in the anterior neural tube (see Fig. S1C, D, M, and K in the supplemental material). At 24 h postelectroporation, this dynamic transcriptional activity persisted and resulted in reporter gene expression detected at high levels in the posterior neural tube and rapidly becoming dorsally restricted at the lumbar axial level (data not shown). This expression pattern was reminiscent of that of endogenous Gli3, which is first expressed throughout the neural tube before becoming restricted to a dorsal domain as the neural tube develops (14) (see Fig. S1A in the supplemental material), suggesting that some of these HCNEs may indeed control Gli3 activation in vivo.
HCNE1 is an intronic element in the mouse embryo.
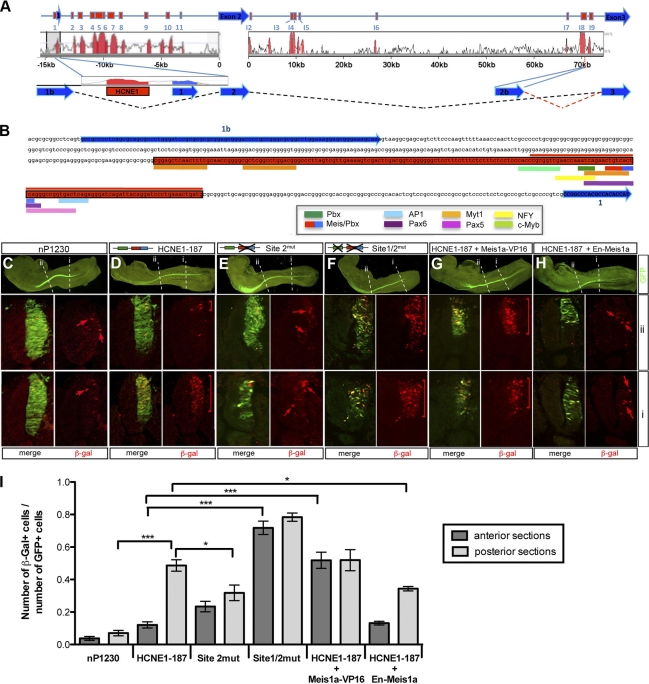
The location of HCNE1 immediately upstream of exon 1 suggested that HCNE1 could belong to a promoter element, although predictive tools did not recognize HCNE1 as a putative promoter. Thus, we performed 5′ RACE on E9.5 mouse cDNA, using a primer embedded in exon 3 to determine which transcriptional start site Gli3 utilizes in mouse embryos. Surprisingly, none of the cDNAs identified contains exon 1 (Fig. 2 A). Instead, we find that transcripts expressed in E9.5 mouse embryos include a novel exon, exon 1b, located upstream of exon 1, which contains a consensus 5′ splice donor site and splices directly to exon 2 (Fig. 2A). The transcript generated is consistent with an expressed sequence tag (EST) present in the EBI database (accession no. AK144851) and would not affect the protein product, as the translational start site is located in exon 2. In light of these data, we inferred that HCNE1 is an intragenic element with putative enhancer activity (Fig. 2B).
Fig. 2.
HCNE1 is an intronic element with transcriptional activity in the chick neural tube. (A) Schematic representation of the various 5′ RACE products identified from mouse embryo cDNAs in relation to the 5′ end of the Gli3 genomic locus as reported in Ensembl and the different HCNEs identified. (B) Sequence of the genomic region between exons 1 and 1b. Exons are indicated in blue and HCNE1 in red. Predicted transcription factor binding sites found in mouse HCNE1 are shown with colored thick lines below the HCNE1 sequence. HCNE1-78, which lacks all Myt1 binding sites, is indicated by a red line above the sequence. Site 1, which contains a Pbx binding site, is indicated in green, and site 2, which contains a compound Meis/Pbx binding site, is indicated in red/blue. (C to H) β-Galactosidase (β-Gal) protein expression following in ovo electroporation in the chick neural tube of nP1230 (C), HCNE1-187 (D), site 2mut (E), site 1/2mut (F), HCNE1-187 with Meis1a-VP16 (G), and HCNE1-187 with En-Meis1a (H). Coelectroporation with a control GFP plasmid served as an internal control (left panels). β-Gal expression was examined by immunofluorescence on cryosections at the anterior and posterior axial levels as indicated by “i” and “ii.” Note that electroporation of HCNE1-187 results in strong β-Gal expression (D) compared to the level for nP1230 (C), with an anteroposterior pattern reminiscent of Gli3 expression. Mutations in site 2 (E) or coelectroporation with a Meis1a-En construct (H) reduces HCNE1-187 activity, whereas mutations is both site 1 and site 2 (F) or coelectroporation with a Meis1a-VP16 construct (G) impairs HCNE1-187-controlled anteroposterior patterning and results in continuous reporter activity along the axis. Brackets and arrows indicate the domain of β-Gal expression. Magnification is ×25 (left panels) and ×400 (sections). (I) Quantification of HCNE1 in vivo transcriptional activity in the anterior and posterior neural tube. The ratio of the number of β-Gal-positive cells over the number of GFP-positive cells per section was plotted for each electroporation condition. Error bars indicate the standard error of the mean. Statistical significance is indicated above the bars and was evaluated using t test analysis.
HCNE1 contains TALE protein binding sites that mediate HCNE1 activity along the anteroposterior axis of the neural tube.
HCNE1 is of interest because it is uncharacterized, presents a strong transcriptional activity, and drives reporter gene expression with a spatiotemporal pattern similar to that of endogenous Gli3 (Fig. 2D). Furthermore, HCNE1 displays a high degree of conservation in amniotes but is not conserved in the zebrafish, Xenopus frog, or fugu genomes. Mouse HCNE1 is 231 bp long, contains a number of putative binding sites within the first 187 bp, and includes a 78-bp sequence with 86% identity to the human sequence (Fig. 2B). To determine the minimal sequence required for HCNE1 transcriptional activity, we tested various deletion constructs and found that HCNE1-187 displayed similar reporter gene expression upon electroporation in the chick neural tube, as does HCNE1 (Fig. 2D; compare with Fig. S1C in the supplemental material). Predicted binding sites for MyT1 (myeloid transcription factor 1), a zinc finger transcription factor expressed predominantly in the CNS (9), were found at the 5′ extremity of HCNE1-187. Further deletion analyses confirmed that they are not necessary for HCNE1 transcriptional activity, as a 78-bp fragment lacking the MyT1 binding sites was still sufficient to drive β-Gal activity in the neural tube (n = 13/18; data not shown). HCNE1-78, which retains all HCNE1 transcriptional activity, contains two TALE (three-amino-acid loop extension) protein binding sites, including site 1, which contains a putative Pbx binding site, and site 2, which contains a compound Meis-Pbx binding site (Fig. 2B and 3 A). In addition, it contains putative binding sites for the paired-box transcription factors Pax6 and Pax5, for activating protein 1 (AP-1), and for nuclear factor Y (NFY) (Fig. 2B). However, sequence comparison of the human, mouse, rat, and chick HCNE1-78 elements reveals that binding sites for Pax6, Pax5, and AP-1 are not conserved to the same degree as those for TALE proteins in the chick genome (Fig. 3A), suggesting that TALE proteins may mediate HCNE1 transcriptional activity. The TALE family of transcription factors is composed of the PBC (PBX and CEH-20), Meis, Prep, Iro (Iroquois), Mkx (Mohawk), and Tgif homeodomain transcription factors, although members of the Iro and Mkx gene families are evolutionarily more distant (51). Pbx proteins bind the consensus sequence AATCA (present in site 1) as monomers with low affinity (20) but bind DNA with high affinity as heterodimers with members of the Meis/Prep family or with Hox proteins (12, 18, 19). Meis, Prep, and Tgif homeodomain proteins bind a consensus motif, CTGTCA, which is present at site 2 (13).
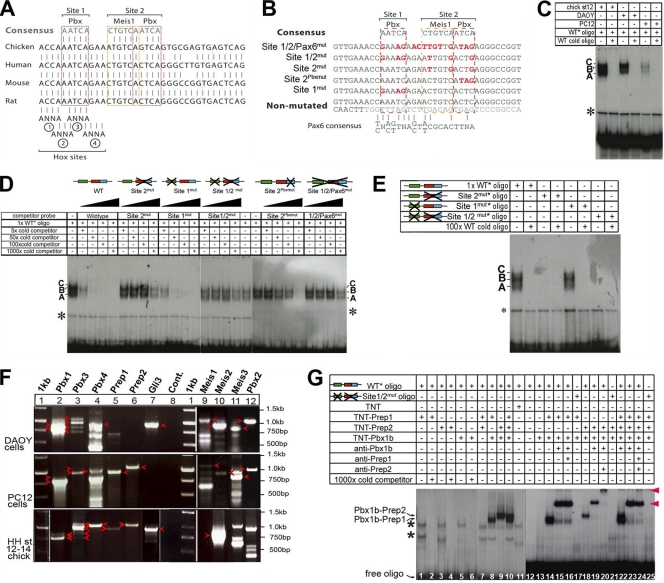
Fig. 3.
TALE proteins bind to HCNE1. (A) Sequence alignments of human, mouse, chicken, and rat genomic DNA show a high degree of conservation at sites 1 and 2, which contain binding sites for Pbx and Meis-Pbx, respectively. Four putative Hox binding site core sequences are also indicated, although no Pbx-Hox compound site was identified in databases. (B) Sequence of wild-type and mutated constructs and oligonucleotides used in EMSA and electroporation studies. Mutated nucleotides are indicated in red. (C) EMSA using a 35-bp oligonucleotide containing site 1 and site 2 incubated with nuclear extracts from chicken embryos and DAOY and PC12 cells in the presence/absence of a 1,000-fold excess of cold oligonucleotide. The asterisk indicates nonspecific binding. Complexes are labeled A, B, and C. (D) EMSA using a 35-bp oligonucleotide containing site 1 and site 2 incubated with nuclear extracts from chicken embryos in the presence of increasing amounts (5-, 50-, 100-, and 1,000-fold) of cold wild-type or mutated oligonucleotides. (E) EMSA using a 35-bp oligonucleotide containing no mutation or mutations in site 1, site 2, or sites 1 and 2 incubated with nuclear extracts from chicken embryos in the presence or absence of a 100-fold excess of cold wild-type oligonucleotide. (F) Expression of Pbx, Meis, and Prep genes was assessed using RT-PCR on mRNA extracted from DAOY cells, PC12 cells, and chicken embryos. Loading and expected sizes are as follows: lane 1, 1-kb DNA ladder; lane 2, 901 bp (Pbx1a) and 788 bp (Pbx1b); lane 3, 1,073 bp (Pbx3a), 960 bp (Pbx3b), 831 bp (Pbx3c), and 718 bp (Pbx3d); lane 4, 983 bp (Pbx4); lane 5, 959 bp (Prep1); lane 6, 1,059 bp (Prep2); lane 7, 929 bp (Gli3); lane 8, negative control; lane 9, 1,156 bp (Meis1a); lane 10, 934 bp (Meis2a); lane 11, 766 bp (Meis3); lane 12, 1,146 bp (Pbx2). Bands observed at the expected sizes and confirmed through sequencing are indicated with arrowheads. The other PCR products observed either are nonspecific bands or upon sequencing were found to be a paralogue (as is the case for the RT-PCR bands present in lane 12 that proved to not contain Pbx2 upon sequencing). Arrowheads indicate bands at the expected sizes. (G) EMSA using a 35-bp oligonucleotide containing either wild-type site 1 and site 2 or mutated site 1 and site 2 that was radiolabeled and incubated with in vitro-translated Pbx1b, Prep1, and Prep2 proteins in the presence or absence of anti-Pbx1b, anti-Prep1, or anti-Prep2 antisera. Asterisks indicate nonspecific binding bands.
To address whether site 1 and site 2 play a role in HCNE1 transcriptional activity, we electroporated wild-type, site 1mut, site 2mut, and site 1/2mut reporter constructs in the chick neural tube (Fig. 2C to F). Electroporation of the site 1mut construct does not affect reporter gene expression (n = 21/21; data not shown), whereas a reporter gene construct in which site 2 is mutated causes a consistent reduction of reporter gene activation in the posterior neural tube (n = 15/23) (Fig. 2I; also compare Fig. 2Ei and 2Di), suggesting that TALE protein binding on site 2, but not on site 1, is necessary for HCNE1 transcriptional activity. Interestingly, mutation of site 2 does not affect reporter gene dorsalization in the anterior neural tube (Fig. 2Eii). As the TALE binding site in site 2 can be recognized by any of the three protein families (Meis, Prep, or Tgif), we used constitutively active or repressive Meis constructs to assess whether binding of a TALE protein could affect HCNE1 transcriptional activity. Coelectroporation of wild-type HCNE1-187 with an En-Meis1a construct that acts in a dominant-negative manner (75) causes a downregulation of reporter gene expression similar to that caused by mutations in site 2 (n = 15/20) (Fig. 2I; also compare Fig. 2H and 2D). In contrast, coelectroporation of a Meis1a-VP16 (n = 26/29) construct with HCNE1-187 has little or no effect on reporter gene activation in the posterior neural tube but interferes with the normal dorsalization of reporter gene expression in the anterior neural tube (Fig. 2I; also compare Fig. 2Gii and 2Dii). Together, these data suggest that TALE proteins positively regulate HCNE1 transcriptional activity through their binding to site 2. Our results also imply that dorsalization of reporter gene expression in the anterior neural tube is the consequence of either the exhaustion of TALE proteins or the inactivity of TALE proteins in the anterior neural tube, as ectopic expression of a constitutively active TALE protein is sufficient to maintain high levels of HCNE1 activity in the anterior neural tube. Thus, site 2 is required for HCNE1 activity, and levels of TALE protein activity are likely to affect the transcriptional output from HCNE1. Together with our recent findings that TALE genes are dynamically expressed during neural development (see Fig. 6A) (25), these data are consistent with the hypothesis that TALE proteins control how HCNE1-mediated expression is patterned along the anteroposterior axis of the neural tube.
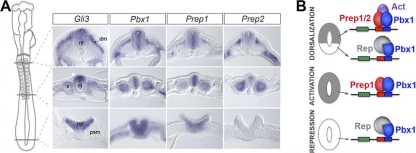
Fig. 6.
Model for the control of HCNE1 transcriptional activity and Gli3 expression by TALE proteins. (A) Expression pattern of Gli3, Pbx1, Prep1, and Prep2 in HH stage 12 chick embryos. Transverse sections shown correspond to the axial level indicated on the cartoon (left). psm, presomitic mesoderm; np, neural plate; nt, neural tube; s, somite; dm, dermomyotome. Note Gli3 and Pbx/Prep overlapping expression domains in the developing neural tube and somites. Note that the posterior-most section of Pbx1 is slightly more posterior than indicated while that of Prep2 is slightly more anterior than indicated. (B) Working model for Gli3 control in the neural tube. We propose that distinct complexes form on HCNE1 along the anteroposterior axis and that availability of Prep (or Meis) proteins dictates the steady state of HCNE1 complexes. We hypothesize that in its inactive state, HCNE1 is bound by Pbx1b (or Pbx4) associated with a repressor. Prep and Meis proteins can displace this complex, and thus, as Prep1 is broadly expressed in the posterior neural plate, Prep1 associates with Pbx1b (or Pbx4) at site 2 to induce Gli3 expression. As neurogenesis proceeds, Gli3 becomes dorsally restricted in the anterior neural tube. This may be triggered by the formation of new heterodimeric complexes between Pbx1 (or Pbx4) and Prep2 (or members of the Meis gene family) and could be aided by the formation of heterotrimeric complexes with other transcriptional activators (Act). In addition, we propose that repression of Gli3 transcription in the ventral neural tube is mediated/reinforced via the specific interaction of Pbx with a repressor (Rep).
Surprisingly, we observe that mutation of both site 1 and site 2 (n = 9/13) does not eradicate reporter gene expression but results in the maintenance of reporter gene expression in the anterior neural tube (Fig. 2F). We ruled out the possibility that mutations in sites 1 and 2 do not completely eliminate site 1/2, as radiolabeled site 1/2mut oligonucleotides fail to bind Pbx1b and Prep1 or Prep2 in an electrophoretic mobility shift assay (EMSA) (Fig. 3G). Thus, possible explanations for this somewhat contradicting result are either the creation of new putative binding sites in the site 1/2mut construct or the unmasking and serendipitous activity of additional binding sites in HCNE1-187 that do not encompass the region of our EMSA oligonucleotides in the absence of TALE complexes. Consistent with the former possibility, longer exposure of an EMSA autoradiography using radiolabeled site 2mut and site 1/2mut oligonucleotides confirms the existence of additional faint complexes (see Fig. S2 in the supplemental material).
TALE protein heterodimers bind cooperatively to HCNE1 in vitro.
To test whether TALE protein members bind to HCNE1, we performed EMSAs using a 36-bp radioactively labeled oligonucleotide encompassing both site 1 and site 2 (Fig. 3B). Three specific complexes (termed A, B, and C) form in the presence of nuclear extract from HH stage 12 to 14 chick embryos, whereas two complexes form in the presence of nuclear extract from DAOY medulloblastoma cells that express Gli3 (Fig. 3F, lane 7) and are competed by an excess of unlabeled oligonucleotide (Fig. 3C). In contrast, we observe a weak formation of complexes in the presence of nuclear extract from PC12 neuronal cells (Fig. 3C) that do not express Gli3 (Fig. 3F, lane 7). To address whether complexes A, B, and C form on site 1 or site 2, we assessed the ability of various mutant oligonucleotides (shown in Fig. 3B) to compete with radiolabeled wild-type oligonucleotides for the formation of complexes in the presence of embryonic nuclear extract (Fig. 3D). Our data show that site 1mut oligonucleotides compete as efficiently as wild-type oligonucleotides, indicating that the upstream Pbx binding site is dispensable for the formation of complexes A, B, and C (Fig. 3D). In contrast, all other mutated oligonucleotides displayed little (site 2Pbxmut) or no (site 2mut, site 1/2mut, and site 1/2/Pax6mut) competition with the wild-type radiolabeled oligonucleotide, suggesting that complexes form on site 2 (Fig. 3D). Moreover, competition was greatly affected by site 2Pbxmut oligonucleotides in which the Pbx, but not the Meis binding site of site 2, is mutated (Fig. 3B), suggesting that Pbx proteins may be an important component of complexes A, B, and C. Consistent with these observations, radiolabeled site 1mut oligonucleotides form three complexes in the presence of chicken embryo nuclear extracts, and these complexes can be competed with unlabeled wild-type oligonucleotides (Fig. 3E). In contrast, neither site 2mut nor site 1/2mut radiolabeled oligonucleotides are able to form a complex with nuclear proteins (Fig. 3E), confirming that mutations in site 2 disrupt its interaction with nuclear proteins.
We next performed RT-PCR assays to establish which members of the TALE gene family were expressed in DAOY cells, PC12 cells, and HH stage 12 to 14 chick embryos using specific primers that detect multiple isoforms of Pbx, Meis, and Prep genes (data available on request). All PCR products were sequenced to confirm that the correct gene family member had been amplified. Interestingly, DAOY cells, which express Gli3, contain all Pbx and Meis transcripts but do not express Prep1 or Prep2 (Fig. 3F, lanes 1 to 6 and 9 to 12). In contrast, both Prep1 and Prep2 are present in PC12 cells (Fig. 3F, lanes 5 and 6), while PC12 cells lack Pbx1a, Pbx3c, and Pbx3d (Fig. 3F, lanes 2 and 3) and express Meis genes at low levels (Fig. 3F, lanes 9 to 11). In HH stage 12 chick embryos, we found that Pbx1b, Pbx3b, and Pbx4 were the predominant Pbx isoforms present but could not amplify Pbx2 (Fig. 3F, lanes 1 to 4). HH stage 12 chick embryos also express Meis1, Meis2, Prep1, Prep2, Tgif1, and Tgif2 but not Meis3 (Fig. 3F) (25). As the CTGTCA core in site 2 can bind members of the Meis, Prep, or Tgif families and both Meis and Prep proteins can form heterodimers with Pbx proteins, we tested whether in vitro-translated Pbx1b, Prep1, and Prep2 bind to radiolabeled wild-type oligonucleotides. No binding was observed in the presence of monomers of Pbx1b, Prep1, and Prep2 alone or in the presence of Prep1 and Prep2 together (Fig. 3G, lanes 1 to 7). However, cooperative binding was observed when Pbx1b was combined with Prep1, Prep2, or both (Fig. 3G, lanes 8 to 10, 14, 18, and 22), indicating that Pbx1b binds HCNE1 as heterodimers of Pbx1b-Prep1 or Pbx1b-Prep2. This binding was specific, as no complex formed on radiolabeled site 1/2mut oligonucleotides (Fig. 3G, lanes 17, 21, and 25). Furthermore, Pbx1b-Prep1 and Pbx1b-Prep2 complexes migrated with an electromobility similar to that of complexes A and B observed in the presence of embryo extract (data not shown), suggesting that Pbx1b forms different heterodimeric complexes with Prep proteins in vivo. Finally, antibodies directed against Pbx1b and Prep2 caused a supershift or super-supershift of Pbx1b-Prep1 and Pbx1b-Prep2 complexes (indicated by red arrowheads in Fig. 3G, lanes 15, 16, 19, 20, 23, and 24), while anti-Prep1 antibodies appeared unable to shift Pbx1b-Prep1 complexes, possibly because the epitope was masked. Together, these data indicate that Pbx proteins bind site 2 as heterodimers with Prep proteins.
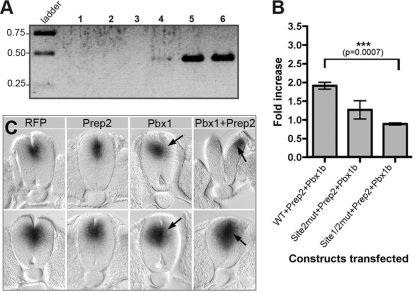
Consistent with these data, chromatin immunoprecipitation (ChIP) analyses of E9.5 mouse embryos show a specific amplification of a DNA fragment containing site 1 and site 2 with anti-Pbx antibodies (Fig. 4 A, lane 5) and to a lesser extent with anti-Meis antibodies (Fig. 4A, lane 4) but not with control anti-β-Gal antibodies (Fig. 4A, lane 3). Furthermore, cotransfection of HeLa cells with expression vectors coding for Pbx1b and Prep2 causes a 2-fold increase in reporter gene activity compared with the level for cells transfected with the HCNE1 reporter construct alone (Fig. 4B). Mutations in site 2 or in both site 1 and site 2 significantly reduce the transcriptional activity of the HCNE1 reporter construct in the presence of Pbx1b and Prep2 (Fig. 4B), confirming that Pbx and Prep proteins can synergistically enhance HCNE1 activity. Finally, although electroporation of a red fluorescent protein (RFP; n = 2) expression construct or a Prep2 (n = 3) expression construct alone in the chick neural tube has no effect on endogenous Gli3 expression, we found that ectopic expression of Pbx1 together with Prep2 caused precocious and increased expression of Gli3 in the dorsal neural tube (n = 4/6) (Fig. 4C). Interestingly, ectopic expression of Pbx1 alone had a converse effect and led to a decrease in Gli3 activation (n = 4/5) (Fig. 4C), suggesting that in the absence of heterodimer formation with a member of the Meis/Prep protein family, Pbx1 may associate with repressor factors that impact negatively on Gli3 transcription.
Fig. 4.
Pbx-containing heterodimers bind to and transactivate HCNE1 in vivo. (A) A chromatin immunoprecipitation (ChIP) assay was performed on E9.5 mouse embryos by use of antibodies against β-Gal (lane 3), Meis (lane 4), or Pbx (lane 5). Immunoprecipitated DNA was amplified by semiquantitative PCR. Lane 1, negative PCR control containing no DNA; lane 2, ChIP protocol performed with no antibody; lane 6, input chromatin (one-third less product was loaded on the gel). (B) HCNE1 transcriptional activity is enhanced by Pbx1b and Prep2. HeLa cells were cotransfected with a wild-type, site 2mut, or site 1/2mut HCNE1 reporter construct in the presence or absence of equal amounts of the Pbx1b and Prep2 expression constructs. The fold increase of relative luciferase activity was measured as the ratio of HCNE1-dependent luciferase activity normalized to the level for Renilla luciferase obtained in the presence of Pbx1b and Prep2, compared to that obtained in the absence of Pbx1b and Prep2. (C) Effect of Pbx1b and Prep2 on endogenous Gli3 expression. Prep2, Pbx1b, or Prep2+Pbx1b expression constructs were coelectroporated with a RFP plasmid in the chick neural tube, and effect on Gli3 expression was assayed by whole-mount in situ hybridization, followed by Vibratome sectioning. Whole-mount photographs of RFP and Gli3 expression are shown in Fig. S3 in the supplemental material. Top panels show caudal transverse sections, whereas bottom panels show rostral transverse sections along the neural tube. Arrows indicate up- or downregulation on the electroporated side (right). Note that the top panel for Pbx1+Prep2 electroporation shows a more posterior section to illustrate the precocious activation of Gli3 in these embryos. At this axial level, Gli3 would normally not be activated yet (or at very low level), as attested by expression on the nonelectroporated side.
Loss of Pbx1 affects Gli3 expression levels in the developing neural tube of the mouse embryo.
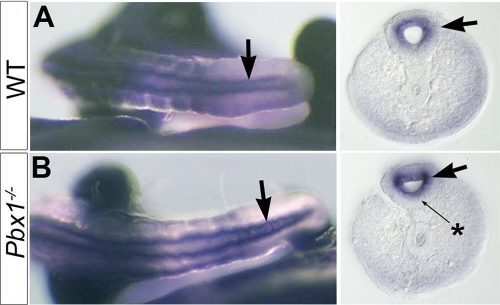
To further establish whether binding of Pbx protein to HCNE1 plays a role in Gli3 regulation in vivo, we examined E10.5 mouse embryos with a targeted mutation in the Pbx1 locus (62). Although the overall expression of Gli3 appeared unaffected in Pbx1−/− embryos (data not shown), we observed that Gli3 expression was strongly upregulated in the posterior neural tube and expressed in the floor plate, where it is normally excluded (Fig. 5). Together with our previous observation that ectopic expression of Pbx1 downregulates Gli3 expression in the chick neural tube, this result suggests that levels of Pbx1 and the absence or presence of Meis/Prep proteins are critical for the regulation of Gli3 transcriptional output. Remarkably, loss of Pbx1 affected Gli3 expression only in the posterior neural tube, suggesting the existence of compensatory roles of other Pbx genes elsewhere in the embryo (16, 17). Indeed, previous studies showed that the Gli3 expression domain expands in Pbx1−/−; Pbx2+/− limbs (16), although, in this case, it has been suggested that Gli3 expansion occurs as a consequence of Shh downregulation.
Fig. 5.
Gli3 is upregulated in the rostral neural tube of Pbx1−/− embryos. Tail Gli3 expression is detected by whole-mount in situ hybridization of E10.5 wild-type (A) and Pbx1−/− (B) mouse embryos, followed by Vibratome sectioning (right panels). Arrows indicate Gli3 upregulation in the neural tube. Note Gli3 transcripts in the floor plate (star) in the absence of Pbx1.
DISCUSSION
Gli3 is an essential transcription factor that antagonizes or mediates Shh signaling in a context-dependent manner during embryogenesis. Thus, Gli3 expression is highly regulated both temporally and spatially, and disruption in this expression pattern is often associated with developmental defects. To gain insight into the mechanisms that control Gli3 expression, we have used phylogenetic footprinting to identify a highly conserved noncoding element (HCNE1) located immediately upstream of Gli3 exon 1. This element is distinct from highly conserved noncoding elements previously identified by others (1–3, 54). We show here that HCNE1 contains a compound Meis-Pbx binding site, bound in vitro and in vivo by members of the Pbx and Meis/Prep protein families. Mutations in this Meis-Pbx binding site cause loss of reporter gene expression, while ectopic expression of Pbx and Meis/Prep causes precocious and prolonged expression of Gli3 and the reporter gene. Together, our data suggest that Pbx and Meis/Prep proteins may play an essential role in the control of the Shh response by regulating the transcription levels of Gli3 through their specific association with cofactors. This observation provides an attractive model for fine-tuning the spatiotemporal control of Gli3 expression during embryogenesis.
We show that Pbx1b associates with Prep1 and Prep2 and to a lesser extent with Meis proteins and that, together, these proteins induce HCNE1 transcriptional activity and Gli3 expression. As Prep1 and Prep2 are differentially expressed along the anteroposterior and dorsoventral axes of the neural tube (Fig. 6 A) (25), we propose that Pbx1b/Prep1 complexes control the initiation of Gli3 expression and that Pbx1b/Prep2 complexes are involved at the time Gli3 becomes dorsalized (Fig. 6B). Moreover, as overexpression of Pbx1b alone in the chick neural tube or loss of Pbx1 function in the mouse embryo elicits repression or ventral expansion of Gli3 expression, respectively, we propose that binding of Prep proteins to Pbx1b displaces and competes with the binding of a repressor to Pbx1b (Fig. 6B). Thus, complexes forming on HCNE1 are dynamic, and competitive cooperative binding between activators or repressors and Pbx1 on HCNE1 is likely to yield different transcriptional outputs. This model could provide a molecular basis for our observation that reporter gene expression mediated by HCNE1 presents a pattern similar to that presented by Gli3 and that manipulation of Pbx1b and Prep protein levels has a differential effect on Gli3 expression.
Our study has essentially focused on the interaction of Pbx1b with Prep1 or Prep2. However, other TALE proteins could be involved in HCNE1 activity. Our RT-PCR data reveal that in addition to Pbx1b, Pbx3b and Pbx4 are also abundantly expressed in HH stage 12 to 14 chick embryos, although Pbx3 is not expressed in the neural tube (Fig. 3F) (25). This indicates that the most likely candidate for binding of the Pbx half-site of site 2 is Pbx1, Pbx4, or both. What are the partners of Pbx in Gli3 regulation? Our data point to the existence of heterodimeric and perhaps heterotrimeric complexes forming on site 2, and we have shown that Pbx1b-Prep1 and Pbx1b-Prep2 heterodimeric complexes have mobilities similar to those of embryonic complexes A and B, respectively. However, our data also show that a Meis protein is weakly bound in vivo to HCNE1, and our previous expression analysis of Tgif genes suggest that they may also be candidate proteins for association with Pbx proteins on HCNE1 (25). In support of the possible involvement of Tgif is the fact that ectopic Tgif1 expression in the chick neural tube causes a downregulation of dorsal neural tube markers similar to that caused by ectopic Gli3 expression (38, 56). Furthermore, mutations in human and mouse Tgif disrupt the dorsoventral patterning of the neural tube and cause holoprosencephaly, a phenotype initially attributed to aberrant retinoic acid signaling (27, 29, 39), although our data could suggest a connection with loss of Shh signaling. Thus, various Pbx-Meis, Pbx-Prep, and Pbx-Tgif heterodimeric complexes could form on site 2. This is of importance, as previous studies have shown that different Pbx paralogues have distinct affinities for partner proteins. For instance, Pbx1, but not Pbx2 or Pbx3, is unable to bind DNA alone and requires association with a Meis/Prep partner (53). In addition, Pbx splice variants can have opposing transcriptional activities. One example of this is Pbx1, whose “b” isoforms can form complexes that activate transcription (5, 28), whereas Pbx1a can inhibit transcription through its association with histone deacetylase (HDAC) proteins and the corepressors NcoR and SMRT (5, 60). The ability of Pbx1, as well as Tgif, to interact with the chromatin remodeling protein HDAC to generate repressive complexes provides a possible mechanism for our proposed inhibition of Gli3 in the absence of Prep proteins (8, 10, 60). Such mechanism has already been reported for Hoxb-controlled genes (22). Further studies are required to establish whether such interaction exists on HCNE1 and participates in the control of Gli3 expression.
Pbx proteins can also form heterotrimeric complexes containing a member of the Meis protein family and another transcriptional regulator, as in the case of the myogenin promoter for which binding of Pbx-Meis is required for subsequent activation by MyoD and recruitment of the SWI/SNF chromatin remodeling complex (10, 11). The presence of higher-molecular-weight complexes in EMSAs (complex C) attests to the possibility that heterotrimeric complexes form on site 2. This is a very attractive possibility that would allow integration of multiple outputs from distinct signaling pathways toward the transcriptional control of Gli3 expression. For instance, BMP signaling is required for Gli3 maintenance in the neural tube (49). Smads, the transcriptional effectors of BMP signaling, can bind Pbx1-Prep1 and Pbx1-Tgif complexes (8, 73). As BMP signaling is restricted to the dorsal neural tube, Pbx1-Prep1-Smad heterotrimeric complexes could thus participate in the maintenance of Gli3 in the dorsal spinal cord. Future studies will be required to identify whether other transcription factors, such as Smads, are implicated in heterotrimeric complexes on HCNE1.
While HCNE1 in isolation is sufficient to faithfully mimic the Gli3 expression pattern, in the context of the whole locus Gli3 expression is presumably governed by the combinatorial effects of multiple HCNEs. Indeed, other studies have reported additional elements implicated in the control of Gli3 expression in the CNS (1–3, 54). Interestingly, computing analyses reveals that CNE13-14 (HCNRE2 in reference 3), which also displays strong transcriptional activity in the neural tube, contains two conserved Pbx binding sites, suggesting a mechanism whereby TALE proteins could globally regulate the transcription of Gli3 via multiple binding sites spread over several CNEs. Such regulation may also apply to other sites of Gli3 expression. Indeed, there is a striking overlap between the expression domains of Gli3 and that of Pbx1, Prep1, and Prep2 in the paraxial mesoderm (Fig. 6A). Further studies will establish whether HCNE1 plays a role in mesodermal Gli3 expression.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to A. Furley and J. Weiss for providing us PC12 and DAOY cell lines, to E. Smythe for providing us with the pcDNA3 expression vector, to M. Torres for providing us with cMeis1 and cMeis2 plasmids, and to R. Maas for providing us with En-Meis1a and Meis1a-VP16 constructs. We thank C. Nicolas for technical support and Jeremy Sanderson at the Sheffield Light Microscopy facility for his technical assistance. We thank A. Furley and A. Brendolan for their critical reading of the manuscript and suggestions.
This work was supported by grants from the EU-FP6 program (Cells into Organs contract LSHM-CT-2003-504468 and MYORES contract 511978), by the BBSRC (grant number 50/G16004) to A.-G.B., and by grants from the EU-FP6 program MYORES (contract 511978) and the MRC (contract G0401199) to J.C. S.C. was funded by a BBSRC Ph.D. fellowship and by the EU-FP6 grants. The Sheffield Light Microscopy facility was funded by a grant from the Wellcome Trust (grant number GR077544AIA).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
Published ahead of print on 24 January 2011.
REFERENCES
- 1. Abbasi A. A., et al. 2010. Human intronic enhancers control distinct sub-domains of Gli3 expression during mouse CNS and limb development. BMC Dev. Biol. 10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abbasi A. A., et al. 2007. Human GLI3 intragenic conserved non-coding sequences are tissue-specific enhancers. PLoS One 2:e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alvarez-Medina R., Cayuso J., Okubo T., Takada S., Marti E. 2008. Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development 135:237–247 [DOI] [PubMed] [Google Scholar]
- 4. Anderson C., Winder S. J., Borycki A. G. 2007. Dystroglycan protein distribution coincides with basement membranes and muscle differentiation during mouse embryogenesis. Dev. Dyn. 236:2627–2635 [DOI] [PubMed] [Google Scholar]
- 5. Asahara H., Dutta S., Kao H. Y., Evans R. M., Montminy M. 1999. Pbx-Hox heterodimers recruit coactivator-corepressor complexes in an isoform-specific manner. Mol. Cell. Biol. 19:8219–8225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aza-Blanc P., Lin H. Y., Ruiz i Altaba A., Kornberg T. B. 2000. Expression of the vertebrate Gli proteins in Drosophila reveals a distribution of activator and repressor activities. Development 127:4293–4301 [DOI] [PubMed] [Google Scholar]
- 7. Bai C. B., Stephen D., Joyner A. L. 2004. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev. Cell 6:103–115 [DOI] [PubMed] [Google Scholar]
- 8. Bailey J. S., Rave-Harel N., McGillivray S. M., Coss D., Mellon P. L. 2004. Activin regulation of the follicle-stimulating hormone beta-subunit gene involves Smads and the TALE homeodomain proteins Pbx1 and Prep1. Mol. Endocrinol. 18:1158–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellefroid E. J., et al. 1996. X-MyT1, a Xenopus C2HC-type zinc finger protein with a regulatory function in neuronal differentiation. Cell 87:1191–1202 [DOI] [PubMed] [Google Scholar]
- 10. Berkes C. A., et al. 2004. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol. Cell 14:465–477 [DOI] [PubMed] [Google Scholar]
- 11. Berthelsen J., Kilstrup-Nielsen C., Blasi F., Mavilio F., Zappavigna V. 1999. The subcellular localization of PBX1 and EXD proteins depends on nuclear import and export signals and is modulated by association with PREP1 and HTH. Genes Dev. 13:946–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berthelsen J., Zappavigna V., Mavilio F., Blasi F. 1998. Prep1, a novel functional partner of Pbx proteins. EMBO J. 17:1423–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bertolino E., Reimund B., Wildt-Perinic D., Clerc R. G. 1995. A novel homeobox protein which recognizes a TGT core and functionally interferes with a retinoid-responsive motif. J. Biol. Chem. 270:31178–31188 [DOI] [PubMed] [Google Scholar]
- 14. Borycki A., Brown A. M., Emerson C. P., Jr 2000. Shh and Wnt signaling pathways converge to control Gli gene activation in avian somites. Development 127:2075–2087 [DOI] [PubMed] [Google Scholar]
- 15. Bray N., Dubchak I., Pachter L. 2003. AVID: a global alignment program. Genome Res. 13:97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Capellini T. D., et al. 2006. Pbx1/Pbx2 requirement for distal limb patterning is mediated by the hierarchical control of Hox gene spatial distribution and Shh expression. Development 133:2263–2273 [DOI] [PubMed] [Google Scholar]
- 17. Capellini T. D., et al. 2008. Pbx1/Pbx2 govern axial skeletal development by controlling Polycomb and Hox in mesoderm and Pax1/Pax9 in sclerotome. Dev. Biol. 321:500–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang C. P., Brocchieri L., Shen W. F., Largman C., Cleary M. L. 1996. Pbx modulation of Hox homeodomain amino-terminal arms establishes different DNA-binding specificities across the Hox locus. Mol. Cell. Biol. 16:1734–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang C. P., et al. 1997. Meis proteins are major in vivo DNA binding partners for wild-type but not chimeric Pbx proteins. Mol. Cell. Biol. 17:5679–5687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang C. P., et al. 1995. Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev. 9:663–674 [DOI] [PubMed] [Google Scholar]
- 21. Chen Y. X., Krull C. E., Reneker L. W. 2004. Targeted gene expression in the chicken eye by in ovo electroporation. Mol. Vis. 10:874–883 [PubMed] [Google Scholar]
- 22. Choe S. K., Lu P., Nakamura M., Lee J., Sagerstrom C. G. 2009. Meis cofactors control HDAC and CBP accessibility at Hox-regulated promoters during zebrafish embryogenesis. Dev. Cell 17:561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cooper G. M., Sidow A. 2003. Genomic regulatory regions: insights from comparative sequence analysis. Curr. Opin. Genet. Dev. 13:604–610 [DOI] [PubMed] [Google Scholar]
- 24. Couronne O., et al. 2003. Strategies and tools for whole-genome alignments. Genome Res. 13:73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coy S. E., Borycki A. G. 2010. Expression analysis of TALE family transcription factors during avian development. Dev. Dyn. 239:1234–1245 [DOI] [PubMed] [Google Scholar]
- 26. Dai P., et al. 1999. Sonic hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J. Biol. Chem. 274:8143–8152 [DOI] [PubMed] [Google Scholar]
- 27. Gongal P. A., Waskiewicz A. J. 2008. Zebrafish model of holoprosencephaly demonstrates a key role for TGIF in regulating retinoic acid metabolism. Hum. Mol. Genet. 17:525–538 [DOI] [PubMed] [Google Scholar]
- 28. Goudet G., Delhalle S., Biemar F., Martial J. A., Peers B. 1999. Functional and cooperative interactions between the homeodomain PDX1, Pbx, and Prep1 factors on the somatostatin promoter. J. Biol. Chem. 274:4067–4073 [DOI] [PubMed] [Google Scholar]
- 29. Gripp K. W., et al. 2000. Mutations in TGIF cause holoprosencephaly and link NODAL signalling to human neural axis determination. Nat. Genet. 25:205–208 [DOI] [PubMed] [Google Scholar]
- 30. Hamburger V., Hamilton H. L. 1951. A series of normal stages in the development of the chick embryo. J. Morphol. 88:49–92 [PubMed] [Google Scholar]
- 31. Hatzis P., Talianidis I. 2002. Dynamics of enhancer-promoter communication during differentiation-induced gene activation. Mol. Cell 10:1467–1477 [DOI] [PubMed] [Google Scholar]
- 32. Hubbard T. J., et al. 2007. Ensembl 2007. Nucleic Acids Res. 35:D610–D617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jacob J., Briscoe J. 2003. Gli proteins and the control of spinal-cord patterning. EMBO Rep. 4:761–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalff-Suske M., et al. 1999. Point mutations throughout the GLI3 gene cause Greig cephalopolysyndactyly syndrome. Hum. Mol. Genet. 8:1769–1777 [DOI] [PubMed] [Google Scholar]
- 35. Kang S., Graham J. M., Jr, Olney A. H., Biesecker L. G. 1997. GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat. Genet. 15:266–268 [DOI] [PubMed] [Google Scholar]
- 36. Kinzler K. W., et al. 1987. Identification of an amplified, highly expressed gene in a human glioma. Science 236:70–73 [DOI] [PubMed] [Google Scholar]
- 37. Kinzler K. W., Ruppert J. M., Bigner S. H., Vogelstein B. 1988. The GLI gene is a member of the Kruppel family of zinc finger proteins. Nature 332:371–374 [DOI] [PubMed] [Google Scholar]
- 38. Knepper J. L., James A. C., Ming J. E. 2006. TGIF, a gene associated with human brain defects, regulates neuronal development. Dev. Dyn. 235:1482–1490 [DOI] [PubMed] [Google Scholar]
- 39. Kuang C., et al. 2006. Intragenic deletion of Tgif causes defects in brain development. Hum. Mol. Genet. 15:3508–3519 [DOI] [PubMed] [Google Scholar]
- 40. Lee J., Platt K. A., Censullo P., Ruiz i Altaba A. 1997. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development 124:2537–2552 [DOI] [PubMed] [Google Scholar]
- 41. Lei Q., Zelman A. K., Kuang E., Li S., Matise M. P. 2004. Transduction of graded Hedgehog signaling by a combination of Gli2 and Gli3 activator functions in the developing spinal cord. Development 131:3593–3604 [DOI] [PubMed] [Google Scholar]
- 42. Litingtung Y., Chiang C. 2000. Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nat. Neurosci. 3:979–985 [DOI] [PubMed] [Google Scholar]
- 43. Liu F., Massague J., Ruiz i Altaba A. 1998. Carboxy-terminally truncated Gli3 proteins associate with Smads. Nat. Genet. 20:325–326 [DOI] [PubMed] [Google Scholar]
- 44. Margulies E. H., Blanchette M., Haussler D., Green E. D. 2003. Identification and characterization of multi-species conserved sequences. Genome Res. 13:2507–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marigo V., Johnson R. L., Vortkamp A., Tabin C. J. 1996. Sonic hedgehog differentially regulates expression of GLI and GLI3 during limb development. Dev. Biol. 180:273–283 [DOI] [PubMed] [Google Scholar]
- 46. Marine J.-C., Bellefoid E. J., Pendeville H., Martial J. A., Pieler T. 1997. A role for Xenopus Gli-type zinc-finger proteins in the early embbryonic paterning of the mesoderm and neuroectoderm. Mech. Dev. 63:211–225 [DOI] [PubMed] [Google Scholar]
- 47. McDermott A., et al. 2005. Gli2 and Gli3 have redundant and context-dependent function in skeletal muscle formation. Development 132:345–357 [DOI] [PubMed] [Google Scholar]
- 48. McMahon A. P., Ingham P. W., Tabin C. J. 2003. Developmental roles and clinical significance of hedgehog signaling. Curr. Top. Dev. Biol. 53:1–114 [DOI] [PubMed] [Google Scholar]
- 49. Meyer N. P., Roelink H. 2003. The amino-terminal region of Gli3 antagonizes the Shh response and acts in dorsoventral fate specification in the developing spinal cord. Dev. Biol. 257:343–355 [DOI] [PubMed] [Google Scholar]
- 50. Mo R., et al. 1997. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development 124:113–123 [DOI] [PubMed] [Google Scholar]
- 51. Mukherjee K., Burglin T. R. 2007. Comprehensive analysis of animal TALE homeobox genes: new conserved motifs and cases of accelerated evolution. J. Mol. Evol. 65:137–153 [DOI] [PubMed] [Google Scholar]
- 52. Mullor J. L., Dahmane N., Sun T., Ruiz i Altaba A. 2001. Wnt signals are targets and mediators of Gli function. Curr. Biol. 11:769–773 [DOI] [PubMed] [Google Scholar]
- 53. Neuteboom S. T., Murre C. 1997. Pbx raises the DNA binding specificity but not the selectivity of antennapedia Hox proteins. Mol. Cell. Biol. 17:4696–4706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Paparidis Z., et al. 2007. Ultraconserved non-coding sequence element controls a subset of spatiotemporal GLI3 expression. Dev. Growth Differ. 49:543–553 [DOI] [PubMed] [Google Scholar]
- 55. Park H. L., et al. 2000. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 127:1593–1605 [DOI] [PubMed] [Google Scholar]
- 56. Persson M., et al. 2002. Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev. 16:2865–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Radhakrishna U., et al. 1999. The phenotypic spectrum of GLI3 morphopathies includes autosomal dominant preaxial polydactyly type-IV and postaxial polydactyly type-A/B; no phenotype prediction from the position of GLI3 mutations. Am. J. Hum. Genet. 65:645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Radhakrishna U., Wild A., Grzeschik K. H., Antonarakis S. E. 1997. Mutation in GLI3 in postaxial polydactyly type A. Nat. Genet. 17:269–271 [DOI] [PubMed] [Google Scholar]
- 59. Ruppert J. M., et al. 1988. The GLI-Kruppel family of human genes. Mol. Cell. Biol. 8:3104–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saleh M., Rambaldi I., Yang X. J., Featherstone M. S. 2000. Cell signaling switches HOX-PBX complexes from repressors to activators of transcription mediated by histone deacetylases and histone acetyltransferases. Mol. Cell. Biol. 20:8623–8633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sasaki H., Nishizaki Y., Hui C.-C., Nakafuku M., Kondoh H. 1999. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 126:3915–3924 [DOI] [PubMed] [Google Scholar]
- 62. Selleri L., et al. 2001. Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development 128:3543–3557 [DOI] [PubMed] [Google Scholar]
- 63. Swartz M., Eberhart J., Mastick G. S., Krull C. E. 2001. Sparking new frontiers: using in vivo electroporation for genetic manipulations. Dev. Biol. 233:13–21 [DOI] [PubMed] [Google Scholar]
- 64. Tagle D. A., et al. 1988. Embryonic epsilon and gamma globin genes of a prosimian primate (Galago crassicaudatus). Nucleotide and amino acid sequences, developmental regulation and phylogenetic footprints. J. Mol. Biol. 203:439–455 [DOI] [PubMed] [Google Scholar]
- 65. te Welscher P., Fernandez-Teran M., Ros M. A., Zeller R. 2002. Mutual genetic antagonism involving GLI3 and dHAND prepatterns the vertebrate limb bud mesenchyme prior to SHH signaling. Genes Dev. 16:421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tyurina O. V., et al. 2005. Zebrafish Gli3 functions as both an activator and a repressor in Hedgehog signaling. Dev. Biol. 277:537–556 [DOI] [PubMed] [Google Scholar]
- 67. Ulloa F., Itasaki N., Briscoe J. 2007. Inhibitory Gli3 activity negatively regulates Wnt/beta-catenin signaling. Curr. Biol. 17:545–550 [DOI] [PubMed] [Google Scholar]
- 68. Vokes S. A., Ji H., Wong W. H., McMahon A. P. 2008. A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog-mediated patterning of the mammalian limb. Genes Dev. 22:2651–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. von Mering C., Basler K. 1999. Distinct and regulated activities of human Gli proteins in Drosophila. Curr. Biol. 9:1319–1322 [DOI] [PubMed] [Google Scholar]
- 70. Vortkamp A., Gessler M., Grzeschik K. H. 1991. GLI3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature 352:539–540 [DOI] [PubMed] [Google Scholar]
- 71. Wang B., Fallon J. F., Beachy P. A. 2000. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100:423–434 [DOI] [PubMed] [Google Scholar]
- 72. Wild A., et al. 1997. Point mutations in human GLI3 cause Greig syndrome. Hum. Mol. Genet. 6:1979–1984 [DOI] [PubMed] [Google Scholar]
- 73. Wotton D., Lo R. S., Swaby L. A., Massague J. 1999. Multiple modes of repression by the Smad transcriptional corepressor TGIF. J. Biol. Chem. 274:37105–37110 [DOI] [PubMed] [Google Scholar]
- 74. Yee S. P., Rigby P. W. 1993. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 7:1277–1289 [DOI] [PubMed] [Google Scholar]
- 75. Zhang X., Friedman A., Heaney S., Purcell P., Maas R. L. 2002. Meis homeoproteins directly regulate Pax6 during vertebrate lens morphogenesis. Genes Dev. 16:2097–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.