Abstract
In patients with hyperkalemic periodic paralysis (HyperKPP), attacks of muscle weakness or paralysis are triggered by K+ ingestion or rest after exercise. Force can be restored by muscle work or treatment with β2-adrenoceptor agonists. A missense substitution corresponding to a mutation in the skeletal muscle voltage-gated Na+ channel (Nav1.4, Met1592Val) causing human HyperKPP was targeted into the mouse SCN4A gene (mutants). In soleus muscles prepared from these mutant mice, twitch, tetanic force, and endurance were markedly reduced compared with soleus from wild type (WT), reflecting impaired excitability. In mutant soleus, contractility was considerably more sensitive than WT soleus to inhibition by elevated [K+]o. In resting mutant soleus, tetrodotoxin (TTX)-suppressible 22Na uptake and [Na+]i were increased by 470 and 58%, respectively, and membrane potential was depolarized (by 16 mV, P < 0.0001) and repolarized by TTX. Na+,K+ pump–mediated 86Rb uptake was 83% larger than in WT. Salbutamol stimulated 86Rb uptake and reduced [Na+]i both in mutant and WT soleus. Stimulating Na+,K+ pumps with salbutamol restored force in mutant soleus and extensor digitorum longus (EDL). Increasing [Na+]i with monensin also restored force in soleus. In soleus, EDL, and tibialis anterior muscles of mutant mice, the content of Na+,K+ pumps was 28, 62, and 33% higher than in WT, respectively, possibly reflecting the stimulating effect of elevated [Na+]i on the synthesis of Na+,K+ pumps. The results confirm that the functional disorders of skeletal muscles in HyperKPP are secondary to increased Na+ influx and show that contractility can be restored by acute stimulation of the Na+,K+ pumps. Calcitonin gene-related peptide (CGRP) restored force in mutant soleus but caused no detectable increase in 86Rb uptake. Repeated excitation and capsaicin also restored contractility, possibly because of the release of endogenous CGRP from nerve endings in the isolated muscles. These observations may explain how mild exercise helps locally to prevent severe weakness during an attack of HyperKPP.
INTRODUCTION
Hyperkalemic periodic paralysis (HyperKPP) is a rare hereditary disease seen in human subjects and horses. It is an autosomal dominant disorder characterized by episodic attacks of flaccid weakness or paralysis and myotonia, lasting from minutes to hours (Gamstorp et al., 1957). The attacks are often (but not always) associated with hyperkalemia, hyponatremia (Streeten et al., 1971), and depolarization of the skeletal muscle cells (Brooks, 1969; Layzer, 1982). In the early phase of attacks, there is a drop in plasma Na+, probably reflecting an increased influx of Na+ into the skeletal muscle cells (Streeten et al., 1971; Clausen et al., 1980). This would initiate and explain the depolarization of the muscle cells and the subsequent hyperkalemia. Magnetic resonance recordings of intracellular 23Na in vivo showed that in patients with HyperKPP, attacks were associated with a significant increase in intracellular Na+, and in muscle fibers prepared from these patients, the membrane potential was depolarized by ∼30 mV (Weber et al., 2006). Also, in between the attacks, the resting muscle cells were shown to be depolarized in patients (Creutzfeldt et al., 1963; McComas et al., 1968) and in horses with a similar disorder (Pickar et al., 1991), where it was proposed to reflect an increased permeability of Na+. Experiments with isolated intercostal muscle fibers from patients with HyperKPP showed that exposure to elevated [K+]o (7 mM) triggered a noninactivating inward Na+ current leading to depolarization and increased intracellular Na+. This Na+ current could be blocked by tetrodotoxin (TTX), leading to repolarization (Ricker et al., 1989). Also, intercostal muscle fibers from horses with HyperKPP were found to be depolarized, and TTX induced repolarization to the level measured in normal horses (Pickar et al., 1991). More recent studies have identified molecular anomalies in the voltage-gated Na+ channels (Nav1.4), which are likely to be the cause of the disorder (Hanna et al., 1998; Lehmann-Horn et al., 2004). The loss of force is usually universal, except in muscle cells that maintain activity, e.g., respiratory muscles and muscles active in, for instance, operating the hand dynamometer used to record changes in force during a paralytic attack (Clausen et al., 1980). That study also showed that in 15 patients, attacks could be abolished or prevented by treatment with the β2-adrenergic agonist salbutamol, which led to the introduction of this treatment for paralytic attacks in HyperKPP (Wang and Clausen, 1976). Because salbutamol was found to stimulate the Na+,K+ pumps in rat soleus muscle, its effect in HyperKPP may be related to improved clearance of K+ from the extracellular space in muscles (Wang and Clausen, 1976). In rat muscle, the activity of the Na+,K+ pumps can also be stimulated by exposure to calcitonin gene-related peptide (CGRP; Andersen and Clausen, 1993) or release of endogenous CGRP from nerve endings by capsaicin or by repeated electrical stimulation (Nielsen et al., 1998), but the effect of these treatments on muscle function in HyperKPP has not been tested.
With the development of a mutant mouse model targeting the skeletal muscle voltage-gated Na+ channels (Nav1.4) gene, it has become possible to characterize the mechanisms of muscle paralysis in isolated muscles in vitro (Hayward et al., 2008). The present study explores the anomalies in contractility, excitability, and endurance of the soleus muscles from the knock-in mice expressing a missense substitution corresponding to the human Met1592Val HyperKPP mutation (Hayward et al., 2008). This substitution is located near the cytoplasmic face of the channel α subunit and has been observed in ∼30% of kindreds. In particular, we analyze the effects of electrical stimulation, increasing extracellular K+, salbutamol, CGRP, and Na+ loading with the Na+ ionophore monensin. The following working hypotheses are tested: (a) in soleus muscles prepared from mutant mice, contractile force and endurance are reduced. This is associated with increased sensitivity to elevated [K+]o, possibly reflecting reduced excitability. (b) The loss of contractile force in soleus and extensor digitorum longus (EDL) from mutant mice can be restored upon addition of salbutamol. (c) In soleus from mutant mice, repeated stimulation or capsaicin augments contractile force. (d) The improvement of contractile function induced by salbutamol, rat CGRP (rCGRP), capsaicin, and by repeated electrical stimulations is at least partly caused by a stimulation of the Na+,K+ pumps of the muscle fibers and may be mimicked by increasing intracellular Na+ with monensin. (e) Soleus from mutant mice show increased Na+ influx, intracellular Na+ concentration, and Na+,K+ pump–mediated 86Rb uptake. Muscles from mutant mice are depolarized but completely restore their resting membrane potential upon exposure to TTX. In addition, their content of Na+,K+ pumps ([3H]ouabain binding sites) is increased.
MATERIALS AND METHODS
Animals
Construction of the targeting vector for homologous recombination is described in the supplemental methods of Hayward et al., 2008). In brief, a 10.6-kb fragment of genomic DNA including exons 17–24 of the mouse SCN4A gene encoding NaV1.4 protein was obtained from clone 50E15 of the Citb/CJ7 BAC library (Shizuya et al., 1992) derived from 129/Sv mouse embryonic stem (ES) cell line CJ7 (Research Genetics). Two point mutations were introduced into exon 24: (1) a missense A→G transition encoding the Met→Val substitution at a position corresponding to residue 1,592 in the human gene and (2) a silent C→T substitution located four nucleotides 5′ to the first mutation that introduced a unique HpaI restriction site. A selection marker, the PGKneo gene (Soriano et al., 1991) flanked on each end by a 42-bp LoxP sequence (5′-CAACAACTTCGTATAATGTATGCTATACGAAGTTATCAGTAC-3′), was inserted into intron 23 as described previously (Hayward et al., 2008). The linearized DNA was electroporated into mouse 129/SvJae J1 ES cells at the Massachusetts General Hospital Knock-in Mouse Core facility. After selection with G418, correctly targeted ES cell clones were injected into blastocysts to produce male chimeras, and offspring with germline integration were crossed to C57BL/6J mice for >10 generations to produce the previously reported strain B6.129S4-Scn4atm1Ljh (Hayward et al., 2008). To minimize the possibility that gene expression or splicing of the Na+ channel mRNA might be altered by the PGKneo marker within intron 23, we developed a parallel line of knock-in mice reported here in which the floxed PGKneo sequence was excised by expression of Cre recombinase (Gu et al., 1993). Two correctly targeted and amplified ES clones were transfected with the 6.1-kb plasmid PGK-hygro/pMC-CRE (a gift from T.J. Ley, Washington University, St. Louis, MO) and selected for Cre expression with hygromycin. Of 48 hygromycin-resistant ES cell clones screened by Southern blotting, two showed excision of the PGKneo sequence with retention of the targeted mutations. These clones were then amplified and injected as described above to produce male chimeras. Offspring with germline integration were crossed to C57BL/6J mice for several generations and then to FVB mice for more than eight generations to establish the present knock-in mutant NaV1.4 mice with PGKneo removed. This strain, FVB.129S4(B6)-Scn4atm1.1Ljh/J, is available at The Jackson Laboratory with the JAX Stock No. 011033 (http://jaxmice.jax.org/query).
No experiments were performed on live animals, and all handling and use of animals complied with Danish animal welfare regulations (permission 2005/562-25), including the euthanasia, which, in addition, was approved by the University Animal Welfare Officer of Aarhus University. Most experiments were performed using 10.5–15-wk-old female or male mutant and wild-type (WT) mice. In a few experiments, muscles from younger (5.5 wk) or older (12 mo) mice were examined. Age-matched WT and mutant mice weighed on average 27.5 ± 1.1 (n = 8) and 27.4 ± 0.8 (n = 6) g, respectively (P > 0.90). Animals were kept in a thermostated environment at 21°C with a 12-h light/12-h dark cycle and fed ad libitum.
Genotyping of mice
All animals used for this study were genotyped using the tip of the tail. A 2-mm tail segment was lysed by incubation with 50 µl lysis reagent (0.2 mM disodium EDTA and 25 mM NaOH, pH 12.31) for 30 min at 65°C and 15 min at 98°C. This was followed by the addition of 50 µl of neutralizing agent (40 mM Tris-HCl, pH. 4.86), mixing in the pipette 10–15 times, and spinning for 10 s in a microfuge. 1 µl of the top layer was taken with 9 µl of water for PCR amplification followed by restriction analysis of the PCR products, taking advantage of the novel HpaI restriction site introduced in the allele encoding mutant NaV1.4 (Hayward et al., 2008). For the PCR, the forward and reverse primers used were 5′-TCGCCTACGTCAAGAAAGAGTC-3′ and 5′-ACCCTGAGCACAATCTCCATTT-3′, respectively. 100 ng genomic DNA was added to a mixture of 0.8 mM deoxy-NTP (0.2 mM each of deoxy-ATP, -GTP, -CTP, and -TTP), 0.4 µM of each primer, 1× Cloned Pfu DNA polymerase reaction buffer (Agilent Technologies), and 1.25 U Pfu Turbo DNA polymerase (Agilent Technologies) in a total volume of 50 µl. PCR was performed on a thermal cycler (MJ Research PTC-200 Peltier; VWR Scientific Products) with initial denaturing for 120 s at 95°C followed by 40 cycles of denaturing, annealing, and extension (30 s at 95°C, 60 s at 59°C, and 90 s at 72°C, respectively) and a final 10-min extension at 72°C. 10 µl of the PCR product was then digested for 16 h at 37°C with 2 U HpaI (Sigma-Aldrich) in 1× Restriction Buffer SA (Sigma-Aldrich), and the DNA fragments were separated by 1.5% agarose gel electrophoresis.
Preparation and incubation of muscles for measurements of force, Na+ contents, and the uptake of 22Na or 86Rb
The animals were killed by cervical dislocation followed by decapitation. Most of the experiments were performed using soleus muscles, which were found to show more pronounced functional response to the mutation than EDL. For the sake of comparison, the contractile performance of EDL muscles was also examined. Intact soleus and EDL muscles were prepared and incubated in standard Krebs–Ringer bicarbonate buffer (KR) containing the following: 122.1 mM NaCl, 25.1 mM NaHCO3, 2.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1.3 mM CaCl2, and 5.0 mM d-glucose. Buffers were continuously bubbled with a humidified mixture of 95% O2 and 5% CO2 so as to maintain a pH of 7.4. The wet weight of soleus from WT was 5.2 ± 0.2 mg (n = 36) and from mutants was 5.0 ± 0.2 mg (n = 34), and the weights were not significantly different (P > 0.40).
Most experiments were performed using muscles mounted vertically with their tendons intact in force transducers in chambers containing 5–8 ml KR, adjusted to optimal length for measurement of isometric contractions, and exposed to direct field stimulation via platinum wire electrodes placed centrally on either side of the muscle. In all experiments, the temperature of the chambers was maintained at 30°C by perfusing their hollow glass walls with distilled water circulating from a thermostated water bath. In soleus, electrical stimulation was applied, generally using 2-s trains of 0.2–1-ms 12-V pulses (supramaximal voltage for WT) and a frequency of 120 Hz. In this muscle, maximum force and a smooth tetanic contraction were obtained after ∼1 s of stimulation at 120 Hz (using 0.2- as well as 1-ms pulses). In some experiments, the excitability was examined by varying the pulse duration from 0.02 to 1.0 ms and the voltage from 7 to 12 V. Isometric force development was measured with force displacement transducers (Grass FTO3; Grass-Telefactor) and recorded with a computer using chart 5.4 software (ADInstruments). Endurance was examined using continuous stimulation at 120 Hz. In EDL muscles, maximum tetanic force was obtained using 0.5-s stimulations at 120 Hz and12-V pulses with a duration of 0.2 ms. In some experiments, 7-V pulses were used. The majority of experiments were performed on soleus, whereas EDL was only tested in a limited series of typical experiments.
In some experiments, the Na+ contents and the uptake of 22Na and 86Rb in soleus muscles were determined with or without salbutamol or rCGRP. After incubation in KR with isotopes, the muscles were blotted on dry filter paper followed by wet weight determination and soaking overnight in 0.3 M trichloroacetic acid (TCA). Samples of this extract were taken for flame photometry and counting of isotopes. In other experiments, extracellular Na+ or 86Rb was removed by washing the muscles during continuous gassing with air four times for 15 min in ice-cold Na+-free Tris-sucrose buffer followed by blotting on dry filter paper, wet weight determination, soaking in TCA, and counting (Everts and Clausen, 1992). In one experiment, incubation was terminated by washing in KR twice for 5 min at 30°C to remove extracellular 22Na. The Na+-free Tris-sucrose had the following composition: 263 mM sucrose, 10 mM Tris-HCl, 2.8 mM KCl, 1.3 mM CaCl2, 1.2 mM MgSO4, and 1.2 mM KH2PO4, pH 7.4. We have previously shown that during wash in this ice-cold Na+-free Tris-sucrose buffer, K+ contents of rat soleus muscles remain constant for 150 min, whereas the intracellular Na+ content undergoes a slow decrease that can be corrected for by multiplying with a constant (1.46 per hour of washout; Everts and Clausen, 1992). Thus, by washing away extracellular Na+ as well as the 86Rb taken up into the extracellular space, this procedure allows the determination of the total cellular contents of Na+ and 86Rb taken up into the intracellular space without causing further loss of intracellular K+. 22Na was used as a tracer for Na+ and 86Rb as a tracer for K+. Thus, the measurement of the uptake of K+ was based on the assumption that 86Rb is representative for K+. Therefore, the specific activity was calculated by dividing 86Rb activity per milliliter of buffer by the K+ contents in nanomoles per milliliter of buffer.
Recording of membrane potentials
The resting membrane potentials of soleus muscle fibers from 12-wk-old WT and mutant mice were recorded with standard glass microelectrode techniques using a setup that has been described previously (Pedersen et al., 2005). In brief, muscles were pinned out in an experimental chamber that was perfused using a circulation pump, and recordings were performed with an Axoclamp-2A amplifier (Axon Instruments). The ∼20-MΩ electrodes were filled with 2 M K-citrate. For experiments, two WT and two mutant muscles were pinned out side by side and allowed to rest for at least 30 min in 30°C KR before 15–20 recordings were performed from each muscle. Separate experiments were performed in the absence and presence of 2 × 10−7 M TTX. Recordings were accepted if the electrodes abruptly penetrated the fibers, giving stable recordings, and returned sharply to bath potential upon withdrawal of fiber without a marked change in electrode resistance. The experimenter was unaware of the genotype of the muscle while performing the recordings.
[3H]Ouabain binding sites
The contents of [3H]ouabain binding sites were determined using the vanadate-facilitated [3H]ouabain binding method, in which muscles are sectioned before incubation (Nørgaard et al., 1983; Hansen and Clausen, 1988). Previously, it has been reported that the values for [3H]ouabain binding obtained using this method are not significantly different from the values obtained in intact muscles (Nørgaard et al., 1983; McKenna et al., 2003). Combined with the finding that [3H]ouabain only binds to the outer membranes of intact muscle fibers (Clausen and Hansen, 1974), this shows that the vanadate-facilitated [3H]ouabain binding method provides a reliable measure of the [3H]ouabain binding sites in the outer membranes of the muscles (for details and discussion, see Hansen and Clausen, 1988; Clausen, 2003). For the assay, ∼5 mg of muscle was washed twice for 10 min at 37°C in Tris-vanadate buffer (250 mM sucrose, 10 mM Tris-HCl, 3 mM MgSO4, and 1 mM Na3VO4, pH 7.25–7.3). Muscle samples were then incubated for 120 min at 37°C in the same buffer with [3H]ouabain (10−6 M, 2 µCi/ml) and were then washed four times for 30 min in ice-cold Tris-vanadate buffer to remove unbound [3H]ouabain. After washout, samples were blotted, weighed, and soaked overnight in 1 ml of 0.3 M TCA containing 0.1 mM ouabain as a carrier. After overnight soaking, 0.5 ml TCA extract was taken for counting with 2.5 ml scintillation cocktail (Opti-Fluor; PerkinElmer). The content of [3H]ouabain binding sites was calculated on the basis of the sample wet weight and the specific activity of [3H]ouabain in the incubation medium. The final [3H]ouabain binding site content was then calculated by subtracting the nonspecific [3H]ouabain uptake measured using vanadate buffer containing an excess of unlabeled ouabain (Nørgaard et al., 1983) and multiplying by a correction factor of 1.33 to allow for impurity of the [3H]ouabain, loss of specifically bound [3H]ouabain during washout, and incomplete saturation during the equilibration of muscle samples with [3H]ouabain. Previous measurements on rat muscles (Murphy et al., 2008) showed that the [3H]ouabain binding site content intra-assay coefficient of variation was 1.30 and 1.47% for the soleus and EDL muscles, respectively (n = 68 samples). It should be noted that the results obtained using this assay for [3H]ouabain binding sites are very reproducible not only in the same laboratory, but also when comparing several different laboratories. The assay has allowed the detection and quantification of several regulatory changes in the content of Na+,K+ pumps in skeletal muscle (for discussion and details, see Clausen, 2003).
Chemicals and isotopes
All chemicals were of analytical grade. Capsaicin, monensin, ouabain, salbutamol, and TTX were obtained from Sigma-Aldrich, and rCGRP was purchased from Bachem. [3H]Ouabain, 22Na, and 86Rb were purchased from PerkinElmer.
Statistics
All data are presented as means with SEM. The statistical significance of a difference between two groups was ascertained using the Student’s two-tailed t test for nonpaired observations. The significance of differences between curves showing repeated force recordings during continuous stimulation was assessed using ANOVA followed by Bonferroni posttests.
RESULTS
Force and fatigue
In 43 mutant soleus muscles, the maximal force measured during stimulation with 2-s 120-Hz trains of 0.2-ms pulses at 12 V was 83 ± 4 mN compared with 156 ± 8 mN in 26 WT muscles (P < 0.001). In the mutant soleus muscles, twitch force (unpublished data) was 66% lower than in WT soleus (n = 11 vs. 11, P < 0.001).
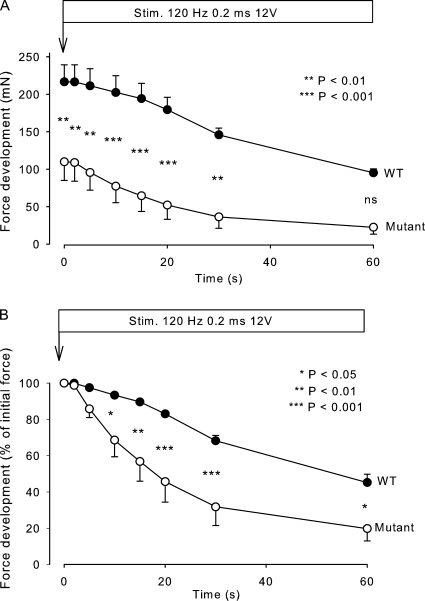
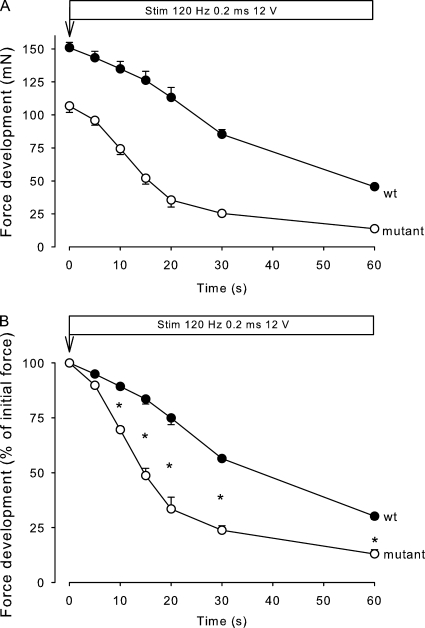
Fig. 1 shows the time course of force development during continuous stimulation at 120 Hz in soleus muscles obtained from 12.5-wk-old mice. As illustrated in Fig. 1 A, muscles from mutants had a considerably lower (49%) initial absolute tetanic force than WT and remained significantly lower during 30 s of the stimulation (P < 0.01). As shown in Fig. 1 B, the rate of force decline during 120-Hz continuous stimulation, as estimated from the decline in relative force over the first 20 s, was 220% faster in mutant muscles than in WT muscles (n = 4 vs. 4, P < 0.02; Fig. 1 B). In human HyperKPP patients, the paralytic attacks start in the first decade of life. At the outset, attacks are usually mild but become more frequent and severe later in childhood (Carson and Pearson, 1964; Layzer et al., 1967). To examine whether the loss of force and endurance was also less pronounced earlier in life in the mutant mice, similar experiments were performed using soleus muscles from 5.5-wk-old mice. As illustrated in Fig. 2, also in these experiments, the muscles from the mutants showed lower (30%) initial absolute tetanic force than those from WT mice, the difference between the two genotypes being highly significant (P < 0.001) throughout the 60-s stimulation period. The rate of decline in relative force as measured over the first 20 s of 120-Hz stimulation was 153% faster in the mutant muscles than in WT, which is similar to the difference in the rate of force decline (220%) observed between mutant and WT soleus from 12.5-wk-old mice. The difference between the relative loss of force was highly significant from 10 to 60 s after the onset of contractions (n = 3 vs. 3, P < 0.001).
Figure 1.
Effect of continuous stimulation on force development in mouse soleus. Muscles from 12.5-wk-old WT and mutant mice were mounted in force transducers for isometric contractions and stimulated for the indicated time using 0.2-ms pulses at 12 V and 120 Hz. Each point represents the mean of observations on four muscles, with error bars denoting SEM. (A) Force in milliNewtons; (B) force in percentage of the initial value. The difference between mutants and WT was significant at the times indicated by the asterisks and p-values.
Figure 2.
Effect of continuous stimulation on force development in soleus from 5.5-wk-old mice. Experimental conditions as described in the legend to Fig. 1. Each point shows the mean of observations on three muscles, with error bars denoting SEM. The asterisks indicate the significance of differences between WT and mutant muscles (P < 0.001).
Effects of elevated [K+]o with and without salbutamol
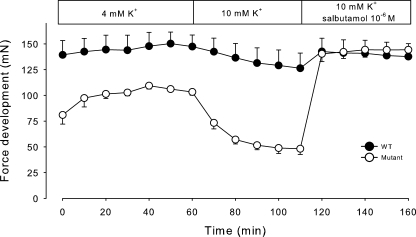
It is well established that elevation of extracellular K+ induced by the ingestion of KCl may trigger attacks of weakness or paralysis in patients with HyperKPP. Therefore, we explored the effects of buffer containing 10 mM K+ on force development in soleus muscles. As shown in Fig. 3, the force development induced by stimulation at 120 Hz for 2 s was reduced both in muscles from WT and mutant mice. In muscles from mutant mice, the rate of force reduction as measured over the first 10 min after exposure to 10 mM K+ was 760% faster than in muscles from WT. Also, when tested with continuous stimulation at 120 Hz, initial force in mutant soleus was clearly lower than in WT, and the rate of force decline was 70% faster (unpublished data). Collectively, these observations indicate that the sensitivity to elevated K+ was more pronounced in soleus muscles from mutant mice. When added to muscles exposed to 10 mM K+, 10−6 M salbutamol restored tetanic force to the same level as measured at 4 mM K+ both in WT and in mutant mice (Fig. 3). In the muscles of the mutant mice, salbutamol augmented tetanic force by 200% in 10 min.
Figure 3.
Effect of elevated [K+]o and salbutamol on force development in mouse soleus. Muscles from 12.5-wk-old WT and mutant mice were mounted in force transducers for isometric contractions in standard KR buffer and stimulated as indicated every 10 min using 2-s trains of 120-Hz pulses of 0.2-ms duration and 12 V. After 60 min, the buffer was changed to a KR in which K+ was increased by 6 mM and Na+ was decreased by 6 mM to maintain isoosmolarity. After 50 min, 10−6 M salbutamol was added to all muscles, and stimulation every 10 min continued. Each point represents the mean of observations on four muscles, with error bars denoting SEM.
Effects of changes in voltage and pulse duration
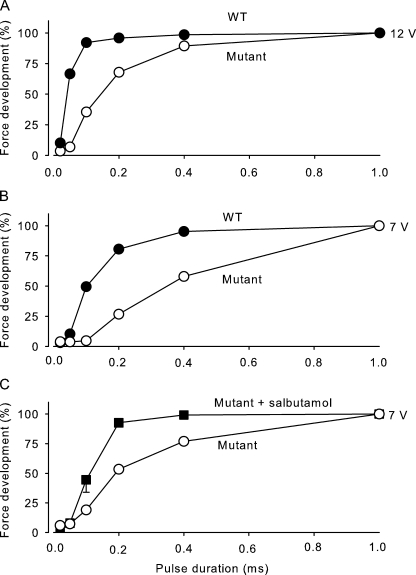
To examine for differences in excitability between muscles from mutants and WT mice, the force response to electrical pulses with varied voltage (7 and 12 V) and duration (0.02–1 ms) was determined. To ascertain that this evaluated the excitability of the muscle fibers rather than that of nerves or nerve endings, these experiments were performed in the presence of 10−5 M tubocurarine. As shown in Fig. 4 (A–C), the pulse duration required to obtain 50 or 100% of maximal force was at both 7 and 12 V substantially longer in mutant muscles than in WT muscles, demonstrating that activation of mutant muscles required stronger pulses than in WT muscles. These observations indicate that in muscles from the mutants, excitability is lower than in muscles from WT.
Figure 4.
Effects of changes in voltage, pulse duration, and salbutamol on tetanic force in soleus from WT and mutant mice in the presence of 10−5 M tubocurarine. (A–C) Muscles were mounted in force transducers, and tetanic force was determined using 120-Hz pulse trains of 12 V (A), 7 V (B), and 7 V in the presence of 10−5 M salbutamol added 15 min before the onset of measurements (C). Pulse durations in the range of 0.02–1 ms as indicated. Each point represents the mean of observations on two muscles in A and B and the mean of four muscles in C, with error bars denoting SEM.
Effects of salbutamol, CGRP, capsaicin, and monensin on tetanic force
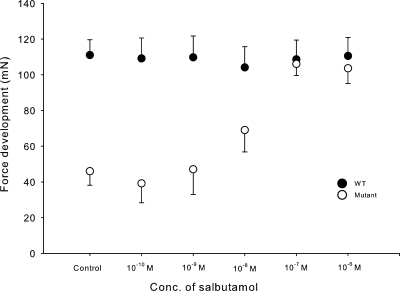
These experiments were performed using 2-s 120-Hz trains of submaximal pulses (7 V of 0.2-ms duration) and, as in Fig. 4, in the presence of 10−5 M tubocurarine. As shown in Fig. 5, 10−8–10−6 M salbutamol produced a significant increase in tetanic force in soleus muscles from mutant mice. At the highest concentration of salbutamol, tetanic force was increased to the same level as that observed in soleus from WT mice. In contrast, the tetanic force of soleus from WT mice showed no response to salbutamol. In mutant muscles, the effect of salbutamol was associated with an increase in excitability as indicated by a large reduction in the pulse length required for 7-V pulses to produce 50 or 100% of maximal tetanic force during tetanic stimulation (Fig. 4 C).
Figure 5.
Effects of salbutamol on tetanic force in soleus obtained from WT and mutant mice. Muscles were stimulated using 2-s 120-Hz trains of 0.2-ms pulses at 7 V in the presence of 10−5 M tubocurarine and salbutamol at the concentrations indicated. Each point represents the mean of observations on four muscles, with error bars denoting SEM. In the absence of salbutamol, the difference between tetanic force in WT and mutants was highly significant (P < 0.001). In the mutant muscles, salbutamol induced a significant increase in tetanic force at 10−8 M (P < 0.05), 10−7 M (P < 0.001), and 10−6 M (P < 0.001).
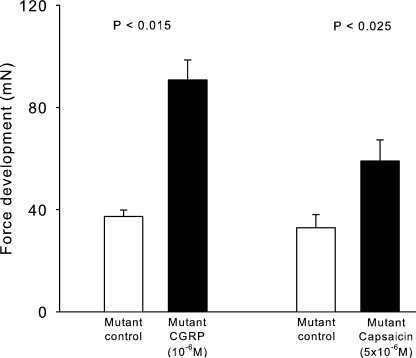
In skeletal muscle, excitation is causing a release of CGRP from the nerve endings. CGRP acts on receptors in the motor endplate, inducing force recovery in rat soleus inhibited by elevated [K+]o (Andersen and Clausen, 1993; Nielsen et al., 1998; Macdonald et al., 2008). As shown in Fig. 6, 10−6 M rCGRP increased tetanic force in soleus muscles from mutant mice by 143% (n = 4 vs. 4, P < 0.015), almost reaching the force developed by soleus muscles from WT. We have previously shown that capsaicin, which induces release of CGRP from nerve endings, gives rise to force recovery in rat soleus muscles inhibited by exposure to elevated [K+]o (Andersen and Clausen, 1993; Nielsen et al., 1998; Macdonald et al., 2008). As shown in Fig. 6, in mutant mouse soleus muscles incubated with 10−5 M tubocurarine, contractions induced by 2-s 120-Hz trains of pulses (7 V and 0.2 ms) were increased by 79% with 5 × 10−6 M capsaicin (n = 3 vs. 3, P < 0.025).
Figure 6.
Effects of rCGRP and capsaicin on tetanic force in soleus muscles obtained from 12-wk-old mutant mice. Experimental conditions as described in the legend to Fig. 5. The columns show the effects of 10−6 M rCGRP (n = 4 vs. 4) and 5 × 10−6 M capsaicin (n = 3 vs. 3), with error bars denoting SEM.
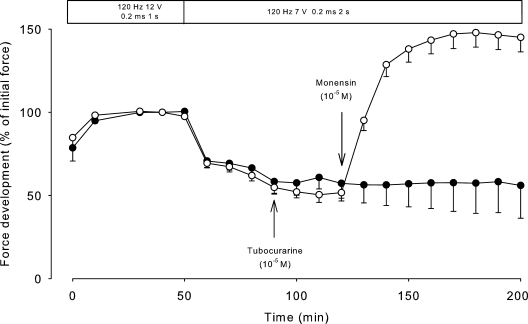
To test the possible effects of Na+,K+-pump stimulation by increasing intracellular Na+, soleus muscles were exposed to the Na+ ionophore monensin, a carboxylic ionophore that forms a complex with Na+, K+, and H+ and in skeletal muscle mediates an electroneutral influx of Na+ accompanied by an equimolar efflux of K+ and H+ (Hoya and Venosa, 1992). We have previously demonstrated that at a concentration of 10−5 M, monensin induces highly significant increases in intracellular Na+ and ouabain-suppressible 86Rb uptake of rat soleus muscle (Everts and Clausen, 1992; Buchanan et al., 2002). Fig. 7 shows that in soleus muscles obtained from mutant mice and exposed to the same stimulation paradigm as in Fig. 5, 10−5 M monensin induced a rapid increase in tetanic force, reaching a plateau 186% higher than the previous level (n = 4, P < 0.001). In contrast, in soleus from WT mice, 10−5 M monensin produced no significant increase in force (4.9 ± 2.2%, n = 3 vs. 3, P > 0.5).
Figure 7.
Effects of monensin on tetanic force in soleus muscles obtained from 10.5-wk-old mutant mice. At the intervals indicated, the muscles were stimulated using 1-s trains of 120-Hz pulses of 0.2-ms duration and 12 V. When steady state had been reached, voltage was reduced to 7 V, and the duration of the trains increased to 2 s. 90 min after the onset of stimulation, 10−5 M tubocurarine was added to all muscles. At 120 min, 10−5 M monensin was added to four muscles (open circles), and the contralateral muscles served as controls (closed circles). Each point represents the mean of observations on four muscles, with errors bars denoting SEM.
Effects of repeated stimulation on tetanic force
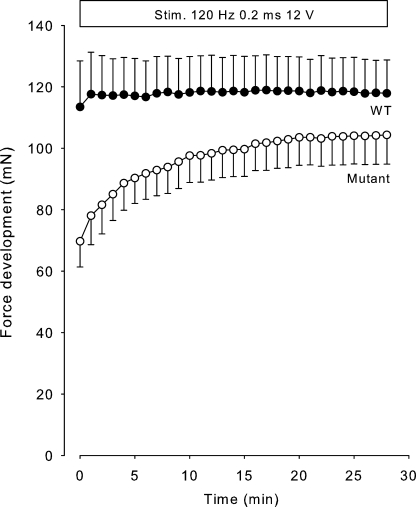
It is well documented that in patients with HyperKPP, the paralytic attacks can be fended off by physical activity, even in localized muscle groups (Clausen et al., 1980). This would suggest that repeated stimulation of soleus muscles from mutant mice might restore contractility. Indeed, as shown in Fig. 8, tetanic stimulation every minute caused a progressive and highly significant force increase of 48% in the soleus of mutant mice (P < 0.001) but no significant change in soleus of WT mice. It seems likely that this reflects stimulation induced by CGRP released from endogenous stores in the nerve endings of the muscles (Nielsen et al., 1998).
Figure 8.
Effects of repeated stimulation on tetanic force in soleus muscles obtained from 12.5-wk-old WT and mutant mice. Muscles were mounted in force transducers for isometric contractions and stimulated at 120 Hz using 2-s trains of 0.2-ms 12-V pulses every minute. Each curve represents the mean of observations on four WT and eight mutant muscles, with error bars denoting SEM. During the initial contractions, the difference between tetanic force in WT and mutants was significant (P < 0.02). In the mutants, the last tetanic contraction was significantly larger than the first (P < 0.001).
Effects of electrical stimulation on EDL muscles
In EDL muscles, tetanic force induced by stimulation with 0.5-s 120-Hz trains of 0.2-ms pulses at 12 V in WT and mutants was 181 ± 22 and 124 ± 9 mN, respectively, and significantly different (P < 0.03). When stimulated at 7 V in the presence of 10−5 M tubocurarine, tetanic force of WT and mutant EDL was 169 ± 17 and 99 ± 11 mN, respectively, and significantly different (n = 6 vs. 6, P < 0.01). In EDL muscles from mutants stimulated at 7 V in the presence of 10−5 M tubocurarine, 10−6 M salbutamol increased force by 64%, from 97 ± 12 to 159 ± 13 mN (n = 6 vs. 6, P < 0.001). In EDL from WT, stimulation at 7 V induced a force of 165 ± 18 mN, and in the presence of 10−6 M salbutamol, force reached 152 ± 15 mN, thus causing no significant change. It is interesting that in the mutants, the addition of salbutamol increased force to the same level as in the WT.
22Na uptake
The 22Na uptake was measured in resting soleus muscles from 12-wk-old mice in the absence and presence of 10−6 M TTX. As shown in Table I, total 22Na uptake in resting muscles from mutant mice was 67% higher than in those from WT mice (P < 0001). Preincubation with TTX for 15 min caused significant inhibition of 22Na uptake both in muscles from WT (−12%, P < 0.015) and in those from mutants (−43%, P < 0.001). The TTX-suppressible 22Na uptake was 172 nmol/g wet wt/min in mutants and 30 nmol/g wet wt/min in WT, corresponding to a 5.7-fold higher level in the mutants.
Table I.
Effects of 10−6 M TTX on 22Na uptake in soleus muscles from WT and mutant mice
| Genotype | 22Na uptake | Significance of difference between controls and TTX (p-value) |
| nmol/g wet wt/min | ||
| WT | 242 ± 6 (8) | <0.012 |
| WT + TTX | 213 ± 8 (8) | |
| Mutant | 403 ± 13 (8) | <0.001 |
| Mutant + TTX | 231 ± 11 (8) |
Soleus muscles were prepared from 12-wk-old mice and mounted on electrodes at resting length, equilibrated for 30 min in KR buffer at 30°C, transferred to tubes containing 0.5 µCi/ml 22Na, and incubated for 5 min. To remove 22Na from the extracellular space, all muscles were washed twice for 5 min in KR at 30°C, blotted, weighed, and taken for counting. 10−6 M TTX, when added, was present during the last 15 min of the equilibration period. The uptake of 22Na was calculated from the specific activity of the isotope in the incubation medium. Results are expressed as nanomoles of 22Na/gram of wet weight/minute ± SEM in soleus of WT and mutants and their contralateral muscles exposed to TTX. The number of muscles is given in parentheses.
Membrane potential recordings
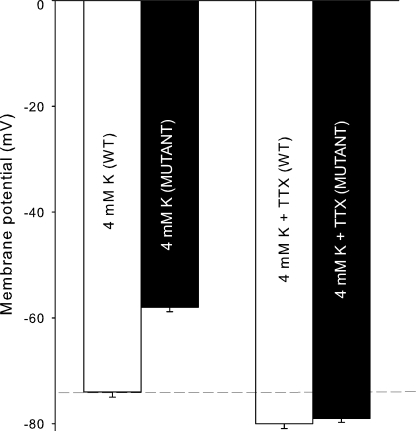
Recordings of membrane potentials showed that mutant mice were depolarized by 16 mV (P < 0.0001 when compared with WT; Fig. 9). The addition of 2 × 10−7 M TTX caused hyperpolarization in the fibers of both genotypes (P < 0.0001). However, in the presence of 2 × 10−7 M TTX, there was no significant difference between the resting membrane potentials of WT and mutant muscles. This is compatible with a higher resting permeability for Na+ in the mutant mice through altered NaV1.4 function, as also suggested by the larger TTX-suppressible 22Na uptake in mutants.
Figure 9.
Effects of 2 × 10−7 M TTX on the resting membrane potentials of soleus muscle fibers. Muscles were prepared from 12-wk-old WT and mutant mice, and membrane potentials were recorded with standard glass microelectrodes as described in the Materials and methods section. Each column represents the mean of membrane potentials recorded in the fibers of four soleus muscles from mutants and four from WT, with error bars indicating SEM. In soleus from WT, 94 recordings were made before and 80 after the addition of TTX. In soleus from mutants, 101 recordings were made before and 71 after the addition of TTX. The dashed line represents the resting membrane potential of WT controls.
86Rb uptake and Na+ contents
The role of the Na+,K+ pumps was characterized by measuring 86Rb uptake and Na+ contents in soleus muscles exposed to the same stimulation paradigm as in the measurements of force stimulation by salbutamol (Fig. 5). As shown in Table II, 86Rb uptake as measured over a 40-min incubation was significantly (P < 0.02) higher (12%) in the mutants than in WT. Total Na+ content was also significantly (P < 0.02) larger (16%) in the mutants than in WT. 10−6 M salbutamol induced a significant stimulation (18%) of 86Rb uptake in WT but no significant change in total Na+ content. Salbutamol induced a significant (P < 0.005) stimulation (10%) of 86Rb uptake in mutants and a significant (P < 0.001) decrease (15%) in total Na+ content. In contrast, rCGRP caused no significant change in 86Rb uptake or total Na+ contents in mutants. In WT, however, rCGRP induced a significant (P < 0.02) stimulation (19%) of 86Rb uptake but no significant change in Na+ content.
Table II.
Effects of 10−6 M salbutamol and 10−6 M rCGRP on total 86Rb uptake and total Na+ contents in WT and mutant mouse soleus
| Genotype | 86Rb uptake | P-value | Total Na+ content | P-value |
| nmol/g wet wt/min | µmol/g wet wt | |||
| WT | 697 ± 27 (6) | <0.02 | 42.6 ± 2.1 (6) | >0.5 |
| WT + salbutamol | 821 ± 36 (6) | 41.1 ± 0.9 (6) | ||
| Mutant | 783 ± 15 (6) | <0.005 | 49.5 ± 0.9 (6) | <0.001 |
| Mutant + salbutamol | 858 ± 14 (6) | 41.8 ± 1.3 (6) | ||
| WT | 650 ± 24 (4) | <0.02 | 45.1 ± 2.1 (4) | >0.15 |
| WT + rCGRP | 776 ± 28 (4) | 40.9 ± 1.6 (4) | ||
| Mutant | 815 ± 17 (8) | >0.9 | 45.4 ± 1.4 (8) | >0.08 |
| Mutant + rCGRP | 818 ± 30 (8) | 41.9 ± 1.4 (8) |
The muscles were mounted at resting length on stimulation electrodes, equilibrated for 30 min in KR with 10−5 M tubocurarine, transferred to KR with 0.2 µCi/ml 86Rb, incubated for 40 min with or without 10−6 M salbutamol or 10−6 M rCGRP, blotted, weighed, and taken for counting of 86Rb and measurement of Na+ content. All muscles were stimulated every 10 min with 2-s 120-Hz trains of 0.2-ms pulses of 7 V. All values are given as means with SEM and the number of muscles in parentheses. Controls are compared with contralateral muscles exposed to salbutamol or to rCGRP.
To reduce the contribution from 86Rb and Na+ residing in the extracellular space and gain information about the early phase of 86Rb uptake in resting muscles, these experiments were also performed using shorter incubation with 86Rb (10 min) and followed by a 15-min washout performed four times in ice-cold Na+-free Tris-sucrose buffer (Everts and Clausen, 1992; Buchanan et al., 2002). As shown in Table III, in soleus from mutants, 86Rb uptake was 54% higher than in those from WT (P < 0.001). In soleus from mutants, ouabain-suppressible 86Rb uptake was 83% larger than in those from WT. Salbutamol increased 86Rb uptake both in WT (by 35%) and in mutants (by 19%). Intracellular Na+ content was 58% higher in the muscles from the mutants than in those from WT (P < 0.003). When incubated with 10−3 M ouabain, intracellular Na+ content of soleus from WT and mutants was clearly elevated in comparison to controls (by 518 and 500%, respectively). In the presence of ouabain, the muscles from the mutants showed a clearly higher (51%) intracellular Na+ content (P < 0.001) than in those from WT. Conversely, stimulation of the Na+,K+ pumps with salbutamol reduced intracellular Na+ content by 44% in mutants and by 39% in WT. In the presence of ouabain, salbutamol caused no decrease in the elevated intracellular Na+. However, salbutamol increased 86Rb uptake, indicating that the active Na+,K+ transport had not been completely suppressed by the 10-min preincubation with ouabain, leaving some Na+,K+ pumps operative. Because salbutamol augments the affinity of the Na+,K+ pumps for intracellular Na+ (Buchanan et al., 2002), the elevated intracellular Na+ could allow salbutamol to stimulate 86Rb uptake.
Table III.
Effects of 10−6 M salbutamol and 10−3 M ouabain on 86Rb uptake and intracellular Na+ content in soleus muscles from WT and mutant mice
| Genotype | 86Rb uptake | P-value | Intracellular Na+ content | P-value |
| nmol/g wet wt/min | µmol/g wet wt | |||
| WT | 652 ± 66 (6) | <0.02 | 7.4 ± 0.4 (6) | <0.001 |
| WT + salbutamol | 883 ± 45 (6) | 4.5 ± 0.2 (6) | ||
| WT + ouabain | 186 ± 9 (5) | <0.002 | 30.9 ± 0.5 (5) | >0.3 |
| WT + ouabain + salbutamol | 252 ± 12 (6) | 32.0 ± 1.0 (4) | ||
| Mutant | 1,005 ± 39 (6) | <0.005 | 11.7 ± 1.0 (6) | <0.005 |
| Mutant + salbutamol | 1,198 ± 34 (6) | 6.6 ± 0.6 (6) | ||
| Mutant + ouabain | 154 ± 7 (4) | <0.005 | 46.8 ± 1.2 (4) | >0.15 |
| Mutant + ouabain + salbutamol | 207 ± 10 (6) | 48.6 ± 0.6 (6) |
The muscles were mounted at resting length and equilibrated for 30 min at 30°C in KR buffer. Then, the muscles were incubated at rest for 10 min in buffer containing 0.2 µCi/ml 86Rb with or without salbutamol, washed four times for 15 min in ice-cold Na+-free Tris-sucrose buffer during gassing with air, blotted, weighed, and taken for flame photometry and counting. 86Rb uptake was calculated on the basis of the specific activity of the isotope in the buffer (see Materials and methods), and the results are expressed in nanomoles/gram of wet weight/minute. The ouabain-suppressible component of 86Rb uptake was determined by deducting the uptake of 86Rb measured in muscles preexposed to 10−3 M ouabain for 10 min before the incubation with 86Rb.
To examine whether the increase in Na+ content was the result of changes induced by the incubation procedures, we also measured the total Na+ content in fresh muscles prepared from the animals and extracted in TCA without any incubation. In tibialis anterior from 12-wk-old mutant and WT mice, total Na+ contents were 18.3 ± 0.3 and 16.3 ± 0.6 µmol/g wet wt, respectively (n = 8 vs. 8, P < 0.03). In tibialis anterior from 12-mo-old mutant and WT mice, total Na+ contents were 17.6 ± 0.5 and 15.1 ± 0.3 µmol/g wet wt, respectively (n = 4 vs. 5, P < 0.005). In EDL muscles from 17–19-wk-old mutant and WT mice, total Na+ content was 30.5 ± 1.1 and 25.3 ± 1.2 µmol/g wet wt, respectively (n = 6 vs. 8, P < 0.02). This indicates that the increase in Na+ content is seen in vivo and is not a result of the incubation procedures. Moreover, it may persist for 12 mo.
It should be noted that the total Na+ content measured without washout in Na+-free buffer (Table II) is clearly higher than that measured after the muscles had been washed (Table III). This can be attributed to the Na+ residing in the extracellular space after the incubation (∼30 µmol/g wet wt). Measurements of [14C]sucrose space show that after incubation, this amounts to ∼20% of the wet weight (Clausen et al., 2004). In the fresh muscles, total Na+ contents were also higher than in those that had been washed in Na+-free buffer. However, the difference was smaller because the extracellular space in vivo is smaller than that reached during incubation in vitro (Neville and White, 1979).
[3H]Ouabain binding
As shown in Table IV, the content of [3H]ouabain binding sites in soleus muscles of 12.5-wk-old mice was 28% higher in mutants than in WT. In the EDL muscle of the same mice, the content of [3H]ouabain binding sites was 62% higher in the mutants than in WT. In tibialis anterior of mutants, the content of [3H]ouabain binding sites was 33% higher than in WT.
Table IV.
[3H]Ouabain binding sites in soleus, EDL, and tibialis anterior muscles of WT and mutant mice
| Muscle | [3H]Ouabain binding | P-value |
| pmol/g wet wt | ||
| WT soleus | 746 ± 28 (6) | <0.004 |
| Mutant soleus | 952 ± 43 (10) | |
| WT EDL | 647 ± 14 (4) | <0.001 |
| Mutant EDL | 1,047 ± 37 (4) | |
| WT tibialis anterior | 417 ± 28 (8) | <0.001 |
| Mutant tibialis anterior | 554 ± 22 (8) |
[3H]Ouabain binding in 12.5-wk-old WT and mutant mice. Whole frozen soleus and EDL muscles or biopsies of tibialis anterior muscles were incubated for 2 h at 37°C in Tris-vanadate buffer containing 10−6 M [3H]ouabain. After the incubation, the muscles were washed four times for 30 min in ice-cold Tris-vanadate buffer to remove unbound [3H]ouabain, blotted, and taken for counting and calculation of specific [3H]ouabain binding as described in the Materials and methods section.
DISCUSSION
The soleus and EDL muscles isolated from mutant mice offer models for the study of the relationship between contractility, Na+,K+ fluxes, and Na+,K+-pump activity in HyperKPP. Our results emphasize that the pathophysiology of HyperKPP is caused by anomalies in skeletal muscle cells, primarily increased TTX-sensitive Na+ influx and depolarization, leading to reduced excitability, contractility, and endurance. Moreover, these muscles allow the testing of pharmaceuticals of potential value in the treatment of HyperKPP in patients.
The major results of this study are as follows: (a) soleus muscles of mutant mice showed reduced force and endurance that could be related to reduced excitability. (b) The defect in force production in vitro could be restored by addition of salbutamol, rCGRP, capsaicin, or monensin and by repeated electrical stimulation. Also, in EDL muscles, mutants showed reduced force that could be restored to WT level by salbutamol. (c) Salbutamol induced significant stimulation of the Na+,K+ pumps in both mutant and WT soleus muscles. (d) Na+ loading with monensin improves contractile function, indicating that this can be attributed to stimulation of the Na+,K+ pumps induced primarily by Na+. (e) Resting mutant muscles showed increased intracellular Na+ that could be related to markedly increased Na+ influx via Na+ channels, causing pronounced depolarization. The increase in intracellular Na+ was associated with increased 86Rb uptake and up-regulation of the content of Na+,K+ pumps.
Force, fatigue, and excitability
When stimulated with single pulses or tetanic trains of pulses, the isolated soleus muscles from mutant mice were generally weaker than those of WT and showed markedly reduced endurance during continuous tetanic stimulation. Because previous studies have demonstrated that loss of excitability contributes significantly to the loss of force when isolated muscles are fatigued by continuous high frequency stimulation (Clausen et al., 2004), these differences in contractility and endurance are most likely related to the lower excitability of mutant muscles (Fig. 3), as already suggested by Creutzfeldt et al. (1963). Similarly, the difference in excitability is a likely explanation for the larger loss of force in mutant soleus muscles when they were exposed to elevated [K+]o (Fig. 3) and for their 70% faster rate of force decline when they were stimulated continuously at 10 mM K+. The larger loss of force in mutant soleus muscles when they were exposed to elevated [K+]o is in keeping with the results of experiments on the effects of elevated [K+]o on tetanic force in EDL muscles from mice with the same mutation (Hayward et al., 2008). However, in contrast to soleus, the mutant EDL muscles in that study showed increased contractile endurance during continuous stimulation (Hayward et al., 2008). In the present study, tetanic force of mutant EDL was reduced in comparison to WT, although the reduction was less than in soleus. These results suggest that the pathophysiological changes causing HyperKPP are somewhat more pronounced in slow-twitch muscle fibers than in fast-twitch fibers. The difference between EDL and soleus may be related to the fact that in EDL, the resting membrane potential is hyperpolarized in comparison to soleus (Cairns et al., 1997).
In human subjects with HyperKPP, attacks start in the first decade of life and become more frequent in the second decade (Carson and Pearson, 1964; Layzer et al., 1967). In keeping with this, we find that in the mutants, the reduction in tetanic force is slightly more pronounced in soleus muscles from 12.5-wk-old mutants (49%) than in muscles from 5.5-wk-old mice (30%). Thus, the 12.5-wk-old mice might be more prone to develop attacks than the 5.5-wk-old mice.
Na+ contents, Na+,K+ fluxes, and membrane potential
Resting soleus muscles from the mutants showed higher intracellular Na+ content than those from the WT. This difference could not be attributed to a reduction in the content or activity of the Na+,K+ pumps in the mutants. In contrast, both soleus, EDL, and tibialis anterior muscles from the mutants contained significantly more Na+,K+ pumps, and soleus showed a 83% faster ouabain-suppressible 86Rb uptake than the WT muscles. The elevated Na+,K+ pump–mediated 86Rb uptake is most likely a result of the increased intracellular Na+ (Everts and Clausen, 1992; Buchanan et al., 2002). The increase in Na+ content was not only seen in the muscles that had been incubated, but also in muscles prepared directly from mutant mice without incubation. This indicates that also in vivo, there is a significant increase in intracellular Na+.
The simplest explanation for the elevated intracellular Na+ in the mutant muscles seems to be increased resting Na+ influx. Thus, in the soleus from the mutants, resting TTX-suppressible 22Na influx was 5.7-fold larger than in the WT. This reflects higher Na+ channel activity caused by defective slow inactivation of Nav1.4 channels, which is in keeping with previous experiments (Hayward et al., 1999). Increased Na+ influx also explains the rapid early drop in plasma Na+ seen at the onset of attacks of paralysis in the HyperKPP patients (Streeten et al., 1971; Clausen et al., 1980) as well as the repeated observation of depolarization in resting skeletal muscle of patients with HyperKPP (Creutzfeldt et al., 1963; McComas et al., 1968) and horses with a similar disorder (Pickar et al., 1991). In the present study, the markedly augmented resting TTX-suppressible influx of 22Na gave rise to a pronounced TTX-sensitive depolarization that explains the lower excitability of muscle from mutant mice. Because depolarization in earlier experiments has been shown to cause a reduction in both tetanic force and contractile endurance (Clausen and Nielsen, 2007), it is most likely that the compromised contractile function of muscles from HyperKPP is the result of depolarization.
Force recovery by salbutamol, rCGRP, capsaicin, monensin, and repeated electrical stimulation
In this study, 10−8–10−6 M salbutamol caused a graded restoration of contractile force in soleus muscles from mutant mice, and at the highest concentration of salbutamol examined, force reached the same level as recorded in muscles from WT mice. These results are in keeping with the clinical experience that salbutamol given as inhalation or tablets suppresses or prevents paralytic attacks in patients with HyperKPP (Wang and Clausen, 1976; Hanna et al., 1998). In the present study, salbutamol also caused a significant increase in Na+,K+-pump activity as demonstrated by an increase in both the total and the ouabain-suppressible uptake of 86Rb and a reduced intracellular Na+ content. Also, in mutant EDL muscles, 10−6 M salbutamol caused a significant increase (64%) in tetanic force, indicating that the fast-twitch fibers contribute to the restoration of force induced by administration of the β2 agonist to HyperKPP patients. It is intriguing that both in mutant soleus and EDL, salbutamol restores tetanic force to almost that of the WT muscles.
Previously, it had been established that similar stimulation of the Na+,K+ pumps with salbutamol and other β2 agonists can produce a large increase in excitability and contractile function in isolated skeletal muscles in which excitability is compromised by depolarization (Clausen, 2003). This effect is related to hyperpolarization, increased uptake of K+, and decreased intracellular Na+ induced by the increase in Na+,K+-pump activity (Kuba, 1970; Wang and Clausen, 1976; Clausen and Flatman, 1977; Ballanyi and Grafe, 1988; Overgaard et al., 1999; Clausen, 2003). It is most likely, therefore, that the increase in Na+,K+-pump activity after addition of salbutamol to muscles from HyperKPP mice is the main reason for the improvement of contractile function observed in the present study. This conclusion is supported by two sets of observations. First, when the Na+,K+-pump activity was increased by loading the muscles with Na+ using monensin, muscles of mutant mice showed pronounced force recovery, indicating that repolarization induced by stimulation of the electrogenic Na+,K+ pumps itself is sufficient to improve excitability. Second, when isolated intercostal muscles from a patient with HyperKPP were depolarized by exposure to 7 mM K+, epinephrine induced repolarization and a decrease in intracellular Na+ activity (Ricker et al., 1989).
Epinephrine and salbutamol also induced hyperpolarization in normal rat soleus muscle in vivo (Flatman and Clausen, 1979), and in the human forearm, intraarterial injection of the β2 agonist terbutalin increased the uptake of K+ in skeletal muscle (Ford et al., 1995). Likewise, in horses with HyperKPP, epinephrine induced hyperpolarization in the muscle cells (Pickar et al., 1991). Together with the observations that even low concentrations of ouabain (0.5–2.0 µM) induce substantial loss of force in EDL muscles from mutant mice (Hayward et al., 2008), these findings provide evidence that the activity of the Na+,K+ pumps is important in maintaining contractile performance of muscles from mutant mice. Therefore, the beneficial effects of salbutamol on muscle paralysis during hyperkalemic attacks in human subjects with HyperKPP is primarily the result of direct Na+,K+-pump stimulation in the muscles, inducing force recovery in the paralyzed muscles (Clausen, 2003). In addition, because the muscles in HyperKPP patients are more sensitive to K+, the general hypokalemic effect of salbutamol contributes to force recovery.
The present study also showed that rCGRP could induce substantial force recovery in the soleus of mutant mice. As for salbutamol, rCGRP has been shown to stimulate the Na+,K+ pumps in rat muscle (Andersen and Clausen, 1993), and in the present study, rCGRP was found to stimulate 86Rb uptake in WT mice. Increased Na+,K+-pump activity could, therefore, be envisaged to contribute to the effect of CGRP on force in muscles from mutant mice. Based on measurements of 86Rb uptake and intracellular Na+ content, however, this mechanism seemed to be of limited importance in this case, indicating that other mechanisms must have contributed to the increase in excitability after addition of CGRP. The present study does not allow for an identification of such other mechanisms, but a similar conclusion was reached in an earlier study on isolated rat muscles in which, based on action potential configuration, it was suggested that CGRP could improve the excitability of muscles via an effect on the voltage-gated Na+ channels (Macdonald et al., 2008).
In addition to the improvement of contractile function seen after addition of salbutamol and rCGRP to muscles from mutant mice, we here demonstrate that repeated excitation itself can elicit a substantial increase in the tetanic force production. Importantly, this suggests that the recurrent observation that patients with HyperKPP can fend off attacks of paralysis by exercising their muscles rather is related to local factors within the working muscles than to systemic factors like circulating hormones. Because repeated stimulation of depolarized rat soleus muscle causes a local release of CGRP from the nerve endings that is sufficient to produce a substantial force recovery (Nielsen et al., 1998; Macdonald et al., 2008), it is possible that the peptide released from nerve endings contributes to the excitation-induced protection of muscle function in HyperKPP. Indeed, after treatment with capsaicin, which induces a release of CGRP from the nerve endings in the muscle, soleus muscles from the mutant mice showed a 79% increase in contractile force. These observations indicate that the beneficial effect of exercise on paralysis in patients with HyperKPP is mediated via a release of CGRP from the endogenous stores of CGRP in nerve endings. This may be localized to the exercising muscles and is not caused by changes in plasma K+.
Content of Na+,K+ pumps
Previous studies have shown that in horses with HyperKPP, the content of [3H]ouabain binding sites in skeletal muscles showed no difference from clinically normal horses (Pickar et al., 1993). At variance with that, the present results demonstrate that in soleus, EDL, and tibialis anterior muscles of mutant mice, the content of [3H]ouabain binding sites was significantly higher than in WT mice. This might reflect that long-term exposure to elevated intracellular Na+ stimulates the synthesis of Na+,K+ pumps, which is in keeping with the well-documented up-regulation of Na+,K+ pumps in isolated cells (myotubes) exposed to Na+ loading (Brodie and Sampson, 1989) or thyroid hormone (Harrison and Clausen, 1998). In the mutant mice, the up-regulation of Na+,K+ pumps was clearly larger in EDL than in soleus, perhaps because the excitation-induced Na+ influx is considerably larger in EDL than in soleus (Clausen et al., 2004). The content of [3H]ouabain binding sites observed in soleus muscle obtained from WT mice (746 ± 28 pmol/g wet wt) was only slightly higher than the ∼600 pmol/g wet wt measured in the soleus of NMRI mice in the same age range (Kjeldsen et al., 1984).
It should be noted that in patients with HyperKPP, 42K uptake and the content of [3H]ouabain binding sites in lymphocytes were not increased, indicating that an up-regulation of the Na+,K+ pumps in HyperKPP is not a general cellular phenomenon in this disease (Clausen et al., 1980). It has been proposed that HyperKPP might be caused by impairment of the Na+,K+ pumps (Brooks, 1969). In view of the evidence available now, this seems unlikely. On the other hand, increased physical activity is known to up-regulate the content of Na+,K+ pumps in skeletal muscles, which is likely to contribute to contractile endurance (Clausen, 2003). Here, we show that the muscles of the mutant mice contain more Na+,K+ pumps than those of the WT. This up-regulation may be important for the ability to fend off paralytic attacks. Therefore, it would be of interest to measure the content of Na+,K+ pumps in the muscles of HyperKPP patients before and after exercise training and to relate this to the frequency and severity of attacks.
Acknowledgments
We thank Tove Lindahl Andersen, Lene Hornbæk Holm, Marianne Stürup-Johansen, and Vibeke Uhre for skilled technical assistance.
This study was supported by grants from The Lundbeck Foundation (J. No. R31-A2536), Aarhus Universitets Forskningsfond, and the Karen Elise Jensen Foundation.
Lawrence G. Palmer served as editor.
Footnotes
Abbreviations used in this paper:
- CGRP
- calcitonin gene-related peptide
- EDL
- extensor digitorum longus
- ES
- embryonic stem
- HyperKPP
- hyperkalemic periodic paralysis
- rCGRP
- rat CGRP
- TCA
- trichloroacetic acid
- TTX
- tetrodotoxin
- WT
- wild type
References
- Andersen S.L.V., Clausen T. 1993. Calcitonin gene-related peptide stimulates active Na+-K+ transport in rat soleus muscle. Am. J. Physiol. 264:C419–C429 [DOI] [PubMed] [Google Scholar]
- Ballanyi K., Grafe P. 1988. Changes in intracellular ion activities induced by adrenaline in human and rat skeletal muscle. Pflugers Arch. 411:283–288 10.1007/BF00585116 [DOI] [PubMed] [Google Scholar]
- Brodie C., Sampson S.R. 1989. Regulation of the sodium-potassium pump in cultured rat skeletal myotubes by intracellular sodium ions. J. Cell. Physiol. 140:131–137 10.1002/jcp.1041400116 [DOI] [PubMed] [Google Scholar]
- Brooks J.E. 1969. Hyperkalemic periodic paralysis. Intracellular electromyographic studies. Arch. Neurol. 20:13–18 [DOI] [PubMed] [Google Scholar]
- Buchanan R., Nielsen O.B., Clausen T. 2002. Excitation- and beta2-agonist-induced activation of the Na+-K+ pump in rat soleus muscle. J. Physiol. 545:229–240 10.1113/jphysiol.2002.023325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns S.P., Hing W.A., Slack J.R., Mills R.G., Loiselle D.S. 1997. Different effects of raised [K+]o on membrane potential and contraction in mouse fast- and slow-twitch muscle. Am. J. Physiol. 273:C598–C611 [DOI] [PubMed] [Google Scholar]
- Carson M.J., Pearson C.M. 1964. Familial hyperkalemic periodic paralysis with myotonic features. J. Pediatr. 64:853–865 10.1016/S0022-3476(64)80643-0 [DOI] [PubMed] [Google Scholar]
- Clausen T. 2003. Na+-K+ pump regulation and skeletal muscle contractility. Physiol. Rev. 83:1269–1324 [DOI] [PubMed] [Google Scholar]
- Clausen T., Flatman J.A. 1977. The effect of catecholamines on Na-K transport and membrane potential in rat soleus muscle. J. Physiol. 270:383–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Hansen O. 1974. Ouabain binding and Na+-K+ transport in rat muscle cells and adipocytes. Biochim. Biophys. Acta. 345:387–404 10.1016/0005-2736(74)90200-4 [DOI] [Google Scholar]
- Clausen T., Nielsen O.B. 2007. Potassium, Na+,K+-pumps and fatigue in rat muscle. J. Physiol. 584:295–304 10.1113/jphysiol.2007.136044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Wang P., Ørskov H., Kristensen O. 1980. Hyperkalemic periodic paralysis. Relationships between changes in plasma water, electrolytes, insulin and catecholamines during attacks. Scand. J. Clin. Lab. Invest. 40:211–220 10.3109/00365518009095569 [DOI] [PubMed] [Google Scholar]
- Clausen T., Overgaard K., Nielsen O.B. 2004. Evidence that the Na+-K+ leak/pump ratio contributes to the difference in endurance between fast- and slow-twitch muscles. Acta Physiol. Scand. 180:209–216 10.1111/j.0001-6772.2003.01251.x [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O.D., Abbott B.C., Fowler W.M., Pearson C.M. 1963. Muscle membrane potentials in episodic adynamia. Electroencephalogr. Clin. Neurophysiol. 15:508–519 10.1016/0013-4694(63)90071-3 [DOI] [PubMed] [Google Scholar]
- Everts M.E., Clausen T. 1992. Activation of the Na-K pump by intracellular Na in rat slow- and fast-twitch muscle. Acta Physiol. Scand. 145:353–362 10.1111/j.1748-1716.1992.tb09375.x [DOI] [PubMed] [Google Scholar]
- Flatman J.A., Clausen T. 1979. Combined effects of adrenaline and insulin on active electrogenic Na+-K+ transport in rat soleus muscle. Nature. 281:580–581 10.1038/281580a0 [DOI] [PubMed] [Google Scholar]
- Ford G.A., Dachman W.D., Blaschke T.F., Hoffman B.B. 1995. Effect of aging on beta2-adrenergic receptor-stimulated flux of K+, PO4, FFA, and glycerol in human forearms. J. Appl. Physiol. 78:172–178 [DOI] [PubMed] [Google Scholar]
- Gamstorp I., Hauge M., Helweglarsen H.F., Mjones H., Sagild U. 1957. Adynamia episodica hereditaria: a disease clinically resembling familial periodic paralysis but characterized by increasing serum potassium during the paralytic attacks. Am. J. Med. 23:385–390 10.1016/0002-9343(57)90318-2 [DOI] [PubMed] [Google Scholar]
- Gu H., Zou Y.R., Rajewsky K. 1993. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 73:1155–1164 10.1016/0092-8674(93)90644-6 [DOI] [PubMed] [Google Scholar]
- Hanna M.G., Stewart J., Schapira A.H., Wood N.W., Morgan-Hughes J.A., Murray N.M. 1998. Salbutamol treatment in a patient with hyperkalaemic periodic paralysis due to a mutation in the skeletal muscle sodium channel gene (SCN4A). J. Neurol. Neurosurg. Psychiatry. 65:248–250 10.1136/jnnp.65.2.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen O., Clausen T. 1988. Quantitative determination of Na+-K+-ATPase and other sarcolemmal components in muscle cells. Am. J. Physiol. 254:C1–C7 [DOI] [PubMed] [Google Scholar]
- Harrison A.P., Clausen T. 1998. Thyroid hormone-induced upregulation of Na+ channels and Na+-K+ pumps: implications for contractility. Am. J. Physiol. 274:R864–R867 [DOI] [PubMed] [Google Scholar]
- Hayward L.J., Sandoval G.M., Cannon S.C. 1999. Defective slow inactivation of sodium channels contributes to familial periodic paralysis. Neurology. 52:1447–1453 [DOI] [PubMed] [Google Scholar]
- Hayward L.J., Kim J.S., Lee M.Y., Zhou H., Kim J.W., Misra K., Salajegheh M., Wu F.F., Matsuda C., Reid V., et al. 2008. Targeted mutation of mouse skeletal muscle sodium channel produces myotonia and potassium-sensitive weakness. J. Clin. Invest. 118:1437–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoya A., Venosa R.A. 1992. Ionic movements mediated by monensin in frog skeletal muscle. Biochim. Biophys. Acta. 1104:123–131 10.1016/0005-2736(92)90140-H [DOI] [PubMed] [Google Scholar]
- Kjeldsen K., Nøgaard A., Clausen T. 1984. The age-dependent changes in the number of 3H-ouabain binding sites in mammalian skeletal muscle. Pflugers Arch. 402:100–108 10.1007/BF00584838 [DOI] [PubMed] [Google Scholar]
- Kuba K. 1970. Effects of catecholamines on the neuromuscular junction in the rat diaphragm. J. Physiol. 211:551–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzer R.B. 1982. Periodic paralysis and the sodium-potassium pump. Ann. Neurol. 11:547–552 10.1002/ana.410110602 [DOI] [PubMed] [Google Scholar]
- Layzer R.B., Lovelace R.E., Rowland L.P. 1967. Hyperkalemic periodic paralysis. Arch. Neurol. 16:455–472 [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Rüdel R., Jurkat-Rott K. 2004. Nondystrophic myotonias and periodic paralyses. Myology: Basic and Clinical. Engel A.G., Franzini-Armstrong C., Third edition. McGraw-Hill, New York: 1257–1300 [Google Scholar]
- Macdonald W.A., Nielsen O.B., Clausen T. 2008. Effects of calcitonin gene-related peptide on rat soleus muscle excitability: mechanisms and physiological significance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295:R1214–R1223 10.1152/ajpregu.00893.2007 [DOI] [PubMed] [Google Scholar]
- McComas A.J., Mrozek K., Bradley W.G. 1968. The nature of the electrophysiological disorder in adynamia episodica. J. Neurol. Neurosurg. Psychiatry. 31:448–452 10.1136/jnnp.31.5.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna M.J., Gissel H., Clausen T. 2003. Effects of electrical stimulation and insulin on Na+-K+-ATPase ([3H]ouabain binding) in rat skeletal muscle. J. Physiol. 547:567–580 10.1113/jphysiol.2003.034512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K.T., Nielsen O.B., Clausen T. 2008. Analysis of exercise-induced Na+-K+ exchange in rat skeletal muscle in vivo. Exp. Physiol. 93:1249–1262 10.1113/expphysiol.2008.042457 [DOI] [PubMed] [Google Scholar]
- Neville M.C., White S. 1979. Extracellular space of frog skeletal muscle in vivo and in vitro: relation to proton magnetic resonance relaxation times. J. Physiol. 288:71–83 [PMC free article] [PubMed] [Google Scholar]
- Nielsen O.B., Hilsted L., Clausen T. 1998. Excitation-induced force recovery in potassium-inhibited rat soleus muscle. J. Physiol. 512:819–829 10.1111/j.1469-7793.1998.819bd.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nørgaard A., Kjeldsen K., Hansen O., Clausen T. 1983. A simple and rapid method for the determination of the number of 3H-ouabain binding sites in biopsies of skeletal muscle. Biochem. Biophys. Res. Commun. 111:319–325 10.1016/S0006-291X(83)80154-5 [DOI] [PubMed] [Google Scholar]
- Overgaard K., Nielsen O.B., Flatman J.A., Clausen T. 1999. Relations between excitability and contractility in rat soleus muscle: role of the Na+-K+ pump and Na+/K+ gradients. J. Physiol. 518:215–225 10.1111/j.1469-7793.1999.0215r.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen T.H., de Paoli F., Nielsen O.B. 2005. Increased excitability of acidified skeletal muscle: role of chloride conductance. J. Gen. Physiol. 125:237–246 10.1085/jgp.200409173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickar J.G., Spier S.J., Snyder J.R., Carlsen R.C. 1991. Altered ionic permeability in skeletal muscle from horses with hyperkalemic periodic paralysis. Am. J. Physiol. 260:C926–C933 [DOI] [PubMed] [Google Scholar]
- Pickar J.G., Spier S.J., Harrold D., Carlsen R.C. 1993. [3H]ouabain binding in skeletal muscle from horses with hyperkalemic periodic paralysis. Am. J. Vet. Res. 54:783–787 [PubMed] [Google Scholar]
- Ricker K., Camacho L.M., Grafe P., Lehmann-Horn F., Rüdel R. 1989. Adynamia episodica hereditaria: what causes the weakness? Muscle Nerve. 12:883–891 10.1002/mus.880121103 [DOI] [PubMed] [Google Scholar]
- Shizuya H., Birren B., Kim U.J., Mancino V., Slepak T., Tachiiri Y., Simon M. 1992. Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc. Natl. Acad. Sci. USA. 89:8794–8797 10.1073/pnas.89.18.8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. 1991. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 64:693–702 10.1016/0092-8674(91)90499-O [DOI] [PubMed] [Google Scholar]
- Streeten D.H.P., Dalakos T.G., Fellerman H. 1971. Studies on hyperkalemic periodic paralysis. Evidence of changes in plasma Na and Cl and induction of paralysis by adrenal glucocorticoids. J. Clin. Invest. 50:142–155 10.1172/JCI106468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Clausen T. 1976. Treatment of attacks in hyperkalaemic familial periodic paralysis by inhalation of salbutamol. Lancet. 307:221–223 10.1016/S0140-6736(76)91340-4 [DOI] [PubMed] [Google Scholar]
- Weber M.A., Nielles-Vallespin S., Essig M., Jurkat-Rott K., Kauczor H.-U., Lehmann-Horn F. 2006. Muscle Na+ channelopathies: MRI detects intracellular 23Na accumulation during episodic weakness. Neurology. 67:1151–1158 10.1212/01.wnl.0000233841.75824.0f [DOI] [PubMed] [Google Scholar]