Abstract
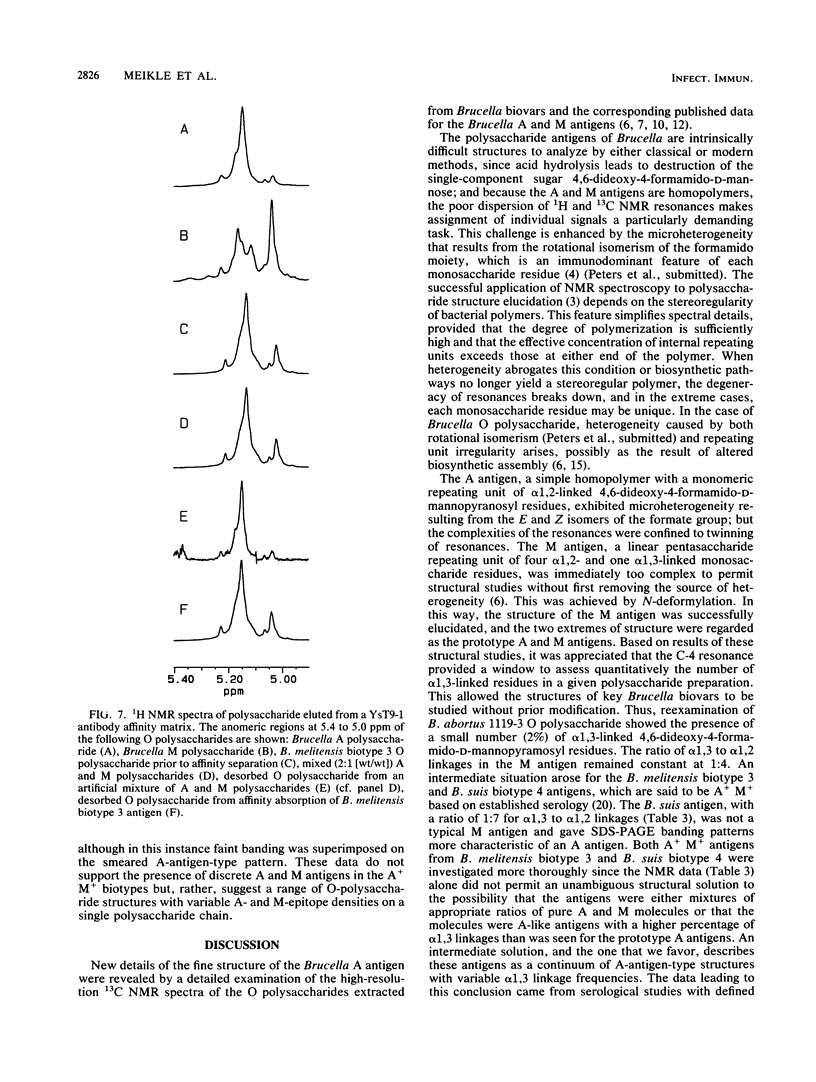
Brucella A and M epitopes were found on single O-polysaccharide chains of all biotype strains of this species. Lipopolysaccharides from the type and reference strains of five of the six Brucella species, B. abortus, B. melitensis, B. suis, B. canis, and B. neotomae, were extracted and purified. Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, in conjunction with silver staining and immunoblotting developed by monoclonal antibodies, showed bands characteristic of A, M, or mixed A and M antigens. The A antigen previously described as an exclusively alpha 1,2-linked homopolymer of 4,6-dideoxy-4-formamido-D-mannopyranose was shown by 1H and 13C nuclear magnetic resonance spectroscopy to possess a fine structure consistent with the low-frequency occurrence of alpha 1, 3-linked 4,6-dideoxy-4-formamido-D-mannopyranose residues. This feature was previously attributed only to the M antigen, which is also a homopolymer of the same sugar. B. melitensis biotype 3 and B. suis biotype 4 lipopolysaccharides showed characteristics of mixed A and M antigens. Immunoabsorption of these O polysaccharides on a column of immobilized A-antigen-specific monoclonal antibody enriched polymer chains with A-antigen characteristics but did not eliminate M epitopes. Composite A- and M-antigen characteristics resulted from O polysaccharides in which the frequency of alpha 1,3 linkages, and hence, M-antigen characteristics, varied. All biotypes assigned as A+ M- expressed one or two alpha 1,3-linked residues per polysaccharide O chain. M antigens (M+ A-) also possessed a unique M epitope as well as a tetrasaccharide determinant common to A-antigen structures. B. canis and B. abortus 45/20, both rough strains, expressed low-molecular-weight A antigen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahvonen P., Jansson E., Aho K. Marked cross-agglutination between Brucellae and a subtype of Yersinia enterocolitica. Acta Pathol Microbiol Scand. 1969;75(2):291–295. [PubMed] [Google Scholar]
- Bundle D. R., Cherwonogrodzky J. W., Gidney M. A., Meikle P. J., Perry M. B., Peters T. Definition of Brucella A and M epitopes by monoclonal typing reagents and synthetic oligosaccharides. Infect Immun. 1989 Sep;57(9):2829–2836. doi: 10.1128/iai.57.9.2829-2836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundle D. R., Cherwonogrodzky J. W., Perry M. B. Characterization of Brucella polysaccharide B. Infect Immun. 1988 May;56(5):1101–1106. doi: 10.1128/iai.56.5.1101-1106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundle D. R., Cherwonogrodzky J. W., Perry M. B. Structural elucidation of the Brucella melitensis M antigen by high-resolution NMR at 500 MHz. Biochemistry. 1987 Dec 29;26(26):8717–8726. doi: 10.1021/bi00400a034. [DOI] [PubMed] [Google Scholar]
- Bundle D. R., Cherwonogrodzky J. W., Perry M. B. The structure of the lipopolysaccharide O-chain (M antigen) and polysaccharide B produced by Brucella melitensis 16M. FEBS Lett. 1987 Jun 1;216(2):261–264. doi: 10.1016/0014-5793(87)80702-0. [DOI] [PubMed] [Google Scholar]
- Bundle D. R., Gidney M. A., Perry M. B., Duncan J. R., Cherwonogrodzky J. W. Serological confirmation of Brucella abortus and Yersinia enterocolitica O:9 O-antigens by monoclonal antibodies. Infect Immun. 1984 Nov;46(2):389–393. doi: 10.1128/iai.46.2.389-393.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundle D. R., Perry M. B. Structure and serology of the Brucella abortus O-antigen. Biochem Soc Trans. 1985 Dec;13(6):980–982. doi: 10.1042/bst0130980. [DOI] [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B., Cherwonogrodzky J. W., Duncan J. R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 1984 Nov;46(2):384–388. doi: 10.1128/iai.46.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B. Structure of the O-chain of the phenol-phase soluble cellular lipopolysaccharide of Yersinia enterocolitica serotype O:9. Eur J Biochem. 1984 Feb 15;139(1):195–200. doi: 10.1111/j.1432-1033.1984.tb07994.x. [DOI] [PubMed] [Google Scholar]
- Diaz R., Jones L. M. The immuno-diffusion method for the identification of cattle vaccinated with Brucella abortus strain 45-20. Vet Rec. 1973 Sep 15;93(11):300–302. doi: 10.1136/vr.93.11.300. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]