IKKa associates with the il17a locus and is required in T cells for Th17-mediated CNS inflammation in vivo.
Abstract
Th17 cells are a subset of T cells that play crucial roles in the pathogenesis of many inflammatory diseases. We report here the identification of IKKα (inhibitor of NF-κB kinase-α) as a key transcriptional regulator of the Th17 lineage. T cells expressing a nonactivatable form of IKKα were significantly compromised in their ability to produce IL-17 and to initiate neural inflammation. IKKα is present in the nuclei of resting CD4+ T cells. Upon Th17 differentiation, IKKα selectively associated with the Il17a locus, and promoted its histone H3 phosphorylation and transcriptional activation in a NF-κB–independent manner. These findings indicate that nuclear IKKα maintains the Th17 phenotype by activating the Il17a gene.
For many years, CD4+ T helper (Th) cells have been classified into two major types, Th1 and Th2 cells (Romagnani, 1997). Th1 cells express IFN-γ and control cellular immunity, whereas Th2 cells produce IL-4, IL-5, and IL-13 and regulate humoral immunity. Recently, a new helper T cell subset, Th17 (also known as Thi), which produces IL-17A, IL-17F, IL-21, and IL-22, but not IFN-γ or IL-4, has been defined (Cua et al., 2003; Langrish et al., 2005; Veldhoen and Stockinger, 2006; Ivanov et al., 2006; Weaver et al., 2006; Bettelli et al., 2006; Sutton et al., 2006). Unlike Th1 and Th2 cells, Th17 cells are considered to be “proinflammatory” because they are involved primarily in mediating inflammatory diseases and immune defense against extracellular bacteria (Langrish et al., 2005; Bettelli et al., 2006; Ivanov et al., 2006; Veldhoen and Stockinger, 2006; Weaver et al., 2006; Sutton et al., 2006). Th17 cells can be generated in vitro by activating naive T cells in the presence of IL-6/IL-21 and TGF-β (Weaver et al., 2006; Bettelli et al., 2007). IL-6 acts in a signal transducer and activator of transcription 3 (Stat3)-dependant manner to induce IL-21, IL-23 receptor, retinoid-related orphan receptor (ROR) γT, and RORα expression (Yang et al., 2007; Dong, 2008; Yang et al., 2008). Upon binding to IL-23, which is normally produced by macrophages and dendritic cells, IL-23 receptor promotes the survival of Th17 cells and maintains its differentiated phenotype (Cua et al., 2003; Langrish et al., 2005). Transcriptionally, RORγT and RORα are considered to be master regulators of Th17 differentiation, as T-bet and GATA3 are to Th1 and Th2 cells, respectively (Ivanov et al., 2006; Dong, 2008; Yang et al., 2008). Moreover, similar to Th1 and Th2 cells in which the Ifng and Il4 loci are selectively activated, respectively, differentiated Th17 cells exhibit unique epigenetic modifications of the Il17a locus (Akimzhanov et al., 2007). However, the nuclear factors that are responsible for Il17a locus activation are not well understood.
The inhibitor of nuclear factor-κB kinase-α (IKKα) is a member of the IKK family, which regulates multiple biological processes through either NF-κB–dependent or –independent mechanisms (Häcker and Karin, 2006). IKKα can phosphorylate NF-κB2 (p100), leading to the generation of p52, which dimerizes with RelB, to activate target genes involved in lymphoid organ development (Senftleben et al., 2001). However, it has recently been recognized that IKKα can also regulate gene expression in an NF-κB–independent manner. Unlike IKKβ, IKKα contains a nuclear localization sequence. It was suggested that in the nucleus, IKKα phosphorylates histone H3 at serine (Ser) 10 position, a prerequisite event for subsequent histone acetylation and gene transcription (Anest et al., 2003; Yamamoto et al., 2003). However, H3 Ser10 phosphorylation may simply serve as an indicator of an active “open” chromatin structure, and its dependence on IKKα may indicate that IKKα is required for establishment of the active chromatin state. More recently, IKKα kinase activity was shown to be required in the nucleus for repression of certain genes (Sil et al., 2004; Luo et al., 2007). Additionally, IKKα can also regulate epidermal keratinocyte differentiation through a kinase-independent mechanism (Hu et al., 2001). The NF-κB-independent functions of IKKα remain to be fully established.
To determine whether IKKα is required for T cell differentiation and T cell–mediated autoimmunity, we studied myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis (EAE) in IkkαAA knock-in mice (Senftleben et al., 2001). The knock-in allele specifies expression of a variant IKKα protein, in which the activating phosphorylation sites, Ser176 and Ser180, are replaced by two alanines (AA), thereby abolishing the activation of its kinase activity (Bonizzi et al., 2004; Lawrence et al., 2005). We found that IkkαAA mutant mice were refractory to EAE, and IkkαAA CD4+ T cells were defective in their Th17 cell differentiation. We then discovered that IKKα controls Th17 lineage commitment by maintaining the activation state of the Il17a locus, in an NF-κB–independent manner.
RESULTS
Autoimmune encephalomyelitis is markedly reduced in IkkαAA knock-in mice
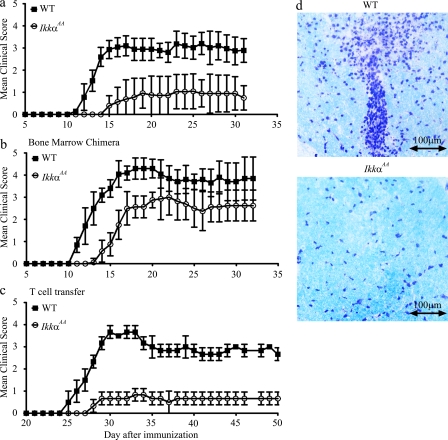
To determine the roles of IKKα in T cell–mediated inflammation, we immunized WT and IkkαAA C57BL/6 mice with MOG peptide, and monitored daily for clinical signs of EAE (Fig. 1 a). Although all WT mice developed EAE, only 77.8% of IkkαAA mice developed the disease. The disease severity was also significantly reduced in the IkkαAA group (maximal clinical score, 1.2 ± 0.8) as compared with the WT group (maximal clinical score, 3.3 ± 0.4; P < 0.05). The day of disease onset was increased from 11.9 ± 0.9 d in the WT group to 15 ± 1.8 d in the IkkαAA group (P < 0.01; Fig. 1 a). Consistent with these clinical findings, histological examination of spinal cord sections revealed significant differences in the degree of inflammation between the two groups. In the WT group, multiple inflammatory foci were observed, with extensive leukocyte infiltration in the white matter (Fig. 1 d, top). In contrast, leukocyte infiltration in IkkαAA spinal cords was much less pronounced (Fig. 1 d, bottom). Thus, IKKα kinase activity contributes to the development of EAE.
Figure 1.
IKKα expressed by T cells is required for the development of autoimmune encephalomyelitis. WT (n = 9) and IkkαAA (n = 6) C57BL/6 mice (a), WT C57BL/6 mice that received WT (n = 8) or IkkαAA (n = 8) bone marrow (b), and Rag1−/− C57BL/6 mice that received WT (n = 3) or IkkαAA (n = 3) CD4+ T cells (c) were immunized with MOG to induce EAE as described in the Materials and methods. Data presented are means ± SD of EAE scores. The differences between two groups are statistically significant (P < 0.01) for all panels. (d) Mice were sacrificed at the end of the experiments, and their spinal cords and brains were sectioned and stained with luxol fast blue and cresyl violet. (d, top) The spinal cord of a WT mouse from panel a with a clinical score of 4; (bottom) the spinal cord of an IkkαAA mouse with no signs of EAE. Data presented are representative of three independent experiments.
Loss of IKKα kinase activity in CD4+ T cells is responsible for the abrogated EAE development in IkkαAA mice
IkkαAA mice have severe defects in secondary lymphoid organogenesis and develop only rudiments of certain lymph nodes (Senftleben et al., 2001). This defect is caused by the loss of IKKα activity in nonhematopoietic stromal cells, other than hematopoietic cells (Senftleben et al., 2001; Bonizzi et al., 2004). To separate the effect of IKKα on lymphoid organogenesis from its effect on EAE, we studied the disease in irradiated WT C57BL/6 mice that had received bone marrow from either WT or IkkαAA mice (Fig. 1 b). In the chimeric mice, 80–90% of leukocytes were derived from donor bone marrow as determined by flow cytometry (unpublished data). Importantly, mice that received IkkαAA bone marrow developed significantly less severe EAE than those reconstituted with WT cells (maximal disease score, 3.0 ± 0.5 vs. 4.3 ± 0.5; P < 0.01). Disease onset was also delayed from 11.0 ± 0 in the WT to 14.3 ± 0.9 d (P < 0.01) in the IkkαAA group (Fig. 1 b). Therefore, loss of IKKα kinase activity in hematopoietic cells alone is sufficient to compromise the development of EAE.
Although MOG-induced EAE is a T cell–dependent disease, other hematopoietic cell types also contribute to the development of the disease. To directly test the T cell–specific function of IKKα in EAE, we studied disease development in Rag1−/− mice that had received WT or IkkαAA CD4+ T cells (Fig. 1 c). Rag1−/− mice receiving WT CD4+ T cells started to develop clinical signs of EAE at 25.3 ± 0.6 d after MOG immunization, and reached a maximal disease score of 3.7 ± 0.3. Remarkably, although Rag1−/− mice received IkkαAA CD4+ T cells developed EAE with only a slight delay relative to mice reconstituted with WT cells (28.3 ± 0.5 d; P < 0.01), the severity of the disease was significantly reduced (maximal disease score: 0.7 ± 0.3; P < 0.01). All mice in this experiment developed EAE, but no mice died of the disease. Splenic T cell numbers of RAG1 knockout recipient mice were determined 22 d after the disease onset. No significant differences were observed between mice that received WT or IKKαAA cells. Collectively, these results establish that IKKα kinase activity in CD4+ T cells is essential for the development of EAE. It is to be noted that in addition to IKKα expressed by T cells, IKKα expressed by non–T cells (hematopoietic and nonhematopoietic cells) may also play a role in EAE. However, this issue is difficult to address because of the following: (a) IKKα expressed by nonhematopoietic cells may indirectly affect EAE by controlling lymphoid organogenesis, in addition to its potential direct effects in the CNS; (b) the difference in EAE between bone marrow chimeras (Fig. 1 b) and their parental mice (Fig. 1 a) is small. Thus, in this study we focused on the roles of IKKα in T cells.
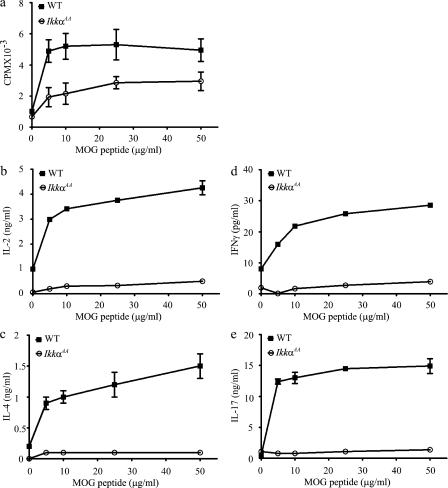
To measure the anti-MOG response of transferred T cells, Rag1−/− mice were sacrificed 22 d after disease onset, and their splenocytes were cultured in the presence of MOG peptide (Fig. 2). Total splenic T cell and splenocyte numbers were not significant different between mice received WT and IkkαAA cells. Splenocytes isolated from mice receiving WT T cells proliferated vigorously in response to MOG peptide and produced high levels of IL-2, IL-4, IL-17A, and IFN-γ. Strikingly, splenocytes from mice reconstituted with IkkαAA T cells exhibited significantly reduced proliferation (P < 0.05) and cytokine production (P < 0.01; Fig. 2). Similar reduction in MOG-induced responses was observed in IkkαAA splenocytes isolated from the mice tested in Fig. 1 (a and b; and not depicted). To determine whether IKKα mutation affects the survival of CD4+ T cells, we performed flow cytometry analysis of blood samples collected from mice reconstituted with T cells before they were immunized for EAE. We observed no significant differences in the number and frequency of T cells between WT and IkkαAA groups. Additionally, WT and IkkαAA T cells, when cultured in the presence of plate-bound anti-CD3 and soluble anti-CD28, did not have significant differences in survival as determined by flow cytometry after staining the cells with Annexin V. In contrast, IKKβ-deficient T cells had a significantly increased rate of death under the same culture condition (unpublished data).
Figure 2.
Reduced cytokine production by IkkαAA CD4+ T cells primed in vivo. WT or IkkαAA CD4+ T cells, 107 cells/mouse, were transferred into Rag1−/− recipients through tail vein (n = 3). EAE was induced by immunizing mice with MOG peptide 24 h later. Mice were sacrificed on day 22 after disease onset, and their splenocytes, 0.5 × 106/well, were cultured in complete DME with or without 5–50 µg of MOG peptide. [3H]thymidine was added 48 h after the initiation of the cell culture. Cells were harvested 18 h later, and [3H]thymidine incorporation was measured (a). (b–e) Cytokine concentrations were measured by ELISA 40 h after the initiation of the culture. Results shown are means ± SD of triplicate cultures. The differences between the two groups are statistically significant for all cultures with MOG peptide (P < 0.01). CPM, count per minute. Data are representative of two independent experiments.
IKKα kinase activity is required for Th17 responses
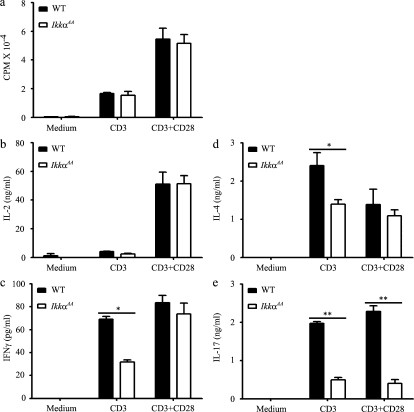
The markedly reduced anti-MOG response of IkkαAA CD4+ T cells indicates that the IKKα kinase activity may be required for T cell activation and/or differentiation. To test this theory, we isolated CD4+ T cells from naive WT and IkkαAA mice and measured their responses to anti-CD3 and anti-CD28 stimulation (Fig. 3). We found that IkkαAA mutant CD4+ T cells proliferated to the same extent as WT CD4+ T cells after anti-CD3 or anti-CD3 plus anti-CD28 stimulation (Fig. 3 a). Furthermore, upon stimulation with anti-CD3 and anti-CD28, IkkαAA CD4+ T cells produced normal levels of IL-2, IL-4, and IFN-γ as compared with WT cells; however, when stimulated with anti-CD3 alone, they produced moderately less IL-4 and IFN-γ (Fig. 3). In contrast, IkkαAA CD4+ T cells produced significantly less IL-17A than WT cells upon stimulation with anti-CD3 or anti-CD3 plus anti-CD28 (Fig. 3 e). IL-17A, but not IL-2, mRNA expression in IkkαAA CD4+ T cells was also significantly reduced (Fig. S1). The viability of T cells in the two groups was not significantly different, as determined by trypan blue and/or Annexin V staining. Similar results were obtained when unfractionated WT and IkkαAA splenocytes were stimulated with anti-CD3 and anti-CD28 (unpublished data). These results are in contrast to the global defect of the IkkαAA CD4+ T cells to MOG restimulation as shown in Fig. 2. Because T cells tested in those experiments were isolated from mice with different degrees of EAE, the effect of the disease on T cell responsiveness could not be excluded. IkkαAA mice did not show general defects in T cell activation, and similar percentages of CD4+CD44+ T cells (15.5 ± 1.3% vs. 16.3 ± 1.7%) and CD4+CD62Llow T cells (22.6 ± 2.1% vs. 24.6 ± 1.6%) were detected in spleens of naive WT and IkkαAA mice, respectively.
Figure 3.
Selective defect in cytokine production of IkkαAA CD4+ T cells activated in vitro. CD4+ T cells isolated from naive WT (n = 5) and IkkαAA (n = 5) mice were cultured in 96-well plates coated with 1 µg/ml anti-CD3 in the presence or absence of 2 µg/ml soluble anti-CD28. Proliferation was determined by [3H]thymidine incorporation at 48 h (a), and cytokine production was measured by ELISA at 40 h (b–e). Data are presented as means ± SD of triplicate cultures and are representative of two independent experiments. *, P < 0.05; **, P < 0.001. CD3, cultured with anti-CD3; CD28, cultured with anti-CD28.
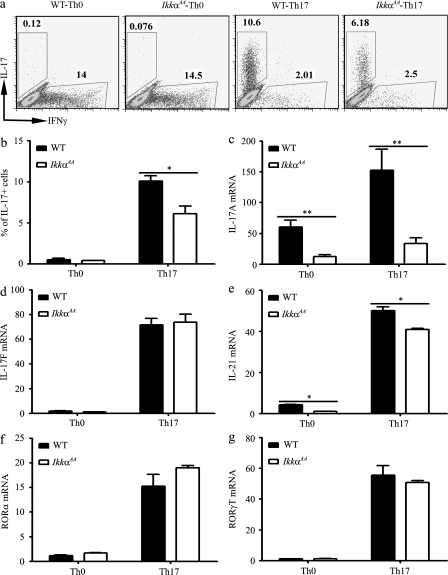
These results indicate that IKKα may selectively regulate Th17 cell differentiation. To test this possibility, we compared the ability of WT and IkkαAA CD4+ T cells to differentiate into Th17 cells in vitro. Differentiated Th17 cells were identified by flow cytometry after intracellular staining of IL-17A. When cultured under Th0 conditions, very few Th17 cells were spontaneously generated, but ∼14% of WT or IkkαAA CD4+ T cells produced IFN-γ (Fig. 4 a). In contrast, when cultured under Th17-inducing conditions, 9.9 ± 0.6% of WT CD4+ T cells produced IL-17; but only 5.6 ± 0.8% of IkkαAA T cells were IL-17+ (P < 0.01; Fig. 4 b). Therefore, IKKα kinase activity is required for the development of the Th17 response.
Figure 4.
Defective IL-17 production and Th17 differentiation of IkkαAA CD4+ T cells. CD4+ T cells from WT (n = 4) and IkkαAA (n = 4) mice were cultured under either neutral (Th0) or Th17-inducing condition as indicated. (a) 72 h later, IL-17– and/or IFN-γ–producing cells were measured by flow cytometry after intracellular staining of cytokines. (b) Quantification of data shown in a. (c–g) 24 h after the initiation of the culture, total RNA was isolated and mRNA levels of the Th17 lineage genes were assessed by real-time RT-PCR. The lowest expression level of each gene was set to 1. The experiments were repeated at least three times with similar results. *, P < 0.05; **, P < 0.001.
IKKα specifically regulates Il17a gene expression
To determine whether IKKα regulates Th17 response at the transcriptional level, we examined the expression of a panel of Th17-related genes by quantitative real-time PCR. Il17a expression in IkkαAA CD4+ T cells was significantly reduced as compared with WT cells when cultured under either Th0 or Th17 condition (Fig. 4 c and Fig. S2). In contrast, Il17f (Fig. 4 d) and Il23R (not depicted) expressions were not affected by the loss of IKKα kinase activity. Il21 expression was only marginally reduced in IkkαAA CD4+ T cells (Fig. 4 e). Additionally, IL-17A heterogenous nuclear RNA (hnRNA) could be readily detected in activated WT CD4+ T cells; in contrast, a much reduced signal was detected in IKKαAA T cells under the same condition (Fig. S3). The Th17 differentiation signals elicited by TGF-β and IL-6 eventually converge onto the induction of two Th17 lineage-specific transcription factors, RORα and RORγT (Ivanov et al., 2006, Dong, 2008). However, the expression of Rora and Rorgt mRNAs and RORγ and RORγT proteins was not affected by the IKKα mutation (Fig. 4, f and g, and Fig. S4). These data indicate that the effect of IKKα on Th17 lineage is Il17a specific and independent of RORα and RORγT.
IKKα selectively binds to the Il17a promoter
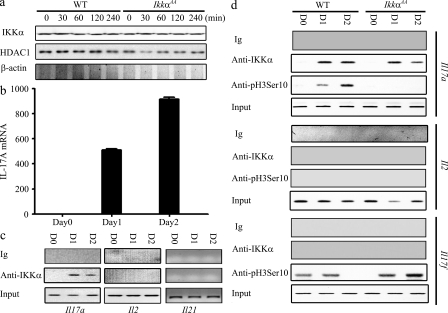
IKKα has been previously reported to be present in the nuclei of several nonhematopoietic cell types, such as keratinocytes, squamous epithelial cells, and fibroblasts (Anest et al., 2003; Yamamoto et al., 2003; Fernández-Majada et al., 2007). In the nucleus, IKKα can regulate target gene expression through a variety of mechanisms, some of which correlate with promoter-associated histone H3 phosphorylation (Anest et al., 2003; Yamamoto et al., 2003). Interestingly, we found that IKKα was not only present in the cytoplasm, but also constitutively expressed at high levels in the nuclei of resting CD4+ T cells (Fig. 5 a). The level of nuclear IKKα was ∼30% of the cytoplasmic IKKα, and was not regulated by anti-CD3 or anti-CD28 stimulation. To test whether IKKα regulates Il17a expression by a direct association with its promoter, we performed chromatin immunoprecipitation (ChIP) with anti-IKKα (Fig. 5). IKKα did not bind to the Il17a promoter in resting WT CD4+ T cells, but in cells that had been cultured in the Th17 differentiation medium for 1–2 d, IKKα was readily detected on the Il17a promoter, coinciding with the appearance of the IL-17A mRNA (Fig. 5, b and c). The Il17a promoter was selectively targeted by IKKα, as IKKα was not detected on promoters of Il21, Il17f, and Il2 (Fig. 5 c). In WT T cells, IKKα binding to the Il17a promoter correlated with its histone H3 phosphorylation (Fig. 5 d). In contrast, in IkkαAA T cells, mutant IKKα also bound to the Il17a promoter under Th17 differentiation conditions, but histone H3 phosphorylation did not occur (Fig. 5 d). On the other hand, histone H3 on Il17f promoter also underwent phosphorylation at serine10 position, 1 and 2 d after cells were cultured in Th17 differentiation medium, but this event was IKKα-independent (Fig. 5 d). As expected, IKKα mutation selectively affected PollII binding to Il17, but not to Il21 and Il22 (Fig. S5). These results indicate that IKKα kinase activity is selectively required for driving the Il17a locus into an active state marked by H3 serine 10 phosphorylation.
Figure 5.
Epigenetic regulation of the Il17a locus by IKKα. (a) Purified CD4+ T cells from WT (n = 4) and IkkαAA (n = 4) mice were stimulated with 10 µg/ml plate-bound anti-CD3 and 10 µg/ml plate-bound anti-CD28 for the indicated times. Nuclear extract was blotted with anti-IKKα (mol wt 85 kD), anti-HDAC1 (anti-histone deacetylase-1, mol wt 60 kD), and anti–β-actin (mol wt 42kD). (b) Purified CD4+ T cells from WT mice (n = 4) were cultured under Th17-inducing condition. Total RNA was prepared at the indicated times. IL-17A mRNA levels were assessed by real-time RT-PCR. The expression level at day 0 was set to 1. (c) Purified WT CD4+ T cells were cultured as in b. ChIP was performed using anti-IKKα or control Ig at days (D) 0, 1, and 2, as indicated. Precipitated DNA was analyzed by PCR using Il17a, Il2, and Il21 promoter-specific primers that gave rise to products of 167bp, 256bp, and 150bp, respectively. (d) Purified CD4+ T cells from WT (n = 4) and IkkαAA (n = 4) mice were cultured under the Th17-inducing condition. ChIP was performed using anti-IKKα, anti-pH3Ser10 (serine 10 phosphorylated histone H3), or control Ig, at the indicated times. Precipitated DNA was analyzed by PCR using Il17a, Il2, and Il17f promoter-specific primers, as in c. Data are representative of three independent experiments.
IKKα regulates Th17 response independent from NF-κB
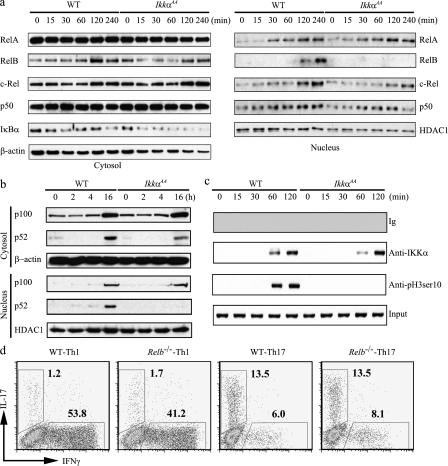
In addition to directly acting on the Il17a promoter, IKKα may also regulate Th17 response through NF-κB–dependent mechanisms. To test this possibility, we first examined IκBα degradation in WT and IkkαAA T cells. In WT CD4+ T cells, upon anti-CD3 and anti-CD28 stimulation, IκBα was quickly degraded followed by a recovery caused by resynthesis of IκBα (Fig. 6 a, left). The early degradation of IκBα in IkkαAA CD4+ T cells was not affected, but IκBα did not return to the prestimulation level at later time points. Similar results were obtained when cells were stimulated with PMA plus ionomycin (unpublished data). To test whether IKKα regulates the IkBa promoter as previously reported (Anest et al., 2003; Yamamoto et al., 2003), we performed ChIP-PCR analyses using CD4+ T cells stimulated with anti-CD3 and anti-CD28. We found that IKKα was recruited to IkBa promoter in both WT and IkkαAA CD4+ T cells after stimulation (Fig. 6 c). However, H3 Ser10 phosphorylation on the Ikba promoter was detected only in WT, but not in mutant, T cells. These results indicate that IKKα kinase activity is required for IκBα resynthesis, but not for its stimulation-induced degradation in T cells.
Figure 6.
IKKα may not regulate Th17 gene expression through NF-κB. (a) Purified CD4+ T cells from WT (n = 4) and IkkαAA (n = 4) mice were stimulated with 10 µg/ml plate-bound anti-CD3 and 10 µg/ml plate-bound anti-CD28 for the indicated times. Nuclear and cytosolic protein lysates were blotted with anti-RelA (mol wt 65 kD), anti-RelB (mol wt 68 kD), anti–c-Rel (mol wt 75 kD), anti-p50 (mol wt 50 kD), anti-IκBα (mol wt 36 kD), anti-β-actin (mol wt 42 kD), or anti-HDAC1 (mol wt 60 kD). (b) Purified CD4+ T cells from WT (n = 4) and IkkαAA (n = 4) mice were stimulated for the indicated times, as in a. Nuclear and cytosolic protein lysates were blotted with anti-p100/p52 (mol wt of p100 and p52 are 100 and 52 kD, respectively), anti–β-actin (mol wt 42 kD), and anti-HDAC1 (mol wt 60 kD). (c) Purified CD4+ T cells from WT (n = 4) and IkkαAA (n = 4) mice were stimulated for the indicated times, as in a. ChIP was performed using anti-IKKα, anti-pH3Ser10, or control Ig. Precipitated DNA was analyzed by PCR using Iκbα promoter-specific primers that gave rise to a 230-bp product. (d) Purified CD4+ T cells from WT (n = 4) and Relb−/− (n = 4) mice were cultured under the Th1- or Th17-inducing condition. 72 h later, IL-17A– and IFN-γ–producing cells were measured by flow cytometry. Results are representative of two independent experiments.
Next, we measured nuclear translocation of NF-κB subunits in T cells stimulated with anti-CD3 and anti-CD28. RelA, RelB, c-Rel, and p50 all migrated into the nucleus of WT CD4+ T cells upon activation (Fig. 6 a). As expected (Bonizzi et al., 2004), the IKKα mutation did not affect the nuclear translocation of RelA, c-Rel, or p50, but completely blocked nuclear localization of RelB (Fig. 6 a), indicating that IKKα selectively controls RelB activation. The lack of an effect of IKKα mutation on nuclear RelA activity was also confirmed by RelA ELISA (Fig. S6). As the key kinase of the noncanonical (alternative) NF-κB pathway, IKKα mediates the processing of p100 and, consequently, the generation of the RelB-p52 heterodimer (Bonizzi et al., 2004). Defective RelB nuclear translocation in IkkαAA CD4+ T cells may be caused by impaired IKKα-regulated p100 processing. We therefore examined p100 processing and p52 nuclear translocation in anti-CD3– and anti-CD28–activated T cells (Fig. 6 b). In WT T cells, p100 and p52 were up-regulated in both the cytosolic and nuclear fractions upon stimulation. In IkkαAA T cells, however, p100 was also up-regulated in the cytosol and nucleus, but p52 expression was reduced in the cytosol and was undetectable in the nucleus after anti-CD3 and anti-CD28 stimulation. These data confirm that IKKα kinase activity is important for activation-induced p100 processing and is required for p52 and RelB nuclear translocation in T cells. Similar results were obtained when WT and IkkαAA T cells were stimulated with PMA and ionomycin (unpublished data).
These results raise the question of whether IKKα regulates Th17 cell differentiation through p52-RelB heterodimers. To test this, we examined whether RelB was required for Th cell differentiation by culturing WT and Relb−/− CD4+ T cells under Th1- or Th17-inducing conditions (Fig. 6 d). Consistent with previously published data (Corn et al., 2005), Relb−/− cells showed a moderately reduced capacity to differentiate into IFN-γ–producing Th1 cells (53.8 vs. 41.2%). Interestingly, similar percentages of IL-17–producing Th17 cells were generated in Relb−/− and WT cultures (Fig. 6 d). Collectively, these results suggest that IKKα regulates the Th17 response through NF-κB–independent mechanisms.
DISCUSSION
A novel finding from the current study is that IKKα selectively regulates the Th17 cell response. As a result, IkkαAA mutant mice are more resistant to MOG-induced EAE. Although IKKα is important for the activation of the noncanonical NF-κB pathway in CD4+ T cells, it does not seem to regulate Th17 response through NF-κB-dependent mechanisms because RelB−/− CD4+ T cells did not show any defect in Th17 differentiation. Using the TESS program, we were not able to identify any putative NF-κB–binding site in either the murine or human Il17a promoter. In a promoter-transactivating assay, we didn’t detect any effect of p50, p52, p65, p100, RelB, or c-Rel expression construct on the Il17a promoter reporter, whereas the RORγT construct activated the reporter significantly in the same assay (unpublished data). Expression of two Th17 lineage-specific transcription factors, RORα and RORγT was not changed in IkkαAA CD4+ T cells, indicating that the defect may be attributed to another mechanism. This theory is supported by the observation that IKKα is recruited to the Il17a promoter during Th17 differentiation and a specific defect in IL-17A expression in IkkαAA CD4+ T cells. Furthermore, phosphorylation of histone H3 on the Il17a promoter, an activation marker that correlates with Th17 differentiation, was absent in IkkαAA T cells. Therefore, our study suggests a novel nuclear function for IKKα in Th17 differentiation.
In this study, we showed that RelB activation after anti-CD3 plus anti-CD28 stimulation was blocked in IkkαAA CD4+ T cells. Although it has been reported that RelB is required for maximal T cell activation (Corn et al., 2005), we did not observe any defect in IkkαAA CD4+ T cell proliferation and Th1 and Th2 cytokine production after anti-CD3 and anti-CD28 stimulation; this may be caused by additional effects of the IKKα mutation, such as reduced IκBα resynthesis, which may compensate for the loss of RelB function. However, when stimulated in vitro with MOG peptide, splenocytes isolated from IkkαAA mice that had developed EAE showed significantly reduced responses (Fig. 2). There are two possible reasons for this discrepancy. First, IKKα may be required for T cell activation induced by weak, but not strong, ligands. Second, and more likely, the decreased anti-MOG T cell response may be secondary to the effect of IKKα on EAE. The increased severity of EAE in WT mice may help to activate and expand more MOG-specific CD4+ T cells than in IkkαAA mice. As a consequence, there are likely more MOG-responsive T cells in the WT splenocyte culture than in the IkkαAA culture (Fig. 2).
It should be emphasized that IKKα-mediated IL-17 regulation may be only one of the mechanisms whereby IKKα controls EAE, as other cytokines including Th1 cytokines may also be affected by IKKα (Figs. 2 and 3). With regard to IL-17, the most significant IKKα effect appears to be on its mRNA transcription (Figs. 3 and 4). However, because IKKα does not appear to affect the IL-17 protein levels of individual Th17 cells, but increases the frequency of IL-17–producing cells, we propose that IKKα may selectively affect IL-17 mRNA expression in a subpopulation of Th17 cells, but not all Th17 cells. This could be related to the stage of Th17 cell differentiation, the phase of cell cycle, and the microenvironment surrounding the Th17 cells. This may explain why many IkkαAA cells do not produce any IL-17 protein, although others make normal amounts (Fig. 4).
Two subunits of the IKK complex, IKKα and IKKβ, possess kinase activities (Häcker and Karin, 2006). Despite high sequence similarities, it is now evident that the two molecules possess distinct functions (Häcker and Karin, 2006). In T cells, IKKβ mediates NF-κB activation through the canonical pathway (Schmidt-Supprian et al., 2003). Ikkβ−/− CD4+ T cells show significantly less IκBα degradation with severely delayed kinetics (unpublished data). In contrast, IKKα was not required for the activation of the canonical NF-κB pathway in CD4+ T cells. Instead, IKKα is required for the activation of the noncanonical NF-κB pathway through p100 processing (Fig. 6 b). In the cytosol, p100 can still be processed, albeit at a markedly reduced rate in IkkαAA cells. However, p52 nuclear translocation was completely abolished in these cells.
Gene expression is regulated by epigenetic mechanisms involving histone modifications. Dong et al. showed that similar to loci specific to Th1 and Th2 cells, Th17 loci encoding IL-17A and IL-17F were regulated by chromatin remodeling events (Akimzhanov et al., 2007). Transcriptionally permissive histone modifications, such as histone H3 acetylation and Lys-4 tri-methylation, were observed at the Il17a promoter in Th17 cells (Akimzhanov et al., 2007). We showed that during Th17 differentiation, histone H3 on both Il17a and Il17f promoters was phosphorylated at Ser10, a known prerequisite for histone H3 acetylation. However, IKKα was found to be selectively required for histone H3 phosphorylation at the Il17a promoter, but not Il17f promoter.
Recently, several groups have reported nuclear expression of IKK subunits (Anest et al., 2003; Yamamoto et al., 2003; Fernández-Majada et al., 2007; Lubin and Sweatt, 2007). Unlike IKKβ and IKKγ (NEMO), which are exclusively cytoplasmic, IKKα contains a nuclear localization sequence. Furthermore, IKKα was found to be recruited to NF-κB–dependent (e.g., Il8, Il6, and IkBa) and NF-κB–independent (e.g., c-fos) gene promoters, where its presence correlated with H3 phosphorylation, and thus chromatin activation. However, nuclear IKKα was also found to be recruited to genes (e.g., Maspin) that undergo active repression (Luo et al., 2007). Although IKKα was proposed to directly phosphorylate histone H3 at Ser10 (Anest et al., 2003; Yamamoto et al., 2003), its association with gene repression and silencing suggest that IKKα-induced enhancement of H3 phosphorylation can also be mediated by indirect mechanisms. For example, IKKα may be responsible for the recruitment of identified H3 kinases to the promoters it activates. In addition, nuclear IKKα has also been shown to mediate RelA/p65 turnover in macrophages through its phosphorylation of Ser536 (Lawrence et al., 2005). This effect seems to be cell-type specific because we found that IKKα was not required for RelA/p65 phosphorylation on Ser536 in CD4+ T cells (unpublished data). This may partially explain the different roles of IKKα in T cells as compared with macrophages. Thus, the highly specific effect of IKKα on Th17 lineage and the resistance of IkkαAA mice to MOG-induced EAE implicate IKKα as a potential therapeutic target for Th17-mediated autoimmune disorders.
MATERIALS AND METHODS
Mice and cell transfer.
IkkαAA mutant C57BL/6 mice were previously described (Senftleben et al., 2001). To generate bone marrow chimeric mice, C57BL/6 recipient mice were irradiated with 2 doses of 500 rads each. They were then intravenously injected with 107 bone marrow cells collected from either WT or IkkαAA mice. 8 wk after bone marrow reconstitution, mice were immunized for the induction of EAE as described below. For T cell transfer, 107 CD4+ T cells isolated from WT or IkkαAA mice were injected into Rag1−/− C57BL/6 mice through the tail vein. 24 h after the transfer, mice were immunized with MOG for EAE induction. All procedures were preapproved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania.
Cell isolation, cell culture, and reagents.
Naive CD4+CD25−CD44lowCD62L+ T cells were isolated by FACS, whereas total CD4+ T cells were isolated by MACS. The purity of the naive T cells isolated by FACS was >99%, whereas that of T cells isolated by MACS was >95%. Cells were cultured in complete DME containing 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 30 µM β-mercaptoethanol, 1 mM sodium pyruvate, 10 mM Hepes, and 1× nonessential amino acids. Anti-IKKα, anti-RelA, anti-RelB, and anti–c-Rel were purchased from Santa Cruz Biotechnology, Inc. Anti–β-actin was purchased from Sigma-Aldrich. Anti-CD3 and anti-CD28 were purchased from eBioscience. Anti-IκBα, anti-pRelA536, anti-p100/p52, and anti-pSer10H3 were purchased from Cell Signaling Technology. MOG 38–50 peptide was synthesized by Invitrogen. Pertussis toxin was purchased from List Biological Laboratories, Inc. CFA was purchased from DIFCO laboratories.
EAE induction and evaluation.
EAE was induced as previously described (Hilliard et al., 2002). In brief, mice first received a subcutaneous immunization with 300 µg MOG38-50 peptide emulsified in CFA and an intravenous injection of 200 ng pertussis toxin. A second injection of 200 ng pertussis toxin was given 48 h later. Mice were examined daily for clinical signs of EAE and scored as follows: 0, no disease; 1, tail paralysis; 2, hind limb weakness; 3, hind limb paralysis; 4, hind limb plus forelimb paralysis; 5, moribund or dead.
Th cell differentiation.
CD4+ T cells were isolated using autoMACS automatic cell sorter (Miltenyi Biotec). Cells were cultured with 50 U/ml IL-2, 2 µg/ml anti-CD28, and 2 µg/ml anti-CD3 under either Th0- (no more addition of cytokines or antibodies), Th1- (10 ng/ml IL-12 and 20 ng/ml anti–IL-4), or Th17- (0.4 µg/ml anti–IL-4, 10 µg/ml anti–IFN-γ, 20 ng/ml IL-6, and 5 ng/ml TGF-β1) inducing conditions. 5 d later, cells were washed and restimulated with anti-CD3 and anti-CD28 for 12 h, stained with anti–IL-17A and anti–IFN-γ, and examined by flow cytometry.
Real-time PCR.
Total RNA was extracted using TRIzol reagent (Invitrogen). cDNA was synthesized using reverse transcription II (Invitrogen). Real-time quantitative PCR was performed using the SYBR Green master mix (Applied Biosystems). Data were normalized to β-actin mRNA. Primers used are as follows (forward and reverse): IL-17A, 5′-ACCTCACACGAGGCACAAGT-3′ and 5′-CTTCATTGCGGTGGATC-3′; IL-17F, 5′-GAGGATAACACTGTGAGAGTTGAC-3′ and 5′-GAGTTCATGGTGCTGTCTTCC-3′; IL-21, 5′-TCATCATTGACCTCGTGGCCC-3′ and 5′-ATCGTACTTCTCCACTTGCAATCCC-3′; RORα, 5′-TCTCCCTGCGCTCTCCGCAC-3′ and 5′-TCCACAGATCTTGCATGGA-3′; RORγT, 5′-CCGCTGAGAGGGCTTCAC-3′ and 5′-TGCAGGAGTAGGCCACATTACA-3′; IL-23R, 5′-GCCAAGAAGACCATTCCCGA-3′ and 5′-TCAGTGCTACAATCTTCTTCAGAGGACA-3′; p19, 5′-AAGTTCTCTCCTCTTCCCTGTCGC-3′ and 5′-TCTTGTGGAGCAGCAGATGTGAG-3′; β-actin, 5′-GTGGGCCGCTCTAGGCACCAA-3′ and 5′-CTCTTTGATGTCACGCACGATTTC-3′.
ChIP PCR.
ChIP was performed using a Chromatin Immunoprecipitation kit (Millipore) according to the manufacturer’s instructions. In brief, cells were first treated with formaldehyde and sonicated to break up the chromatin. Sonicated chromatinpreparations were immunoprecipitated with specific antibodies. DNA was then eluted after extensive washing. PCR was performed using the following primers (forward and reverse): IL-17A promoter, 5′-GCAGCAGCTTCAGATATGTCC-3′ and 5′-TGAGGTCAGCACAGAACCAC-3′; IL-17F promoter, 5′-CATGTGAATGGCACGATAGG-3′ and 5′-TAATTCCCCCACAAAGCAAC-3′; IL-2 promoter, 5′-CATACAGAAGGCGTTCATTG-3′ and 5′-TACCTGTGTGGCAGAAAGC-3′. A small portion of sonicated chromatin preparations was also amplified by PCR to ensure equal input.
ELISA.
Antibodies used in ELISA were purchased from BD, including purified and biotinylated rat anti–mouse IL-2, IL-4, IL-6, IFN-γ, and IL-17A. Quantitative ELISA was performed using paired mAbs specific for corresponding cytokines according to the manufacturer’s recommendations.
Western blot.
Nuclear protein extract was prepared using a Nuclear Extract kit (Active Motif Inc.) per manufacturer’s instructions. Total cell lysate was prepared by suspending cells in the RIPA buffer containing 150 mM NaCl, 10 mM Tris, pH 7.4, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, 100 µM Na3VO4, 5 mM EDTA, 1 mM PMSF, and protease inhibitors cocktail. Proteins (20 µg per lane) were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and blotted with specific antibodies.
Statistical analyses.
Disease severity, day of onset, and cytokine concentrations were analyzed by Student’s t test. Disease scores were analyzed by Mann-Whitney U test.
Online supplemental material.
Fig. S1 shows reduced IL-17A mRNA expression in IkkαAA CD4+ T cells. Fig. S2 shows defective IL-17A expression in IkkαAA CD4+ T cells during Th17 differentiation. Fig. S3 shows reduced IL-17A hnRNA expression in IkkαAA CD4+ T cells. Fig. S4 shows normal RORγ/RORγt protein expression in IkkαAA CD4+ T cells. Fig. S5 shows the binding of RNA polymerase II to the Il17, Il21, and Il22 promoters. Fig. S6 shows nuclear RelA/p65 activities as determined by ELISA. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091346/DC1.
Acknowledgments
The authors thank Jing Sun, Ruaidhri Carmody, and Christopher Hunter for their valuable discussions and reagents.
This work was supported by grants from the National Institutes of Health (AI50059, DK070691, and AI069289).
The authors have no competing financial interests related to this work.
Footnotes
Abbreviations used:
- ChIP
- chromatin immunoprecipitation
- EAE
- experimental autoimmune encephalomyelitis
- hnRNA
- heterogenous nuclear RNA
- IKKα
- inhibitor of NF-κB kinase-α
- MOG
- myelin oligodendrocyte glycoprotein
- ROR
- retinoid-related orphan receptor
- Stat3
- signal transducer and activator of transcription 3
References
- Akimzhanov A.M., Yang X.O., Dong C. 2007. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J. Biol. Chem. 282:5969–5972 10.1074/jbc.C600322200 [DOI] [PubMed] [Google Scholar]
- Anest V., Hanson J.L., Cogswell P.C., Steinbrecher K.A., Strahl B.D., Baldwin A.S. 2003. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 423:659–663 10.1038/nature01648 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Korn T., Kuchroo V.K. 2007. Th17: the third member of the effector T cell trilogy. Curr. Opin. Immunol. 19:652–657 10.1016/j.coi.2007.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G., Bebien M., Otero D.C., Johnson-Vroom K.E., Cao Y., Vu D., Jegga A.G., Aronow B.J., Ghosh G., Rickert R.C., Karin M. 2004. Activation of IKKalpha target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. EMBO J. 23:4202–4210 10.1038/sj.emboj.7600391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corn R.A., Hunter C., Liou H.C., Siebenlist U., Boothby M.R. 2005. Opposing roles for RelB and Bcl-3 in regulation of T-box expressed in T cells, GATA-3, and Th effector differentiation. J. Immunol. 175:2102–2110 [DOI] [PubMed] [Google Scholar]
- Cua D.J., Sherlock J., Chen Y., Murphy C.A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T., et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748 10.1038/nature01355 [DOI] [PubMed] [Google Scholar]
- Dong C. 2008. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 8:337–348 10.1038/nri2295 [DOI] [PubMed] [Google Scholar]
- Fernández-Majada V., Aguilera C., Villanueva A., Vilardell F., Robert-Moreno A., Aytés A., Real F.X., Capella G., Mayo M.W., Espinosa L., Bigas A. 2007. Nuclear IKK activity leads to dysregulated notch-dependent gene expression in colorectal cancer. Proc. Natl. Acad. Sci. USA. 104:276–281 10.1073/pnas.0606476104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häcker H., Karin M. 2006. Regulation and function of IKK and IKK-related kinases. Sci. STKE. 2006:re13 10.1126/stke.3572006re13 [DOI] [PubMed] [Google Scholar]
- Hilliard B.A., Mason N., Xu L., Sun J., Lamhamedi-Cherradi S.-E., Liou H.-C., Hunter C., Chen Y.H. 2002. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J. Clin. Invest. 110:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Baud V., Oga T., Kim K.I., Yoshida K., Karin M. 2001. IKKalpha controls formation of the epidermis independently of NF-kappaB. Nature. 410:710–714 10.1038/35070605 [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240 10.1084/jem.20041257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T., Bebien M., Liu G.Y., Nizet V., Karin M. 2005. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature. 434:1138–1143 10.1038/nature03491 [DOI] [PubMed] [Google Scholar]
- Lubin F.D., Sweatt J.D. 2007. The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron. 55:942–957 10.1016/j.neuron.2007.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J.L., Tan W., Ricono J.M., Korchynskyi O., Zhang M., Gonias S.L., Cheresh D.A., Karin M. 2007. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 446:690–694 10.1038/nature05656 [DOI] [PubMed] [Google Scholar]
- Romagnani S. 1997. The Th1/Th2 paradigm. Immunol. Today. 18:263–266 10.1016/S0167-5699(97)80019-9 [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M., Courtois G., Tian J., Coyle A.J., Israël A., Rajewsky K., Pasparakis M. 2003. Mature T cells depend on signaling through the IKK complex. Immunity. 19:377–389 10.1016/S10747613(03)00237-1 [DOI] [PubMed] [Google Scholar]
- Senftleben U., Cao Y., Xiao G., Greten F.R., Krähn G., Bonizzi G., Chen Y., Hu Y., Fong A., Sun S.C., Karin M. 2001. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 293:1495–1499 10.1126/science.1062677 [DOI] [PubMed] [Google Scholar]
- Sil A.K., Maeda S., Sano Y., Roop D.R., Karin M. 2004. IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 428:660–664 10.1038/nature02421 [DOI] [PubMed] [Google Scholar]
- Sutton C., Brereton C., Keogh B., Mills K.H., Lavelle E.C. 2006. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203:1685–1691 10.1084/jem.20060285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M., Stockinger B. 2006. TGFbeta1, a “Jack of all trades”: the link with pro-inflammatory IL-17-producing T cells. Trends Immunol. 27:358–361 10.1016/j.it.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Weaver C.T., Harrington L.E., Mangan P.R., Gavrieli M., Murphy K.M. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 24:677–688 10.1016/j.immuni.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Verma U.N., Prajapati S., Kwak Y.T., Gaynor R.B. 2003. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 423:655–659 10.1038/nature01576 [DOI] [PubMed] [Google Scholar]
- Yang X.O., Panopoulos A.D., Nurieva R., Chang S.H., Wang D., Watowich S.S., Dong C. 2007. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282:9358–9363 10.1074/jbc.C600321200 [DOI] [PubMed] [Google Scholar]
- Yang X.O., Pappu B.P., Nurieva R., Akimzhanov A., Kang H.S., Chung Y., Ma L., Shah B., Panopoulos A.D., Schluns K.S., et al. 2008. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 28:29–39 10.1016/j.immuni.2007.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]