IL-4Rα expression on airway smooth muscle cells is sufficient for the development of airway hyperresponsiveness.
Abstract
Production of the cytokines IL-4 and IL-13 is increased in both human asthma and mouse asthma models, and Stat6 activation by the common IL-4/IL-13R drives most mouse model pathophysiology, including airway hyperresponsiveness (AHR). However, the precise cellular mechanisms through which IL-4Rα induces AHR remain unclear. Overzealous bronchial smooth muscle constriction is thought to underlie AHR in human asthma, but the smooth muscle contribution to AHR has never been directly assessed. Furthermore, differences in mouse versus human airway anatomy and observations that selective IL-13 stimulation of Stat6 in airway epithelium induces murine AHR raise questions about the importance of direct IL-4R effects on smooth muscle in murine asthma models and the relevance of these models to human asthma. Using transgenic mice in which smooth muscle is the only cell type that expresses or fails to express IL-4Rα, we demonstrate that direct smooth muscle activation by IL-4, IL-13, or allergen is sufficient but not necessary to induce AHR. Five genes known to promote smooth muscle migration, proliferation, and contractility are activated by IL-13 in smooth muscle in vivo. These observations demonstrate that IL-4Rα promotes AHR through multiple mechanisms and provide a model for testing smooth muscle–directed asthma therapeutics.
Asthma is a pulmonary disorder in which episodic dyspnea is caused by reversible bronchospasm (Steel and Holgate, 2001). Increased susceptibility to bronchospasm presumably results from qualitative and/or quantitative increases in the contractility of airway smooth muscle. Increased contractility could result from changes intrinsic to smooth muscle (increases in the number, size, and/or responsiveness of smooth muscle cells to stimuli that induce contraction) or from changes in other cell types such as neural cells, infiltrating inflammatory cells and epithelial cells that produce stimuli that promote smooth muscle contractility. In human asthmatics and animals with experimental allergic airway disease, the predisposition to bronchospasm is manifested as airway hyperresponsiveness (AHR; Steel and Holgate, 2001; Lewkowich and Wills-Karp, 2008), an increased sensitivity to agents such as acetylcholine and methacholine, which induce smooth muscle contraction in normal individuals.
Studies with mouse models of allergic airway disease indicate that the development and maintenance of AHR depend on Th2 cytokines, particularly IL-13 and to a lesser extent IL-4 (Grünig et al., 1998; Wills-Karp et al., 1998; Perkins et al., 2006), and on the ability of these cytokines to activate the transcription factor Stat6 by signaling through the type 2 IL-4R (Kuperman et al., 1998). Increased IL-4 and IL-13 expression in human asthmatic lungs (Huang et al., 1995; Kotsimbos et al., 1996; Humbert et al., 1997), the association of specific IL-4Rα alleles with risk for atopic asthma (Rosa-Rosa et al., 1999), and observations that inhalation of an IL-4R antagonist improves pulmonary function in human asthmatics (Wenzel et al., 2007) suggest that a similar pathophysiological process is involved in human asthma. However, it is not known whether IL-4 and IL-13 induce AHR through direct effects on airway smooth muscle. Although IL-13 increases smooth muscle contractility when added to cultures of airway smooth muscle cells (Shore and Moore, 2002; Shore, 2004b), airway smooth muscle isolated from human asthmatics is not consistently hypercontractile (Wills-Karp, 1997). Furthermore, IL-13 induces AHR in transgenic mice that only express Stat6 in pulmonary epithelial cells (Kuperman et al., 2002). These observations suggest that AHR might result from changes in the properties of airway epithelium such as increased swelling, permeability, rigidity, and mucus production that decrease airway diameter rather than from changes in smooth muscle. Alternatively, Stat6-mediated effects on epithelial cells could stimulate the production of mediators that affect airway smooth muscle.
Recent studies with mice that selectively lack IL-4Rα on smooth muscle are also consistent with the sufficiency of IL-4 or IL-13 effects on airway epithelium for induction of AHR. Intraperitoneal immunization with ovalbumin bound to alum, followed by ovalbumin inhalation, induced AHR, goblet cell hyperplasia, and airway eosinophilia, as well as Th2 cytokine and IgE secretion, which were similar in mice that expressed IL-4Rα normally or that selectively deleted IL-4Rα from smooth muscle cells, whereas similar immunization of mice totally deficient in IL-4Rα failed to induce any of these effects (Kirstein et al., 2010). The normal responsiveness of mice that selectively lacked smooth muscle IL-4Rα in this model was somewhat surprising, inasmuch as studies with the same mice demonstrated decreased Th2 cytokine expression and airway goblet cell hyperplasia after infection with the nematode parasite Nippostrongylus brasiliensis (Horsnell et al., 2007, 2011).
These results were consistent with some analyses of pulmonary function assays in rodents, which support the view that increased AHR in experimental models of allergic airway disease results predominantly from changes in epithelial rather than smooth muscle cells (Wagers et al., 2004); however, this view is not universal (Glaab et al., 2007). In fact, IL-4R–mediated effects on epithelial cells are not likely to totally account for increased AHR because allergen inhalation still induces AHR in mice in which the signaling IL-4R polypeptide, IL-4Rα, has been selectively deleted from airway epithelial cells (Kuperman et al., 2005). Collectively, the in vivo observations made with IL-4Rα and Stat6 transgenic mice suggest that IL-4R signaling induces AHR through direct effects on more than one cell type and leave open the possibility that AHR development is promoted by direct IL-4R signaling of smooth muscle cells. We have evaluated this possibility by examining the effects of IL-4, IL-13, and allergen immunization in transgenic mice that express IL-4Rα only in smooth muscle, as well as a second set of mice that have selectively deleted IL-4Rα from smooth muscle. Our results demonstrate that selective smooth muscle expression of IL-4R is sufficient for cytokine-dependent induction of AHR and that this occurs in the absence of pulmonary eosinophilia or goblet cell hyperplasia. Our results also identify molecular mechanisms by which IL-4Rα signaling may induce AHR by demonstrating that IL-13 inhalation by mice that express IL-4Rα in smooth muscle induces five genes already known to increase smooth muscle proliferation, migration, and contractility. Finally, we confirm that direct smooth muscle responsiveness to IL-4/IL-13 is not required for induction of AHR by demonstrating that AHR develops, along with airway eosinophilia and goblet cell hyperplasia, in IL-13– and allergen-inoculated mice that selectively lack IL-4Rα on smooth muscle. These observations demonstrate that direct effects of IL-4 and IL-13 on smooth muscle are sufficient but not necessary for induction of AHR, provide a tool that can be used to study how effects of these cytokines on smooth muscle influence AHR in human asthma, and identify novel smooth muscle–associated targets for asthma therapy.
RESULTS
Generation of mice that selectively express IL-4Rα on smooth muscle cells
To determine whether IL-4Rα signaling restricted to smooth muscle cells can induce responses characteristic of murine allergic airway disease, we produced mice that express a transgene for IL-4Rα controlled by the smooth muscle–specific promoter SMP8 (see Materials and methods) and bred these mice for more than eight generations to BALB/c IL-4Rα–deficient mice (Mountford et al., 2001) to produce mice that expressed a single allele of the SMP8–IL-4Rα transgene and two null alleles for the normal IL-4Rα gene (IL-4Rα−/−/SMP8–IL-4Rα+/−).
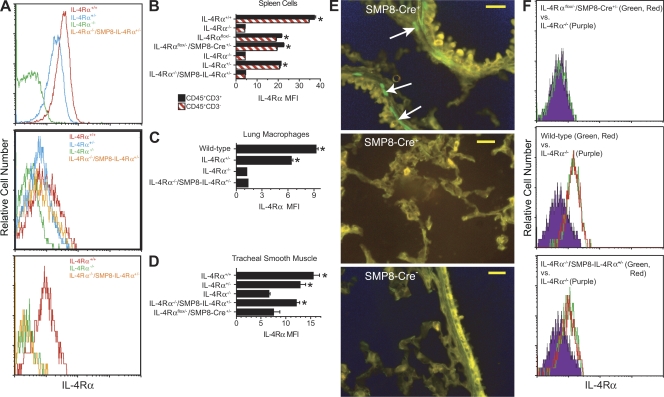
Four approaches were tried to determine whether IL-4Rα−/−/SMP8–IL-4Rα+/− mice express IL-4Rα only in smooth muscle. Staining tissue sections with a probe specific for IL-4Rα messenger RNA or with an anti–IL-4Rα mAb proved unsatisfactory because of a low signal to noise ratio. Instead, we prepared single cell suspensions of spleen cells, tracheal smooth muscle cells, lung macrophages, and tracheal epithelial cells from wild-type (IL-4Rα+/+) mice, IL-4Rα+/− mice, IL-4Rα−/− mice, and IL-4Rα−/−/SMP8–IL-4Rα+/− mice, stained these cells with TO-PRO-3 iodide (Invitrogen) to eliminate dead cells and with anti–IL-4Rα mAb, and analyzed IL-4Rα staining by flow cytometry. We observed greater staining of spleen cells and lung macrophages from IL-4Rα+/+ mice than IL-4Rα−/− mice, with approximately half as much staining by spleen cells from IL-4Rα+/− mice (Fig. 1, A [top], B, and C). Spleen cells from IL-4Rα−/−/SMP8–IL-4Rα+/− mice stained similarly to spleen cells from IL-4Rα−/− mice (Fig. 1, A [top] and B). Tracheal smooth muscle cells from IL-4Rα+/+ mice stained approximately twice as brightly as tracheal smooth muscle cells from either IL-4Rα+/− or IL-4Rα−/−/SMP8–IL-4Rα+/− mice, and these stained more brightly than tracheal smooth muscle cells from IL-4Rα−/− mice (Fig. 1, A [middle] and D). Tracheal epithelial cells from IL-4Rα−/−/SMP8–IL-4Rα+/− mice stained no more brightly than tracheal epithelial cells from IL-4Rα−/− mice and less brightly than tracheal epithelial cells from IL-4Rα+/− mice (Fig. 1 A, bottom). Thus, the SMP8–IL-4Rα transgene induces approximately the same amount of IL-4Rα expression as the normal IL-4Rα gene on smooth muscle cells and does not induce IL-4Rα expression on spleen cells or on pulmonary epithelial cells.
Figure 1.
Evaluation of IL-4Rα expression in IL-4Rα−/−/SMP8–IL-4Rα+/− mice. (A) Expression of IL-4Rα by IL-4Rα+/+, IL-4Rα+/−, IL-4Rα−/−, and IL-4Rα−/−/SMP8–IL-4Rα+/− mice in spleen (top), tracheal smooth muscle cells (middle), or tracheal epithelial cells (bottom). Representative results of three to four replicate experiments with one mouse/group are shown. (B) IL-4Rα expression by CD45+CD3+ and CD45+CD3− spleen cells from mice of the indicated genotype. Single experiment with four mice/group. *, P < 0.05 by one-tailed Student’s t test compared with IL-4Rα−/− spleen cells. (C) IL-4Rα expression by macrophages in lung digests from the indicated genotype. Macrophages were identified as CD45+CD11b+ cells that displayed light scatter characteristics typical for macrophages. Single experiment with four mice/group. *, P < 0.05 by one-tailed Student’s t test compared with lung macrophages from IL-4Rα−/− mice. (D) IL-4Rα expression by tracheal smooth muscle cells from mice of the indicated genotype. Two experiments with two mice/group each. *, P < 0.05 by one-tailed Student’s t test compared with tracheal smooth muscle cells from IL-4Rα−/− mice. (B–D) Error bars indicate SEs. (E) YFP (green) expression in smooth muscle cells in bronchioles (top) or alveoli (middle) in Rosa/SMP8-Cre+ lung. Arrows point to YFP+ cells. Lack of YFP (green) expression in bronchioles from control (Rosa/SMP8-Cre−) lung (bottom) is also shown. Bars, 25 µm. (F) Comparison of IL-4Rα expression by tracheal smooth muscle cells from wild-type, SMP8-Cre/IL-4Rαflox/−, IL-4Rα−/−, and IL-4Rα−/−/SMP8–IL-4Rα+/− mice. Histograms of IL-4Rα expression of cells from IL-4Rα−/− mice are shown as purple-filled black lines in all three panels; histograms of IL-4Rα expression by cells from two individual IL-4Rαflox/−/SMP8-Cre+/− mice (top), wild-type mice (middle), and IL-4Rα−/−/SMP8–IL-4Rα+/− mice (bottom) are as red and green lines. Data are from one of the two experiments used to prepare the graph in D.
Because it was not possible to determine IL-4Rα expression on all cell types by immunofluorescence staining and flow cytometry, we investigated the specificity of the SMP8 promoter for smooth muscle by breeding SMP8-Cre mice with B6.129 × 1-Gt(ROSA)26.Sortm1(EYFP)Cos/J mice (Rosa-YFP mice; Druckenbrod and Epstein, 2005), in which YFP is produced by cells that express Cre, and evaluating frozen sections of lung, intestine, and liver for YFP expression by fluorescence microscopy. YFP expression was restricted to smooth muscle cells in the lung (Fig. 1 E), small intestine, and artery (Fig. S1).
Effects of inhaled IL-13 and IL-4 on mice that selectively express IL-4Rα on smooth muscle
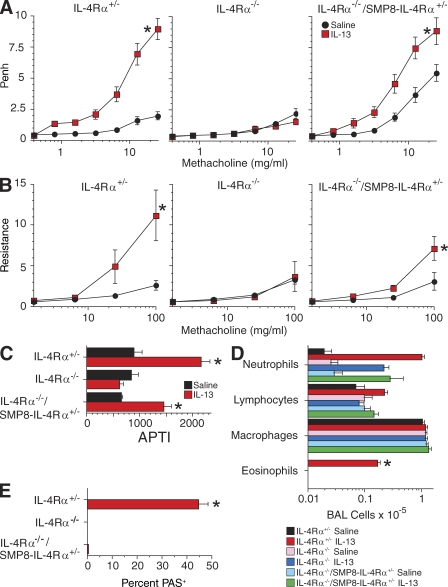
IL-4Rα+/−, IL-4Rα−/−, and IL-4Rα−/−/SMP8–IL-4Rα+/− mice were inoculated intratracheally (i.t.) with IL-13 or saline (2–3 µg/day for 7 d) and tested for responsiveness to cholinergic stimulation by noninvasive barometric plethysmography, the invasive air pressure–time index (APTI) technique, and the invasive flexiVent technique (Fig. 2, A–C). Mice were then sacrificed, bronchoalveolar lavage (BAL) and BAL cell analysis were performed (Fig. 2 D), and lung sections were prepared and stained for goblet cells (Fig. 2 E). As expected, IL-13 had no detectable effects in IL-4Rα−/− mice and induced BAL eosinophilia and goblet cell hyperplasia only in the IL-4Rα+/− mice. Responsiveness to methacholine was somewhat greater in saline-stimulated IL-4Rα−/−/SMP8–IL-4Rα+/− mice than IL-4Rα+/− mice as measured by barometric plethysmography but was similar as measured by the two invasive techniques. IL-13 inhalation increased airway responsiveness in both IL-4Rα−/−/SMP8–IL-4Rα+/− and IL-4Rα+/− mice but had a greater effect in the IL-4Rα+/− mice. This difference was observed with both the invasive and noninvasive techniques but was most apparent when the invasive techniques were used.
Figure 2.
i.t. inoculation of IL-4Rα−/−/SMP8–IL-4Rα+/− mice with IL-13 induces AHR but not goblet cell hyperplasia or BAL fluid eosinophilia. (A–C) IL-4Rα+/−, IL-4Rα−/−, and IL-4Rα−/−/SMP8–IL-4Rα+/− mice were inoculated daily i.t. with saline or 2 or 3 µg IL-13 for a consecutive 7 d and tested for responsiveness to methacholine by barometric plethysmography (A; three experiments, pooled data, n = 11–13/group), flexiVent (B; one experiment, n = 4–7/group), and APTI (C; one experiment, n = 3–5/group). (D) Numbers of BAL neutrophils, lymphocytes, macrophages, and eosinophils (three experiments, pooled data, n = 11–12/group). (E) Lung sections were PAS stained and evaluated for percentage of PAS+ bronchial epithelial cells (one of three experiments with similar results shown, n = 4–5/group). Error bars indicate SEs. *, P < 0.05 for IL-13 versus saline treatment.
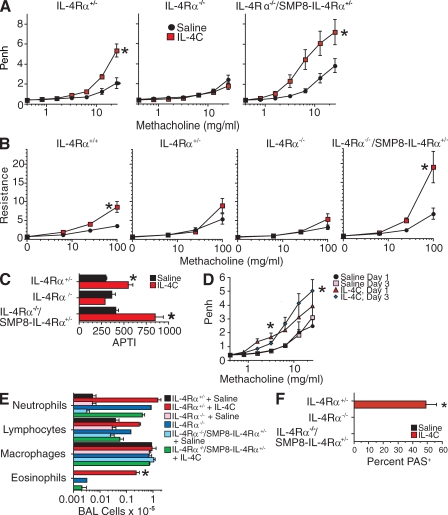
i.t. inoculation of all three mouse strains with IL-4C (a long-acting form of IL-4) also stimulated AHR in IL-4Rα+/−, IL-4Rα+/+, and IL-4Rα−/−/SMP8–IL-4Rα+/− mice but not IL-4Rα−/− mice (Fig. 3, A–C). However, in contrast to IL-13, IL-4C had a greater effect on airway responsiveness in the IL-4Rα−/−/SMP8–IL-4Rα+/− mice than in IL-4Rα+/− or even IL-4Rα+/+ mice. IL-4C inhalation induced some increase in airway responsiveness in IL-4Rα−/−/SMP8–IL-4Rα+/− mice in as little as 24 h, as determined by barometric plethysmography, although greater effects at higher doses of methacholine were seen after 3 d (Fig. 3 D). IL-4C resembled IL-13 more closely in that it induced BAL fluid eosinophilia (Fig. 3 E) and goblet cell hyperplasia (Fig. 3 F) only in the mice that expressed a wild-type IL-4Rα allele. None of the IL-4C– or IL-13–inoculated mice showed convincing airway smooth muscle hyperplasia or hypertrophy by histological examination (unpublished data).
Figure 3.
i.t. inoculation of IL-4Rα−/−/SMP8–IL-4Rα+/− mice with IL-4C induces AHR but not goblet cell hyperplasia or BAL fluid eosinophilia. (A–C) IL-4Rα+/−, IL-4Rα−/−, and IL-4Rα−/−/SMP8–IL-4Rα+/− mice were inoculated daily i.t. with saline or IL-4C (2 µg IL-4 plus 10 µg BVD4-1D11) every other day for 7 d and tested for responsiveness to methacholine by barometric plethysmography (A; two pooled experiments, n = 8–10/group), flexiVent (B; one experiment, n = 4–8/group), and APTI (C; one experiment, n = 4/group). (D) In a separate experiment, IL-4Rα+/−, IL-4Rα−/−, and IL-4Rα−/−/SMP8–IL-4Rα+/− mice were inoculated daily with saline or IL-4C and evaluated by barometric plethysmography 1 d after the first and third inoculations for responsiveness to methacholine (two pooled experiments, n = 12/group). (E) Numbers of BAL neutrophils, lymphocytes, macrophages, and eosinophils (two pooled experiments, n = 8–9/group). (F) Lung sections were PAS stained and evaluated for percentage of PAS+ bronchial epithelial cells (two pooled experiments, n = 8–9/group). Data shown in B and C are from independent experiments. Error bars indicate SEs. *, P < 0.05 for IL-4C versus saline treatment.
Effects of allergen inhalation on mice that selectively express IL-4Rα on smooth muscle
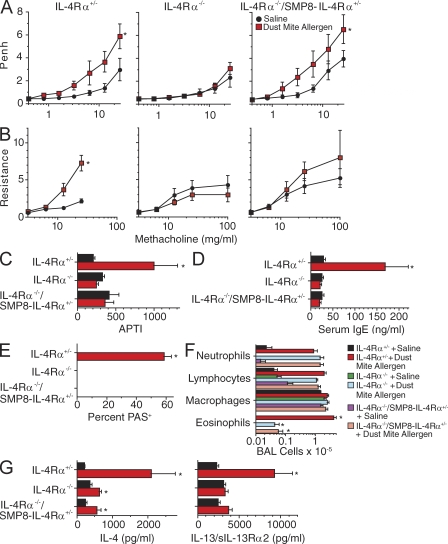
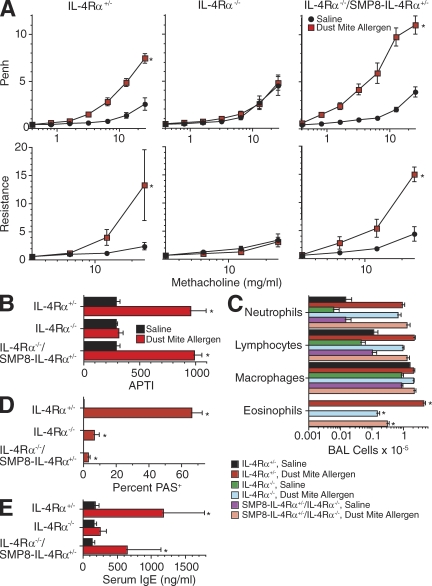
To determine whether AHR could be induced by endogenously produced IL-4/IL-13 in IL-4Rα−/−/SMP8–IL-4Rα+/− mice, these mice as well as IL-4Rα+/− and IL-4Rα−/− mice were inoculated i.t. thrice weekly for 3 wk with dust mite allergen, which induces allergic airway disease in wild-type BALB/c mice without a requirement for systemic priming with allergen plus adjuvant (Johnson et al., 2004; Lewkowich et al., 2005). Dust mite inoculation induced AHR in IL-4Rα+/− mice but not in IL-4Rα−/− mice, as determined by barometric plethysmography and the APTI and flexiVent techniques (Fig. 4, A–C). In the IL-4Rα−/−/SMP8–IL-4Rα+/− mice, dust mite allergen inoculation induced increased responsiveness to methacholine as measured by barometric plethysmography (Fig. 4 A) but not as measured by APTI (Fig. 4 B), whereas flexiVent measurements suggested a slight but insignificant increase in responsiveness (Fig. 4 C). As expected, dust mite inoculation also induced an IgE response in IL-4Rα+/− mice but not in IL-4Rα−/− or IL-4Rα−/−/SMP8–IL-4Rα+/− mice (Fig. 4 D), much greater eosinophil responses in IL-4Rα+/− mice than in IL-4Rα−/− or IL-4Rα−/−/SMP8–IL-4Rα+/− mice (Fig. 4 E), and goblet cell responses only in IL-4Rα+/− mice (Fig. 4 F).
Figure 4.
Effect of i.t. allergen administration on IL-4Rα−/−/SMP8–IL-4Rα+/− mice. (A–C) IL-4Rα+/−, IL-4Rα−/−, and IL-4Rα−/−/SMP8–IL-4Rα+/− mice were inoculated three times per week i.t. for 3 wk with saline or dust mite allergen and then tested for responsiveness to methacholine by barometric plethysmography (A; two pooled experiments, n = 9–12/group), flexiVent (B; two pooled experiments, n = 9–12/group), and APTI (C; one separate experiment, n = 4–6/group). (D) Serum IgE levels were determined by ELISA (two pooled experiments, n = 8–11/group). (E) Lung sections were PAS stained and evaluated for percentage of PAS+ bronchial epithelial cells (two pooled experiments, n = 9–11/group). (F) Numbers of BAL neutrophils, lymphocytes, macrophages, and eosinophils (two pooled experiments, n = 9–12/group). (G) Production of IL-4 and serum levels of IL-13–sIL-13Rα2 complexes (two pooled experiments, n = 11–14/group). Error bars indicate SEs. *, P < 0.05 for dust mite allergen versus saline treatment.
Because the failure of IL-4Rα−/−/SMP8–IL-4Rα+/− mice to develop AHR as demonstrated by the invasive techniques might result from a poor IL-4/IL-13 response to dust mite allergen by the IL-4Rα–deficient T cells in these mice (Fig. 4 G) rather than from the inability of their lung epithelial cells to respond to these cytokines, we performed an additional experiment in which the same mouse strains were injected with bronchial lymph node cells from dust mite–immunized wild-type BALB/c mice before i.t. inoculation of the recipients with dust mite allergen. This protocol induced the development of AHR in both IL-4Rα+/− and IL-4Rα−/−/SMP8–IL-4Rα+/− mice but not in IL-4Rα−/− mice, as determined by barometric plethysmography, flexiVent analysis, and APTI (Fig. 5, A and B). Both the IL-4Rα−/− and IL-4Rα−/−/SMP8–IL-4Rα+/− recipients of allergen-primed wild-type bronchial lymph node cells developed more BAL eosinophilia and goblet cell hyperplasia than mice that were not immunized but considerably less than allergen-inoculated IL-4Rα+/− mice (Fig. 5, C and D), whereas most IL-4Rα+/− mice and some IL-4Rα−/−/SMP8–IL-4Rα+/− mice developed increased serum IgE levels (Fig. 5 E).
Figure 5.
Effect of i.t. allergen administration on IL-4Rα−/−/SMP8–IL-4Rα+/− mice reconstituted with IL-4Rα+/+ allergen–immune lymphoid cells. Wild-type BALB/c mice were immunized i.p. with dust mite allergen adsorbed to alum once a week for 3 wk and then inoculated i.t. with dust mite allergen every other day for 18 d. (A and B) 5 × 106 bronchial lymph node cells prepared from these mice were injected i.v. into IL-4Rα+/−, IL-4Rα−/−, and IL-4Rα−/−/SMP8–IL-4Rα+/− mice, which were then inoculated three times per week i.t. for 3 wk with saline or dust mite allergen and tested for responsiveness to methacholine by barometric plethysmography (A; two pooled experiments, n = 8–12/group), flexiVent analysis (A; one experiment, n = 3–6/group), or APTI (B; one separate experiment, n = 3/group for saline and 5–6/group for house dust mite). (C) BAL was performed, and numbers of BAL neutrophils, lymphocytes, macrophages, and eosinophils were determined (two pooled experiments, n = 6–11/group). (D) Lung sections were PAS stained and evaluated for percentage of PAS+ bronchial epithelial cells (two pooled experiments, n = 9–13/group). (E) Serum IgE levels were determined by ELISA (two pooled experiments, n = 5–13/group). Error bars indicate SEs. *, P < 0.05 for dust mite allergen versus saline treatment.
IL-13 induction of pulmonary gene expression
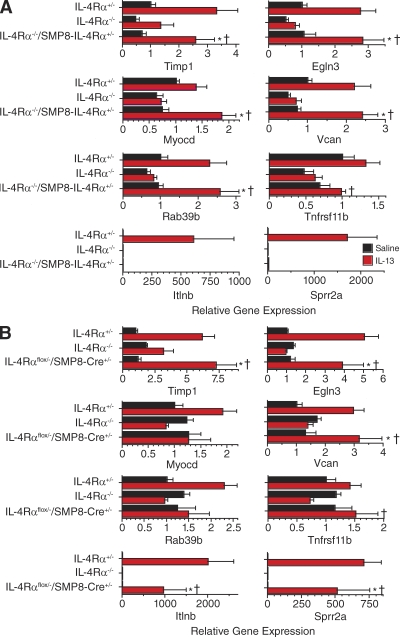
These observations demonstrated that AHR can be induced through direct, in vivo effects of IL-4R signaling on smooth muscle and were consistent with previous in vitro evidence that IL-4R signaling can increase smooth muscle contractility (Laporte et al., 2001; Tliba et al., 2003; Shore, 2004b; Akiho et al., 2005; Kellner et al., 2007; Bossé et al., 2008; Farghaly et al., 2008; Martin et al., 2008; Ohta et al., 2008; Woodruff, 2008). However, they offered no insight into the mechanisms through which IL-4R signaling induces these effects. To shed light on possible mechanisms, a comprehensive gene scan was performed to identify genes in lung whose expression was increased or decreased by a factor >1.5 by IL-13 inhalation in IL-4Rα+/− and IL-4Rα−/−/SMP8–IL-4Rα+/− mice but not in IL-4Rα–deficient mice. Results (GEO DataSets accession no. GSE26476), confirmed by real-time PCR, revealed five genes, Timp1, Egln3, Myocd, Vcan, and Rab39b, whose expression was significantly increased in IL-4Rα−/−/SMP8–IL-4Rα+/− mice by IL-13 inhalation and which were expressed at a greater level in IL-13–inoculated IL-4Rα−/−/SMP8–IL-4Rα+/− mice than in IL-4Rα−/− mice (Fig. 6 A, top six panels). Expression of a sixth gene, Tnfrsf11, which was originally identified by our gene scan, was significantly greater in IL-13–treated IL-4Rα−/−/SMP8–IL-4Rα+/− than IL-4Rα−/− mice but not significantly greater in IL-13–treated than in saline-treated IL-4Rα−/−/SMP8–IL-4Rα+/− mice (P = 0.07). Remarkably, all of these genes were already known to be expressed in smooth muscle and to contribute to smooth muscle cell differentiation, proliferation, contractility, and/or migration (Table I). An additional 12 genes were identified by gene scan to decrease lung expression in response to inhaled IL-13 in IL-4Rα−/−/SMP8–IL-4Rα+/− but not in IL-4Rα−/− mice; however, this was not confirmed by real-time PCR (unpublished data). As expected, several genes known to be induced by IL-4R signaling in epithelial cells and macrophages were strongly induced by IL-13 inhalation in IL-4Rα+/− mice but not in IL-4Rα−/− or IL-4Rα−/−/SMP8–IL-4Rα+/− mice (results are shown in Fig. 6 A for Itlnb and Sprr2a; Arg, Muc5ac, Mmp12, Chi3l3, Retnlb, Chia, and Clca3 were also expressed at least 40-fold more in IL-4Rα+/− mice than in IL-4Rα−/−/SMP8–IL-4Rα+/− mice that had inhaled IL-13). These observations suggest several potential mechanisms for induction of AHR by IL-4 and IL-13 and provide an independent confirmation of the smooth muscle specificity of IL-4Rα expression in IL-4Rα−/−/SMP8–IL-4Rα+/− mice.
Figure 6.
IL-13–induced pulmonary smooth muscle gene expression. (A) Mice of the indicated genotype were inoculated i.t. with normal saline or 3 µg IL-13 for a consecutive 7 d. Lung RNA was prepared and analyzed in an initial experiment by gene scan to identify genes that were increased at least twofold in IL-13– versus saline-treated IL-4Rα−/−/SMP8–IL-4Rα+/− mice and at least twofold in IL-13–treated IL-4Rα−/−/SMP8–IL-4Rα+/− versus IL-4Rα−/− mice and for genes that were expressed >10-fold more in IL-13–treated IL-4Rα+/− versus IL-4Rα−/−/SMP8–IL-4Rα+/− mice (gene scan performed with two separate groups of four mice each for each genotype and treatment). Results of the gene scan were verified by real-time PCR with the same RNA samples and with RNA prepared from lungs from two additional experiments. Total of 18–22 mice for each genotype/treatment group. To pool data from different experiments, the mean value for each gene in the IL-4Rα+/−/saline group in each experiment was given an arbitrary value of 1, and all individual values were adjusted accordingly. Geometric means and SEs were then determined. Log values were converted back to linear values for graphing. A one-tailed Student’s t test was used to test the hypotheses that IL-13 induced greater gene expression than saline in IL-4Rα−/−/SMP8–IL-4Rα+/− mice and that IL-13 induced greater gene expression in IL-4Rα−/−/SMP8–IL-4Rα+/− than in IL-4Rα−/− mice. *, P < 0.05 for IL-13– versus saline-treated IL-4Rα−/−/SMP8–IL-4Rα+/− mice; †, P < 0.05 for IL-13–treated IL-4Rα−/−/SMP8–IL-4Rα+/− versus IL-4Rα−/− mice. (B) Age- and sex-matched IL-4Rα+/−, IL-4Rα−/−, and SMP8-Cre+/−/IL-4Rαflox/− mice were inoculated with saline or IL-13 as in A. RNA was prepared from their lungs and assayed for expression levels of the genes shown by real-time PCR. Data were pooled from two experiments with similar results (n = 13–14 mice/group). A one-tailed Student’s t test was used to compare gene expression in saline- versus IL-13–treated IL-4Rα−/−/SMP8–IL-4Rα+/− mice and in IL-13–treated IL-4Rα+/− versus IL-4Rα−/− and SMP8-Cre+/−/IL-4Rαflox/− mice. *, P < 0.05 for IL-13– versus saline-treated IL-4Rα+/− or SMP8-Cre+/−/IL-4Rαflox/− mice; †, P < 0.05 for IL-13–treated SMP8-Cre+/−/IL-4Rαflox/− versus IL-4Rα−/− mice.
Table I.
Pulmonary genes induced by i.t. IL-13 in IL-4Rα−/−/SMP8–IL-4Rα+/− mice
| Gene | Alternate name | Description | Reference |
| Egln3 | EGL nine homologue 3 | A prolyl hydroxylase, involved in muscle differentiation, most highly expressed in muscle. | Fu et al., 2007 |
| Vcan | Versican, chondroitin sulfate glycoprotein 2 (Cspg2) | A large chondroitin sulfate proteoglycan that is deposited in the airway smooth muscle layer in patients with asthma; promotes proliferation and migration of vascular smooth muscle cells. | Huang et al., 2006 |
| Myocd | Myocardin | The dominant driver of smooth muscle differentiation and the smooth muscle contractile phenotype | Pipes et al., 2006 |
| Rab39b | Ras-related protein Rab 39B | A member of the RAS oncogene family; small molecular weight GTPases that control vesicular trafficking. Ras family signaling is associated with differentiation, proliferation, and hypertrophy. | Sugden, 2003 |
| Timp1 | Tissue inhibitor of metalloproteinase 1 | Expressed in dystrophic and regenerating muscle fibers, blocks extracellular matrix degradation. | Untergasser et al., 2005; Sun et al., 2006 |
| Tnfrsf11b | TNF receptor superfamily, member 11b, osteoprotegerin (OPG) | Produced by smooth muscle cells; stimulates proliferation and migration of pulmonary artery smooth muscle cells in vitro. | Lawrie et al., 2008 |
To determine whether any of the five genes that were induced by IL-13 in IL-4Rα−/−/SMP8–IL-4Rα+/− mice are induced selectively in smooth muscle, we evaluated their expression, along with expression of Tnfrsf11, Itlnb, and Sprr2a in SMP8-Cre+/−/IL-4Rαflox/− mice, which were designed to express IL-4Rα on all cell types except for smooth muscle (Fig. 1, B and D–F). As expected, IL-13 greatly up-regulated expression of Itlnb and Sprr2a in these mice (Fig. 6 B). IL-13 also up-regulated expression of Timp1, Egln3, and Vcan in these mice, suggesting that these genes are up-regulated by IL-13 in nonsmooth muscle as well as smooth muscle cells. In contrast, Myocd and Rab39b were not up-regulated by IL-13 in these mice, even though they were up-regulated by IL-13 in IL-4Rα+/− and IL-4Rα−/−/SMP8–IL-4Rα+/− mice (Figs. 6, A and B). This suggests that Myocd and Rab39b are selectively up-regulated by IL-13 in smooth muscle cells and makes them attractive potential therapeutic targets.
Expression of IL-4Rα by smooth muscle is not essential for induction of AHR by IL-13 or allergen
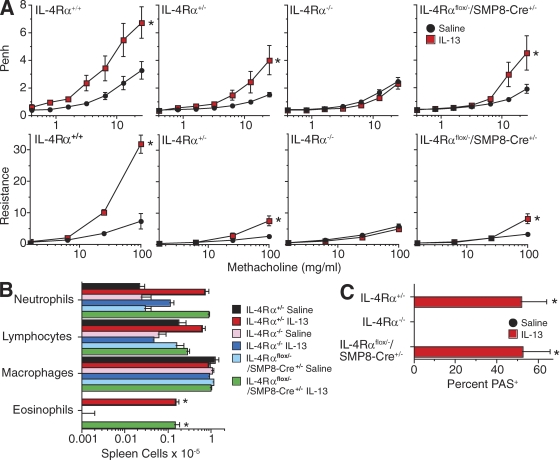
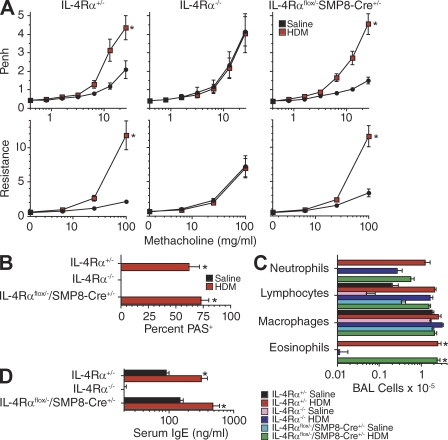
A previous study suggested that IL-4Rα expression by smooth muscle should not be essential for cytokine induction of AHR by demonstrating that AHR develops in mice that overexpress IL-13 in their lungs and express Stat6, which is a transcription factor required for IL-4/IL-13–induced AHR, only in airway epithelium (Kuperman et al., 2002). This result was recently confirmed in a study that used the smooth muscle myosin heavy chain promoter rather than SMP8 to specifically delete IL-4Rα from smooth muscle and inoculated mice with ovalbumin rather than dust mite allergen or IL-13 (Kirstein et al., 2010). To independently evaluate this issue, we compared the effects of IL-13 or dust mite allergen inhalation on the development of airway eosinophilia, goblet cell hyperplasia, and AHR in IL-4Rαflox/−/SMP8-Cre+/− versus IL-4Rα+/− and IL-4Rα−/− mice. AHR, in addition to airway eosinophilia and goblet cell hyperplasia, was induced by inhalation of either IL-13 or dust mite allergen in IL-4Rαflox/−/SMP8-Cre+/− and IL-4Rα+/− mice but not in IL-4Rα−/− mice (Figs. 7 and 8).
Figure 7.
IL-13 induces allergic airway disease in mice that selectively lack IL-4Rα in smooth muscle. (A, top) Mice of the indicated genotype were inoculated i.t. with saline or 3 µg IL-13 thrice weekly for 3 wk and then analyzed for AHR by barometric plethysmography (two pooled experiments, n = 6–14/group). (bottom) Mice were then inoculated with an additional dose of IL-13 and analyzed for AHR with a flexiVent apparatus (two pooled experiments, n = 6–14/group). (B) BAL cell numbers and differential counts (one experiment, n = 7–8/group). (C) Percentage of PAS+ bronchial epithelial cells (one experiment, n = 7–8/group). Error bars indicate SEs. Asterisks indicate a significant increase (*, P < 0.05) for IL-13 versus saline treatment.
Figure 8.
House dust mite allergen induces allergic airway disease in mice that selectively lack IL-4Rα in smooth muscle. (A, top) Mice of the indicated genotype were inoculated i.t. with saline or 50 µg house dust mite allergen thrice weekly for 3 wk and then analyzed for AHR by barometric plethysmography (one experiment, n = 5–8/group). (bottom) Mice were then inoculated with an additional dose of house dust mite allergen and analyzed for AHR with a flexiVent apparatus (one experiment, n = 5–8/group). (B and C) Percentage of PAS+ bronchial epithelial cells (B; one experiment, n = 5–8/group) and BAL cell numbers and differential counts (C; one experiment, n = 5–8/group). (D) Serum IgE levels evaluated by ELISA (one experiment, n = 5–8/group). Error bars indicate SEs. Asterisks indicate a significant increase (*, P < 0.05) for IL-13 versus saline treatment. All data were obtained from the same set of mice. Similar results were observed in a second experiment.
DISCUSSION
AHR, a hallmark of asthma and cause of asthma morbidity, is induced by IL-4 and IL-13 in mice and associated with the same cytokines in humans. Because IL-4/IL-13R expression is ubiquitous, AHR could, in theory, result from IL-4/IL-13 effects on almost any cell type. Stimulation of lymphocytes, macrophages, mast cells, basophils, or dendritic cells, for example, might induce production of vasoactive mediators that promote AHR through their effects on epithelial cells, neurons, or smooth muscle cells or induce production of chemokines that attract inflammatory cells whose activities induce AHR. AHR-inducing effects of IL-4/IL-13 might also be direct. IL-4R stimulation of epithelial cells, which induces AHR in mice (Kuperman et al., 2002), dramatically changes the expression of multiple epithelial cell genes and induces airway goblet cell hyperplasia and increased mucus production, which could contribute to AHR by narrowing airways. IL-4R stimulation of neurons might increase production and/or facilitate release of acetylcholine, which induces smooth muscle contraction (Goldhill et al., 1997). IL-4R stimulation of smooth muscle cells might increase their contractility and/or stimulate them to proliferate or hypertrophy (Wills-Karp, 1997; Laporte et al., 2001; Shore and Moore, 2002; Shore, 2004a).
Although a previous study demonstrated that direct IL-13 activation of Stat6 in airway epithelial cells induces AHR in mice (Kuperman et al., 2002) and AHR has been reported to depend on the products of epithelial cell–expressed genes that are induced by IL-4/IL-13 (Howarth et al., 1995; Goto et al., 2000; Poynter et al., 2002; Zhu et al., 2004; Kawada et al., 2007), a study that demonstrated that AHR still develops in allergen-inoculated mice that have selectively deleted IL-4Rα from airway epithelium (Kuperman et al., 2005) indicates that IL-4/IL-13 effects on other cell types must also be capable of inducing AHR. This justified consideration of smooth muscle, a cell type implicated in AHR by in vitro human and animal studies. These studies demonstrated that IL-13 increases the affinity of intestinal smooth muscle for acetylcholine (Kellner et al., 2007), that IL-4 and IL-13 increase intestinal smooth muscle responses to nerve stimulation through a Stat6-dependent mechanism (Zhao et al., 2003), that IL-13 acts in vitro to increase airway smooth muscle contractility and proliferation and stimulate smooth muscle production of the chemokine eotaxin (Shore, 2004b), and that IL-13 induces calcium transients in cultured mouse airway smooth muscle cells and augments the calcium and contractile responses of these cells to leukotriene D4 (Eum et al., 2005). However, until now, there has been no evidence that direct effects of IL-4/IL-13 on smooth muscle contribute to AHR development in vivo.
Our results indicate that the direct effects of these cytokines on smooth muscle contribute to AHR and are likely to be biologically important. In contrast to IL-4Rα–deficient mice, transgenic mice that express IL-4R only on smooth muscle cells and at approximately the same level as mice that have a single allele of the wild-type IL-4Rα gene developed AHR after i.t. inoculation with IL-4 or IL-13. This was observed despite the lack of development of airway eosinophilia or goblet cell hyperplasia in the smooth muscle–only mice. Consistent results were observed after i.t. inoculation of dust mite allergen instead of IL-4 or IL-13. However, allergen administration induced less AHR in smooth muscle–only mice than in wild-type mice, probably because endogenous IL-4 and IL-13 production was limited in the former strain by the absence of IL-4Rα on lymphoid cells. This conclusion is supported by the more impressive development of AHR in allergen-immunized smooth muscle–only mice that were inoculated with allergen-primed lymph node cells from wild-type mice before their own inoculation with allergen.
Our results also suggest that smooth muscle IL-4R signaling increases AHR by increasing the expression of several genes that likely act in parallel to promote smooth muscle differentiation, proliferation, migration, and contractility and to promote the accumulation of extracellular matrix, which enhances smooth muscle contractility (Table I). Although the extent to which these changes in gene expression affect smooth muscle protein expression and the exact mechanisms by which changes in the expression of these genes promote AHR remain unknown, our observations provide useful leads for further investigation as well as potential targets for asthma therapy. It is also likely that IL-4R signaling increases or decreases the expression of additional genes in smooth muscle but that these changes are not seen in a whole lung analysis because they are obscured by IL-4R–independent expression of the same genes in other cell types.
Although selective smooth muscle IL-4R expression is sufficient for AHR induction by allergen or cytokine inhalation, it is not required. This is consistent with the previous demonstration of AHR in transgenic mice that overexpress IL-13 in their lungs and express Stat6 only in airway epithelial cells (Kuperman et al., 2002). Most likely, AHR can be induced in mice either by airway narrowing caused by epithelial cell enlargement and mucus production, despite normal smooth muscle contractility, or by increased smooth muscle contractility, despite normal airway diameter. Alternatively, it is possible that IL-4/IL-13 effects on airway epithelium indirectly increase smooth muscle contractility.
The significance of AHR in rodents and the best way to determine it has been debated (Finkelman, 2008). There are considerable differences between murine and human lung structure, and it is impossible to characterize maximal voluntary inhalations and exhalations in rodents (these are used to measure airway responsiveness in people). Noninvasive techniques performed with awake mice, such as barometric plethysmography, avoid changes in airway physiology that are induced by anesthesia and intubation but are indirect and can be influenced by upper airway inflammation and factors other than airway diameter and distensibility. Invasive techniques allow more direct measurement of airway characteristics, but the results they provide may be distorted by instrumentation and the use of anesthetics and paralytics. We approached the issues raised by these considerations by using three different techniques to measure responsiveness to cholinergic stimulation. Despite their differences, each technique demonstrated that exogenous and endogenously produced IL-4/IL-13 induce AHR in mice that express IL-4Rα only on smooth muscle cells but not in fully IL-4Rα–deficient mice.
This still leaves open the issue of whether our results with mouse models are applicable to human asthma. Although it is impossible to resolve this issue without performing human studies that are unethical and probably technically impossible, similar in vitro observations made with IL-4/IL-13–stimulated rodent and human smooth muscle cells (Shore, 2004a,b) make it seem likely that the in vivo mouse observations will also extend to humans. Indeed, the much greater airway diameter in humans than in mice and the development of considerable smooth muscle hyperplasia by human asthmatics (Ebina et al., 1993) but not by mice in our study make it likely that effects of IL-4/IL-13 on smooth muscle contribute more to AHR in human asthma than in our mouse models. In this regard, our model in which AHR depends directly on IL-4/IL-13 effects on smooth muscle should be useful for evaluating potential therapeutics for human asthma.
Two other issues raised by our observations remain unresolved and are attractive subjects for future study. First, why do IL-4 and IL-13 differ in their relative effects on airway responsiveness in IL-4Rα+/− versus IL-4Rα−/−/SMP8–IL-4Rα+/− mice? One possible explanation is that IL-4 but not IL-13 stimulates NK and T cells to produce IFN-γ and IL-10 (Finkelman et al., 2005; Morris et al., 2006; unpublished data), cytokines which can suppress airway responsiveness. Failure of IL-4 to induce these cytokines in IL-4Rα−/−/SMP8–IL-4Rα+/− mice might account for the greater IL-4 stimulation of AHR in IL-4Rα−/−/SMP8–IL-4Rα+/− mice than IL-4Rα+/− or wild-type mice. The failure of IL-13 to induce IFN-γ or IL-10 expression would account for the greater effect of IL-13 on AHR in wild-type mice, in which it directly affects epithelial and inflammatory cells, than on IL-4Rα−/−/SMP8–IL-4Rα+/− mice, in which it only directly affects smooth muscle.
Second, do IL-4/IL-13 effects on smooth muscle and epithelial cells totally account for AHR induction by these cytokines, or might effects of these cytokines on other non–bone marrow–derived cell types (neurons, fibroblasts, and vascular endothelium) also contribute? Mice that express a floxed IL-4Rα gene and both smooth muscle– and epithelial cell–specific Cre are currently being bred to address this question.
MATERIALS AND METHODS
Mice.
BALB/c female wild-type and IL-4Rα–deficient mice were purchased from Taconic. Rosa-YFP mice were purchased from the Jackson Laboratory (Druckenbrod and Epstein, 2005). SMP8-Cre transgenic mice on a mixed genetic background were a gift from T. Clemens and J. Fagin (University of Cincinnati, Cincinnati, OH). These mice were bred to Rosa-YFP mice so that Cre expression could be localized by fluorescence microscopy. SMP8-Cre mice were also bred to IL-4Rα−/− mice to generate IL-4Rα−/−/SMP8–IL-4Rα+/− mice, which were then bred to IL-4Rαflox/flox mice to generate SMP8-Cre+/−/IL-4Rαflox/− mice, which selectively delete IL-4Rα in smooth muscle cells. Because these mice have a mixed genetic background, littermates that had an SMP8-Cre−/−/IL-4Rαflox/− genotype (designated, for the sake of simplicity, as IL-4Rα+/− mice) were used as controls, as were mixed genetic background IL-4Rα−/− mice. A PCR approach was used for genotyping. All animal experiments were approved by the Cincinnati Children’s Hospital Medical Center Institutional Animal Care and Use Committee.
Immunological reagents.
M1 (rat IgG2a anti–mouse IL-4Rα mAb; Beckmann et al., 1990) was originally obtained from K. Grabstein (Amgen) and was grown as ascites in Pristane-primed athymic nude mice and purified by ammonium sulfate fractionation and DE-52 cation exchange chromatography. GL117, a control rat IgG2a (anti–Escherichia coli β-galactosidase) was obtained from DNAX and purified in the same way. mAbs were labeled with biotin. Fluorochrome-labeled mAbs to CD4, B220, and CD45 were purchased from BD. Dust mite allergen was purchased from GREER.
Construction of an SMP8–IL-4Rα fusion gene.
We constructed a fusion gene in which IL-4Rα cDNA was controlled by smooth muscle–α-actin regulatory sequences. IL-4Rα cDNA (Mosley et al., 1989; a gift from Amgen) had previously been placed upstream of the terminal SV40 T-antigen intron and polyA signal sequence in a pUC18-based plasmid controlled by the FABPi promoter. The FABPi promoter was excised by digestion with NdeI followed by a partial BamHI digest, which produced a 5.8-kb promoterless fragment containing IL-4Rα cDNA upstream of the SV40 T-intron and polyA signal in a pUC18 backbone. A 3.6-kb NdeI–BamHI fragment containing 1,074 bp of the 5′ flanking region, 63 bp of 5′ untranslated, and the 2.5-kb first intron of smooth muscle–α-actin was released from pSMP8-CAT (Qian et al., 1999), ligated to the IL-4Rα–polyA-pUC18 fragment, and transformed into E. coli TOP 10 cells using standard protocols. Selected clones were PCR screened using sense SMP8 primer 5′-GCCTGTGACACTCCCGCT-3′ located within intron 1 and antisense IL-4Rα primer 5′-TCAGAGAAGCAGGTGGGC-3′ located 92 bases within the IL-4Rα coding sequence. The veracity of the junctions in the pUC18-SMP8up–IL-4Rα–SV40TpolyA construct was confirmed by sequencing across the ligation junction of selected clones.
Generation of SMP8–IL-4Rα transgenic mice.
An NdeI–Not1 digest of pUC18-SMP8up–IL-4Rα–SV40TpolyA liberated a 7.5-kb linear fragment that was gel-purified for pronuclear microinjection by the Cincinnati Children’s Hospital Medical Center Transgenic Mouse Core. The transgenic founder animals were generated on an FVB/N genetic background. The offspring generated after pronuclear microinjection were typed by PCR analysis of tail DNA using the sense SMP8 and antisense IL-4Rα primers described in the previous section and verified by Southern blot using a BamHI IL-4Rα–SV40TpolyA fragment as probe (Horsnell et al., 2007). Offspring that were both PCR- and Southern blot–positive were bred to BALB/c IL-4Rα–deficient mice, and their progeny were screened by PCR for the presence of the SMP8up–IL-4Rα–SV40TpolyA transgene. Transgene-positive offspring were bred a second time to BALB/c IL-4Rα–deficient mice to generate F1 double backcross offspring that were typed by PCR for the presence of the transgene, using sense SMP8 and antisense IL-4Rα primers, and for the absence of the normal IL-4Rα gene using primers to neomycin (Neo forward, 5′-AGACAATCGGCTGCTCTGAT-3′; and Neo reverse, 5′-ATACTTTCTCGGCAGGAGCA-3′) and IL-4Rα exon 7 (Ex7 forward, 5′-CATATGTCCCTGTCTTCCTT-3′; and Ex7 reverse, 5′-GGACTCCACTCACTCCAG-3′), which was deleted during the generation of IL-4Rα–deficient mice. We verified expression of the transgene in RNA isolated from uterus (which is rich in smooth muscle) and gut by RT-PCR using sense primer SMP8 exon 1 5′-GGACACCACCCACCCAGA-3′ combined with antisense IL-4Rα primer, resulting in a 158-bp amplicon specific to SMP8–IL-4Rα messenger RNA.
IL-4–anti–IL-4 complexes.
To extend the in vivo half-life of IL-4, mice were inoculated in some experiments with complexes produced by mixing recombinant mouse IL-4 with a neutralizing anti–IL-4 mAb (BVD4-1D11) at a 2:1 molar (1:5 weight) ratio, which saturates the mAb with IL-4. We have previously demonstrated that these complexes have an in vivo half-life of ∼1 d and slowly dissociate, releasing biologically active IL-4 (Finkelman et al., 1993). Because these complexes contain a single IgG molecule, they neither fix complement nor react more avidly than uncomplexed serum IgG with FcγRs. Because IL-4 cannot bind to IL-4Rs when it is complexed with BVD4-1D11, there is no possibility for these complexes to cross-link IL-4Rs to FcγRs. Uncomplexed IL-4 has similar effects as IL-4C on lung inflammation and airway responsiveness, but higher quantities are required to obtain the same effect (Finkelman et al., 1993).
In vivo cytokine production.
IL-4 production was measured with the in vivo cytokine capture assay (Finkelman et al., 2003). Mice were injected with 10 µg biotin-anti–IL-4 mAb and bled 1 d later, and serum levels of IL-4–biotin-anti–IL-4 mAb complex were determined. Serum levels of IL-13–sIL-13Rα2 complex were measured as described previously (Khodoun et al., 2007).
i.t. inoculation.
Mice were anesthetized by i.p. injection of ketamine and xylazine and allowed to hang vertically with their mouths open, supported by a taut string placed under their canine teeth. Their tongues were gently withdrawn with a blunt forceps to keep them from swallowing, and 40 µl of vehicle (1% autologous serum in PBS) ± IL-4C was pipetted onto the base of the tongue. When the mice had aspirated the pipetted solution, they were placed on their sides in a box flushed with 100% oxygen until they recovered from the anesthesia.
BAL.
Mice were sacrificed, and polyethylene catheters (OD = 0.97 mm; BD) were inserted into their tracheas. Lungs were lavaged three times with 1 ml Ca2+- and Mg2+-free HBSS (Invitrogen), which contained 0.02% EDTA and EGTA. The BAL fluid was centrifuged at 1,000 rpm for 5 min at 4°C. Cell pellets were resuspended in 1.5 ml cold HBSS with 10% newborn bovine serum and 0.2% NaN3 (HNA) and counted with a Beckman Coulter counter. 200,000 cells were centrifuged at 700 rpm for 5 min onto glass microscopic slides with a cytocentrifuge (Shandon Scientific). Slides were stained with Giemsa stain (Sigma-Aldrich). At least 200 cells/slide were examined microscopically and characterized as neutrophils, lymphocytes, macrophages, or eosinophils.
Inoculation protocol.
Mice were inoculated i.t. with saline, IL-4C (2 µg IL-4/10 µg anti–IL-4 mAb) every other day for 7 d, IL-13 (2 or 3 µg) daily for 7 d, or with dust mite allergen, 50 µg three times per week for 3 wk.
Determination of airway responsiveness to methacholine.
For determination of responsiveness to methacholine by barometric plethysmography, mice were placed, unrestrained, in cylindrical Plexiglas plethysmograph chambers that were connected to a nebulized control aerosol delivery system and a Max II apparatus for analyzing barometric plethysmography (Buxco Research Systems). Baseline measurements of enhanced pause (Penh; Hamelmann et al., 1997) were made over a 5-min period. Mice were then challenged for 3 min by inhalation of aerosolized β-methacholine in PBS, produced with a nebulizer (model 5500D-030; DeVilbiss Healthcare), starting at a methacholine concentration of 0.8 mg/ml. Penh measurements were made for 5 min, starting 2 min after completion of exposure to the aerosolized methacholine, and average Penh values for the 5-min period were calculated. The procedure was then serially repeated with methacholine at concentrations of 1.6, 3.2, 6.4, 12.8, and 25.6 mg/ml. Airway responsiveness of anesthetized, intubated mice to acetylcholine by the APTI technique was performed as previously described (Gavett et al., 1994). Airway responsiveness of anesthetized, intubated mice to methacholine was also performed with a flexiVent apparatus (SCIREQ). Mice were anesthetized with xylazine and Na pentobarbital. Their tracheas were then cannulated with an 18-gauge needle, and mice were ventilated at 150 breaths/min, 3.0-cm water positive end expiratory pressure. Mice were paralyzed with pancuronium bromide (Sigma-Aldrich) and allowed to stabilize on the ventilator for 2 min. Two total lung capacity perturbations were performed for airway recruitment before baseline measurement and subsequent methacholine challenges were performed. Dynamic resistance (R) was determined by fitting the data to a single compartment model of airway mechanics where Ptr = RV + EV+ PO (Ptr, tracheal pressure; V, volume; and PO, constant). Resistance measurements were made using a 1.25-s, 2.5-Hz volume driven oscillation applied to the airways by a computer-controlled piston (SnapShot perturbation). Methacholine was aerosolized for 10 s followed by 10 s of ventilation with an ultrasonic nebulizer (Aeroneb; Aerogen), and 20 SnapShot perturbations were performed. The procedure was repeated for 0, 6.25, 25, and 100 mg/ml concentrations of methacholine. The maximum R value with a coefficient of determination of 0.9 or greater (as determined by the flexiVent software) was used to determine the dose–response curve.
Lung histology.
After BAL, lungs were inflated by injecting 1 ml of zinc formalin fixative through the catheter used to perform BAL. Lungs were then removed and fixed in the same solution. Lung sections were stained with periodic acid-Schiff (PAS) and examined microscopically.
Evaluation of goblet cell hyperplasia.
Lung sections stained with PAS were evaluated blindly. Three bronchioles containing 90–130 epithelial cells were identified on each section. PAS+ (goblet) cells and total epithelial cell nuclei per bronchiole section were counted, and the ratio was converted into a percentage.
Preparation of suspensions of tracheal smooth muscle cells.
Tracheas excised from mice were rinsed in antibiotic-supplemented HBSS. Connective tissue was removed under a dissecting microscope. Tracheas were then flushed with 0.02% EDTA and cut longitudinally. The tracheal interior was scraped with a round scalpel blade, and the trachea was transferred to a Petri dish containing 2 ml HBSS and 20 µl each of Liberase Blendzyme 1 and 4 (Roche). Tissue was incubated for 20 min at 37°C in an atmosphere that contained 5% CO2. Dissociated tissue was further cleaned, and tracheal rings were separated into smaller tissue fragments. Three tissue fragments of 2–3 mm2 were transferred, intima side down, to collagen IV–coated Petri dishes (BD). The plates were prewetted with smooth muscle basal medium containing growth supplements human epidermal growth factor, insulin, human fibroblast growth factor B, FBS, and GA-1000 (Lonza). Additional media was added to the plate to just cover the tissue. The plates were incubated at 37°C, 5% CO2 for 4 d undisturbed to allow the tissue to become established to the collagen. Enough media was added for the first 10–14 d to just keep the tissue covered. After 10–14 d of incubation and observing growth of new smooth muscle cells, media was gently pipetted off and replaced with enough new media to just cover the tissue. Media was replaced every 4 d thereafter. Cells were lifted from the plate and stained after 2–3 wk of growth.
Preparation of suspensions of tracheal epithelial cells.
Epithelial cells were isolated from mouse tracheas by enzymatic digestion with 0.1% Pronase (Roche). Detached cells were suspended in bronchial epithelial cell growth medium (Lonza) and plated on Transwell plates (Corning) that had been coated with collagen type 1 (BD) at 1 mg/ml. Cultures were grown at 37°C in a 5% CO2–containing atmosphere until the cells became confluent. Cells were then dissociated from the plates with Liberase (Roche) and analyzed for surface IL-4Rα expression by flow cytometry.
Fluorescence microscopy.
Frozen sections of lung and jejunum were prepared, mounted with Vectashield mounting medium with DAPI, and examined for background and YFP fluorescence with a microscope (Axioskop; Carl Zeiss) equipped with filters for YFP (EX 513 and EM 527). Photographs were taken with a color camera (MRC 5; Carl Zeiss) and processed with Axiovision software version 4.3 (Carl Zeiss) and Photoshop CS (Adobe).
Gene expression experiments.
Gene expression of homogenized whole lung was determined by a gene scan approach, as previously described (Lewis et al., 2009; GEO DataSets accession no. GSE26476). Gene scan results were confirmed by real-time PCR, as previously described (Finkelman et al., 2005), using primers purchased from QIAGEN. IL-13–responsive genes were considered to be induced predominantly or entirely in smooth muscle cells if they were (a) expressed less in IL-13–inoculated SMP8-Cre+/−/IL-4Rαflox/− mice than in IL-13–inoculated IL-4Rα+/− mice and (b) expressed no more in IL-13–inoculated SMP8-Cre+/−/IL-4Rαflox/− mice than in saline-inoculated SMP8-Cre+/−/IL-4Rαflox/− mice and/or were expressed no more in IL-13–inoculated SMP8-Cre+/−/IL-4Rαflox/− mice than in IL-13–inoculated IL-4Rα−/− mice.
Statistics.
Data were analyzed with a one-tailed Student’s t test to test hypotheses that cytokine or allergen treatment increased AHR or did not increase goblet cell hyperplasia or airway eosinophilia in IL-4Rα−/−/SMP8–IL-4Rα+/− mice or induced all three effects in SMP8-Cre/IL-4Rαflox/− mice. A one-tailed Student’s t test was also used to test the hypothesis that IL-13 inhalation increased the expression in IL-4Rα−/−/SMP8–IL-4Rα+/− mice but not in SMP8-Cre/IL-4Rαflox/− mice of six genes that had previously been identified by a gene scan. IL-4Rα+/− and IL-4Rα−/− were used as positive or negative controls, respectively. Values for P < 0.05 were considered significant. Figures show means ± SEs.
Online supplemental material.
Fig. S1 demonstrates that the SMP8-Cre transgene is expressed only in smooth muscle in arteries and intestine. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100023/DC1.
Acknowledgments
We thank James Fagin for providing the SMP8 promoter, Jeffrey Whitsett for help with fluorescence microscopy, Hermine Brunner for advice and help with statistical analysis, and Amgen for providing us with reagents.
This work was supported by the US Department of Veterans Affairs (Merit Award to F.D. Finkelman) and National Institutes of Health (grants PO1 HL076383 to F.D. Finkelman and M. Wills-Karp; RO1 AI052099 to F.D. Finkelman).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- AHR
- airway hyperresponsiveness
- APTI
- air pressure–time index
- BAL
- bronchoalveolar lavage
- i.t.
- intratracheal(ly)
- PAS
- periodic acid-Schiff
References
- Akiho H., Lovato P., Deng Y., Ceponis P.J., Blennerhassett P., Collins S.M. 2005. Interleukin-4- and -13-induced hypercontractility of human intestinal muscle cells-implication for motility changes in Crohn’s disease. Am. J. Physiol. Gastrointest. Liver Physiol. 288:G609–G615 10.1152/ajpgi.00273.2004 [DOI] [PubMed] [Google Scholar]
- Beckmann M.P., Schooley K.A., Gallis B., Vanden Bos T., Friend D., Alpert A.R., Raunio R., Prickett K.S., Baker P.E., Park L.S. 1990. Monoclonal antibodies block murine IL-4 receptor function. J. Immunol. 144:4212–4217 [PubMed] [Google Scholar]
- Bossé Y., Thompson C., Audette K., Stankova J., Rola-Pleszczynski M. 2008. Interleukin-4 and interleukin-13 enhance human bronchial smooth muscle cell proliferation. Int. Arch. Allergy Immunol. 146:138–148 10.1159/000113517 [DOI] [PubMed] [Google Scholar]
- Druckenbrod N.R., Epstein M.L. 2005. The pattern of neural crest advance in the cecum and colon. Dev. Biol. 287:125–133 10.1016/j.ydbio.2005.08.040 [DOI] [PubMed] [Google Scholar]
- Ebina M., Takahashi T., Chiba T., Motomiya M. 1993. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am. Rev. Respir. Dis. 148:720–726 [DOI] [PubMed] [Google Scholar]
- Eum S.Y., Maghni K., Tolloczko B., Eidelman D.H., Martin J.G. 2005. IL-13 may mediate allergen-induced hyperresponsiveness independently of IL-5 or eotaxin by effects on airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 288:L576–L584 10.1152/ajplung.00380.2003 [DOI] [PubMed] [Google Scholar]
- Farghaly H.S., Blagbrough I.S., Medina-Tato D.A., Watson M.L. 2008. Interleukin 13 increases contractility of murine tracheal smooth muscle by a phosphoinositide 3-kinase p110delta-dependent mechanism. Mol. Pharmacol. 73:1530–1537 10.1124/mol.108.045419 [DOI] [PubMed] [Google Scholar]
- Finkelman F.D. 2008. Use of unrestrained, single-chamber barometric plethysmography to evaluate sensitivity to cholinergic stimulation in mouse models of allergic airway disease. J. Allergy Clin. Immunol. 121:334–335 10.1016/j.jaci.2007.11.028 [DOI] [PubMed] [Google Scholar]
- Finkelman F.D., Madden K.B., Morris S.C., Holmes J.M., Boiani N., Katona I.M., Maliszewski C.R. 1993. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J. Immunol. 151:1235–1244 [PubMed] [Google Scholar]
- Finkelman F.D., Morris S.C., Orekhova T., Sehy D. 2003. The in vivo cytokine capture assay (IVCCA) for measurement of in vivo cytokine production in the mouse. Curr. Protoc. Immunol. Chapter 6:Unit 6.28 [DOI] [PubMed] [Google Scholar]
- Finkelman F.D., Yang M., Perkins C., Schleifer K., Sproles A., Santeliz J., Bernstein J.A., Rothenberg M.E., Morris S.C., Wills-Karp M. 2005. Suppressive effect of IL-4 on IL-13-induced genes in mouse lung. J. Immunol. 174:4630–4638 [DOI] [PubMed] [Google Scholar]
- Fu J., Menzies K., Freeman R.S., Taubman M.B. 2007. EGLN3 prolyl hydroxylase regulates skeletal muscle differentiation and myogenin protein stability. J. Biol. Chem. 282:12410–12418 10.1074/jbc.M608748200 [DOI] [PubMed] [Google Scholar]
- Gavett S.H., Chen X., Finkelman F., Wills-Karp M. 1994. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am. J. Respir. Cell Mol. Biol. 10:587–593 [DOI] [PubMed] [Google Scholar]
- Glaab T., Taube C., Braun A., Mitzner W. 2007. Invasive and noninvasive methods for studying pulmonary function in mice. Respir. Res. 8:63 10.1186/1465-9921-8-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhill J., Morris S.C., Maliszewski C., Urban J.F., Jr, Funk C.D., Finkelman F.D., Shea-Donohue T. 1997. Interleukin-4 modulates cholinergic neural control of mouse small intestinal longitudinal muscle. Am. J. Physiol. 272:G1135–G1140 [DOI] [PubMed] [Google Scholar]
- Goto Y., Uchida Y., Nomura A., Sakamoto T., Ishii Y., Morishima Y., Masuyama K., Sekizawa K. 2000. Dislocation of E-cadherin in the airway epithelium during an antigen-induced asthmatic response. Am. J. Respir. Cell Mol. Biol. 23:712–718 [DOI] [PubMed] [Google Scholar]
- Grünig G., Warnock M., Wakil A.E., Venkayya R., Brombacher F., Rennick D.M., Sheppard D., Mohrs M., Donaldson D.D., Locksley R.M., Corry D.B. 1998. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 282:2261–2263 10.1126/science.282.5397.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelmann E., Schwarze J., Takeda K., Oshiba A., Larsen G.L., Irvin C.G., Gelfand E.W. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156:766–775 [DOI] [PubMed] [Google Scholar]
- Horsnell W.G., Cutler A.J., Hoving J.C., Mearns H., Myburgh E., Arendse B., Finkelman F.D., Owens G.K., Erle D., Brombacher F. 2007. Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Ralpha-deficient mice. PLoS Pathog. 3:e1 10.1371/journal.ppat.0030001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsnell W.G., Vira A., Kirstein F., Mearns H., Hoving J.C., Cutler A.J., Dewals B., Myburgh E., Kimberg M., Arendse B., et al. 2011. IL-4Rα-responsive smooth muscle cells contribute to initiation of TH2 immunity and pulmonary pathology in Nippostrongylus brasiliensis infections. Mucosal Immunol. 4:83–92 10.1038/mi.2010.46 [DOI] [PubMed] [Google Scholar]
- Howarth P.H., Redington A.E., Springall D.R., Martin U., Bloom S.R., Polak J.M., Holgate S.T. 1995. Epithelially derived endothelin and nitric oxide in asthma. Int. Arch. Allergy Immunol. 107:228–230 10.1159/000236986 [DOI] [PubMed] [Google Scholar]
- Huang R., Merrilees M.J., Braun K., Beaumont B., Lemire J., Clowes A.W., Hinek A., Wight T.N. 2006. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ. Res. 98:370–377 10.1161/01.RES.0000202051.28319.c8 [DOI] [PubMed] [Google Scholar]
- Huang S.K., Xiao H.Q., Kleine-Tebbe J., Paciotti G., Marsh D.G., Lichtenstein L.M., Liu M.C. 1995. IL-13 expression at the sites of allergen challenge in patients with asthma. J. Immunol. 155:2688–2694 [PubMed] [Google Scholar]
- Humbert M., Durham S.R., Kimmitt P., Powell N., Assoufi B., Pfister R., Menz G., Kay A.B., Corrigan C.J. 1997. Elevated expression of messenger ribonucleic acid encoding IL-13 in the bronchial mucosa of atopic and nonatopic subjects with asthma. J. Allergy Clin. Immunol. 99:657–665 10.1016/S0091-6749(97)70028-9 [DOI] [PubMed] [Google Scholar]
- Johnson J.R., Wiley R.E., Fattouh R., Swirski F.K., Gajewska B.U., Coyle A.J., Gutierrez-Ramos J.C., Ellis R., Inman M.D., Jordana M. 2004. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am. J. Respir. Crit. Care Med. 169:378–385 10.1164/rccm.200308-1094OC [DOI] [PubMed] [Google Scholar]
- Kawada M., Hachiya Y., Arihiro A., Mizoguchi E. 2007. Role of mammalian chitinases in inflammatory conditions. Keio J. Med. 56:21–27 10.2302/kjm.56.21 [DOI] [PubMed] [Google Scholar]
- Kellner J., Gamarra F., Welsch U., Jörres R.A., Huber R.M., Bergner A. 2007. IL-13Ralpha2 reverses the effects of IL-13 and IL-4 on bronchial reactivity and acetylcholine-induced Ca+ signaling. Int. Arch. Allergy Immunol. 142:199–210 10.1159/000097022 [DOI] [PubMed] [Google Scholar]
- Khodoun M., Lewis C.C., Yang J.Q., Orekov T., Potter C., Wynn T., Mentink-Kane M., Hershey G.K., Wills-Karp M., Finkelman F.D. 2007. Differences in expression, affinity, and function of soluble (s)IL-4Ralpha and sIL-13Ralpha2 suggest opposite effects on allergic responses. J. Immunol. 179:6429–6438 [DOI] [PubMed] [Google Scholar]
- Kirstein F., Horsnell W.G., Kuperman D.A., Huang X., Erle D.J., Lopata A.L., Brombacher F. 2010. Expression of IL-4 receptor alpha on smooth muscle cells is not necessary for development of experimental allergic asthma. J. Allergy Clin. Immunol. 126:347–354 10.1016/j.jaci.2010.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsimbos T.C., Ernst P., Hamid Q.A. 1996. Interleukin-13 and interleukin-4 are coexpressed in atopic asthma. Proc. Assoc. Am. Physicians. 108:368–373 [PubMed] [Google Scholar]
- Kuperman D., Schofield B., Wills-Karp M., Grusby M.J. 1998. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J. Exp. Med. 187:939–948 10.1084/jem.187.6.939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman D.A., Huang X., Koth L.L., Chang G.H., Dolganov G.M., Zhu Z., Elias J.A., Sheppard D., Erle D.J. 2002. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 8:885–889 [DOI] [PubMed] [Google Scholar]
- Kuperman D.A., Huang X., Nguyenvu L., Hölscher C., Brombacher F., Erle D.J. 2005. IL-4 receptor signaling in Clara cells is required for allergen-induced mucus production. J. Immunol. 175:3746–3752 [DOI] [PubMed] [Google Scholar]
- Laporte J.C., Moore P.E., Baraldo S., Jouvin M.H., Church T.L., Schwartzman I.N., Panettieri R.A., Jr, Kinet J.P., Shore S.A. 2001. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am. J. Respir. Crit. Care Med. 164:141–148 [DOI] [PubMed] [Google Scholar]
- Lawrie A., Waterman E., Southwood M., Evans D., Suntharalingam J., Francis S., Crossman D., Croucher P., Morrell N., Newman C. 2008. Evidence of a role for osteoprotegerin in the pathogenesis of pulmonary arterial hypertension. Am. J. Pathol. 172:256–264 10.2353/ajpath.2008.070395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C.C., Aronow B., Hutton J., Santeliz J., Dienger K., Herman N., Finkelman F.D., Wills-Karp M. 2009. Unique and overlapping gene expression patterns driven by IL-4 and IL-13 in the mouse lung. J. Allergy Clin. Immunol. 123:795–804: e8 10.1016/j.jaci.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowich I.P., Wills-Karp M. 2008. Animal models of allergen-induced asthma. Middleton’s Allergy: Principles and Practice. Adkinson N.F., Jr, Busse W.W., Bochner B.S., Holgate S.T., Simons F.E., Lemanske R.F., Jr, Elsevier, London: 437–454 [Google Scholar]
- Lewkowich I.P., Herman N.S., Schleifer K.W., Dance M.P., Chen B.L., Dienger K.M., Sproles A.A., Shah J.S., Köhl J., Belkaid Y., Wills-Karp M. 2005. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J. Exp. Med. 202:1549–1561 10.1084/jem.20051506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G., O’Connell R.J., Pietrzykowski A.Z., Treistman S.N., Ethier M.F., Madison J.M. 2008. Interleukin-4 activates large-conductance, calcium-activated potassium (BKCa) channels in human airway smooth muscle cells. Exp. Physiol. 93:908–918 10.1113/expphysiol.2008.042432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S.C., Orekhova T., Meadows M.J., Heidorn S.M., Yang J., Finkelman F.D. 2006. IL-4 induces in vivo production of IFN-γ by NK and NKT cells. J. Immunol. 176:5299–5305 [DOI] [PubMed] [Google Scholar]
- Mosley B., Beckmann M.P., March C.J., Idzerda R.L., Gimpel S.D., VandenBos T., Friend D., Alpert A., Anderson D., Jackson J., et al. 1989. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 59:335–348 10.1016/0092-8674(89)90295-X [DOI] [PubMed] [Google Scholar]
- Mountford A.P., Hogg K.G., Coulson P.S., Brombacher F. 2001. Signaling via interleukin-4 receptor alpha chain is required for successful vaccination against schistosomiasis in BALB/c mice. Infect. Immun. 69:228–236 10.1128/IAI.69.1.228-236.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y., Hayashi M., Kanemaru T., Abe K., Ito Y., Oike M. 2008. Dual modulation of airway smooth muscle contraction by Th2 cytokines via matrix metalloproteinase-1 production. J. Immunol. 180:4191–4199 [DOI] [PubMed] [Google Scholar]
- Perkins C., Wills-Karp M., Finkelman F.D. 2006. IL-4 induces IL-13-independent allergic airway inflammation. J. Allergy Clin. Immunol. 118:410–419 10.1016/j.jaci.2006.06.004 [DOI] [PubMed] [Google Scholar]
- Pipes G.C., Creemers E.E., Olson E.N. 2006. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 20:1545–1556 10.1101/gad.1428006 [DOI] [PubMed] [Google Scholar]
- Poynter M.E., Irvin C.G., Janssen-Heininger Y.M. 2002. Rapid activation of nuclear factor-kappaB in airway epithelium in a murine model of allergic airway inflammation. Am. J. Pathol. 160:1325–1334 10.1016/S0002-9440(10)62559-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J., Lorenz J.N., Maeda S., Sutliff R.L., Weber C., Nakayama T., Colbert M.C., Paul R.J., Fagin J.A., Clemens T.L. 1999. Reduced blood pressure and increased sensitivity of the vasculature to parathyroid hormone-related protein (PTHrP) in transgenic mice overexpressing the PTH/PTHrP receptor in vascular smooth muscle. Endocrinology. 140:1826–1833 10.1210/en.140.4.1826 [DOI] [PubMed] [Google Scholar]
- Rosa-Rosa L., Zimmermann N., Bernstein J.A., Rothenberg M.E., Khurana Hershey G.K. 1999. The R576 IL-4 receptor α allele correlates with asthma severity. J. Allergy Clin. Immunol. 104:1008–1014 10.1016/S0091-6749(99)70082-5 [DOI] [PubMed] [Google Scholar]
- Shore S.A. 2004a. Airway smooth muscle in asthma—not just more of the same. N. Engl. J. Med. 351:531–532 10.1056/NEJMp048139 [DOI] [PubMed] [Google Scholar]
- Shore S.A. 2004b. Direct effects of Th2 cytokines on airway smooth muscle. Curr. Opin. Pharmacol. 4:235–240 10.1016/j.coph.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Shore S.A., Moore P.E. 2002. Effects of cytokines on contractile and dilator responses of airway smooth muscle. Clin. Exp. Pharmacol. Physiol. 29:859–866 10.1046/j.1440-1681.2002.03756.x [DOI] [PubMed] [Google Scholar]
- Steel M.D., Holgate S.T. 2001. Asthma. Samter’s Immunologic Diseases. Sixth edition Austen K.F., Frank M.M., Atkinson J.P., Cantor H., Lippincott Williams & Wilkins, Philadelphia: 836–864 [Google Scholar]
- Sugden P.H. 2003. Ras, Akt, and mechanotransduction in the cardiac myocyte. Circ. Res. 93:1179–1192 10.1161/01.RES.0000106132.04301.F5 [DOI] [PubMed] [Google Scholar]
- Sun G.L., Zhao S., Li P., Jiang H.K. 2006. Expression of tissue inhibitor of metalloproteinase-1 in progression muscular dystrophy. Neurosci. Bull. 22:85–90 [PubMed] [Google Scholar]
- Tliba O., Deshpande D., Chen H., Van Besien C., Kannan M., Panettieri R.A., Jr, Amrani Y. 2003. IL-13 enhances agonist-evoked calcium signals and contractile responses in airway smooth muscle. Br. J. Pharmacol. 140:1159–1162 10.1038/sj.bjp.0705558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untergasser G., Gander R., Lilg C., Lepperdinger G., Plas E., Berger P. 2005. Profiling molecular targets of TGF-β1 in prostate fibroblast-to-myofibroblast transdifferentiation. Mech. Ageing Dev. 126:59–69 10.1016/j.mad.2004.09.023 [DOI] [PubMed] [Google Scholar]
- Wagers S., Lundblad L.K., Ekman M., Irvin C.G., Bates J.H. 2004. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J. Appl. Physiol. 96:2019–2027 10.1152/japplphysiol.00924.2003 [DOI] [PubMed] [Google Scholar]
- Wenzel S., Wilbraham D., Fuller R., Getz E.B., Longphre M. 2007. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 370:1422–1431 10.1016/S0140-6736(07)61600-6 [DOI] [PubMed] [Google Scholar]
- Wills-Karp M. 1997. Smooth muscle as a direct or indirect target accounting for bronchopulmonary hyperresponsiveness. Res. Immunol. 148:59–72 10.1016/S0923-2494(97)86275-X [DOI] [PubMed] [Google Scholar]
- Wills-Karp M., Luyimbazi J., Xu X., Schofield B., Neben T.Y., Karp C.L., Donaldson D.D. 1998. Interleukin-13: central mediator of allergic asthma. Science. 282:2258–2261 10.1126/science.282.5397.2258 [DOI] [PubMed] [Google Scholar]
- Woodruff P.G. 2008. Gene expression in asthmatic airway smooth muscle. Proc. Am. Thorac. Soc. 5:113–118 10.1513/pats.200705-059VS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao A., McDermott J., Urban J.F., Jr, Gause W., Madden K.B., Yeung K.A., Morris S.C., Finkelman F.D., Shea-Donohue T. 2003. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J. Immunol. 171:948–954 [DOI] [PubMed] [Google Scholar]
- Zhu Z., Zheng T., Homer R.J., Kim Y.K., Chen N.Y., Cohn L., Hamid Q., Elias J.A. 2004. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 304:1678–1682 10.1126/science.1095336 [DOI] [PubMed] [Google Scholar]