iNKT cell and pDC cross talk prevents type 1 diabetes by inducing T reg cells in the pancreatic lymph node during viral infection.
Abstract
Type 1 diabetes (T1D) is an autoimmune disease resulting from T cell–mediated destruction of insulin-producing β cells, and viral infections can prevent the onset of disease. Invariant natural killer T cells (iNKT cells) exert a regulatory role in T1D by inhibiting autoimmune T cell responses. As iNKT cell–plasmacytoid dendritic cell (pDC) cooperation controls viral replication in the pancreatic islets, we investigated whether this cellular cross talk could interfere with T1D development during viral infection. Using both virus-induced and spontaneous mouse models of T1D, we show that upon viral infection, iNKT cells induce TGF-β–producing pDCs in the pancreatic lymph nodes (LNs). These tolerogenic pDCs convert naive anti-islet T cells into Foxp3+ CD4+ regulatory T cells (T reg cells) in pancreatic LNs. T reg cells are then recruited into the pancreatic islets where they produce TGF-β, which dampens the activity of viral- and islet-specific CD8+ T cells, thereby preventing T1D development in both T1D models. These findings reveal a crucial cooperation between iNKT cells, pDCs, and T reg cells for prevention of T1D by viral infection.
Type 1 diabetes (T1D) is an autoimmune disease characterized by destruction of insulin-producing β cells by T cells (Mathis et al., 2001). The development of T1D has been associated with defects in immunoregulation (Anderson and Bluestone, 2005). Restoration of efficient regulatory T cell (T reg cell) populations is therefore a promising therapeutic approach to prevent and even cure disease (Nishio et al., 2010). Studies with animal models have revealed the strong potential of two T reg cell subsets, Foxp3+ CD4+ T reg cells and NKT cells, in preventing T1D (Novak et al., 2007b; Tang and Bluestone, 2008). Invariant NKT cells (iNKT cells) are nonconventional T lymphocytes restricted by the CD1d molecule, which presents glycolipid antigens. These cells express a semi-invariant TCR comprising a canonical Vα14-Jα18 (Vα14) chain in the mouse and a Vα24-Jα18 chain in humans (Bendelac et al., 2007). Many studies have shown the protective role of iNKT cells against the development of autoimmune diseases, including T1D (Van Kaer, 2005). T1D can be prevented in nonobese diabetic (NOD) mice both by increasing iNKT cell numbers and by specific iNKT cell stimulation with exogenous ligands such as α-galactosylceramide (α-GalCer; Lehuen et al., 1998; Naumov et al., 2001; Sharif et al., 2001). Relative to control NOD mice, NOD mice protected from T1D by iNKT cell manipulation have weak anti-islet Th1 responses (Laloux et al., 2001). Indeed, iNKT cells can impair the differentiation of anti-islet CD4+ and CD8+ T cells, which eventually become hyporesponsive or anergic (Beaudoin et al., 2002; Chen et al., 2005a).
Converging epidemiological, genetic, and functional studies suggest that viral infections may play a role in T1D (Knip et al., 2005; Dotta et al., 2007; Nejentsev et al., 2009). Initial studies showed that various viruses could enhance or elicit T1D either by direct infection and destruction of target cells or by activating autoimmune T cell responses (Horwitz et al., 1998; Roep et al., 2002; Filippi and von Herrath, 2008). However, virus can also prevent T1D onset (Oldstone, 1988; Tracy et al., 2002; Filippi et al., 2009). This is supported by significant epidemiological data, as there is a concomitant decline in infections and increase in autoimmune diseases in Western countries (Viskari et al., 2005; Filippi and von Herrath, 2008; Tracy et al., 2010). The incidence of most autoimmune diseases such as T1D, inflammatory bowel disease, and multiple sclerosis has been steadily increasing over the last three decades in North America and Europe. Recently, it has been shown in experimental models using NOD mice that T reg cells are involved in the T1D protection by virus (Filippi et al., 2009). However, the mechanism leading to T reg cell expansion remains unknown.
Although iNKT cells inhibit autoimmune responses, they also promote immune responses against viruses (Tupin et al., 2007; Diana and Lehuen, 2009). Upon activation, iNKT cells promptly secrete copious amounts of various cytokines, and they can provide activation/maturation signals to other immune cells such as DCs, NK cells, T cells, and B cells. We recently reported that during lymphochoriomeningitis virus (LCMV) infection, iNKT cells promote plasmacytoid DC (pDC) function locally in the pancreatic islets, leading to enhanced type I IFN production and low viral burden (Diana et al., 2009).
Because iNKT cell–pDC cooperation controls viral infection in the pancreatic islets, we investigated the role of this cooperation in the development of T1D upon viral infection to determine whether both cell types could exert immunoregulatory functions in the pancreatic islets. To address this question, we used two mouse models: a transgenic model of virus-induced T1D, which expresses LCMV nucleoprotein in pancreatic β cells (Ins-NP) of NOD mice (Martinic et al., 2007), and proinsulin 2−/− (Ins2−/−) NOD mice, which is an accelerated model of spontaneous T1D (Thébault-Baumont et al., 2003). To analyze the influence of iNKT cells on diabetogenic CD8+ T cells, mice were either treated with an iNKT cell agonist or crossed to Vα14 transgenic (Lehuen et al., 1998) or to Cd1d−/− NOD mice (Mendiratta et al., 1997; Novak et al., 2007a), which are enriched or devoid of iNKT cells, respectively. Our results revealed that upon viral infection, iNKT cell–pDC interplay inhibited antiviral and anti-islet diabetogenic CD8+ T cell responses in the pancreatic islets, thereby preventing both virus-induced and spontaneous T1D. This local inhibition of the autoimmune effector responses by iNKT cells after viral infection was caused by an increased frequency of CD4+ Foxp3+ T reg cells that produce TGF-β. Importantly, TGF-β–producing pDCs were critical for this iNKT cell–induced accumulation of T reg cells in the pancreatic islets through the conversion of naive T cells in the pancreatic LNs. Using both virally induced and spontaneous models of T1D, this study unveils a new bridge between innate and adaptive immune responses in the prevention of autoimmune disease after viral infection.
RESULTS
iNKT cells promote the accumulation of TGF-β–producing T reg cells in pancreatic islets upon viral infection
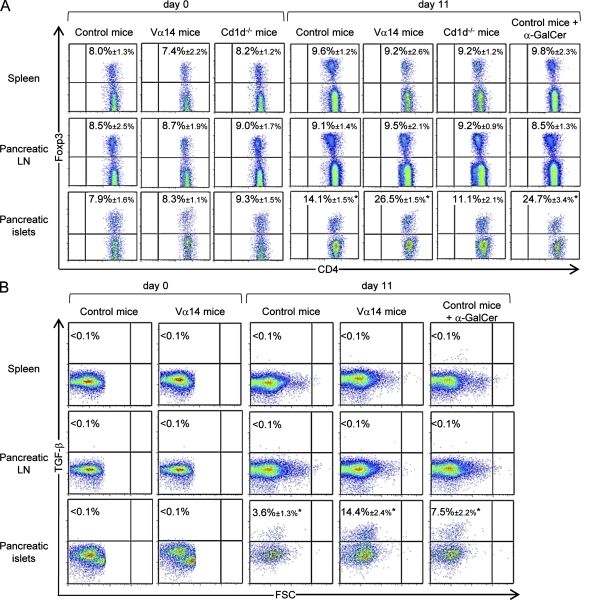
Our previous study showed that in pancreatic islets, iNKT cells cooperate with pDCs to control viral replication, leading to a low effector anti-LCMV CD8+ T cell response. However, in the spleen, iNKT cells do not influence viral replication but promote an adaptive anti-LCMV CD8+ response (Diana et al., 2009). Because these effector T cells could migrate to peripheral tissues, including the pancreatic islets, we further analyzed the frequency and the effector functions of anti-LCMV CD8+ T cells in the pancreatic islets. Although iNKT cells dampened the IFN-γ production and cytotoxicity (CD107a expression) of anti-NP396–404 CD8+ T cell responses, the frequency of tetramer+ staining anti-NP396–404 CD8+ T cells was similar in control, Vα14 mice, and α-GalCer–treated Ins-NP mice (Fig. S1, A–C). These observations suggested a role for regulatory mechanisms acting locally in the pancreatic islets. To address this possibility, we measured the frequency of Foxp3+ CD4+ T reg cells on various days after infection (Fig. 1 A and Fig. S2 A). Interestingly, the frequency of T reg cells increased significantly in the pancreatic islets of control Ins-NP mice at day 11 after infection but not in the spleen and pancreatic LNs. The increase in pancreatic T reg cell frequency was significantly higher in Vα14 Ins-NP mice containing an elevated frequency of iNKT cells as well as in Ins-NP mice treated with the iNKT cell agonist α-GalCer compared with control Ins-NP mice. In contrast, the frequency of T reg cells in the pancreatic islets of Cd1d−/− Ins-NP mice, which lack iNKT cells, was not increased upon infection. The increase in T reg cell frequency was also observed in terms of absolute T cell numbers (Fig. S2 B). Of note, the newly described Foxp3+ iNKT cells (Monteiro et al., 2010) were not detected in any organs before or after LCMV infection (unpublished data). T reg cells exhibited a more activated phenotype in the pancreatic islets than in the lymphoid organs as shown by their higher expression of CD103 and CTLA-4 (Fig. S2 C). To further explore the function of T reg cells, we examined CD4+ T cell expression of TGF-β (Fig. 1 B). Intracellular staining of CD4+ T cells 11 d after infection revealed that TGF-β was significantly expressed in these T cells from pancreatic islets of Ins-NP control mice. In addition, the percentage of these TGF-β+ T cells was much greater in Vα14 and α-GalCer–treated Ins-NP mice. No increase in TGF-β expression was seen in the spleen or pancreatic LNs of any of the mice. Using Foxp3-GFP Ins-NP mice, we confirmed that TGF-β expression was restricted to Foxp3+ CD4+ T cells (Fig. S2 D). Thus, our data suggest that upon viral infection, iNKT cells promote the accumulation of TGF-β–producing T reg cells in the pancreatic islets.
Figure 1.
iNKT cells promote the accumulation of TGF-β–producing T reg cells in the pancreatic islets upon viral infection. (A) Analysis of CD4+ Foxp3+ T cells after LCMV challenge of Ins-NP mice. Spleens and pancreatic LNs and islets were harvested on day 0 or 11 after infection. Cells were stained with anti-CD45, anti–TCR-β, and anti-CD4 mAbs and then stained with anti-Foxp3 mAb for intracellular expression in the CD4+ TCR-β+ population. (B) Analysis of TGF-β expression by CD4+ T cells on days 0 and 11 after infection. Cells were stimulated with PMA-ionomycin over 6 h in the presence of brefeldin A and then surface stained with anti–TCR-β and anti-CD4 mAbs and intracellularly stained with anti–TGF-β mAb. Data are represented as mean values ± SD obtained in four independent experiments with three pooled mice for each group. *, P < 0.05 for day 11 mice compared with day 0 mice.
T reg cells and TGF-β inhibit pancreatic antiviral CD8+ T cell responses and prevent T1D
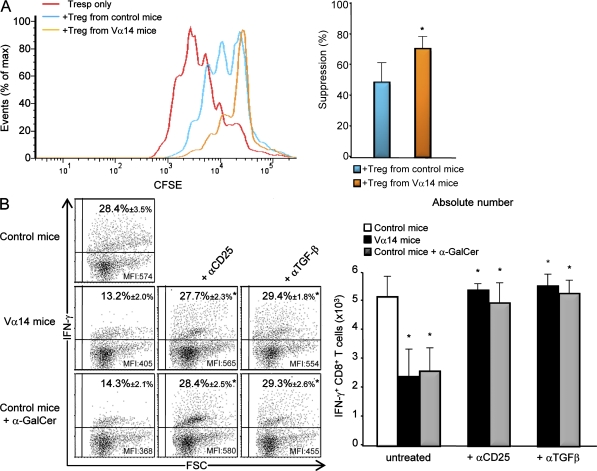
To evaluate the suppressive activity of pancreatic T reg cells, we sorted them from infected Foxp3-GFP Ins-NP control or Vα14 mice and performed co-culture with splenic CD4+ CD25− T responder cells (T resp cells) activated with anti-CD3/CD28 beads (Fig. 2 A). Pancreatic T reg cells strongly inhibited naive T cell proliferation in vitro, and importantly, pancreatic T reg cells from Vα14 Ins-NP mice were more efficient than T reg cells from control Ins-NP mice. These data are in accordance with the higher production of TGF-β detected in T reg cells from Vα14 Ins-NP mice compared with control Ins-NP mice (Fig. 1 B).
Figure 2.
T reg cells and TGF-β inhibit pancreatic antiviral CD8+ T cell response. (A) Proliferation of CD4+ CD25− T resp cells labeled with the cytosolic dye CFSE. In some wells, T reg cells sorted from the pancreatic islets of infected (day 11) Foxp3-GFP control or Vα14 Ins-NP mice were added at a ratio of 1 T reg cell/2 T resp cells. CFSE dilution in T resp cells cultured with or without T reg cells is presented as the percentage of undivided T resp cells. Data are represented as mean values ± SD obtained in three independent experiments with four pooled mice for each group. *, P < 0.05 for Vα14 compared with control Ins-NP mice. (B) Analysis of NP396–404-specific CD8+ T cells upon LCMV infection of Ins-NP mice. 12 d after infection, pancreatic islets were harvested from mice treated with anti-CD25, anti–TGF-β, or no mAb. After in vitro peptide stimulation, cells were stained with anti–TCR-β and anti-CD8 mAbs and stained with anti–IFN-γ mAb for intracellular expression in the CD8+ TCR-β+ population. Data are represented as mean values ± SD obtained in four independent experiments with three pooled mice for each group. *, P < 0.05 compared with untreated mice.
To examine the role of T reg cells in the control of pancreatic anti-LCMV CD8+ T cell response, Vα14 and α-GalCer–treated Ins-NP mice were treated with either anti-CD25 (Kohm et al., 2006) or anti–TGF-β mAbs during LCMV infection. The blockade of T reg cell function by these mAbs led to an increase in IFN-γ+ anti-NP396–404 CD8+ T cells in the pancreatic islets of Vα14 or α-GalCer–treated Ins-NP mice both in terms of frequency and absolute number (Fig. 2 B, left and right, respectively). These mAb treatments did not increase the already high frequency of IFN-γ+ anti-NP396–404 CD8+ T cells in the pancreatic islets of control Ins-NP mice (unpublished data).
We next investigated whether T reg cells were involved in the iNKT cell–mediated protection against T1D. Anti-CD25 mAb treatment abolished this protection, as around 80% of treated Vα14 or α-GalCer–treated Ins-NP mice became diabetic, an incidence similar to that observed in untreated control Ins-NP mice (Table I). Using a viral dose of 104 PFU, all anti-CD25 mAb–treated control Ins-NP mice became diabetic as compared with 40–50% of untreated control mice. Similarly, blockade of TGF-β reversed the protection mediated by iNKT cells. Delayed treatment (day 9) with anti–TGF-β mAb also efficiently abolished iNKT cell–mediated protection (unpublished data). Thus, iNKT cell–induced T reg cells and TGF-β are required in the pancreatic islets to dampen the CD8+ T cell diabetogenic response and to prevent T1D.
Table I.
Diabetes incidences after LCMV infection and various antibody treatments
| Treatment | Control | Vα14 | Control + α-GalCer | Control | Cd1d−/− |
| 105 PFU | 105 PFU | 105 PFU | 104 PFU | 104 PFU | |
| None | 10/12 | 0/12 | 1/12 | 6/12 | 12/12 |
| Anti-CD25 | 12/12 | 11/12a | 10/12a | 12/12a | ND |
| Control isotype | 10/12 | 0/12 | 1/12 | 6/12 | ND |
| Anti–TGF-β | 11/12 | 10/12a | 10/12a | 10/12a | ND |
| Control isotype | 10/12 | 0/12 | 0/12 | 7/12 | ND |
| 120G8 | 11/12 | 10/12a | 10/12a | 10/12a | ND |
| Control isotype | 10/12 | 0/12 | 1/12 | 6/12 | ND |
| Anti-CD62L | 10/12 | 11/12a | 10/12a | ND | ND |
| Control isotype | 10/12 | 1/12 | 0/12 | ND | ND |
Incidence of T1D 20 d after LCMV infection with 104 or 105 PFU of Ins-NP mice treated with anti-CD25, anti–TGF-β, 120G8, or anti-CD62L mAbs or with respective control isotype, as indicated. n = 12 for each group of mice.
P-values <0.05 for mAb-treated mice compared with untreated mice.
iNKT cells promote the accumulation of pDCs in pancreatic LNs, which is critical for T reg cell accumulation in the pancreatic islets and the prevention of T1D
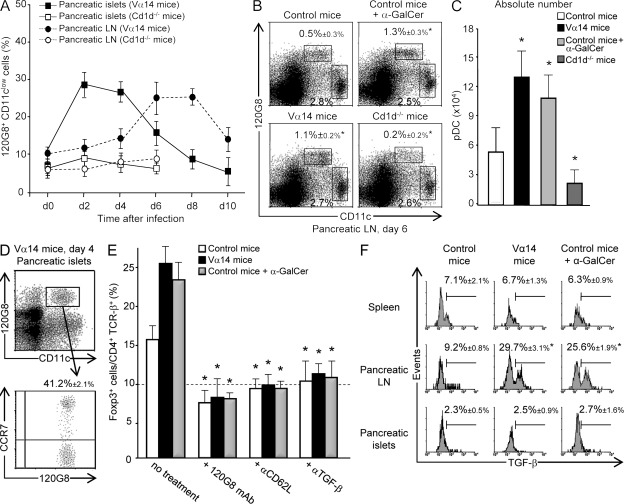
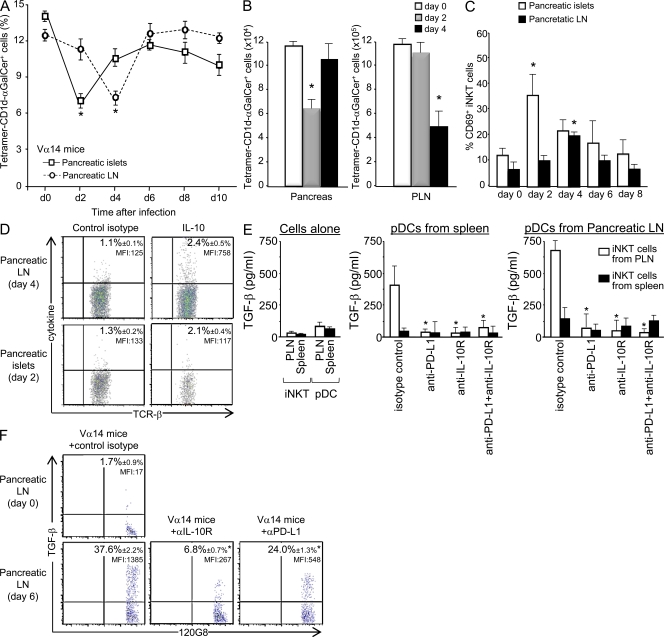
As pDCs can induce T reg cells in a noninfectious context (Ochando et al., 2006; Sharma et al., 2007; Hadeiba et al., 2008), we investigated whether pDCs were involved in the expansion of pancreatic T reg cells after LCMV infection. Kinetics analysis of the pDC population showed that infection induced a rapid recruitment of pDCs to the pancreatic islets of control and Vα14 Ins-NP mice, with the pDC frequency reaching a maximum on day 2 and then progressively declining after 4 d (Fig. 3 A and Fig. S3 A). An increase in pDC frequency appeared later in pancreatic LNs, peaking at day 6–8. In contrast, no increase in pDC frequency was observed in the pancreatic islets and LNs of Cd1d−/− Ins-NP mice (Fig. 3, A–C). PDC frequency upon infection was higher in Vα14 and α-GalCer–treated Ins-NP mice than in control Ins-NP mice (Fig. 3, A–C). Interestingly, ∼40% of pDCs in the pancreatic islets of Vα14 Ins-NP mice on day 2 expressed high levels of CCR7 (Fig. 3 D) and CD62L (not depicted). The kinetics of increase in pDC frequency and the high expression of the CCR7 LN homing receptor suggest that pDCs first recruited to the pancreatic islets subsequently migrate to pancreatic LNs. An analysis of maturation markers (CD40, CD80, CD86, MHC class II, and PD-L1) on DC populations in pancreatic LNs revealed that both conventional DCs (cDCs) and pDCs were activated by day 6 after infection (Table II).
Figure 3.
iNKT cells promote the accumulation of TGF-β–producing pDCs in pancreatic LNs. (A–C) Analysis of pDC frequency in pancreatic LNs and islets of LCMV-infected Ins-NP mice. Cells were harvested at various times after infection and then stained with anti-CD45, anti-CD11c, and 120G8 mAbs. Results represent the percentage of 120G8+ cells among total CD11c+ cells in A or among lymphoid-myeloid cells in B; absolute numbers at day 6 (C) of pDCs in the pancreatic LNs are shown. (D) Analysis of CCR7 expression on pDCs from pancreatic islets of Vα14 Ins-NP mice 2 d after infection. Cells were stained with anti-CD11c, 120G8, and anti-CCR7 mAbs. (E) Analysis of pancreatic T reg cell frequency after 120G8, anti-CD62L mAb, or anti–TGF-β mAb treatment in Ins-NP mice. Cells from pancreatic islets were harvested 11 d after infection and then stained with anti-CD45, anti–TCR-β, anti-CD4, and anti-Foxp3 mAbs. The dashed line indicates the frequency of Foxp3+ cells among CD4+ T cells in uninfected Ins-NP control mice. (F) Analysis of TGF-β production by pDCs after LCMV infection. 6 d after infection, cells from spleen or pancreatic LNs or islets of Ins-NP mice were incubated for 5 h in the presence of brefeldin A and then stained with anti-CD11c and 120G8 mAbs and then intracellularly with anti–TGF-β mAb. Data are represented as mean values ± SD obtained in four independent experiments, each performed with three pooled mice. *, P < 0.05 compared with control mice (B, C, and F) or with untreated mice (E).
Table II.
Phenotype of cDCs and pDCs in pancreatic LNs from LCMV-infected Vα14 Ins-NP mice
| Cell surface marker | cDCs (CD11c+120G8−) | pDCs (CD11c+120G8+) | ||||
| Day 0 | Day 6 | Fold change | Day 0 | Day 6 | Fold change | |
| CD80 | 255 ± 20 | 462 ± 11a | 1.81 | 204 ± 36 | 405 ± 18a | 1.98 |
| CD86 | 325 ± 33 | 543 ± 18a | 1.67 | 285 ± 19 | 475 ± 31a | 1.67 |
| CD40 | 240 ± 18 | 470 ± 41a | 1.95 | 125 ± 12 | 350 ± 20a | 2.80 |
| I-Ag7 (MHC class II) | 824 ± 45 | 1,180 ± 37a | 1.43 | 201 ± 43 | 274 ± 51a | 1.36 |
| PD-L1 | 582 ± 23 | 1,056 ± 27a | 1.81 | 219 ± 25 | 738 ± 35a | 3.37 |
MFI was assessed on DC subsets in pancreatic LNs of Ins-NP Vα14 mice before and 6 d after LCMV infection. Results correspond to means ± SD of values obtained in four independent experiments with three pooled mice per group.
P < 0.05 for day 6 compared with day 0.
To assess the role of pDCs in T reg cell expansion, pDCs were depleted 4 d after infection with 120G8 mAb treatment (Fig. S3 B; Asselin-Paturel et al., 2003). Such depletion abrogated the increased frequency of pancreatic T reg cells normally observed after LCMV infection of control, Vα14, and α-GalCer–treated Ins-NP mice (Fig. 3 E). To demonstrate the essential function of LN homing of pDCs in T reg cell development and tolerance induction (Ochando et al., 2006), anti-CD62L mAb treatment was performed 4 d after infection to block pDC homing to the LNs (Fig. S3 C). Such treatment prevented the increase in T reg cell frequency in the pancreatic islets (Fig. 3 E), thus confirming the critical role of LNs in the expansion of T reg cells in the pancreatic islets. Collectively, these data suggest that upon LCMV infection, pDCs migrate from pancreatic islets to pancreatic LNs, which is critical for T reg cell accumulation in the pancreatic islets.
Next, we examined the effect of pDC depletion and blockade of LN homing on T1D development (Table I). pDC depletion or LN homing blockade 4 d after infection completely abolished T1D protection in Vα14 or α-GalCer–treated Ins-NP mice. Similarly, pDC depletion also increased T1D incidence in control Ins-NP mice infected with 104 PFU of LCMV. These results point to the critical role of pDCs and their migration to pancreatic LNs in the protective effect exerted by iNKT cells against virus-induced diabetes.
pDCs produce TGF-β in pancreatic LNs that converts naive T cells into T reg cells
Several mechanisms have been described to explain how pDCs can induce T reg cells, including ICOS (inducible co-stimulatory)–ICOS ligand molecular interaction, and indoleamine 2,3-dioxygenase (IDO) secretion (Ito et al., 2007; Sharma et al., 2007). We investigated the role of these pathways after LCMV infection. In our model, ICOS deficiency did not influence the pancreatic T reg cell frequency in LCMV-infected Ins-NP mice and did not abolish iNKT cell–mediated T1D protection (Fig. S4, A and B). Blocking ICOS with a specific mAb (Herman et al., 2004) further ruled out the role of this pathway (unpublished data). We next evaluated the involvement of IDO by treating Ins-NP mice with its inhibitor, 1-methyl-d-tryptophan (1-MT; Sharma et al., 2007). This treatment partially inhibited T1D prevention mediated by iNKT cells (Fig. S4, C and D). This was correlated with a slight decrease in the T reg cell frequency in the pancreatic islets of infected Vα14 and control Ins-NP mice; however, these decreases were not statistically significant.
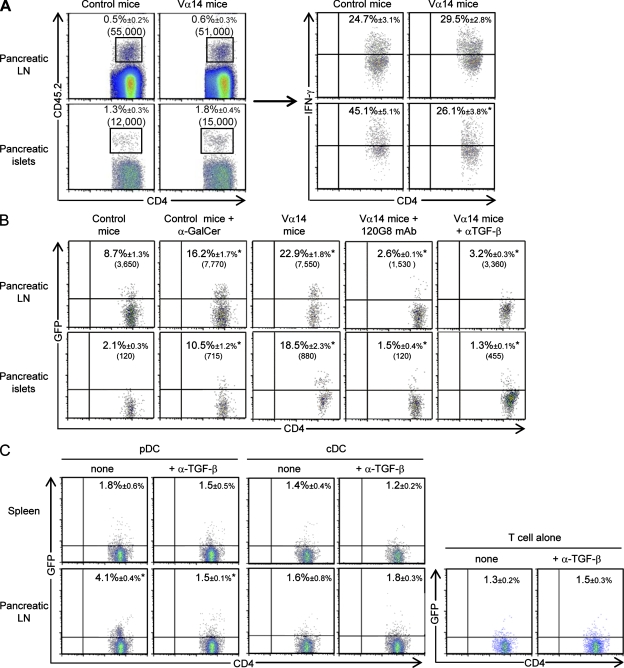
Because TGF-β is involved in T reg cell induction and expansion (Peng et al., 2004; Kretschmer et al., 2005), the role of this cytokine was explored using anti–TGF-β mAb treatment. TGF-β blockade abolished the increased frequency of T reg cells in pancreatic islets of control, Vα14, and α-GalCer–treated Ins-NP mice after infection (Fig. 3 E). To determine whether pDCs could produce TGF-β in pancreatic LNs after infection, its expression was evaluated by intracellular staining. The specificity of the staining was validated by showing that preincubation of the anti–TGF-β mAb with recombinant TGF-β abolished the staining (Fig. S3 D). 6 d after infection, ∼25–30% of pDCs in the pancreatic LNs of Vα14 or α-GalCer–treated Ins-NP mice produced TGF-β compared with 9% in control Ins-NP mice (Fig. 3 F). Only a low level of expression of TGF-β was observed in pDCs from the pancreatic islets and the spleen. To determine whether TGF-β–producing pDCs in the pancreatic LNs could inhibit effector T cell function, anti-islet BDC2.5 T cells were transferred into Vα14 and control Ins-NP mice infected with LCMV 4 d earlier (Fig. 4 A). 6 d later, IFN-γ production by BDC2.5 T cells was analyzed. A similar frequency of IFN-γ+ BDC2.5 T cells was observed in the pancreatic LNs of both mice. In contrast, the frequency of IFN-γ+ BDC2.5 T cells in the pancreatic islets was lower in Vα14 than in control Ins-NP mice. These data suggest that the suppression of the diabetogenic response occurs predominantly in the pancreatic islets.
Figure 4.
pDCs produce TGF-β in pancreatic LNs that converts naive T cells into T reg cells. (A) CD62L+ CD4+ BDC2.5 T cells were isolated from BDC2.5 CD45.2+/+ Cα−/− NOD females, and 106 cells were transferred into Ins-NP mice 4 d after LCMV infection. Cells from pancreatic LNs and islets were recovered 6 d after transfer and then restimulated with PMA-ionomycin in the presence of brefeldin A over 4 h and then stained with anti–TCR-β, anti-CD4, and anti-CD45.2 mAbs and IFN-γ mAb for intracellular expression. The frequency of gated cells is showed. Data are represented as mean values ± SD obtained in three independent experiments, each performed with three pooled mice. *, P < 0.05 compared with control mice. (B) CD62L+ CD4+ GFP− BDC2.5 T cells were isolated from BDC2.5 CD45.2+/+ Foxp3-GFP+/+ Cα−/− NOD females, and 106 cells were transferred into Ins-NP mice 4 d after infection. In some conditions, Vα14 Ins-NP mice were treated with 120G8 or anti–TGF-β mAbs 2 d after infection. Cells from pancreatic LNs and islets were recovered 6 d after transfer and then stained with anti–TCR-β, anti-CD4, and anti-CD45.2 mAbs, and GFP expression was analyzed. The frequency and absolute number of gated cells are shown. *, P < 0.05 compared with control mice or mAb-treated Vα14 mice compared with untreated Vα14 mice. (C) 105 CD62L+ CD4+ GFP− BDC2.5 T cells were co-cultured with 104 pDCs or cDCs sorted from spleen or pancreatic LNs of infected Vα14 Ins-NP mice (4 d earlier). Cells were cultured for 3 d in complete medium with 25 U/ml recombinant human IL-2 with or without 100 µg/ml anti–TGF-β mAb and then stained with anti–TCR-β, anti-CD4, and anti-CD45.2 mAbs, and GFP expression was analyzed. Data are represented as mean values ± SD obtained in four independent experiments. *, P < 0.05 pancreatic LNs compared with spleen or anti–TGF-β mAb–treated culture compared with untreated one.
We next investigated whether a cell conversion mechanism was involved in pancreatic islet T reg cell accumulation upon LCMV infection. To address this question, sorted naive anti-islet BDC2.5 T cells devoid of T reg cells, isolated from Foxp3-GFP+/+ BDC2.5 Cα−/− NOD mice, were transferred into Ins-NP mice that had been infected with LCMV 4 d earlier (Fig. S5). 6 d after transfer, GFP+ BDC2.5 T reg cells were detected in pancreatic LNs and islets, and, importantly, the frequency and absolute number of induced T reg cells were increased in α-GalCer–treated and Vα14 mice as compared with control mice (Fig. 4 B). Moreover, the induction of GFP+ BDC2.5 T reg cells in pancreatic LNs and islets of Vα14 Ins-NP mice was dependent on pDCs and TGF-β, as specific depleting or blocking mAb treatments, respectively, abrogated this conversion (Fig. 4 B). In noninfected mice, treated or not with α-GalCer, very few GFP+ BDC2.5 T reg cells were detected (unpublished data). To demonstrate that pDCs directly induced T reg cells in the pancreatic LNs through TGF-β secretion, we performed co-culture experiments with sorted pDCs from infected Ins-NP mice (day 6 after infection) and anti-islet BDC2.5 T cells devoid of T reg cells (Fig. 4 C). Only pDCs isolated from the pancreatic LNs and not from the spleen of infected mice were able to convert GFP+ T reg cells from naive BDC2.5 T cells. Importantly, the addition of blocking anti–TGF-β mAb in the co-culture abrogated the induction of T reg cells by pDCs isolated from pancreatic LNs. pDCs isolated from pancreatic islets did not induce T reg cell conversion (unpublished data); however, for technical reasons, these experiments could only be performed with twofold less pDCs. In contrast to pancreatic LN pDCs, cDCs from this organ and from the spleen of infected mice did not induce T reg cell conversion (Fig. 4 C). We next addressed whether the proliferation of the existing pool of T reg cells could also contribute to the increase of pancreatic islet T reg cell number after viral infection. 11 d after LCMV challenge, the frequency of proliferating Ki67+ T reg cells increased only marginally in the pancreatic LNs and pancreas from both control and Vα14 Ins-NP mice compared with uninfected mice (Fig. S5 D). Similar data were obtained at day 8 after infection (unpublished data). Thus, T reg cell proliferation could play a minor role compared with the high rate of conversion observed after infection. Altogether, these results support a critical role for TGF-β–producing pDCs in the pancreatic LNs for the conversion of naive T cells into anti-islet–specific T reg cells during LCMV infection.
Induction of tolerogenic pDCs by iNKT cells requires the IL-10 and PD-1–PD-L1 pathways
To investigate how pancreatic LN iNKT cells could induce TGF-β production by pDCs, we characterized iNKT cell phenotype and function after LCMV infection. The frequency of iNKT cells detected in the pancreatic islets and LNs decreased at day 2 and 4, respectively (Fig. 5 A and Fig. S6 A). A similar decrease was also observed in terms of absolute number of iNKT cells (Fig. 5 B). The up-regulation of CD69 and PD-1 and the down-regulation of NK1.1 expression indicated the activation of iNKT cells in both organs (Fig. 5 C and Fig. S6, A and B). In terms of cytokine production, only a low frequency of IL-10–producing NKT cells was observed after infection (Fig. 5 D and Fig. S6 C). However, IL-10 expression was approximately sevenfold higher in terms of mean fluorescence intensity (MFI) in iNKT cells from the pancreatic LNs compared with iNKT cells from the pancreatic islets. iNKT cells were not anergic after LCMV infection, as their ability to produce IFN-γ and IL-4 was unchanged after α-GalCer ex vivo stimulation (Fig. S6 D). Of note, the activation of iNKT cells in the pancreatic islets and LNs of infected mice required neither pDCs nor the CD1d molecule, as iNKT cells up-regulated CD69 even after pDC depletion or in mice expressing CD1d exclusively in the thymus (CD1dpLck mice; Fig. S7 A; Novak et al., 2007a).
Figure 5.
Induction of tolerogenic pDCs by iNKT cells requires IL-10 and PD-1–PD-L1 pathways. Analysis of iNKT cell frequency and phenotype of LCMV-infected Ins-NP mice. Cells from pancreatic LNs (PLN) and islets were harvested at various times after infection. (A–C) Cells were stained with tetramer-CD1d–α-GalCer, anti-CD45, anti–TCR-β, and anti-CD69 mAbs. *, P < 0.05 compared with day 0. (D) Cells were incubated for 4 h in presence of brefeldin A and then stained with tetramer-CD1d–α-GalCer, anti-CD45, and anti–TCR-β mAbs and then stained with anti–IL-10 mAb for intracellular expression. Data are represented as mean values ± SD obtained in two to four independent experiments, each performed with three pooled mice. (E) iNKT cells and 105 pDCs were sorted from spleen or pancreatic LNs of infected Vα14 Ins-NP mice (4 d earlier). Cells were cultured for 90 h in complete medium with 10 µg/ml control isotype, anti–PD-L1, anti–IL-10R, or both mAbs. Supernatants were recovered, and TGF-β production was analyzed by ELISA. Data are represented as mean values ± SD obtained in three independent experiments. *, P < 0.05 compared with isotype control. (F) 6 d after infection, cells from pancreatic LNs of Ins-NP mice treated on day 3 with anti–IL-10R or anti–PD-L1 mAbs or control isotype were incubated for 5 h in presence of brefeldin A and then stained with anti-CD11c and 120G8 mAbs and then intracellularly with anti–TGF-β mAb. Data are representative of three independent experiments with three pooled mice per group. *, P < 0.05 compared with untreated mice.
To determine the molecular pathways involved in the induction of TGF-β–producing pDCs by iNKT cells, we co-cultured both cell types sorted from the spleen or the pancreatic LNs of 4-d infected Vα14 Ins-NP mice. 90 h later, TGF-β released into the supernatant was measured by ELISA. TGF-β was detected only when iNKT cells from the pancreatic LNs were added to the culture with pDCs from either the spleen or pancreatic LNs. TGF-β production was higher with pDCs from pancreatic LNs. This result revealed the critical role of iNKT cells from the pancreatic LNs in the induction of TGF-β production by pDCs. Because we observed that iNKT cells secrete some IL-10 and express PD-1 (Fig. 5 D and Fig. S6 B), whereas pDCs express high levels of PD-L1 (Table II) in the pancreatic LN after LCMV infection, we investigated the role of these molecules in the induction of TGF-β–producing pDCs by iNKT cells. TGF-β secretion was abrogated when anti–PD-L1, anti–IL-10R, or both mAbs were included to the culture. No TGF-β secretion was detected when iNKT cells or pDCs from the spleen or pancreatic LNs were cultured alone. Importantly, treating infected mice with blocking anti–IL-10R or anti–PD-L1 mAbs decreased both the frequency and the MFI of TGF-β produced by pDCs in pancreatic LNs at day 6 (Fig. 5 F). Collectively, these results strongly suggest that, during LCMV infection, iNKT cells in pancreatic LNs induce TGF-β secretion by pDCs through IL-10 signaling and PD-1–PD-L1 interaction.
iNKT cell–pDC cooperation similarly induces T reg cell protective function in a spontaneous model of T1D
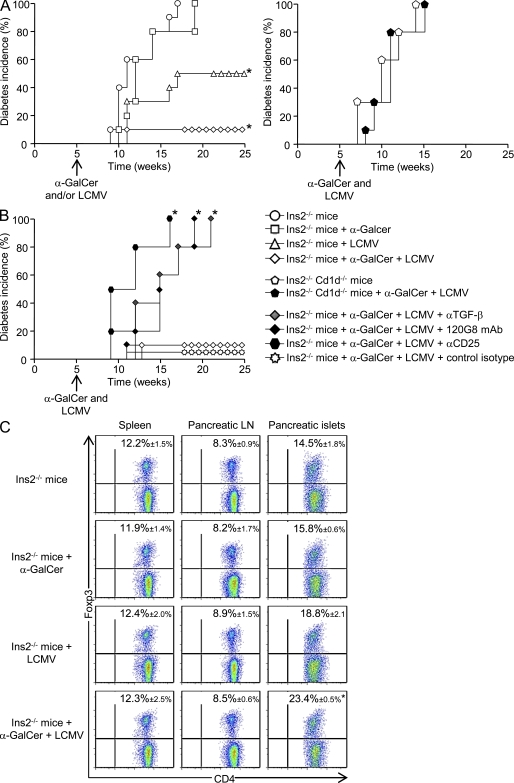
To extend our results obtained using Ins-NP mice to a spontaneous model of T1D, we used Ins2−/− NOD mice, which develop accelerated T1D (Thébault-Baumont et al., 2003). This strain was used in preference to the wild-type NOD as it has a rapid onset of T1D, thus facilitating the choice of timing for LCMV infection, α-GalCer treatment, and disease follow up. To determine the role of iNKT cells in this model, we followed T1D development in Ins2−/− mice after LCMV infection, α-GalCer injection, or a combination of both, all performed at 5 wk of age (Fig. 6 A, left). All Ins2−/− mice, whether or not injected with α-GalCer, developed T1D before 20 wk, whereas only 50% of LCMV-infected Ins2−/− mice were diabetic after 25 wk of age. Interestingly, only 10% of Ins2−/− mice that were both infected and α-GalCer treated became diabetic at 25 wk, revealing a synergistic protective effect of viral infection and iNKT cell stimulation. To further determine whether iNKT cells were required for the LCMV-induced protection against diabetes, Ins2−/− Cd1d−/− mice were infected or not with LCMV (Fig. 6 A, right). T1D development in these mice was not prevented by infection, demonstrating the critical role of iNKT cells in the protection against spontaneous diabetes after LCMV infection.
Figure 6.
iNKT cells prevent T1D in infected Ins2−/− NOD mice and promote pancreatic islet T reg cell accumulation. (A and B) T1D incidence in Ins2−/− mice or Ins2−/− Cd1d−/− mice, infected or not with 105 PFU LCMV and treated or not with α-GalCer at 5 wk of age; in some conditions, mice were treated 4 d after infection with 120G8, anti-CD25, or anti–TGF-β mAbs or with respective control isotype (n = 10–12 mice per group). (C) Analysis of CD4+ Foxp3+ T cells 10 d after LCMV challenge of Ins2−/− mice, infected or not with LCMV and treated or not with α-GalCer. Spleens and pancreatic LNs and islets were harvested, and cells were stained with anti–TCR-β, anti-CD4, and anti-Foxp3 mAbs. Data are represented as mean values ± SD obtained in three independent experiments with three pooled mice for each group. *, P < 0.05 compared with uninfected and untreated Ins2−/− mice.
To address the putative role of T reg cells in this model, we measured their frequency in Ins2−/− mice 10 d after infection, α-GalCer treatment, or combined infection and treatment (Fig. 6 C). Although α-GalCer treatment alone did not impact T reg cell frequency in pancreatic islets, infection alone induced some expansion of this cell population. In line with the stronger protective effect of combined α-GalCer treatment and viral infection, T reg cell frequency in the pancreatic islets was further increased when both stimuli were given. No difference in T reg cell frequency was observed in lymphoid organs. This increased T reg cell frequency in the pancreatic islets was still observed 20 wk after infection and α-GalCer treatment of Ins2−/− mice (Fig. S8 A). To further analyze the role of T reg cells, pDCs, and TGF-β in the prevention of T1D in α-GalCer–treated and infected Ins2−/− mice, treatments with blocking T reg cell, depleting pDCs, and blocking anti–TGF-β mAbs were performed at the time of infection (Fig. 6 B). All of these treatments fully reversed the protection against T1D, confirming the critical role of T reg cells, pDCs, and TGF-β in LCMV-induced T1D protection. Altogether, these data show that iNKT cell activation synergizes with viral infection to expand pancreatic T reg cells and prevent T1D in Ins2−/− mice in a pDC- and TGF-β–dependent fashion.
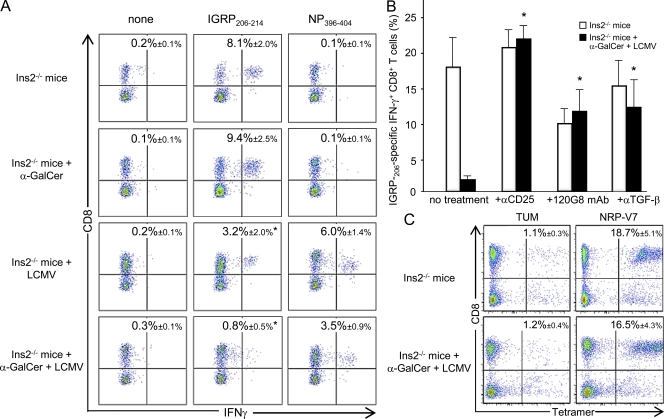
To examine the effect of iNKT cells and LCMV infection on diabetogenic T cell responses, we analyzed the CD8+ T cell IFN-γ response against the dominant β-cell epitope, IGRP206–214, in pancreatic islets of Ins2−/− mice 10 d after α-GalCer injection and/or LCMV infection (Fig. 7 A). The anti-IGRP206–214 CD8+ T cell response was lower in LCMV-infected (3.2 ± 2.0%) compared with uninfected mice (8.1 ± 2.0%). Although α-GalCer injection alone had no effect on the pancreatic anti-IGRP206–214 response (9.4 ± 2.5%), α-GalCer injection synergized with LCMV infection to further decrease the diabetogenic response (0.8 ± 0.5%). The CD8+ T cell response against the viral epitope NP396–404 was also reduced by α-GalCer treatment in LCMV-infected mice (Fig. 7 A).
Figure 7.
iNKT cells dampen the anti-islet CD8+ T cell response in infected Ins2−/− NOD mice through pDCs, T reg cells, and TGF-β. (A–C) Analysis of CD8+ T cells specific for IGRP206–214 (A–C) and NP396–404 (A) in 5-wk-old Ins2−/− mice 10 d after LCMV challenge (and treated or not) with α-GalCer. Pancreatic islets were harvested and cultured for 8 d in complete RPMI medium with recombinant IL-2. Cells were then stimulated for 4 h with IGRP206–214 or NP396–404 peptides and stained with anti–TCR-β, anti-CD8, and IFN-γ mAbs. Values correspond to the frequency of epitope-specific CD8+ T cells in the lymphoid gate. (B) Analysis of IGRP206–214-specific cells among CD8+ T population in pancreatic islets of Ins2−/− mice treated with anti-CD25, 120G8, or anti–TGF-β mAbs. (C) For NRP-V7 (high affinity analogue of IGRP206–214 tetramer staining), cells were stained with tetramers (NRP-17 and TUM control) and then surface stained with anti-CD8 mAb. Values correspond to the frequency of tetramer-specific CD8+ T cells in the lymphoid gate. Data are represented as mean values ± SD obtained in two (B and C) to six (A) independent experiments with three independent mice for each group. *, P < 0.05 compared with untreated/uninfected Ins2−/− mice (A and C) or compared with mAb-untreated mice (B).
To assess the involvement of T reg cells, pDCs, and TGF-β in this dampening of the diabetogenic T cell responses after α-GalCer injection and LCMV infection, Ins2−/− mice were treated with specific mAbs (Fig. 7 B). These treatments abrogated the inhibition of the IFN-γ+ anti-IGRP206–214 CD8+ T cell response in the pancreatic islets of LCMV-infected/α-GalCer–treated Ins2−/− mice. Importantly, the frequency of anti-IGRP206–214 CD8+ T cells measured by tetramer staining was similar in LCMV-infected/α-GalCer–treated and control Ins2−/− mice, supporting a local regulation that dampens the effector function of diabetogenic T cells (Fig. 7 C). Collectively, these data show that iNKT cell activation and LCMV infection synergize to dampen diabetogenic T cell responses in pancreatic islets through pDCs and T reg cells preventing spontaneous T1D.
DISCUSSION
Many studies have described that infections, including viral infections, can prevent T1D (Lehuen et al., 2010); however, the mechanisms remain elusive. This study unveils an important new aspect of the protective role of iNKT cells in T1D during viral infection. We demonstrate that iNKT cells induce tolerogenic pDCs, which convert naive T cells into T reg cells that dampen the diabetogenic T cell responses in the pancreatic islets and thereby prevent T1D.
The ability of pDCs to induce T reg cells was previously described in a noninfectious context (Ochando et al., 2006; Sharma et al., 2007; Hadeiba et al., 2008; Irla et al., 2010; Martín-Gayo et al., 2010) and after TLR9 triggering of human pDCs (Ito et al., 2007). Some of these studies described a role for the IDO and/or the ICOS–ICOS ligand pathways (Ito et al., 2007; Sharma et al., 2007). However, these molecules did not play a key role in the expansion of pancreatic T reg cells by pDCs after LCMV infection. Interestingly, 1-MT treatment did partially interfere with the T1D prevention mediated by iNKT cells. This could be because this assay is more sensitive in revealing some IDO involvement or alternatively because IDO is involved at a different level. T reg cells might initiate an immunoregulatory pathway involving tryptophan catabolism in DCs (Fallarino et al., 2003). Our present study highlights the critical role of TGF-β in the generation of T reg cells because anti–TGF-β mAb treatment abrogated the increase in T reg cell frequency in the pancreatic islets of infected mice. Moreover, a high percentage of pDCs present in the pancreatic LNs after LCMV infection produced TGF-β. In keeping with a role for this cytokine in the generation of T reg cells (Kretschmer et al., 2005; Li and Flavell, 2008), our in vivo and in vitro data clearly demonstrate that in the pancreatic LNs, pDCs induce T reg cell conversion from naive T cells through TGF-β secretion. A similar mechanism has been proposed in lupus prevention after injection of a histone peptide (Kang et al., 2007). In our study, TGF-β–producing pDCs are mainly localized in the pancreatic LNs on day 6 after LCMV infection. This observation suggests that after their iNKT cell–dependent recruitment to the infected islets, activated pDCs leave the pancreatic islets to migrate to the pancreatic LNs. Our results do not exclude that some pDCs may be recruited from the blood. The importance of draining lymphoid tissues in the tolerogenic function of pDCs was recently described in the context of cardiac allografts (Ochando et al., 2006), graft-versus-host disease (Hadeiba et al., 2008), and experimental autoimmune encephalomyelitis (Irla et al., 2010).
We previously demonstrated that pancreatic iNKT cells expressed the OX40 molecule and promoted type I IFN production by pDCs through OX40–OX40 ligand interaction (Diana et al., 2009). Importantly, blocking this pathway using anti-OX40L mAb did not prevent T reg cell expansion in the pancreatic islets (Fig. S7 B). Moreover, the role of CD1d was also ruled out because there was a similar pancreatic T reg cell frequency in mice expressing or not CD1d in the periphery (control vs. CD1dpLck Ins-NP mice; Fig. S7 A). Accordingly, Vα14 CD1dpLck and Vα14 Ins-NP mice treated with a blocking anti-CD1d mAb remained diabetes-free after LCMV infection (Fig. S7 C). Our study reveals a new regulatory function of iNKT cells in the induction of TGF-β–producing pDCs through IL-10 secretion and PD-1–PD-L1 interactions. Indeed, co-culture experiments performed with sorted cells from infected mice showed that iNKT cells from pancreatic LNs incubated with pDCs from spleen or pancreatic LNs result in TGF-β production, which was abrogated by IL-10R and/or PD-L1 blocking mAbs. Furthermore, treating infected mice with blocking anti–IL-10R or anti–PD-L1 mAbs strongly inhibited TGF-β production by pDCs in the pancreatic LNs. Of note, in pancreatic LNs, LCMV infection induces IL-10 production and up-regulation of PD-1 on iNKT cells as well as PD-L1 up-regulation on pDCs. Interestingly, blocking IL-10R in vivo abolished the prevention of LCMV-induced diabetes by iNKT cells (unpublished data). Our data are reminiscent of previous studies suggesting that PD-L1 ligation results in reverse signaling into the DC, thus inhibiting subsequent immune response (Kuipers et al., 2006; Keir et al., 2008). Regarding IL-10, many studies have shown that this cytokine induced regulatory DC functions (Li and Flavell, 2008). Moreover a direct role of PD-L1+ pDCs on T reg cell generation cannot be ruled out because PD-L1 can potentiate and support Foxp3+ T reg cell function and induce Foxp3 expression (Francisco et al., 2009). A previous study described the synergic role of these regulatory pathways during the anti-LCMV CD8+ T cell response, as a combination of blocking mAbs against IL-10R and PD-L1 enhanced the viral clearance in C56BL/6 mice (Brooks et al., 2008). Our previous (Diana et al., 2009) and present data strengthen the role of iNKT cells in promoting dual pDC functions (antiviral and tolerogenic), controlling deleterious immune response in the pancreatic islets during viral infection.
Our study shows that conversion of naive CD4 T cells into T reg cells takes place in pancreatic LNs based on several in vivo and in vitro experiments. However, T reg cells mainly accumulate in pancreatic islets. These data suggest that after their conversion in pancreatic LNs, T reg cells migrate to the pancreatic islets where they harbor a fully activated phenotype and produce TGF-β. Their migration into the inflamed tissue is reminiscent of that described in previous studies (Belkaid and Tarbell, 2009). Therefore, it might not be surprising that T reg cells do not accumulate in pancreatic LNs and could only be detected with sufficient sensitivity after the transfer of Foxp3-GFP–negative BDC2.5 T cells. Our data are also in agreement with a recent study on experimental autoimmune encephalomyelitis (Irla et al., 2010). The induction of T reg cells in the draining LNs does not lead to an increase of T reg cell frequency in this lymphoid tissue, and the authors proposed that induced T reg cells migrate from the secondary lymphoid tissues to the target tissue. We observed a lower Foxp3 expression in T reg cells in the pancreatic islets as compared with lymphoid tissues. This might be related to a later differentiation stage of pancreatic T reg cells, as they exhibit a more activated phenotype (CD103, CTLA-4, and TGF-β).
The involvement of T reg cells in iNKT cell–mediated prevention of T1D upon LCMV infection was first suggested by the absence of islet destruction despite the high frequency of anti-NP396–404 CD8+ T cells in the pancreatic islets of Vα14 and α-GalCer–treated Ins-NP mice. Similarly, protected Ins2−/− mice exhibit the same frequency of pancreatic anti-islet T cells as control Ins2−/− mice. Moreover, blockade of T reg cell function restored a high frequency of IFN-γ+ diabetogenic CD8+ T cells in the pancreatic islets and subsequent T1D in Ins-NP and Ins2−/− mice. This local control of islet inflammation by iNKT and T reg cells is reminiscent of previous studies showing that pancreatic islets are the major site of immune regulation by T reg cells in spontaneous T1D (Chen et al., 2005b; Tang et al., 2008; Feuerer et al., 2009). Analysis of effector diabetogenic T cells in pancreatic LNs did not show immune regulation in this tissue as there was a similar frequency of IFN-γ+ BDC2.5 cells after the transfer of naive BCD2.5 T cells into control and Vα14 infected Ins-NP mice. Consistent with the idea of local control of diabetogenic responses, CD4+ T cells in the pancreatic islets but not in the spleen and pancreatic LNs are activated and produce TGF-β, and blocking of TGF-β at day 9 after infection abolished T1D protection. The key role of TGF-β in our study is in line with previous studies describing the suppressive mechanisms of T reg cells in mouse models of T1D (Green et al., 2003; You et al., 2006; Filippi et al., 2009).
It is striking that an acute viral infection can induce a long-term protection against T1D as observed in the Ins2−/− mice. Moreover, one single injection of α-GalCer strongly potentiated this stable protection without affecting the viral clearance (Fig. S8 B). Analysis of diabetes-free Ins2−/− mice, 20 wk after combined infection and α-GalCer injection, revealed a sustained high frequency of T reg cells in the pancreatic islets. This observation suggests that pancreatic T reg cells might mediate this long-term tolerance. Because TGF-β is critical for the maintenance of the T reg cell pool, TGF-β produced by T reg cells might act in an autocrine manner (Li and Flavell, 2008). However, we did not detect TGF-β by intracellular staining in T reg cells at these later time points (unpublished data). Alternatively, the local suppressive environment could be sustained by the production of other regulatory cytokines and/or by other regulatory cells (Belkaid and Oldenhove, 2008; Belkaid and Tarbell, 2009). Importantly, upon LCMV infection, T reg cells controlled both antivirus- and anti-islet–specific T cell responses. These data fit with the emerging concept that multiple T reg cell functions act either directly or indirectly at the site of inflammation to create a regulatory milieu that promotes bystander suppression (Tang and Bluestone, 2008). Even though LCMV infection in the absence of α-GalCer treatment reduced anti-islet T cell responses and prevented T1D development in Ins2−/− mice, there was only a weak increase in T reg cells in the pancreatic islets. This moderate expansion of T reg cells might be caused by the already high frequency of T reg cells in the pancreatic islets of these mice.
We have recently reported that upon LCMV infection, iNKT cells and pDCs cooperate to induce an efficient innate antiviral type I interferon response (Diana et al., 2009). We now describe that both cell types also cooperate to control the adaptive immune response in the pancreatic islets through the conversion of naive T cells into T reg cells. Thus, iNKT cells and pDCs prevent deleterious inflammation subsequent to viral infection of the pancreatic islets through two different and complementary mechanisms. In contrast, in the spleen, iNKT cells promoted antiviral adaptive immune responses, which are critical for LCMV clearance (Barber et al., 2006). Our data highlight the ambivalent role of iNKT cells depending on their localization and their interaction with various DC subsets (Lehuen et al., 2010). Upon LCMV infection, iNKT cells promote CD8+ T cell response in the spleen by interacting with cDCs producing IL-12 (Diana et al., 2009; unpublished data), whereas in the pancreatic islets and LNs, iNKT cells activate pDCs to produce type I IFN and TGF-β, respectively. iNKT cells exert these various functions through distinct molecular pathways, which must be tightly regulated. The stimulation of adaptive immune responses by iNKT cells has also been observed after parasitic and bacterial infections, in which iNKT cells cooperate with cDCs (Tupin et al., 2007). Interestingly, these infectious agents can also prevent T1D development (Alyanakian et al., 2006; Zaccone et al., 2008), and it would be interesting to further explore the involvement of iNKT cells in the protective role of these infections.
Our findings on the regulatory role of iNKT cells might have broad implications in human diseases, given the marked inter-individual differences in human iNKT cell frequencies (Lee et al., 2002) and the putative role of infections in many autoimmune disorders (von Herrath et al., 2003). Even though some controversy exists regarding the frequency of blood iNKT cells in diabetic patients (Wilson et al., 1998; Lee et al., 2002), only limited analysis of pancreatic LN iNKT cells has been performed so far (Kent et al., 2005). Our study suggests that it may be important to carry out such an analysis because iNKT cells appear to act locally in the pancreatic LNs to protect against T1D. Finally, this study, using both a well-defined model of virus-induced T1D and a spontaneous model of T1D, reveals new regulatory mechanisms by which iNKT cells and pDCs prevent autoimmune diseases during viral infection. These data pave the way to design new clinical approaches combining the manipulation of iNKT cells and pDCs by specific agonists.
MATERIALS AND METHODS
Mice and treatments.
The Vα14 transgenic NOD mouse line expressing the Vα14-Jα18 TCR α chain corresponds to line A14-86 (Beaudoin et al., 2002). The Ins-NP NOD, Cd1d−/−NOD, Cd1dpLck NOD, and Ins2−/− NOD mice were previously described (Thébault-Baumont et al., 2003; Martinic et al., 2007; Novak et al., 2007a). Vα14, Cd1d−/− NOD mice were crossed to Ins-NP NOD mice. Foxp3-GFP+/+ mice (Fontenot et al., 2005) were backcrossed >10 times on a NOD background (from D. Mathis and C. Benoist, Harvard Medical School, Boston, MA). BDC2.5 CD45.2+/+ Foxp3-GFP+/+ Cα−/− NOD mice were generated by backcrossing to BDC2.5 CD45.2+/+ Cα−/− NOD mice. Mice were bred and housed in specific pathogen-free conditions and used at 6–8 wk of age. In some experiments, mice were injected i.p. with 2 µg/mouse α-GalCer (from the Kirin Brewery) on the day of infection (day 0), with 1 mg/mouse depleting 120G8 mAb (from the Schering-Plough Research Institute) or anti-CD62L mAbs on days 4 and 6, with 0.5 mg/mouse of blocking anti-CD25 (PC61) or anti–TGF-β (2G7) on days 3, 6, and 9, or only at day 9, or with 0.25 mg/mouse blocking anti–IL-10R (1B1) or anti–PD-L1 (10F.9G2; BioExpress) mAbs on day 2 or with respective control isotype, after LCMV infection, as mentioned in the figure legend. This study was approved by the local ethics committee on animal experimentation (P2.AL.045.08).
Virus.
LCMV Armstrong clone 53b stocks were prepared by a single passage on baby hamster kidney 21 cells. Mice were infected with a single i.p. dose of 105 or 104 PFU as indicated.
Diabetes diagnosis.
Mice were tested every day for diabetes. Overt diabetes was defined as two positive urine glucose tests, confirmed by a glycemia >200 mg/dl. Glukotest and Heamogokotest kits were purchased from Roche.
Flow cytometry.
Cell suspensions were prepared from spleen and pancreatic LNs and islets. Cells were stained at 4°C in PBS containing 2% FCS and 1% EDTA after blocking Fc-γR with 2.4G2 mAb. Surface staining was performed with antibodies all from BD except 120G8 and anti–TGF-β (2G7) mAbs, which were FITC-conjugated in our laboratory. For CD1d tetramer preparation, biotinylated soluble CD1d was loaded with α-GalCer and then incubated with allophycocyanin-conjugated streptavidin. H2Db-NP396–404 dextramers were purchased from Dako. To detect degranulation, anti-CD107a mAb (1D4B; BD) was added during restimulation with NP396–404 peptide (from J.-P. Briand, Institut de Biologie Moléculaire et Cellulaire, Strasbourg, France). For IFN-γ (XMG1.2; BD) intracellular staining, single-cell suspensions were stimulated with 1 µg/ml of viral NP396–404 peptide for 5 h at 37°C in the presence of 10 U/ml recombinant mouse IL-2 (R&D Systems) and 1 µg/ml brefeldin A. For IL-10 (BD) and TGF-β (2G7) intracellular staining of CD4+ T cells, single-cell suspensions were incubated with PMA, ionomycin, and brefeldin A (all from Sigma-Aldrich) for 5 h at 37°C (Kared et al., 2005). TGF-β (2G7) intracellular staining of pDCs was performed after a 5-h incubation in the presence of brefeldin A without exogenous stimulation. Validation for this assay was performed using an isotype control and staining after blocking the 2G7 mAb with recombinant human TGF-β (R&D Systems). T reg cells were detected using the anti–mouse/rat Foxp3 staining set (FJK-16s; eBioscience). T reg cell proliferation was assessed using an anti–human Ki67 staining kit (BD) containing anti-Ki67 (B56). Foxp3 staining was performed before anti-Ki67 staining. For NRP-V7 tetramer (from the National Institutes of Health tetramer core facility) staining, cells were incubated for 30 min at 37°C in Lck inhibitor buffer (50 nM PBS–2% FBS; EMD) and then stained with tetramers for 45 min at room temperature in Lck inhibitor buffer followed by surface staining with anti-CD8 mAb for 15 min at 4°C. Stained cells were analyzed on a FACS Aria flow cytometer equipped with 488-, 605-, and 405-nm lasers (BD).
Preparation of pancreatic islets.
Mice were sacrificed, and pancreata were perfused with 3 ml of a solution containing 1.5 mg/ml collagenase P (Roche) and then dissected free from surrounding tissues. Pancreata were then digested at 37°C for 10 min. Digestion was stopped by adding PBS–5% FCS followed by extensive washes. Islets were then purified on a Ficoll gradient, picked with a glass pipette, and disrupted by adding 1 ml of cell dissociation buffer (Invitrogen) for 10 min at 37°C. After another wash, cells were resuspended, counted, and used. To measure anti-IGRP206–214 and anti-NP396–404 CD8+ T cell responses in Ins2−/− mice, pancreatic islets were recovered by handpicking under a polarized microscope (M3B; Wild Heerbrugg) without any prior density separation. Islets were then cultured for 8 d in RPMI medium containing 10% FBS, 1% penicillin/streptomycin, and 25 U/ml recombinant human IL-2 (R&D Systems). For IFN-γ intracellular staining, single-cell suspensions were stimulated for 5 h on resting splenocytes previously pulsed with 1 µg/ml peptide in the presence of 1 µg/ml brefeldin A.
Adoptive transfer experiment.
CD62L+ CD4+ GFP− BDC2.5 T cells were isolated from CD45.2+/+ Foxp3-GFP+/+ BDC2.5 Cα−/− NOD females. Splenocytes were enriched in T cells by negative selection (Dynal beads; Invitrogen) and then FACS sorted as CD62L+ CD4+ GFP− cells. These cells were injected i.v. (106 cells/mouse) into Ins-NP mice females LCMV infected 4 d earlier. The same procedure was used for isolation of CD62L+ CD4+ BDC2.5 T cells from CD45.2+/+ BDC2.5 Cα−/− NOD females.
In vitro cultures.
105 CD62L+ CD4+ GFP− BDC2.5 T cells sorted from BDC2.5 CD45.2+/+ Cα−/− NOD females were co-cultured with 104 pDCs sorted from spleen or pancreatic LNs of infected Vα14 Ins-NP mice (4 d earlier). Cells were cultured for 3 d in complete medium with 25 U/ml recombinant human IL-2 and 10 ng/ml peptide 1040-51, a mimotope of BDC2.5 T cells (Judkowski et al., 2001), with or without 100 µg/ml anti–TGF-β mAb and then stained with anti–TCR-β, anti-CD4, and anti-CD45.2 mAbs, and GFP expression was analyzed.
105 sorted cells (iNKT cells and pDCs) from pancreatic LNs or spleen of 4-d-infected Ins-NP Vα14 female mice were cultured in X-Vivo 15 serum-free medium (BioWhittaker and Cambrex) and 1% penicillin/streptomycin. In some wells, anti–PD-L1 (MIH5) or anti–IL-10R (1B1) mAbs or control isotype (rat anti–mouse IgG) was added to the culture at 10 µg/ml. Culture supernatants were collected after 90 h for ELISA analysis. Samples were acidified by addition of HCl 1N for 15 min and neutralized by NaOH 1N, and then the amount of TGF-β1 was measured by the TGF-β1 Emax ImmunoAssay System (Promega).
T reg cell suppression assay.
Splenic CD45.2+ CD4+ CD25− cells labeled with CFSE were used as T resp cells. 104 T resp cells were cultured for 72 h with anti-CD3/anti-CD28 Dynabeads (Invitrogen; according to the manufacturer’s protocol) in the presence or absence of 5 × 103 T reg cells sorted from the pancreatic islets of infected Foxp3-GFP–Ins-NP control or Vα14 mice. The division of T resp cells was assessed by flow cytometry by dilution of CFSE.
Statistical analysis.
Diabetes incidence was plotted according to the Kaplan-Meier method. Incidences between groups were compared with the log-rank test. For other experiments, comparison between means was performed using the nonparametric Mann-Whitney U test. Reported values are means ± SD as indicated. P-values <0.05 were considered statistically significant. All data were analyzed using Prism version 5 software (GraphPad Software).
Online supplemental material.
Fig. S1 shows that iNKT cells alter the anti-LCMV CD8+ response in pancreatic islets of Vα14 or α-GalCer–treated Ins-NP mice. Fig. S2 shows the frequency and phenotype of T reg cells in the spleen and pancreatic LNs and islets of Ins-NP mice after LCMV infection. Fig. S3 shows the kinetics of pDC frequency in the control Ins-NP mice during infection, the efficacy of the pDC depletion by 120G8 mAb treatment, and the validation of intracellular TGF-β staining. Fig. S4 shows T reg frequency and diabetes development in ICOS−/− Ins-NP NOD mice or in Ins-NP mice after 1-MT treatment. Fig. S5 shows the strategy of sorting naive BDC T cells devoid in Foxp3+ T cells and their transfer into Ins-NP mice and the frequencies of proliferating Ki67+ T reg cells determined in the Foxp3+ CD4+ T cell population. Fig. S6 shows the analysis of iNKT cell phenotype and frequency in pancreatic LNs and islets of LCMV-infected Vα14 Ins-NP mice. Fig. S7 shows the analysis of iNKT cell phenotype and frequency in pancreatic LNs and islets of LCMV-infected Vα14 Ins-NP or CD1dpLck Vα14 Ins-NP mice after pDC depletion. Fig. S8 shows the analysis of T reg cell frequency in Ins2−/− mice 20 wk after LCMV infection and α-GalCer treatment. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101692/DC1.
Acknowledgments
We thank D. Mathis and C. Benoist for the gift of Foxp3-GFP+/+ NOD mice, J.-P. Briand for NP396–404 viral peptide, the Kirin Brewery for α-GalCer, the Schering-Plough Research Institute for 120G8 mAb, and the National Institutes of Health tetramer core facility for tetramer NRP-V7. We thank J. Gayet and F. Boutillon for technical assistance, L. Breton and the staff of the mouse facility for help in animal care, G. Naselli for English editing, and R. Monteiro and R. Schwartz for critically reading the manuscript and for suggestions.
This work was supported by funds from the Institut National de la Santé et de la Recherche Médicale and Centre National de la Recherche Scientifique (ANR-05-PCOD009-01, ANR-09-GENO-023, and EFSD/JDRF/NN-2008 to A. Lehuen) and by the Juvenile Diabetes Research Foundation (grant #1-2008-106 to R. Mallone).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- 1-MT
- 1-methyl-d-tryptophan
- α-GalCer
- α-galactosylceramide
- cDC
- conventional DC
- ICOS
- inducible co-stimulatory
- IDO
- indoleamine 2,3-dioxygenase
- iNKT cell
- invariant NKT cell
- LCMV
- lymphochoriomeningitis virus
- MFI
- mean fluorescence intensity
- NOD
- nonobese diabetic
- pDC
- plasmacytoid DC
- T1D
- type 1 diabetes
References
- Alyanakian M.A., Grela F., Aumeunier A., Chiavaroli C., Gouarin C., Bardel E., Normier G., Chatenoud L., Thieblemont N., Bach J.F. 2006. Transforming growth factor-beta and natural killer T-cells are involved in the protective effect of a bacterial extract on type 1 diabetes. Diabetes. 55:179–185 10.2337/diabetes.55.01.06.db05-0189 [DOI] [PubMed] [Google Scholar]
- Anderson M.S., Bluestone J.A. 2005. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 23:447–485 10.1146/annurev.immunol.23.021704.115643 [DOI] [PubMed] [Google Scholar]
- Asselin-Paturel C., Brizard G., Pin J.J., Brière F., Trinchieri G. 2003. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J. Immunol. 171:6466–6477 [DOI] [PubMed] [Google Scholar]
- Barber D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H., Freeman G.J., Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- Beaudoin L., Laloux V., Novak J., Lucas B., Lehuen A. 2002. NKT cells inhibit the onset of diabetes by impairing the development of pathogenic T cells specific for pancreatic beta cells. Immunity. 17:725–736 10.1016/S1074-7613(02)00473-9 [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Oldenhove G. 2008. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 29:362–371 10.1016/j.immuni.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y., Tarbell K. 2009. Regulatory T cells in the control of host-microorganism interactions. Annu. Rev. Immunol. 27:551–589 10.1146/annurev.immunol.021908.132723 [DOI] [PubMed] [Google Scholar]
- Bendelac A., Savage P.B., Teyton L. 2007. The biology of NKT cells. Annu. Rev. Immunol. 25:297–336 10.1146/annurev.immunol.25.022106.141711 [DOI] [PubMed] [Google Scholar]
- Brooks D.G., Ha S.J., Elsaesser H., Sharpe A.H., Freeman G.J., Oldstone M.B. 2008. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc. Natl. Acad. Sci. USA. 105:20428–20433 10.1073/pnas.0811139106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.G., Choisy-Rossi C.M., Holl T.M., Chapman H.D., Besra G.S., Porcelli S.A., Shaffer D.J., Roopenian D., Wilson S.B., Serreze D.V. 2005a. Activated NKT cells inhibit autoimmune diabetes through tolerogenic recruitment of dendritic cells to pancreatic lymph nodes. J. Immunol. 174:1196–1204 [DOI] [PubMed] [Google Scholar]
- Chen Z., Herman A.E., Matos M., Mathis D., Benoist C. 2005b. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J. Exp. Med. 202:1387–1397 10.1084/jem.20051409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana J., Lehuen A. 2009. NKT cells: friend or foe during viral infections? Eur. J. Immunol. 39:3283–3291 10.1002/eji.200939800 [DOI] [PubMed] [Google Scholar]
- Diana J., Griseri T., Lagaye S., Beaudoin L., Autrusseau E., Gautron A.S., Tomkiewicz C., Herbelin A., Barouki R., von Herrath M., et al. 2009. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity. 30:289–299 10.1016/j.immuni.2008.12.017 [DOI] [PubMed] [Google Scholar]
- Dotta F., Censini S., van Halteren A.G., Marselli L., Masini M., Dionisi S., Mosca F., Boggi U., Muda A.O., Prato S.D., et al. 2007. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl. Acad. Sci. USA. 104:5115–5120 10.1073/pnas.0700442104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F., Grohmann U., Hwang K.W., Orabona C., Vacca C., Bianchi R., Belladonna M.L., Fioretti M.C., Alegre M.L., Puccetti P. 2003. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 4:1206–1212 10.1038/ni1003 [DOI] [PubMed] [Google Scholar]
- Feuerer M., Shen Y., Littman D.R., Benoist C., Mathis D. 2009. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 31:654–664 10.1016/j.immuni.2009.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi C.M., von Herrath M.G. 2008. Viral trigger for type 1 diabetes: pros and cons. Diabetes. 57:2863–2871 10.2337/db07-1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi C.M., Estes E.A., Oldham J.E., von Herrath M.G. 2009. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J. Clin. Invest. 119:1515–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341 10.1016/j.immuni.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K., Sharpe A.H. 2009. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206:3015–3029 10.1084/jem.20090847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E.A., Gorelik L., McGregor C.M., Tran E.H., Flavell R.A. 2003. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc. Natl. Acad. Sci. USA. 100:10878–10883 10.1073/pnas.1834400100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadeiba H., Sato T., Habtezion A., Oderup C., Pan J., Butcher E.C. 2008. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat. Immunol. 9:1253–1260 10.1038/ni.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A.E., Freeman G.J., Mathis D., Benoist C. 2004. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J. Exp. Med. 199:1479–1489 10.1084/jem.20040179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M.S., Bradley L.M., Harbertson J., Krahl T., Lee J., Sarvetnick N. 1998. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat. Med. 4:781–785 10.1038/nm0798-781 [DOI] [PubMed] [Google Scholar]
- Irla M., Küpfer N., Suter T., Lissilaa R., Benkhoucha M., Skupsky J., Lalive P.H., Fontana A., Reith W., Hugues S. 2010. MHC class II–restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell–mediated autoimmunity. J. Exp. Med. 207:1891–1905 10.1084/jem.20092627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Yang M., Wang Y.H., Lande R., Gregorio J., Perng O.A., Qin X.F., Liu Y.J., Gilliet M. 2007. Plasmacytoid dendritic cells prime IL-10–producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 204:105–115 10.1084/jem.20061660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judkowski V., Pinilla C., Schroder K., Tucker L., Sarvetnick N., Wilson D.B. 2001. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J. Immunol. 166:908–917 [DOI] [PubMed] [Google Scholar]
- Kang H.K., Liu M., Datta S.K. 2007. Low-dose peptide tolerance therapy of lupus generates plasmacytoid dendritic cells that cause expansion of autoantigen-specific regulatory T cells and contraction of inflammatory Th17 cells. J. Immunol. 178:7849–7858 [DOI] [PubMed] [Google Scholar]
- Kared H., Masson A., Adle-Biassette H., Bach J.F., Chatenoud L., Zavala F. 2005. Treatment with granulocyte colony-stimulating factor prevents diabetes in NOD mice by recruiting plasmacytoid dendritic cells and functional CD4(+)CD25(+) regulatory T-cells. Diabetes. 54:78–84 10.2337/diabetes.54.1.78 [DOI] [PubMed] [Google Scholar]
- Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26:677–704 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S.C., Chen Y., Clemmings S.M., Viglietta V., Kenyon N.S., Ricordi C., Hering B., Hafler D.A. 2005. Loss of IL-4 secretion from human type 1a diabetic pancreatic draining lymph node NKT cells. J. Immunol. 175:4458–4464 [DOI] [PubMed] [Google Scholar]
- Knip M., Veijola R., Virtanen S.M., Hyöty H., Vaarala O., Akerblom H.K. 2005. Environmental triggers and determinants of type 1 diabetes. Diabetes. 54:S125–S136 10.2337/diabetes.54.suppl_2.S125 [DOI] [PubMed] [Google Scholar]
- Kohm A.P., McMahon J.S., Podojil J.R., Begolka W.S., DeGutes M., Kasprowicz D.J., Ziegler S.F., Miller S.D. 2006. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J. Immunol. 176:3301–3305 [DOI] [PubMed] [Google Scholar]
- Kretschmer K., Apostolou I., Hawiger D., Khazaie K., Nussenzweig M.C., von Boehmer H. 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6:1219–1227 10.1038/ni1265 [DOI] [PubMed] [Google Scholar]
- Kuipers H., Muskens F., Willart M., Hijdra D., van Assema F.B., Coyle A.J., Hoogsteden H.C., Lambrecht B.N. 2006. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. Eur. J. Immunol. 36:2472–2482 10.1002/eji.200635978 [DOI] [PubMed] [Google Scholar]
- Laloux V., Beaudoin L., Jeske D., Carnaud C., Lehuen A. 2001. NK T cell-induced protection against diabetes in V alpha 14-J alpha 281 transgenic nonobese diabetic mice is associated with a Th2 shift circumscribed regionally to the islets and functionally to islet autoantigen. J. Immunol. 166:3749–3756 [DOI] [PubMed] [Google Scholar]
- Lee P.T., Putnam A., Benlagha K., Teyton L., Gottlieb P.A., Bendelac A. 2002. Testing the NKT cell hypothesis of human IDDM pathogenesis. J. Clin. Invest. 110:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehuen A., Lantz O., Beaudoin L., Laloux V., Carnaud C., Bendelac A., Bach J.F., Monteiro R.C. 1998. Overexpression of natural killer T cells protects Vα14- Jα281 transgenic nonobese diabetic mice against diabetes. J. Exp. Med. 188:1831–1839 10.1084/jem.188.10.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehuen A., Diana J., Zaccone P., Cooke A. 2010. Immune cell crosstalk in type 1 diabetes. Nat. Rev. Immunol. 10:501–513 10.1038/nri2787 [DOI] [PubMed] [Google Scholar]
- Li M.O., Flavell R.A. 2008. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 28:468–476 10.1016/j.immuni.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Martín-Gayo E., Sierra-Filardi E., Corbí A.L., Toribio M.L. 2010. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood. 115:5366–5375 10.1182/blood-2009-10-248260 [DOI] [PubMed] [Google Scholar]
- Martinic M.M., Juedes A.E., Bresson D., Homann D., Skak K., Huber C., Ling E., Ejrnaes M., Wolfe T., Togher L., et al. 2007. Minimal impact of a de novo-expressed beta-cell autoantigen on spontaneous diabetes development in NOD mice. Diabetes. 56:1059–1068 10.2337/db05-0062 [DOI] [PubMed] [Google Scholar]
- Mathis D., Vence L., Benoist C. 2001. beta-Cell death during progression to diabetes. Nature. 414:792–798 10.1038/414792a [DOI] [PubMed] [Google Scholar]
- Mendiratta S.K., Martin W.D., Hong S., Boesteanu A., Joyce S., Van Kaer L. 1997. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 6:469–477 10.1016/S1074-7613(00)80290-3 [DOI] [PubMed] [Google Scholar]
- Monteiro M., Almeida C.F., Caridade M., Ribot J.C., Duarte J., Agua-Doce A., Wollenberg I., Silva-Santos B., Graca L. 2010. Identification of regulatory Foxp3+ invariant NKT cells induced by TGF-beta. J. Immunol. 185:2157–2163 10.4049/jimmunol.1000359 [DOI] [PubMed] [Google Scholar]
- Naumov Y.N., Bahjat K.S., Gausling R., Abraham R., Exley M.A., Koezuka Y., Balk S.B., Strominger J.L., Clare-Salzer M., Wilson S.B. 2001. Activation of CD1d-restricted T cells protects NOD mice from developing diabetes by regulating dendritic cell subsets. Proc. Natl. Acad. Sci. USA. 98:13838–13843 10.1073/pnas.251531798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejentsev S., Walker N., Riches D., Egholm M., Todd J.A. 2009. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 324:387–389 10.1126/science.1167728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio J., Feuerer M., Wong J., Mathis D., Benoist C. 2010. Anti-CD3 therapy permits regulatory T cells to surmount T cell receptor–specified peripheral niche constraints. J. Exp. Med. 207:1879–1889 10.1084/jem.20100205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J., Beaudoin L., Park S., Griseri T., Teyton L., Bendelac A., Lehuen A. 2007a. Prevention of type 1 diabetes by invariant NKT cells is independent of peripheral CD1d expression. J. Immunol. 178:1332–1340 [DOI] [PubMed] [Google Scholar]
- Novak J., Griseri T., Beaudoin L., Lehuen A. 2007b. Regulation of type 1 diabetes by NKT cells. Int. Rev. Immunol. 26:49–72 10.1080/08830180601070229 [DOI] [PubMed] [Google Scholar]
- Ochando J.C., Homma C., Yang Y., Hidalgo A., Garin A., Tacke F., Angeli V., Li Y., Boros P., Ding Y., et al. 2006. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 7:652–662 10.1038/ni1333 [DOI] [PubMed] [Google Scholar]
- Oldstone M.B. 1988. Prevention of type I diabetes in nonobese diabetic mice by virus infection. Science. 239:500–502 10.1126/science.3277269 [DOI] [PubMed] [Google Scholar]
- Peng Y., Laouar Y., Li M.O., Green E.A., Flavell R.A. 2004. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc. Natl. Acad. Sci. USA. 101:4572–4577 10.1073/pnas.0400810101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roep B.O., Hiemstra H.S., Schloot N.C., De Vries R.R., Chaudhuri A., Behan P.O., Drijfhout J.W. 2002. Molecular mimicry in type 1 diabetes: immune cross-reactivity between islet autoantigen and human cytomegalovirus but not Coxsackie virus. Ann. N. Y. Acad. Sci. 958:163–165 10.1111/j.1749-6632.2002.tb02961.x [DOI] [PubMed] [Google Scholar]
- Sharif S., Arreaza G.A., Zucker P., Mi Q.S., Sondhi J., Naidenko O.V., Kronenberg M., Koezuka Y., Delovitch T.L., Gombert J.M., et al. 2001. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat. Med. 7:1057–1062 10.1038/nm0901-1057 [DOI] [PubMed] [Google Scholar]
- Sharma M.D., Baban B., Chandler P., Hou D.Y., Singh N., Yagita H., Azuma M., Blazar B.R., Mellor A.L., Munn D.H. 2007. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Invest. 117:2570–2582 10.1172/JCI31911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Bluestone J.A. 2008. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 9:239–244 10.1038/ni1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Adams J.Y., Penaranda C., Melli K., Piaggio E., Sgouroudis E., Piccirillo C.A., Salomon B.L., Bluestone J.A. 2008. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 28:687–697 10.1016/j.immuni.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thébault-Baumont K., Dubois-Laforgue D., Krief P., Briand J.P., Halbout P., Vallon-Geoffroy K., Morin J., Laloux V., Lehuen A., Carel J.C., et al. 2003. Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J. Clin. Invest. 111:851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S., Drescher K.M., Chapman N.M., Kim K.S., Carson S.D., Pirruccello S., Lane P.H., Romero J.R., Leser J.S. 2002. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J. Virol. 76:12097–12111 10.1128/JVI.76.23.12097-12111.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S., Drescher K.M., Jackson J.D., Kim K., Kono K. 2010. Enteroviruses, type 1 diabetes and hygiene: a complex relationship. Rev. Med. Virol. 20:106–116 10.1002/rmv.639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupin E., Kinjo Y., Kronenberg M. 2007. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 5:405–417 10.1038/nrmicro1657 [DOI] [PubMed] [Google Scholar]
- Van Kaer L. 2005. alpha-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat. Rev. Immunol. 5:31–42 10.1038/nri1531 [DOI] [PubMed] [Google Scholar]
- Viskari H., Ludvigsson J., Uibo R., Salur L., Marciulionyte D., Hermann R., Soltesz G., Füchtenbusch M., Ziegler A.G., Kondrashova A., et al. 2005. Relationship between the incidence of type 1 diabetes and maternal enterovirus antibodies: time trends and geographical variation. Diabetologia. 48:1280–1287 10.1007/s00125-005-1780-9 [DOI] [PubMed] [Google Scholar]
- von Herrath M.G., Fujinami R.S., Whitton J.L. 2003. Microorganisms and autoimmunity: making the barren field fertile? Nat. Rev. Microbiol. 1:151–157 10.1038/nrmicro754 [DOI] [PubMed] [Google Scholar]
- Wilson S.B., Kent S.C., Patton K.T., Orban T., Jackson R.A., Exley M., Porcelli S., Schatz D.A., Atkinson M.A., Balk S.P., et al. 1998. Extreme Th1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature. 391:177–181 10.1038/34419 [DOI] [PubMed] [Google Scholar]
- You S., Thieblemont N., Alyanakian M.A., Bach J.F., Chatenoud L. 2006. Transforming growth factor-beta and T-cell-mediated immunoregulation in the control of autoimmune diabetes. Immunol. Rev. 212:185–202 10.1111/j.0105-2896.2006.00410.x [DOI] [PubMed] [Google Scholar]
- Zaccone P., Burton O.T., Cooke A. 2008. Interplay of parasite-driven immune responses and autoimmunity. Trends Parasitol. 24:35–42 10.1016/j.pt.2007.10.006 [DOI] [PubMed] [Google Scholar]